Abstract
We have investigated the regulation of hormone secretion from rat pancreatic islets by the GABAB receptors (GABABRs). Inclusion of the specific GABABR antagonist CGP 55845 in the extracellular medium increased glucose-stimulated insulin secretion 1.6-fold but did not affect the release of glucagon and somatostatin. Conversely, addition of the GABABR agonist baclofen inhibited glucose-stimulated insulin secretion by ∼60%. Using RT-PCR, transcription of GABABR1a-c,f and GABABR2 subunits was detected in β-cells. Measurements of membrane currents and cell capacitance were applied to single β-cells to investigate the mechanisms by which GABABR activation inhibits insulin secretion. In perforated-patch measurements, baclofen inhibited exocytosis elicited by 500-ms voltage-clamp depolarizations to 0 mV by ≤ 80% and voltage-gated Ca2+ entry by only ∼30%. Both effects were concentration-dependent with IC50 values of ∼2 μm. The inhibitory action of baclofen was abolished in the presence of CGP 55845. The ability of baclofen to suppress exocytosis was prevented by pre-treatment with pertussis toxin and by inclusion of GDPβS in the intracellular medium, and became irreversible in the presence of GTPγS as expected for a process involving inhibitory G-proteins (Gi/o-proteins). The inhibitory effect of baclofen resulted from activation of the serine/threonine protein phosphatase calcineurin and pre-treatment with cyclosporin A or intracellular application of calcineurin autoinhibitory peptide abolished the effect. Addition of baclofen had no effect on [Ca2+]i and electrical activity in glucose-stimulated β-cells. These data indicate that GABA released from β-cells functions as an autocrine inhibitor of insulin secretion in pancreatic islets and that the effect is principally due to direct suppression of exocytosis.
Pancreatic islets contain high levels of the inhibitory neurotransmitter γ-aminobutyric acid (GABA). GABA is the most important inhibitory neurotransmitter in the mammalian central nervous system (CNS). Three types of GABA-receptors exist. In addition to the iontropic GABAA- and GABAC-receptors, which are ligand-gated Cl− channels, there are also metabotropic G-protein-coupled GABAB-receptors (GABABRs; Chebib & Johnston, 1999). In neurones, GABA reduces electrical excitability via both GABAA- and GABAB-signalling and by a combination of pre- and postsynaptic mechanisms. Several cellular processes are influenced by GABABRs. Examples include inhibition of the voltage-gated Ca2+ channels, modulation of the voltage dependence of a transient A-type K+ current and activation of inwardly rectifying K+ channels (for review see Kerr & Ong, 1995). In addition, it has recently been reported that baclofen reduces synaptic transmission by inhibition of vesicle priming (Sakaba & Neher, 2003).
By contrast, the physiological roles of GABA in the pancreatic islets are not fully understood. GABA is stored in the β-cell in synaptic-like microvesicles (SLMVs), which accumulate GABA by active transport (Thomas-Reetz et al. 1993). We have recently demonstrated that GABA is released by regulated Ca2+-dependent exocytosis of the SLMVs (Braun et al. 2004), and that GABA thus released regulates glucagon secretion from neighbouring α-cells by activation of GABAA-receptors (Wendt et al. 2004). However, there is also evidence suggesting that GABABRs are expressed in pancreatic islet cells (Brice et al. 2002) and application of the GABABR agonist baclofen has been reported to inhibit glucose-induced insulin secretion from rat pancreatic islet (Gu et al. 1993) and clonal MIN6-cells (Brice et al. 2002). Here we have studied the expression and function of GABABRs in rat pancreatic islets using a combination of hormone release measurements, RT-PCR analysis of gene transcription, recordings of the cytoplasmic Ca2+ concentration ([Ca2+]i) and single-cell measurements of exocytosis and voltage-gated Ca2+ currents. We demonstrate that: (1) activation of GABABRs selectively modulates insulin secretion from the pancreatic islets and that release of glucagon and somatostatin is not affected; (2) the effect of GABA on insulin secretion involves inhibition of exocytosis at a late stage and is independent of any changes in the intracellular cAMP levels, Ca2+ influx or [Ca2+]i; and (3) the inhibitory action involves G protein-dependent activation of the protein phosphatase calcineurin.
Methods
Preparation of rat α- and β-cells
Pancreatic islets and single α- and β- cells were prepared from Sprague-Dawley or Wistar rats as previously described for mice (Olofsson et al. 2002). The rats were anaesthetized with sodium pentobarbitone (100 mg kg−1 i.p.) and killed by cervical dislocation or by inhalation of CO2 followed by cervical dislocation. All experimental procedures involving animals were approved by the ethical committees in Lund, the City of Hamburg and the University of Copenhagen. After excision of the pancreas, islets were isolated by collagenase digestion and dispersed into single cells essentially as detailed elsewhere (Ämmälä et al. 1993). The cells were plated on 35 mm diameter Petri dishes and incubated in a humidified atmosphere for up to 5 days in RPMI 1640 tissue culture medium (Gibco BRL, Life Technologies Ltd, Paisley, UK) supplemented with 10% (v/v) heat-inactivated fetal calf serum, 100 i.u. ml−1 penicillin and 100 μg ml−1 streptomycin.
One series of experiments (see Fig. 7) was performed on α-cells obtained by fluorescence-activated cell sorting (FACS; Josefsen et al. 1996). Based on the hormone contents and their glucose sensitivities, we estimate that the preparations contain > 80% α-cells and < 3% β-cells (Josefsen et al. 1996; Gromada et al. 1997). The α-cells could be functionally distinguished from the β-cells by the presence of a prominent rapidly inactivating Na+ current and small cell size (cell capacitance < 2.5 pF).
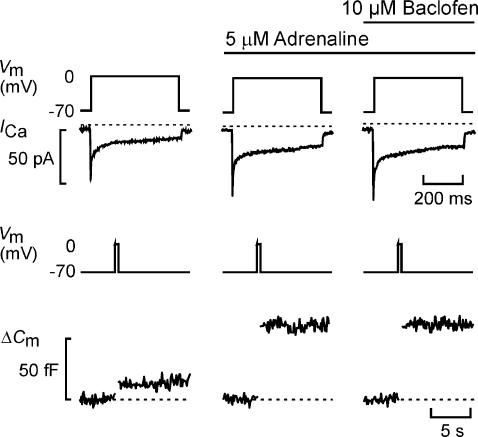
Figure 7. Baclofen does not affect adrenaline-stimulated exocytosis in glucagon-secreting α-cells.
Whole-cell Ca2+ current (ICa) and exocytosis (ΔCm) evoked by membrane depolarizations (Vm; 500 ms) from −70 to 0 mV using the perforated-patch whole-cell configuration in single rat α-cells. Exocytosis was observed under control conditions, 2 min after addition of adrenaline (5 μm) and in the simultaneous presence of both adrenaline and baclofen (10 μm, applied for 2 min). The dotted lines indicate the zero current level and the pre-stimulatory capacitance level.
