Abstract
Using tight-seal recordings from rat spinal cord slices, intracellular labelling and computer simulation, we analysed the mechanisms of spike frequency adaptation in substantia gelatinosa (SG) neurones. Adapting-firing neurones (AFNs) generated short bursts of spikes during sustained depolarization and were mostly found in lateral SG. The firing pattern and the shape of single spikes did not change after substitution of Ca2+ with Co2+, Mg2+ or Cd2+ indicating that Ca2+-dependent conductances do not contribute to adapting firing. Transient KA current was small and completely inactivated at resting potential suggesting that adapting firing was mainly generated by voltage-gated Na+ and delayed-rectifier K+ (KDR) currents. Although these currents were similar to those previously described in tonic-firing neurones (TFNs), we found that Na+ and KDR currents were smaller in AFNs. Discharge pattern in TFNs could be reversibly converted into that typical of AFNs in the presence of tetrodotoxin but not tetraethylammonium, suggesting that lower Na+ conductance is more critical for the appearance of firing adaptation. Intracellularly labelled AFNs showed specific morphological features and preserved long extensively branching axons, indicating that smaller Na+ conductance could not result from the axon cut. Computer simulation has further revealed that down-regulation of Na+ conductance represents an effective mechanism for the induction of firing adaptation. It is suggested that the cell-specific regulation of Na+ channel expression can be an important factor underlying the diversity of firing patterns in SG neurones.
The dorsal horn of the spinal cord is the first relay station processing the sensory input from primary afferent terminals. The diversity of sensory modalities in dorsal horn is encoded by the type of peripheral afferent fibre, synaptic connectivity and intrinsic firing properties of dorsal horn neurones (Brown, 1981; Cervero, 1987; Willis & Coggeshall, 1991). The substantia gelatinosa (SG) is the dorsal horn region where most fine-calibre C- and Aδ-fibres terminate (Rethelyi, 1977; LaMotte, 1977; Light & Perl, 1977; Sugiura et al. 1986) and is therefore considered to be a key element in the nociceptive processing system.
Several classes of SG neurones are distinguished on the basis of their intrinsic firing properties (Yoshimura & Jessell, 1989; Thomson et al. 1989; Lopez-Garcia & King, 1994; Grudt & Perl, 2002). A major criterion widely used for such a classification is a degree of spike frequency adaptation observed during sustained membrane depolarization. While some neurones were characterized by a tonic firing, the others exhibited a strong adaptation generating short bursts of spikes or just a single spike. A degree of spike frequency adaptation in a neurone correlates with a type of its cutaneous afferent input (Lopez-Garcia & King, 1994) and therefore the cell-specific regulation of firing adaptation can underlie diverse modalities of sensory encoding in SG. In spite of its importance, the mechanism of spike frequency adaptation in SG neurones is not well understood.
Recently we described the ion basis of tonic (non-adapting) firing in a group of SG neurones and created a computer model of the SG neurone (Melnick et al. 2004). The voltage-gated Na+ and delayed-rectifier K+ channels were shown to generate the basic pattern of tonic firing, while the Ca2+-dependent conductances stabilized firing and regulated discharge frequency. The present study was carried out to elucidate the major factors responsible for the appearance of spike frequency adaptation in SG neurones.
Methods
Tight-seal recordings were performed using both 200 μm transverse and 300 μm parasagittal slices prepared from the lumbar enlargement of the spinal cord of 2- to 7-week-old rats (Edwards et al. 1989; Bentley & Gent, 1994; Chery & De Konink, 2000). The animals were killed in accordance with national guidelines (Direcção Geral de Veterinária, Ministério da Agricultura). After anaesthesia by intraperitoneal injection of sodium pentobarbital (30 mg kg−1) the vertebral column was quickly cut out and immersed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF). The 5–7 mm segment of the lumbar enlargement was dissected and the slices were prepared using a tissue slicer (Leica VT 1000S). The slices were then incubated for 30–60 min in ACSF at 33°C. SG (lamina II) was identified as a translucent band in the dorsal horn (Fig. 2A). Neurones were localized during recording according to a position of the pipette tip on the video-image of SG.