Electrophysiology
Patch pipettes were pulled from borosilicate glass (tip resistance 3–4 MΩ when filled with the pipette solution), coated with Sylgard and fire-polished before use. The zero-current potential was adjusted before establishment of the seal with the pipette in the bath. The holding potential in all experiments was −70 mV.
Exocytosis was monitored in single β-cells as changes in cell membrane capacitance using either the standard or the perforated-patch whole-cell configuration. The patch-clamp recordings were made using EPC-7 (List Elektronik, Darmstadt, Germany; Figs 3 and 5–7) or EPC-9 (Heka Electronics, Lamprecht/Pfalz, Germany; Figs 4 and 8B) patch-clamp amplifiers. Exocytosis was elicited by 500-ms voltage-clamp depolarizations from −70 to 0 mV. Changes in cell capacitance were detected using in-house software written in Axobasic (Axon Instruments, Union City, CA, USA; Ämmälä et al. 1993) or the software Pulse (Heka, version 8.41). During the electrophysiological experiments, the cells were placed in an experimental chamber with a volume of 0.4 ml, which was continuously superfused at a rate of 1.5 ml min−1 to maintain the temperature at 33°C. Experiments commenced when two successive depolarizations or trains of pulses applied at a 1–2 min interval elicited exocytotic responses of the same amplitude (± 10%) to ascertain that the observed changes were not simply attributable to spontaneous long-term changes of the secretory capacity. Membrane potential recordings (Fig. 8B) were performed in β-cells in intact rat islets as previously described for mouse islets (Göpel et al. 1999).
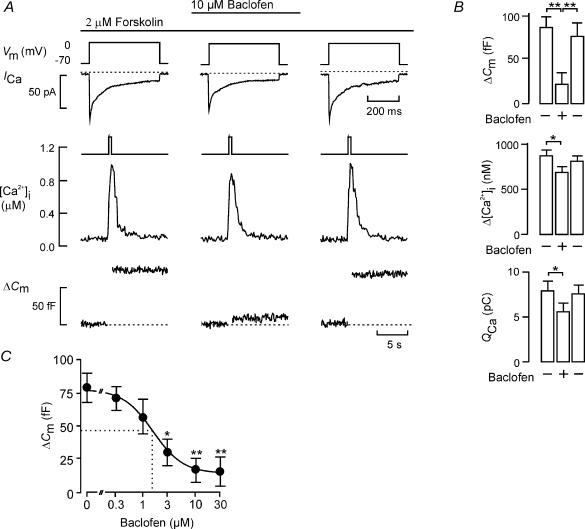
Figure 3. Baclofen reduces Ca2+ currents, [Ca2+]i and exocytosis.
A, whole-cell Ca2+ current (ICa), cytoplasmic Ca2+ concentration ([Ca2+]i) and exocytosis (ΔCm) evoked by membrane depolarizations (Vm; 500 ms) from −70 to 0 mV using the perforated-patch whole-cell configuration in single rat β-cells. Exocytosis was observed under control conditions, 2 min after addition of baclofen (10 μm) and 4 min after wash-out of the agonist. The dotted lines indicate the zero current level and the pre-stimulatory capacitance level. B, histograms summarizing effects on changes of cell capacitance (ΔCm), cytoplasmic Ca2+ levels (Δ[Ca2+]i) and integrated Ca2+ current (QCa). Data are mean values ± s.e.m. of six cells. *P < 0.05; **P < 0.01. C, dose–inhibition curve for the effect of baclofen on exocytosis. Data are the mean ± s.e.m. (n = 5–7) of the responses to 500-ms depolarizations from −70 mV to 0 mV at concentrations of baclofen between 0 and 30 μm; *P < 0.01 and **P < 0.001 versus control (no baclofen). The curve was obtained by fitting the mean data points to the Hill equation. The dotted lines indicate the concentration of baclofen at which exocytosis is reduced by 38% (i.e. exocytosis is 1/1.6 of that observed in the absence of baclofen; cf. Fig. 1).
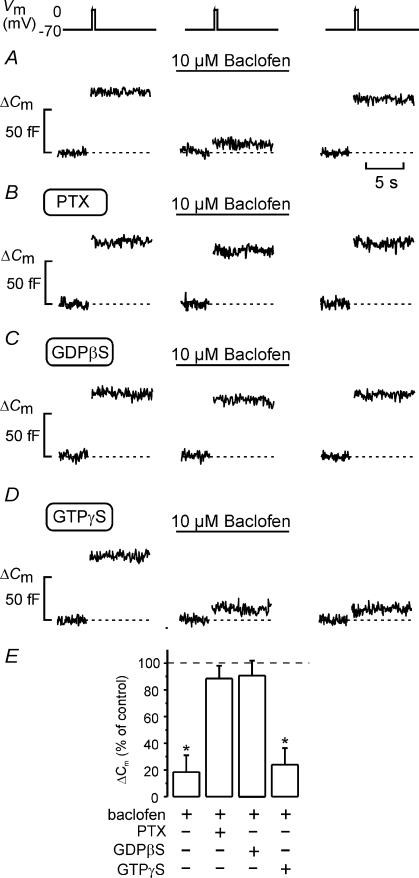
Figure 5. Baclofen-induced inhibition of exocytosis is mediated by activation of a Gi/o protein.
A–E, effects of 10 μm baclofen on exocytosis (ΔCm) elicited by 500 ms voltage-clamp depolarizations of the membrane potential (Vm) from −70 to 0 mV applying the standard whole-cell configuration to rat β-cells. Changes in cell capacitance were measured before and 2 min after the addition of baclofen, and 4 min after wash-out of the agonist under control conditions (A), in cells treated for > 20 h with 100 ng ml−1 pertussis toxin (PTX; B), with 0.5 mm GDPβS included in the pipette solution (C) and in the presence of 0.1 mm intracellular GTPγS (D). Cyclic AMP (0.1 mm) was included in all pipette solutions. E, histogram showing changes in cell capacitance (ΔCm) normalized to the respective controls (before addition of baclofen). Data are the mean ± s.e.m. (n = 5–6 cells; *P < 0.01).
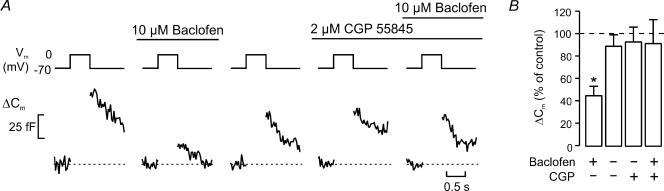
Figure 4. Baclofen-induced inhibition of exocytosis can be antagonized by CGP 55845.