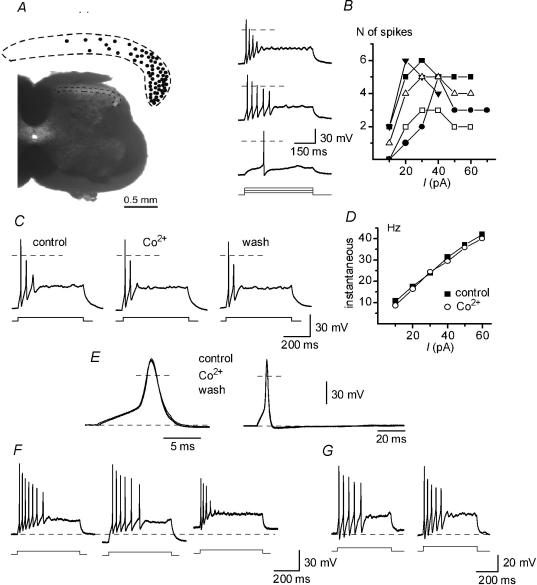
Figure 2. Discharge properties of adapting-firing neurones (AFNs) in ACSF (2 m+) and Ca2+-free solutions.
A, localization of AFNs (n = 65) in SG. Transverse section of the spinal cord of 19-day-old rat. B, bursts of spikes evoked in an AFN by 0.5 s depolarizing current pulses of 10, 30 and 50 pA in ACSF. Dashed lines, 0 mV. Right, the number of spikes as a function of injected current plotted for 5 AFNs. C, firing patterns in ACSF (control and wash) and Co2+-containing solution. The stimulation strength was 20 pA in all recordings. D, instantaneous frequency–current (f–I) characteristics of AFNs in ACSF and Co2+-containing solution. E, single spikes activated by 10 ms current pulses in ACSF and Co2+-containing solution. F, discharge patterns in an AFN at varying membrane potentials, −70 mV (dashed line), −80 and −60 mV. G, firing patterns recorded in ACSF with external 2.5 mm K+ at room temperature (left) and 35–37°C (right). Current pulses: 20 and 30 pA, respectively.
ACSF contained (mm): NaCl 115, KCl 5.6, CaCl2 2, MgCl2 1, glucose 11, NaH2PO4 1, NaHCO3 25 (pH 7.4 when bubbled with a 95% O2–5% CO2). To block Ca2+-dependent conductances Co2+ (2 mm), Cd2+ (0.1 mm) and Mg2+ (5 mm) were used. Co2+ was equimolarly substituted for Ca2+. In experiments with Cd2+, Ca2+ was omitted and [Mg2+] was increased to 3 mm. In 5 mm Mg2+ solution the concentration of Ca2+ was reduced to 0.1 mm. The last solution was also used for studying Na+ and K+ channels and is referred to as ACSF*. Standard pipette solution contained (mm): NaCl 15, KCl 120, MgCl2 1, EGTA 10 and Hepes 10 (pH 7.3 adjusted with KOH, final [K+] was 151 mm). The solution with low Ca2+ buffering capacity contained (mm): NaCl 5, KCl 147, MgCl2 1, EGTA 1 and Hepes 10 (pH 7.3, adjusted with KOH, final [K+] was 155 mm). The pipette solution for studying Na+ channels contained (mm): NaCl 4, CsCl 131, MgCl2 2, EGTA 10 and Hepes 10 (pH 7.3 adjusted by CsOH and NaOH, final [Cs+] = 153 mm and [Na+] = 6 mm). All chemicals were purchased from Sigma.
The patch pipettes were pulled from thick-walled borosilicate glass tubes (Modulohm, Denmark) and had after fire-polishing a resistance of 3–5 MΩ. The EPC-9 amplifier (HEKA, Lambrecht, Germany) was used in all experiments. The effective corner frequency of the low-pass filter was 3 kHz. The frequency of digitization was 10 kHz. Transients and leakage currents were digitally subtracted using standard P/n protocol. Offset potentials were nulled directly before formation of a seal. Liquid junction potentials were calculated and corrected for in all experiments. In neurones subjected to detailed analysis the series resistance measured in the whole-cell mode was 6–20 MΩ and was compensated by at least 60%. Input resistance was measured in both current- and voltage-clamp modes. Ion channels were studied in nucleated patches excised from somatic membrane (Sather et al. 1992). To calculate the density of somatic channels, the diameter of each patch was measured.
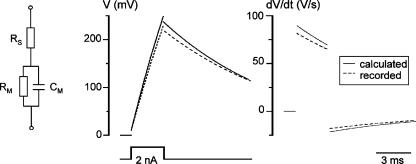
Action potentials were recorded using the fast current-clamp mode of the EPC-9 amplifier. The accuracy of the voltage measurements done with this patch-clamp amplifier was tested in the experiments shown in Fig. 1. A current pulse (2 nA, 3 ms) was applied to a model circuit containing a capacitor (Cm, 22 pF) and a resistor (Rm, 500 MΩ) connected in series with another resistor (RS, 5.1 MΩ). A theoretically calculated response (continuous line) was compared with the averaged trace of 500 recordings (dashed line). Time derivatives of both theoretical and recorded voltage traces (dV/dt) are shown on the right. In the initial phase of depolarization, the fast current-clamp mode of the EPC-9 amplifier produced less distortion of the recorded signal than the normal (slow) current-clamp mode of the EPC-7 amplifier (see Fig. 3C from Magistretti et al. 1996, 1998) and it appears to be more suitable for measurements of voltage changes and polarization velocities.
Figure 1. Test of the voltage change measurements made using the fast current-clamp mode of the EPC-9 amplifier.
Parameters of the model circuit: Cm = 22 pF, Rm = 500 MΩ and RS = 5.1 MΩ. Current pulse: 2 nA, 3 ms. Continuous line, a theoretically calculated response; dashed line, an average of 500 recorded traces. Right, time derivatives of calculated and recorded voltage traces (dV/dt). Points corresponding to instantaneous voltage changes on RS at the beginning and end of the pulse are removed from both dV/dt graphs.
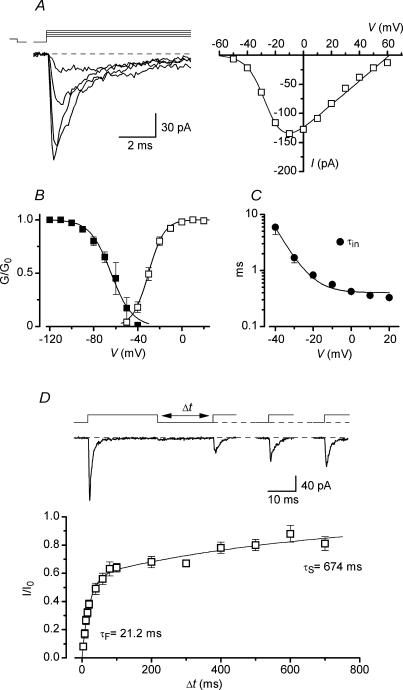
Figure 3. Properties of Na+ channels in nucleated patches from AFNs.
A, Na+ currents activated by depolarization to −40, −30, −20 and −10 mV following a 50 ms prepulse to −120 mV. Holding potential, −80 mV. I–V curve is shown for the same neurone. B, steady-state activation and inactivation characteristics. Fitting parameters are given in the text. C, the inactivation time constant (τin) as a function of membrane potential. D, kinetics of Na+ channel recovery from inactivation. Na+ currents activated by pairs of 25 ms pulses from −80 to −30 mV with varying intervals. The time course of Na+ channel recovery from inactivation was fitted using a double-exponential function.
The current–voltage (I–V) relationship for Na+ channels was fitted using the equation: G0/(1 + exp((V50 − V)/k))(V − Vrev), where G0 is the maximum conductance, V50 is the potential of half-maximum channel activation, k is a steepness factor and Vrev is the reversal potential obtained by fitting for each I–V curve. K+ conductances were calculated assuming Vrev = EK = −84 mV, where EK is the equilibrium potential for K+. The activation and steady-state inactivation characteristics were fitted with a Boltzmann function: 1/(1 + exp((V50 − V)/k)). The time course of Na+ channel recovery from inactivation was fitted using a two-exponential function: 1 − A exp(−t/τF) − (1 − A) exp(−t/τS), where τF and τS are the fast and slow time constants, A is the relative amplitude of the fast component.
All numbers are given as mean ± standard error of the mean (s.e.m.). The values obtained by data fitting with a linear or non-linear least-squares procedures are given as mean ± standard error (s.e.m.). In all figures the error bars are shown when exceeding the symbol size. The parameters were compared by paired or independent Student's t test. The present study is based on recordings from 113 adapting-firing neurones (AFNs), 232 tonic-firing neurones (including 187 from Melnick et al. 2004) and 68 nucleated patches obtained from AFNs. All experiments, except those in Fig. 2G (right), were carried out at room temperature 22–24°C.