A, exocytosis (ΔCm) elicited by 500-ms depolarizations of membrane potential (Vm) from −70 mV to 0 mV under control conditions, in the presence of 10 μm baclofen, following the wash-out of baclofen, in the presence of 2 μm CGP 55845 and in the simultaneous presence of 2 μm CGP 55845 and 10 μm baclofen (left to right). Pulses were applied with a 2-min interval. B, exocytotic responses normalized to control (before addition of baclofen) as the mean ± s.e.m (n = 7; *P < 0.01 versus control).
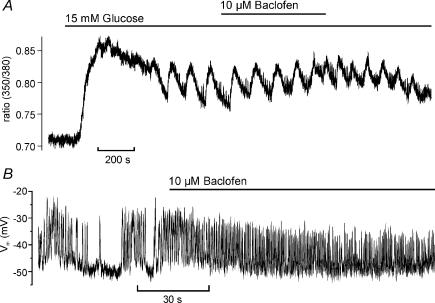
Figure 8. Baclofen does not affect glucose-induced intracellular Ca2+ signalling and electrical activity of β-cells.
A, [Ca2+]i was measured in an intact islet by dual-wavelength microfluorimetry using fura-2 AM as the Ca2+ indicator. [Ca2+]i responses are expressed as fura-2 fluorescence (F) ratios (F350/F380). The islet was superfused with a solution containing 15 mm glucose to generate the characteristic biphasic [Ca2+]i response. At the time indicated, 10 μm baclofen was included in the bath solution. The trace is representative of nine experiments. B, membrane potential recording from a β-cell in an intact rat islet obtained using the perforated-patch whole-cell configuration. Electrical activity was induced by addition of 20 mm glucose to the bath solution. Baclofen (10 μm) was applied as indicated by the horizontal line.
Solutions for electrophysiology
The pipette solution for standard whole-cell experiments (Figs 5 and 6) contained (mm): caesium glutamate 125, CsCl 10, NaCl 10, MgCl2 1, Hepes 5, EGTA 0.05, MgATP 3, cAMP 0.1 and GTP 0.01; pH was adjusted to 7.15 with CsOH. In perforated-patch whole-cell experiments, the pipette solution contained (mm): NaCl 10, KCl 10, MgCl2 1, Cs2SO4 76 (Figs 3–4 and 7) or 76 K2SO4 (Fig. 8B) and Hepes 5; pH was adjusted to 7.35 with CsOH or KOH. Electrical contact with the cell interior was established by adding 0.24 mg ml−1 amphotericin B to the pipette solution. Perforation required a few minutes and the voltage clamp was considered satisfactory when the series conductance (Gseries) was constant and exceeded 35–40 nS. The extracellular medium usually contained (mm): NaCl 118, tetraethylammonium (TEA) chloride 20, KCl 5.6, MgCl2 1.2, CaCl2 2.6, d-glucose 5 (Figs 3–7) and Hepes 5; pH was adjusted to 7.40 using NaOH. TEA was included to block outward rectifying K+ currents, which persist even after replacement of intracellular K+ by Cs+. For measuring glucose-induced electrical acitivity (Fig. 8B), TEA was replaced by an equal concentration of NaCl, and the glucose concentration was increased to 20 mm. Forskolin (2 μm) was included in the extracellular solution in all experiments involving measurements of exocytosis in intact β-cells (perforated-patch recordings) to increase the exocytotic capacity. Calcineurin autoinhibitory peptide was supplied by Calbiochem (La Jolla, CA, USA) and CGP 55845 was purchased from Tocris (Bristol, UK). All other chemicals were purchased from Sigma.
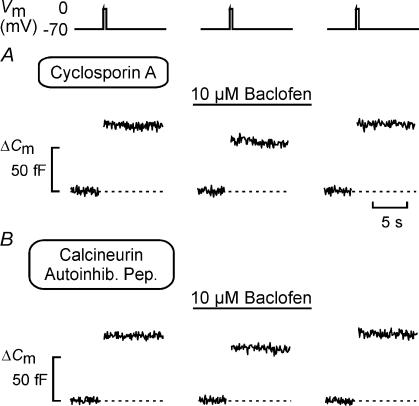
Figure 6. Baclofen-induced inhibition of exocytosis involves activation of the protein phosphatase calcineurin.
A and B, exocytosis measured as increases in cell capacitance (ΔCm), elicited by 500 ms voltage-clamp depolarizations of the membrane potential (Vm) from −70 to 0 mV using the standard whole-cell configuration before and 2 min after the addition of baclofen (10 μm), and 4 min after wash-out of baclofen from the medium in cells pre-treated with cyclosporin A (A; 1 μm for > 20 min) or when 0.1 mm of the calcineurin autoinhibitory peptide was included in the intracellular solution (B).
Measurements of [Ca2+]i
The [Ca2+]i measurements in single cells (Fig. 3) were made using an Axiovert 135 inverted microscope equipped with a Plan-Neofluar × 100, 1.30 NA objective (Carl Zeiss, Oberkochen, Germany) and an Ionoptix (Milton, MA, USA) fluorescence imaging system as described elsewhere (Bokvist et al. 1995). The experiments were conducted using the perforated-patch whole-cell configuration with the pipette-filling solution specified above. Prior to the experiments, the cells were loaded with the acetoxymethyl ester of fura-2 (fura-2 AM; 0.2 μm; Molecular Probes, Eugene, OR, USA) for 16–18 min. Calibration of the fluorescence ratios was performed using the standard whole-cell configuration to infuse fura-2 AM with different mixtures of Ca2+ and EGTA to obtain a known [Ca2+]i.
[Ca2+]i in intact islets (Fig. 8A) was recorded by dual-wavelength microfluorimetry using a D104 PTI microfluorimetry system (Monmouth Junction, NJ, USA) as described elsewhere (Olofsson et al. 2002). In short, the cells were loaded with 3 μm fura-2 AM in the presence of 0.007% w/v pluronic acid (Molecular Probes) at 37°C for 30–40 min prior to measurements. During the experiment, the islet was continuously superfused with a solution containing (mm): NaCl 140, KCl 3.6, NaHCO3 2, NaH2PO4 0.5, MgSO4 0.5, Hepes 5, CaCl2 2.6 and glucose 5 or 15; pH was adjusted to 7.4 with NaOH.
Hormone release measurements
Insulin, glucagon and somatostatin release were determined by radioimmunoassay (RIA) as described elsewhere (Vonen et al. 1989; Salehi et al. 1999). Briefly, batches of 8–10 islets were pre-incubated in 1 ml of Krebs-Ringer buffer (KRB) supplemented with 1 mm glucose for 30 min followed by 1 h incubation in 1 ml KRB containing 1 or 20 mm glucose. The specific GABABR agonist baclofen, the GABABR antagonist CGP 55845 and the ATP-dependent K+ (KATP) channel blocker tolbutamide (Sigma, St Louis, MO, USA) were included in the extracellular medium as indicated. At the end of the incubation, duplicate aliquots (25–100 μl) of the medium were removed and frozen pending the radioimmunoassays.