Ten AFNs were filled with 0.5% biocytin during recording for later cell visualization. Following the recording session, the slices with biocytin-filled neurones were transferred into a fixative containing 4% paraformaldehyde, 0.3% picric acid in 0.1 m phosphate buffer (pH 7.4) for one night. The slices were then washed in 0.1 m phosphate buffer saline (PBS) and treated with 30% sucrose for one night. After re-sectioning at 50 μm, slices were serially collected in PBS. After repeated washing (10 min each) in PBS containing 0.3% Triton X-100 (PBST), slices were incubated in an Alexa Streptavidin 594 antibody solution (1: 1000 PBST, Molecular Probes, The Netherlands) at 4°C for 48 h. Slices were mounted in glycerol/PBS (3: 1). Serial confocal optical sections were obtained with 1 μm steps at maximum pixel intensity. Images of labelled cells show the summed Z projection.
Computer simulations were done using NEURON software (Hines, 1993; Hines & Carnevale, 1997) and a model of a TFN described previously (Melnick et al. 2004). This universal model was created for SG neurones with diverse dendritic organizations. The input resistance and membrane time constants of the model were close to those described here for AFNs. Only three parameters of the model were varied in the present study: the Na+ conductance (gNa) in the axon initial segment and the voltage dependencies of Na+ channel activation and inactivation. The gNa was changed from a control value of 1800 mS cm−2 (1.0) to 730 mS cm−2 (0.41) and 521 mS cm−2 (0.29). The voltage dependencies were modified by uniformly shifting all equations describing Na+ channel activation and inactivation by +5 and +11 mV, respectively.
Results
Adapting-firing neurones (AFNs) were mostly found in the lateral part of SG (Fig. 2A). In ACSF they showed a resting potential of −69.9 ± 0.6 mV (n = 102), an input resistance of 1.3 ± 0.1 GΩ (n = 102) and a membrane time constant of 84.5 ± 5.0 ms (n = 20). Unless otherwise stated, in the following current-clamp experiments the membrane potential was adjusted to −70 mV. AFNs were usually able to support firing only during the first 100–250 ms of depolarization evoked by an injection of 0.5 s pulses of inward current (Fig. 2B). The number of generated spikes depended on the pulse strength. In a narrow range of 10–40 pA it increased to a maximum of 3–6 spikes, but then became lower at stronger stimulation.
Ca2+-dependent conductances
A contribution of Ca2+-dependent conductances to firing in AFNs was studied by using the inorganic blockers of Ca2+ channels Co2+, Cd2+ and Mg2+. The concentration of internal EGTA in these experiments was reduced to 1 mm. In the presence of 2 mm Co2+ (n = 10), 0.1 mm Cd2+ (n = 8) or 5 mm Mg2+ (n = 9), no changes in firing pattern or the instantaneous frequency–current (f–I) characteristic, calculated for the first interspike interval, were observed (Fig. 2C and D, shown for Co2+). The shape of single spikes was also unchanged by the blockers (Fig. 2E, shown for Co2+). For 10 neurones recorded in control and Co2+-containing solutions, several parameters of the single spike were compared and no significant difference was found (Table 1). The amplitude of after-hyperpolarization (fast and slow) was also not reduced by Co2+ (n = 10). Thus, it could be concluded that Ca2+-dependent conductances do not contribute to discharge pattern in AFNs. In the following experiments we used the 10 mm EGTA pipette solution and both ACSF and ACSF* (0.1 mm Ca2+ and 5 mm Mg2+) as bath solution.
Table 1.
Comparison of single spike parameters in control and 2 m+
| Spike parameters | Units | Control | Co2+ | Wash | t test |
|---|---|---|---|---|---|
| Width at 0 mV | ms | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | n.s. |
| Width at −30 mV | ms | 2.3 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.2 | n.s. |
| Amplitudea | mV | 95.2 ± 3.0 | 90.5 ± 2.9 | 86.3 ± 2.7 | n.s. |
| AHP, maximumb | mV | 68.1 ± 1.2 | 69.2 ± 1.1 | 69.6 ± 1.1 | n.s. |
| AHP, at 40 msc | mV | 67.1 ± 1.0 | 67.8 ± 0.7 | 68.6 ± 0.9 | n.s. |
The spike amplitude was measured from a membrane potential of −70 mV.