RT-PCR
Total RNA from rat islets and from FACS purified rat α- and β-cells was isolated using RNeasy Mini Kit (Qiagen). Total RNA 1 μg was used for reverse transcription using random hexamer primers and Superscript II (Invitrogen). In a negative control Superscript II was omitted from the reaction. PCR was performed under the following conditions: 2 min 94°C followed by 35 cycles of 45 s 94°C, 45 s 54°C or 56°C and 60 s 72°C. Products were analysed on a 1% agarose gel and by sequencing. The following primers were used:
Insulin: 5′CTGTGGATGCGCTTCCTGCC3′ and 5′CGGGACTTGGGTGTGTAGAAG3′ (expected fragment 158 bp);
Glucagon: 5′ATGCTGGTACAAGGCAGCTGGCAG3′ and 5′CAAGTAAGAACTCACATCACTGG3′ (expected fragment 297 bp);
*GABABR1a-d,f: 5′GCTGGATGGTTACCACATAG3′ and 5′GGTCACAGGAGCAGTGATG3′ (expected fragment sizes were 525 bp and 618 bp for a,b,d,f and c, respectively);
GABABR1a,f: 5′GCCTGTGGACTATGAGATCG3′ and 5′TTCGATTCACCTGGCAGTGG3′ (expected fragment: 313 bp);
*GABABR1c,f: 5′GTGAGTAGTGATGTTCAGCG3′ and 5′GCTTGATCCTTCTCCATGC3′ (expected fragment: 671 bp);
*GABABR1d: 5′CCTTCGATAGAGGTTTGAG3′ and 5′CTGGAGGAAGAAACACAATC3′ (expected fragment: 506 bp);
GABABR2: 5′CAACGACAGCAAGTACATC3′ and 5′CAGCTCTGTGATCTTCATTC3′ (expected fragment: 357 bp).
Except for GABABR2 and GABABR1d, it was not possible to design primers specific for the individual isoforms. Primer sequences indicated by the asterisks were designed as previously described (Brice et al. 2002).
Data analysis
Results are presented as means ± s.e.m. for the indicated number of experiments. Significant differences were evaluated using Student's t test for paired data except for Fig. 1 where Student's t test for unpaired data was used.
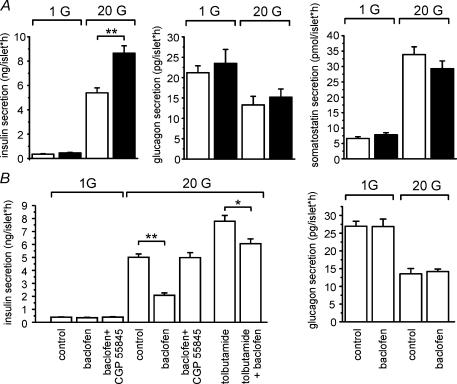
Figure 1. Modulation of hormone release from isolated rat pancreatic islets by GABABRs.
A and B, hormone release from isolated rat pancreatic islets was measured in the presence of 1 mm glucose (1 G) or 20 mm glucose (20 G) in the medium. Data are mean values ± s.e. of 10 experiments (*P < 0.01, **P < 0.001). A, insulin (left panel), glucagon (middle) and somatostatin release (right) in the absence (open bars) or presence (filled bars) of the GABABR antagonist CGP 55845 (10 μm). B, effects of the GABABR agonist baclofen (10 μm), the GABABR antagonist CGP 55845 (10 μm) and the KATP channel blocker tolbutamide (100 μm) as indicated on insulin secretion (left panel) and glucagon secretion (right panel).
Results
GABABR antagonism increases glucose-induced insulin secretion but has no effect on glucagon and somatostatin release
To study the involvement of GABABRs in the regulation of hormone release from isolated rat pancreatic islets, we used the GABABR antagonist CGP 55845 (Fig. 1A). Increasing glucose from 1 mm to 20 mm stimulated insulin secretion 15-fold. Addition of CGP 55845 resulted in a further 61% enhancement of glucose-induced insulin secretion whereas basal release was not affected. Glucagon secretion was suppressed by glucose (−38%) but it was not affected by GABABR antagonism. Finally, glucose stimulated somatostatin release 5.1-fold. Neither basal, nor glucose-induced somatostatin secretion were affected by the antagonist. It should be noted that the observed effects of CGP 55845 in these experiments are likely to reflect the removal of GABABR-signalling due to endogenous GABA as no exogenous GABA was added.
The GABABR agonist baclofen inhibits glucose-stimulated insulin secretion
We next tested the effects of the selective GABABR agonist baclofen on insulin and glucagon secretion (Fig. 1B). Basal insulin secretion was unaffected by 10 μm baclofen. In this series of experiments, glucose (20 mm) stimulated insulin secretion 13-fold. The latter effect was partially suppressed (−63%) by baclofen. The inhibitory action of baclofen on glucose-induced insulin secretion was fully antagonized by the GABABR antagonist CGP 55845. It is important that the amount of insulin secreted in the presence of baclofen, CGP 55845 and 20 mm glucose is less than that released from islets exposed to glucose and CGP 55845 (compare Fig. 1A and B). The KATP channel blocker tolbutamide stimulated insulin secretion in excess of that elicited by glucose alone (+55%). Baclofen remained capable of inhibiting insulin secretion under the latter conditions but the effect was limited to a reduction by 23%. Whereas 20 mm glucose inhibited glucagon secretion by ∼50%, addition of baclofen had no statistically significant effect on glucagon secretion (Fig. 1B). Taken together, the hormone release measurements suggest that GABABR activation selectively modulates insulin secretion and that it has no effect on the release of the two other major islet hormones.
Expression of GABABRs in pancreatic islet cells
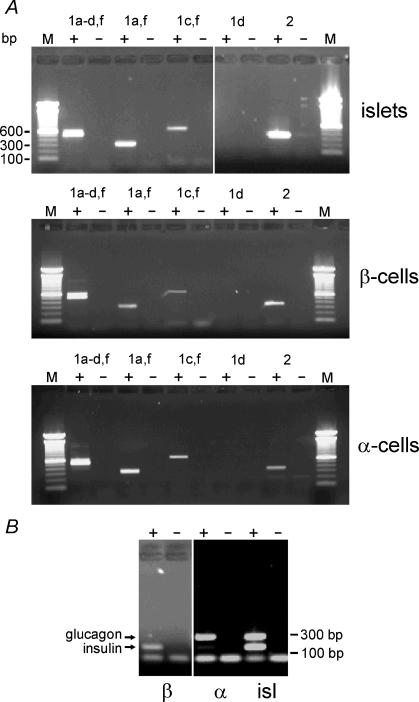
We explored expression of metabotropic GABABR isoforms in rat islets and purified rat α- and β-cells (Fig. 2A). We thus confirmed expression of GABABR1a and/or GABABR1b and GABABR2 in pancreatic islets. We also observed expression of GABABR1c and/or GABABR1f. The same expression pattern was observed for whole rat islets, purified rat α-cells and purified rat β-cells. Both α- and β-cell cDNA preparations were checked for purity using insulin and glucagon primers in a separate multiplex RT-PCR (Fig. 2B). Insulin and glucagon mRNAs were only detected in the β- and α-cell fractions, respectively.
Figure 2. RT-PCR on purified α- and β-cells with specific primers against different GABABR subunits.
A, total RNA from rat islets as well as FACS-purified α- and β-cells was reverse transcribed into cDNA. PCR analysis was performed using specific primers against GABABR1a-d,f, GABABR1a,f, GABABR1c,f, GABABR1d and GABABR2 (+). In the negative control reaction (−) reverse transcriptase was omitted. Molecular standards are shown to the left and the right. B, multiplex RT-PCR on islets (isl) and purified α- and β-cell with specific primers against insulin and glucagon.
The GABABR agonist baclofen inhibits depolarization-evoked exocytosis in rat β-cells
Figure 3A shows parallel recordings of Ca2+ current (ICa), associated increase in cytoplasmic free Ca2+ concentration ([Ca2+]i) and exocytosis (ΔCm) in a single rat β-cell in response to 500-ms depolarization from −70 mV to 0 mV applied at 2 min intervals. Under control conditions, the depolarization evoked an integrated Ca2+ current of ∼7 pC, produced a [Ca2+]i increase from a basal concentration of 0.1 μm to a peak concentration of ∼1 μm and a elicited a capacitance increase of ∼80 fF. Following the addition of 10 μm baclofen to activate the GABABR, both the Ca2+ current and the associated increase in [Ca2+]i were moderately (∼20%) reduced, whereas exocytosis was more strongly (83%) inhibited. All the effects of baclofen were readily reversible and 4 min after the removal of the agonist, the parameters had returned to their control values.
In a series of six experiments, the average inhibition of exocytosis produced by baclofen amounted to 79 ± 14% (P < 0.01; Fig. 3B). The effect was associated with an average reduction of the integrated Ca2+ current (QCa) of 28 ± 9% and a decrease in the amplitude of the depolarization-evoked peak [Ca2+]i (Δ[Ca2+]i) of 22 ± 8%. All effects were reversed upon withdrawal of baclofen. The inhibition of exocytosis in β-cells produced by baclofen is comparable to the reduction of neurotransmitter release at the calyx of Held (Takahashi et al. 1998; Sakaba & Neher, 2003).
Dose-dependent inhibition of exocytosis by baclofen
The relationship between baclofen concentration and the amplitude of the exocytotic responses is shown in Fig. 3C. The inhibitory effect of baclofen on exocytosis was concentration-dependent. Little inhibition of exocytosis was observed at 0.3 μm baclofen, and 10 μm produced maximal inhibition. Approximating the mean data points to the Hill equation yielded a half-maximal inhibition of 1.7 μm and co-operativity factor of 1.1. Our value for the IC50 of baclofen-induced inhibition of exocytosis in β-cells (1.7 μm) is close to the 0.8 μm obtained for the inhibition of neurotransmitter release in the calyx of Held (Takahashi et al. 1998). Similarly, the integrated Ca2+ current was half-maximally inhibited by baclofen at a concentration of 1.6 μm (not shown).
The inhibitory action of baclofen is antagonized by CGP 55845
We next ascertained that CGP 55845 is capable of antagonizing the inhibitory action of baclofen on exocytosis. Exocytosis was elicited by 500-ms depolarizations from −70 mV to 0 mV, applied at 2-min intervals. Figure 4A shows recordings of exocytosis (from left to right) under control conditions, after addition of baclofen, following washout of baclofen, after application of CGP 55845 alone and in the combined presence of CGP 55845 and baclofen. It can be seen that whereas baclofen in this experiment inhibited exocytosis by ∼70% when applied on its own, it lacked inhibitory action in the presence of the antagonist. Data from a series of seven experiments are summarized in Fig. 4B.
The inhibitory action of baclofen on exocytosis is mediated by activation of an inhibitory G-protein
The GABAB receptor is a member of the large group of G protein-linked membrane receptors. We made use of the standard whole-cell configuration, which has the advantage of permitting the cell interior to be dialysed by the pipette solution (Hamill et al. 1981), to elucidate the involvement of G proteins in the effects of baclofen on exocytosis. Figure 5A demonstrates that the ability of baclofen to inhibit exocytosis is maintained in the standard whole-cell configuration and when the intracellular cAMP levels are clamped at 0.1 mm. In a series of six experiments, 10 μm baclofen reversibly inhibited exocytosis (ΔCm) elicited by individual 500-ms pulses from −70 mV to 0 mV by 82 ± 13% (Fig. 5E). Consistent with the involvement of an inhibitory G protein (Gi/o), the ability of baclofen to inhibit exocytosis was lost following overnight treatment with 100 ng ml−1 pertussis toxin (Fig. 5B and E) or when 0.5 mm of the stable GDP analogue GDPβS was included in the intracellular solution (Fig. 5C and E). By contrast, when the experiment was conducted in the intracellular presence of 100 μm GTPγS (a stable GTP analogue that causes irreversible activation of the GTP-binding proteins), application of baclofen resulted in permanent inhibition of exocytosis (Fig. 5D and E).
Baclofen mediates its inhibitory action on exocytosis via activation of calcineurin
We have previously reported that the inhibitory action of somatostatin, galanin and adrenaline on exocytosis from β-cells is secondary to activation of the serine/threonine protein phosphatase calcineurin (Renström et al. 1996a). We explored whether this also applies to baclofen-induced inhibition of exocytosis. Indeed, baclofen failed to suppress exocytosis following inhibition of calcineurin with cyclosporin A (1 μm for > 20 min; Fig. 6A). A similar abolition was observed after intracellular application (through the patch electrode during whole-cell recordings) of calcineurin autoinhibitory peptide (100 μm), which is a highly selective inhibitor of calcineurin (Perrino et al. 1995) (Fig. 6B). Exocytosis measured after addition of baclofen averaged 87 ± 18% (n = 5) and 89 ± 13% (n = 5) of that observed under control conditions when the experiments were conducted in the presence of cyclosporin A and calcineurin autoinhibitory peptide, respectively. Under otherwise identical experimental conditions but in the absence of the calcineurin inhibitors, baclofen suppressed exocytosis by ∼80% (Fig. 5A).