The largest after-hyperpolarization (AHP, maximum) was measured within the first 8–15 ms after beginning of the spike.
The second measurement of AHP was 40 ms after the beginning of the spike. Data from 10 neurones. Student's paired t test was used to compare control parameters with those obtained in Co2+. n.s., non-significant difference with P > 0.25.
The effect of membrane potential on the firing pattern was studied in experiments where it was changed from −70 to −80 or −60 mV. Varying the potential in this range did not change the adapting firing patterns in either ACSF or ACSF* (Fig. 2F, shown for ACSF*, n = 34).
In 11 AFNs we tested whether lower external K+ and increase in temperature to 35–37°C can change the basic pattern of intrinsic firing. In all these neurones a typical pattern of adaptation was seen in external 2.5 mm K+ at room temperature (Fig. 2G, left) as well as after increasing the temperature to 35–37°C (Fig. 2G, right).
Na+ channels
Na+ channels were studied using the Cs+-containing pipette solution. The identification of a firing pattern in a neurone was done during the first few seconds after membrane rupture, before Cs+ replaced intracellular K+ (Melnick et al. 2004). Na+ currents were recorded in nucleated patches in ACSF* containing 1 mm TEA to reduce outward K+ current (Fig. 3A). Their activation curve fitted with a Boltzmann equation had V50 = −30.3 ± 0.2 mV and k = 7.1 ± 0.2 mV (Fig. 3B, n = 10). The steady-state inactivation of Na+ channels, studied with 50 ms conditioning prepulses, revealed a half-maximum inactivation at −64.2 ± 0.9 mV and k = −9.6 ± 0.8 mV (Fig. 3B, n = 10, test pulses to −30 or −20 mV). The inactivation kinetics of Na+ channels could be fitted using a mono-exponential function with the time constant changing from 5.9 ± 1.5 ms at −40 mV to less than 0.5 ms at positive potentials (Fig. 3C, n = 7). Recovery of Na+ channels from inactivation at −80 mV was studied by applying two 25 ms voltage pulses to −30 mV with varying intervals (Fig. 3D). The time course of recovery was double-exponential (Safronov & Vogel, 1995; Martina & Jonas, 1997; Melnick et al. 2004) with time constants of 21.2 ± 2.2 ms (57%) and 674 ± 95 ms (43%) (n = 7). The density of somatic Na+ current in AFNs was 0.84 ± 0.16 pA μm−2 (n = 8).
Voltage-gated K+ channels
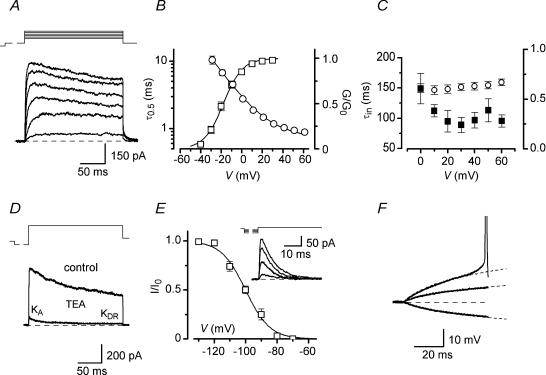
The major K+ current found in AFNs was a slowly inactivating delayed-rectifier (KDR) current (Fig. 4A). For its recording the patch was held at −80 mV and depolarizing voltage pulses were applied after a 150 ms prepulse to −60 mV inactivating a fast transient K+ (KA) current. Half-maximum activation of KDR conductance was observed at V50 = −18.0 ± 0.6 mV (k = 9.3 ± 0.5 mV,M n = 11, Fig. 4B). The activation kinetics of the current was described by plotting the rise time of a half-maximum current (τ0.5) as a function of potential (Fig. 4B). The τ0.5 value became close to 1 ms at potentials positive to +30 mV (Fig. 4B, n = 11), indicating involvement of KDR current in spike repolarization. Starting from −20 mV a partial inactivation of KDR current developed. The time course and degree of inactivation were only weakly voltage dependent (Fig. 4C, n = 11). The reversal potential for KDR current, estimated from instantaneous tail currents, was close to EK of −84 mV (n = 5, not shown). In 10 mm TEA the current was blocked to 7.9 ± 1.0% (n = 9, not shown). The density of somatic KDR current in AFNs at +60 mV was 3.7 ± 0.3 pA μm−2 (n = 18).
Figure 4. Voltage-gated K+ channels in AFNs.
A, KDR currents in patches activated in ACSF* by 200 ms depolarizing pulses (from −30 mV to +20 mV in a 10 mV step) following a 150 ms prepulse to −60 mV. Holding potential, −80 mV. These traces were off-line low-pass filtered at 1 kHz. B, steady-state activation curve (□) and the rise time of a half-maximum current (τ0.5, ○). C, the time constant of inactivation (▪) and the ratio of currents measured at peak and at the end of the pulse (○) at different potentials. D, separation of transient KA currents in patches. Total K+ current activated in a nucleated patch by a voltage step from −120 (150 ms) to +60 mV (200 ms) in the presence and absence of 10 mm TEA. Holding potential, −80 mV. E, steady-state inactivation of KA current. Whole-cell KA currents were recorded at −50 mV after a 150 ms prepulse to varying potentials (−130, −110, −100 and −90 mV). Holding potential, −80 mV. F, analysis of subthreshold current-clamp responses to injection of current pulses. AFN was stimulated by −10, +10 and +20 pA currents. A response to −10 pA current was considered as passive. It was digitally multiplied by factors of −1 and −2 (shown by dashed curves) and shown superimposed on the other two traces.
The KA component was not seen in nucleated patches when K+ current was activated by a voltage step from −120 to +60 mV, but it could be separated after addition of 10 mm TEA as the fast inactivating component of the remaining current (Fig. 4D). The inactivation time constant of KA current was 10.5 ± 1.2 ms (n = 10). The amplitude of KA current, estimated from the double-exponential fitting of the inactivation kinetics of the remaining current, gave the somatic current density of 0.60 ± 0.17 pA μm−2 at +60 mV (n = 10). Because of its low density in nucleated patches, the steady-state inactivation of KA current was studied in whole-cell mode. The whole-cell KA current evoked after a conditioning prepulse to −120 mV by a voltage step to potentials subthreshold for Na+ channel activation (between −60 and −50 mV) ranged from 0 to 220 pA. The steady-state inactivation of KA current (V50 = −96.3 ± 1.2 mV; k = −7.2 ± 0.8 mV; n = 5) showed that it was completely inactivated at −70 mV (Fig. 4E). To reveal a possible contribution of KA current to setting the firing threshold in AFNs, the current-clamp responses of AFNs to depolarization were compared with membrane passive responses (Fig. 4F). A hyperpolarization evoked by a −10 pA current injection was considered as a passive response, which was appropriately scaled (dashed curves) and compared with cell responses to +10 and +20 pA depolarization (Fig. 4F). In agreement with our data about complete KA current inactivation at resting potential, a subthreshold depolarization was found to be passive and not affected by activation of KA conductance (n = 28).
By comparing the present results for AFNs with those recently reported for tonic-firing neurones (TFNs) from Melnick et al. (2004), it was possible to conclude that both neurone types possess Na+ and KDR currents as well as negligible KA current. The ranges of KDR channel activation were very similar (V50, −18.0 mV for AFNs versus −19.8 mV for TFNs). The kinetics of Na+ channel recovery from inactivation in both cell types were two-exponential with the time constants: 21.2 ms (57%) and 674 ms (43%) for AFNs versus 21.8 ms (63%) and 793 ms (37%) for TFNs. The activation characteristics of Na+ channels were insignificantly different (V50, −30.3 mV for AFNs versus −35.7 mV for TFNs; P < 0.1). However, the density of somatic Na+ and KDR currents in AFNs was lower and the Na+ channel inactivation was shifted to more positive potentials by 11 mV (V50, −64.2 mV for AFNs versus −75.5 mV for TFNs; P < 0.02). The following experiments were done to find out which of these factors played a critical role in the appearance of firing adaptation.