Baclofen does not inhibit adrenaline-stimulated exocytosis in α-cells
We have previously reported that somatostatin in addition to its effects on β-cells (Renström et al. 1996a) also inhibits exocytosis in α-cells by a G protein-mediated action involving activation of calcineurin (Gromada et al. 2001). Given the similarity between the effects of somatostatin and baclofen in β-cells and the detection of GABABR mRNA in the α-cell fraction (Fig. 2), we next investigated the ability of baclofen to modulate exocytosis in rat α-cells exposed to adrenaline (Fig. 7). In a series of five experiments, adrenaline increased exocytosis by 312 ± 31% (P < 0.01), an effect that was associated with a moderate increase in the integrated Ca2+ current (40 ± 17%; P < 0.01). Baclofen had no effect on either parameter, and the mean amplitude of the exocytotic responses was 95 ± 9% (n = 5) of that observed in the presence of adrenaline alone. The failure of baclofen to affect exocytosis is in agreement with the lack of effect of the agonist on glucagon secretion (Fig. 1).
Baclofen has no effect on [Ca2+]i or β-cell electrical activity in intact islets
To investigate the effect of GABABR stimulation on Ca2+ signalling we measured [Ca2+]i in glucose stimulated islets before and after the addition of baclofen (Fig. 8A). The agonist was added to the islet in the steady state after a regular [Ca2+]i oscillatory pattern had established. In a series of nine experiments, the fura-2 AM fluorescence (F) ratio at 350 and 380 nm (F350/F380), as a measure of [Ca2+]i, was 0.71 ± 0.02 in the presence of 5 mm glucose. In the presence of 15 mm glucose, the average ratio before addition of baclofen was 0.83 ± 0.03. F350/F380 was 0.85 ± 0.03 4 min after addition of baclofen, and 0.86 ± 0.03 4 min after washout. Oscillations in [Ca2+]i were observed in 8 out of 9 islets. The oscillations were temporary in six islets and stable throughout the experiment in two islets (see also Fig. 8A). No consistent correlation between addition of baclofen and the appearance or disappearance of oscillations or the oscillatory pattern was observed.
We also investigated the effect of baclofen on β-cell membrane potential. Recordings were performed in the perforated-patch whole cell configuration on functionally identified β-cells in intact rat islets. Electrical activity was elicited by increasing the glucose concentration of the extracellular medium from 3 to 20 mm. Application of baclofen had no effect on action potential firing and membrane potential (Fig. 8B; n = 5). In the same experiments the membrane conductance, principally reflecting KATP channel activity, was 1.24 ± 0.16 nS in the presence of 20 mm glucose alone and 1.35 ± 0.11 nS after addition of baclofen (n.s.; n = 5).
Discussion
Pancreatic islet cells interact with each other via hormonal and electrical signals. It is well established that the blood sugar-regulating hormones also act as paracrine/autocrine regulators of hormone release from islets (Samols et al. 1986). In addition to peptide hormones, islet cells contain and release several other bioactive compounds, including classical neurotransmitters, which constitute potential local signalling molecules (Hayashi et al. 2003; Rorsman & Renström, 2003). We have recently demonstrated that Ca2+-dependent exocytosis of the SLMVs leads to GABA release into the islet interstitium that, via activation of ionotropic GABAA receptors in the α-cells, inhibits glucagon secretion (Braun et al. 2004; Wendt et al. 2004). In this paper we focus on the possible role of metabotropic GABAB receptors in the regulation of pancreatic hormone secretion.
Endogenous GABA release selectively modulates insulin secretion in intact islets
The GABABR antagonist CGP 55845 when added on its own stimulated glucose-induced insulin secretion whilst not affecting glucagon and somatostatin secretion (Fig. 1A). The fact that the antagonist was effective suggests that biologically active concentrations of GABA are present in the islet interstitium. The observed 1.6-fold enhancement of glucose-induced insulin secretion can be used to estimate the interstitial GABA concentration. As shown in Fig. 3C, exocytosis is influenced by baclofen in a concentration-dependent fashion with an IC50 value of ∼2 μm. If we assume that insulin secretion in the intact islet and in the presence of the antagonist is equivalent to exocytosis measured in isolated β-cells in the complete absence of GABA, then we can estimate that the average interstitial concentration of GABA experienced by the β-cell in situ is equivalent to 2 μm baclofen (75 fF/1.6 = 48 fF; see dotted line in Fig. 3C). We have estimated the intravesicular GABA content to be 0.4 amol (Braun et al. 2004). A pancreatic β-cell has a volume of ∼1 pl (estimated using spherical geometry and a diameter of 12 μm). If we assume that the interstitial space is about 10% of the intracellular volume as suggested by electron microscopy (Dean, 1973), then an islet containing 1000 β-cells has an interstitial volume of 0.1 nl. An average interstitial concentration of 1 μm will accordingly arise if as little as 250 SLMVs are released in the entire islet (i.e. 0.25 SLMV per β-cell). For comparison, the basal rate of GABA release has been estimated to be one vesicle per second (Smismans et al. 1997), and the release rate increases up to 10-fold upon stimulation (Braun et al. 2004). Even when allowance is made for the fact that baclofen is more potent than GABA (Takahashi et al. 1998), these considerations make it likely that physiologically relevant concentrations of GABA do indeed occur in the islet interstitium during β-cell electrical activity. The fact that neither glucagon, nor somatostatin secretion was affected under these conditions suggests that the effect on insulin secretion was indeed mediated by activation of GABABRs and not unspecific interactions with other effector proteins. The failure to demonstrate an effect of baclofen on hormone release from mouse islets in an earlier study (Gilon et al. 1991) possibly reflects the low GABA concentration found in mouse islets (Michalik & Erecinska, 1992). Thus, the importance of GABAergic signalling in islets is likely to vary between different species. It may seem surprising that CGP 55845 has no effect on depolarization-evoked exocytosis (Fig. 4A and B). This we attribute to GABA being quickly diluted/washed away in the experiments on isolated β-cells, whereas it is trapped in the interstitium in the insulin release experiments conducted on intact pancreatic islets.
Mechanisms of baclofen action
It has been reported that baclofen leads to a reduction in the cytoplasmic Ca2+ concentration in rat β-cells (Gu et al. 1993). We did not observe any effect of baclofen on the glucose-induced Ca2+ signal in islets (Fig. 8A). Accordingly, baclofen had no effect on glucose-induced electrical activity and KATP currents measured in β-cells in intact islets (Fig. 8B). This suggests that baclofen, unlike other inhibitory agonists (Rorsman et al. 1991; Renström et al. 1996a), does not inhibit insulin secretion by influencing electrical activity and cytoplasmic Ca2+ signalling.
In the calyx of Held, acute inhibition of synaptic transmission by baclofen has been found to be principally determined by the suppression of pre-synaptic Ca2+ entry, and exocytosis itself is unaffected (Takahashi et al. 1998). It is unlikely that the small decrease in Ca2+ current we observe is responsible for the inhibition of exocytosis. Indeed, reducing the β-cell Ca2+ current by 20–25% only marginally reduces the exocytotic responses (Renström et al. 1996b). We therefore conclude that more distal actions are responsible for the suppression of exocytosis in the β-cell. Recently, it has been demonstrated that activation of GABABRs also interferes with the replenishment of the ‘readily releasable pool’ (RRP) of synaptic vesicles (Sakaba & Neher, 2003). In the present study, baclofen inhibited exocytosis elicited by individual pulses, which principally reflects release of RRP granules (Renström et al. 1997; Barg et al. 2001). This suggests that the effect of baclofen is exerted distal to granule priming in β-cells. Given the similarities of exocytosis in various cells, it is possible that a direct effect on the exocytotic process itself also contributes to GABABR modulation of synaptic transmission; however, this remains to be confirmed experimentally.
Considering that baclofen appears to inhibit insulin secretion via a direct effect on exocytosis, it was surprising that the KATP channel blocker tolbutamide (which acts via increasing electrical activity and [Ca2+]i) partially antagonized the inhibitory effect of baclofen on insulin secretion (Fig. 1B). Sulphonylureas have been shown to stimulate exocytosis in β-cells not only via closure of KATP channels, but also by a direct effect on insulin granule exocytosis (Eliasson et al. 1996). Tolbutamide might therefore counteract the baclofen effect via a direct effect on the secretory machinery. Alternatively, the ability of tolbutamide to antagonize the inhibitory action of baclofen may be apparent. The stimulatory effect of the GABAB antagonist CGP 55845 on insulin secretion (Fig. 1A) indicates the presence of a substantial concentration of GABA in the islet interstitium. Compared to 20 mm glucose alone, tolbutamide further increased insulin secretion ∼1.6-fold, and a similar effect can be expected on GABA release from β-cells (Braun et al. 2004). It is therefore conceivable that release of endogenous GABA is sufficient to achieve near saturation of the GABABR and addition of baclofen therefore only leads to a moderate further reduction.
Baclofen mediates its inhibitory action by activation of the protein phosphatase calcineurin
As cAMP has been shown to enhance Ca2+-dependent exocytosis in β-cells by both protein kinase A (PKA)-dependent and PKA-independent mechanisms, it could be argued that suppression of exocytosis by baclofen results from Gi/o-mediated reduction of adenylate cyclase activity (Kerr & Ong, 1995; Bowery et al. 2002). Although we cannot exclude the possibility that baclofen inhibits adenylate cyclase in β-cells, the finding that the inhibitory action of baclofen persisted in standard whole-cell recordings when the cytoplasmic cAMP concentration was clamped at 100 μm by inclusion of the nucleotide in the pipette solution, argues that inhibition of cAMP generation is not required for the effect on exocytosis. Our data instead suggest that the ability of baclofen to inhibit Ca2+-dependent exocytosis involves activation of the protein phosphatase calcineurin. These results are consistent with the results previously obtained in mouse pancreatic β-cells (Renström et al. 1996a), rat α-cells (Gromada et al. 2001), bovine chromaffin cells (Craig et al. 2003) and nerve terminals (Hens et al. 1998). The data underscore the importance of phosphorylation/dephosphorylation in the control of exocytosis in a variety of secretory cells and suggest that the actions of the inhibitors converge at calcineurin. Clearly, it is now important to identify the molecular target involved. Recent observations made in chromaffin cells suggest that phosphorylation/dephosphorylation of SNAP-25 by PKA/calcineurin determines vesicle release competence (Nagy et al. 2004).
GABABRs in α-cells
In RT-PCR experiments, expression of GABABR subunit mRNA was detected in both purified β-cells and α-cells (Fig. 2). However, baclofen neither affected glucagon secretion from isolated islets (Fig. 1) nor adrenaline-stimulated exocytosis in single α-cells (Fig. 7). We have previously shown that the effects of adrenaline on exocytosis in α-cells are mimicked by forskolin (Gromada et al. 1997), which suggests that the experiments on α- and β-cells were conducted under comparable conditions. The reason for the discrepancy between gene expression and function in α-cells is not clear. Although gene expression is commonly regulated at the level of mRNA synthesis, it is also known to be controlled at more distal stages such as RNA transport and localization, mRNA translation or protein activity. Another possible explanation is that we have only examined exocytosis of glucagon in this study. Therefore we can not rule out the possibility that GABABRs regulate other processes in α-cells such as the release of SLMVs (Yamada et al. 2001). The exocytosis of these vesicles would contribute little to the capacitance changes (< 1%; cf. Braun et al. 2004) and therefore easily escape detection.
Physiological significance of autocrine suppression of insulin secretion
Pancreatic β-cells are constantly exposed to the microenvironment within the islet. The cells are exposed to the secretions of the neighbouring α- and δ-cells, including the islet hormones glucagon and somatostatin that exert strong effects on insulin secretion (Pipeleers et al. 1985a,b). Exocytosis of the insulin granules is associated with the co-release of adenine nucleotides, which have been reported to activate KATP channels and inhibit exocytosis in β-cells (Poulsen et al. 1999). It is also surprising that insulin itself has been reported to suppress β-cell electrical activity at concentrations only slightly higher than those found in the plasma (Khan et al. 2001; Persaud et al. 2002). Here we demonstrate that GABA by activation of GABABRs inhibits insulin secretion by suppression of exocytosis. Together these mechanisms provide powerful feedback systems safeguarding against hypoglycaemia and may contribute to the pulsatility of insulin secretion in vivo.
Acknowledgments
This study was supported by the Swedish Diabetes Association, The Juvenile Diabetes Research Foundation International, the Knut and Alice Wallenbergs Stiftelse, the Swedish Research Council (grant 8647), the European Commission (Growbeta), the Novo Nordisk Foundation and the Goran Gustafssons Foundation for Research in the Natural Sciences and Medicine.