Block of Na+ rather than KDR channels in TFNs induces adaptation typical of AFNs
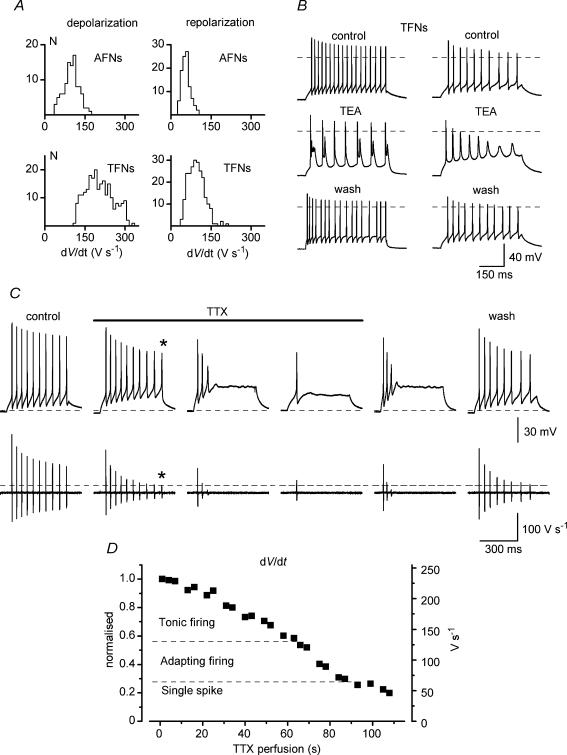
Since somatic Na+ current represents only few per cent of the total Na+ current in a spinal neurone (Safronov et al. 1997, 1999; Alessandri-Haber et al. 1999), a comparison of total Na+ conductances in AFNs and TFNs was done by measuring maximum spike depolarization rates. The histogram of distributions of maximum de- and repolarization rates obtained by digital differentiation of the first spike in a train are shown in Fig. 5A for 102 AFNs and 232 TFNs. The mean maximum depolarization rates of 103.7 ± 2.8 V s−1 for AFNs (n = 102) and 208.3 ± 3.4 V s−1 for TFNs (n = 232) were significantly different (P < 0.0001). Since the depolarization rate is proportional to the inward current charging the membrane according to the equation: I = C(dV/dt), where C is membrane capacitance, our results imply relatively lower Na+ conductance in AFNs in comparison with TFNs. In addition, the velocity of repolarization was also lower in AFNs (61.2 ± 1.5 V s−1, n = 102, for AFNs versus 99.9 ± 1.9 V s−1, n = 232, for TFNs, P < 0.0001).
Figure 5. TTX but not TEA can induce adaptation in tonic-firing neurones (TFNs).
A, histograms of distribution of de- and repolarization rates in AFNs and TFNs. Bin width, 10 V s−1. B, modifications of discharge patterns in TFNs by 1 mm TEA. C, induction of adaptation in a TFN during perfusion of the slice with 40 nm TTX. Bottom traces are derivatives of voltage traces (dV/dt). The baselines are 0 V s−1. Dashed line shows the maximum depolarization rate for the reduced spike with the overshoot close to 0 mV (indicated by asterisks). D, modification of firing pattern from tonic to adapting as a function of Na+ channel block during slice perfusion with 40 nm TTX. The maximum depolarization rate of the first spike in a train (right axis) is also shown as normalized to the control value (left axis).
In 10 TFNs, the effect of partial KDR current block on firing pattern was studied in ACSF*. In 5 of them an addition of 1 mm TEA resulted in the appearance of spike doublets in the train (Fig. 5B, left). In the other 5 TFNs, 1 mm TEA prolonged spikes, reduced the after-hyperpolarization and stability of firing, so that the amplitude of spikes progressively decreased during the train (Fig. 5B, right). However, both modified firing patterns were obviously different from those of AFNs.
In 21 TFNs the effect of TTX on pattern was studied in ACSF*. In all TFNs tested, the tonic firing changed to adapting and then to strongly adapting with a single spike when perfusion of the slice with 40 nm TTX-containing solution was started (Fig. 5C). Wash out of TTX was done just after the pattern modification occurred (before 40 nm TTX completely diffused into the slice). Differentiation of the voltage traces has shown that the appearance of adaptation correlated with a reduction of Na+ current (Fig. 5C, bottom). In control solution, Na+ current (estimated from derivatives) decreased during tonic firing due to the channel inactivation (Melnick et al. 2004) but still remained sufficiently large to support firing. After a partial Na+ current block by TTX, only the first few spikes could be generated before inactivation further reduced Na+ current to the level below that necessary for the spike generation (dashed line). It should be noted that the block of Na+ conductance also resulted in a reduction in the rate of spike repolarization, probably because of lesser activation of KDR channels by spikes with reduced overshoots. A transition from tonic to adapting firing occurred at 40–60% block of Na+ conductance (Fig. 5D). Thus, block of Na+ rather than KDR channels in TFNs modified a firing pattern to that typical of AFNs.