References
- Ämmälä C, Eliasson L, Bokvist K, Larsson O, Ashcroft FM, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic β-cells. J Physiol. 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, Thevenod F, Renström E. Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J Cell Sci. 2001;114:2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- Bokvist K, Eliasson L, Ämmälä C, Renström E, Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic β-cells. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology XXXIII Mammalian γ-aminobutyric acid (B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Braun M, Wendt A, Birnir B, Broman J, Eliasson L, Galvanovskis J, Gromada J, Mulder H, Rorsman P. Regulated exocytosis of GABA-containing synaptic-like microvesicles in pancreatic β-cells. J Gen Physiol. 2004;123:191–204. doi: 10.1085/jgp.200308966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice NL, Varadi A, Ashcroft SJ, Molnar E. Metabotropic glutamate and GABA (B) receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic β-cells. Diabetologia. 2002;45:242–252. doi: 10.1007/s00125-001-0750-0. [DOI] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- Craig TJ, Evans GJ, Morgan A. Physiological regulation of Munc18/nSec1 phosphorylation on serine-313. J Neurochem. 2003;86:1450–1457. doi: 10.1046/j.1471-4159.2003.01955.x. [DOI] [PubMed] [Google Scholar]
- Dean PM. Ultrastructural morphometry of the pancreatic β-cell. Diabetologia. 1973;9:115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Renström E, Ämmälä C, Berggren PO, Bertorello AM, Bokvist K, Chibalin A, Deeney JT, Flatt PR, Gabel J, Gromada J, Larsson O, Lindström P, Rhodes CJ, Rorsman P. PKC-dependent stimulation of exocytosis by sulfonylureas in pancreatic β-cells. Science. 1996;271:813–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC. The influence of γ-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology. 1991;129:2521–2529. doi: 10.1210/endo-129-5-2521. [DOI] [PubMed] [Google Scholar]
- Göpel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from β-cells in intact mouse pancreatic islets. J Physiol. 1999;521:717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renström E, Rorsman P. Adrenaline stimulates glucagon secretion in pancreatic α-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Hoy M, Buschard K, Salehi A, Rorsman P. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by Gi2-dependent activation of calcineurin and depriming of secretory granules. J Physiol. 2001;535:519–532. doi: 10.1111/j.1469-7793.2001.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XH, Kurose T, Kato S, Masuda K, Tsuda K, Ishida H, Seino Y. Suppressive effect of GABA on insulin secretion from the pancreatic β-cells in the rat. Life Sci. 1993;52:687–694. doi: 10.1016/0024-3205(93)90229-v. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Secretory granule-mediated co-secretion of l-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003;278:1966–1974. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- Hens JJ, De Wit M, Ghijsen WE, Leenders AG, Boddeke HW, Kissmehl R, Wiegant VM, Weller U, Gispen WH, De Graan PN. Role of calcineurin in Ca2+-induced release of catecholamines and neuropeptides. J Neurochem. 1998;71:1978–1986. doi: 10.1046/j.1471-4159.1998.71051978.x. [DOI] [PubMed] [Google Scholar]
- Josefsen K, Stenvang JP, Kindmark H, Berggren PO, Horn T, Kjaer T, Buschard K. Fluorescence-activated cell sorted rat islet cells and studies of the insulin secretory process. J Endocrinol. 1996;149:145–154. doi: 10.1677/joe.0.1490145. [DOI] [PubMed] [Google Scholar]
- Kerr DI, Ong J. GABAB receptors. Pharmacol Ther. 1995;67:187–246. doi: 10.1016/0163-7258(95)00016-a. [DOI] [PubMed] [Google Scholar]
- Khan FA, Goforth PB, Zhang M, Satin LS. Insulin activates ATP-sensitive K+ channels in pancreatic β-cells through a phosphatidylinositol 3-kinase-dependent pathway. Diabetes. 2001;50:2192–2198. doi: 10.2337/diabetes.50.10.2192. [DOI] [PubMed] [Google Scholar]
- Michalik M, Erecinska M. GABA in pancreatic islets: metabolism and function. Biochem Pharmacol. 1992;44:1–9. doi: 10.1016/0006-2952(92)90030-m. [DOI] [PubMed] [Google Scholar]
- Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sorensen JB. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–429. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Göpel SO, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic β-cells. Pflugers Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- Perrino BA, Ng LY, Soderling TR. Calcium regulation of calcineurin phosphatase activity by its β subunit and calmodulin. Role of the autoinhibitory domain. J Biol Chem. 1995;270:340–346. doi: 10.1074/jbc.270.1.340. [DOI] [PubMed] [Google Scholar]
- Persaud SJ, Asare-Anane H, Jones PM. Insulin receptor activation inhibits insulin secretion from human islets of Langerhans. FEBS Lett. 2002;510:225–228. doi: 10.1016/s0014-5793(01)03268-9. [DOI] [PubMed] [Google Scholar]
- Pipeleers DG, Schuit FC, In't Veld PA, Maes E, Hooghe-Peters EL, Van De Winkel M, Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985 a;117:824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- Pipeleers DG, Schuit FC, Van Schravendijk CF, Van De Winkel M. Interplay of nutrients and hormones in the regulation of glucagon release. Endocrinology. 1985 b;117:817–823. doi: 10.1210/endo-117-3-817. [DOI] [PubMed] [Google Scholar]
- Poulsen CR, Bokvist K, Olsen HL, Hoy M, Capito K, Gilon P, Gromada J. Multiple sites of purinergic control of insulin secretion in mouse pancreatic β-cells. Diabetes. 1999;48:2171–2181. doi: 10.2337/diabetes.48.11.2171. [DOI] [PubMed] [Google Scholar]
- Renström E, Ding WG, Bokvist K, Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting β-cells by activation of calcineurin. Neuron. 1996 a;17:513–522. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- Renström E, Eliasson L, Bokvist K, Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic β-cells by suppression of granule mobilization. J Physiol. 1996 b;494:41–52. doi: 10.1113/jphysiol.1996.sp021474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renström E, Eliasson L, Rorsman P. Protein kinase A-dependent and – independent stimulation of exocytosis by cAMP in mouse pancreatic β-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Bokvist K, Ämmälä C, Arkhammar P, Berggren PO, Larsson O, Wahlander K. Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic β cells. Nature. 1991;349:77–79. doi: 10.1038/349077a0. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Renström E. Insulin granule dynamics in pancreatic β-cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Salehi A, Chen D, Hakanson R, Nordin G, Lundquist I. Gastrectomy induces impaired insulin and glucagon secretion: evidence for a gastro-insular axis in mice. J Physiol. 1999;514:579–591. doi: 10.1111/j.1469-7793.1999.579ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols E, Bonner-Weir S, Weir GC. Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab. 1986;15:33–58. doi: 10.1016/s0300-595x(86)80041-x. [DOI] [PubMed] [Google Scholar]
- Smismans A, Schuit F, Pipeleers D. Nutrient regulation of γ-aminobutyric acid release from islet β-cells. Diabetologia. 1997;40:1411–1415. doi: 10.1007/s001250050843. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Reetz A, Hell JW, During MJ, Walch-Solimena C, Jahn R, De Camilli P. A γ-aminobutyric acid transporter driven by a proton pump is present in synaptic-like microvesicles of pancreatic β-cells. Proc Natl Acad Sci U S A. 1993;90:5317–5321. doi: 10.1073/pnas.90.11.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonen B, Florholmen J, Giaever AK, Burhol P. Radioimmunoassay of somatostatin from isolated rat pancreatic islets. Scand J Clin Lab Invest. 1989;49:135–138. doi: 10.3109/00365518909105411. [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Yamada H, Otsuka M, Hayashi M, Nakatsuka S, Hamaguchi K, Yamamoto A, Moriyama Y. Ca2+−dependent exocytosis of l-glutamate by αTC6, clonal mouse pancreatic α-cells. Diabetes. 2001;50:1012–1020. doi: 10.2337/diabetes.50.5.1012. [DOI] [PubMed] [Google Scholar]