Anatomy of AFNs
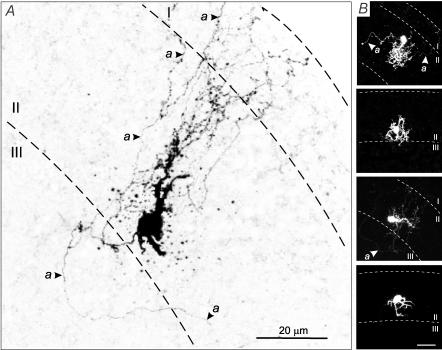
In order to test whether the smaller Na+ conductance observed in AFNs might result from the cutting of the axon during the preparation of slices, AFNs were labelled by including biocytin in the patch pipette (n = 10). Because of their predominant location in the lateral part of SG (Fig. 2A), AFNs were rarely seen in sagittal sections typically used for anatomical studies. Therefore, the labelling was performed in transverse slices. All AFNs had small rounded somata and dense dendritic trees mostly staying within SG. Dendritic arbors exhibited spines and a limited spread in the medio-lateral or dorso-ventral dimensions. The axon was identified as a thinner process with constant diameter lacking spines. The axons branched extensively and entered laminae I and III. In some AFNs labelled axon branches had a total length of several hundreds of micrometres (Fig. 6A and B). Thus it could be concluded that the axon initial segment in AFNs was not cut during the preparation of slices.
Figure 6. Confocal images of AFNs labelled with biocytin.
A, inverted image of AFN with extensive axonal collaterals indicated by arrows. B, images of another four AFNs two of which have extensive axonal collaterals. Calibration bar, 20 μm. Dashed lines represent the borders between laminae I, II (SG) and III.
Computer simulation
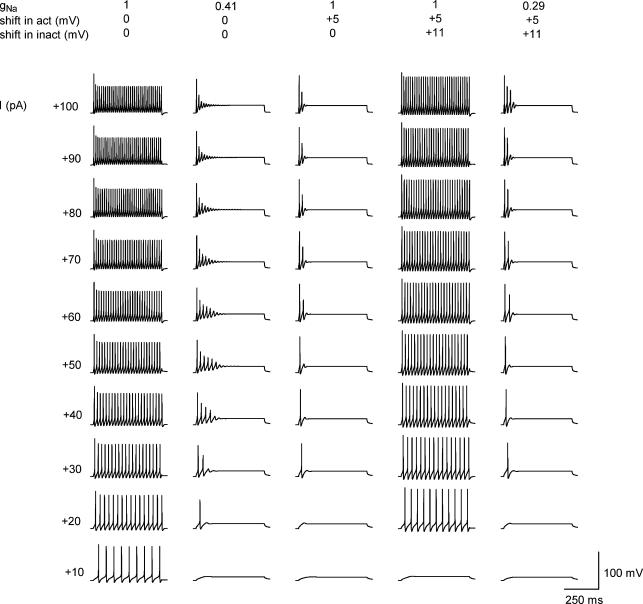
Computer simulations were done to study how a reduction in Na+ current and a shift in its activation and inactivation characteristics to more positive potentials can influence firing in a model neurone (Fig. 7). A control model of a TFN (Melnick et al. 2004) generated sustained firing with frequency increasing with stimulation intensity (Fig. 7, 1/0/0). The maximum depolarization rate of the first spike in a train was 231 V s−1 when measured at +30 pA stimulation. By reducing the Na+ conductance (gNa) to 0.41 it was possible to induce firing adaptation (0.41/0/0). The maximum depolarization rate of the first spike in a burst was reduced to 132 V s−1 (+30 pA stimulation). If the activation characteristics of Na+ channels were shifted by +5 mV, a strong adaptation appeared in the model already at the control gNa value (1/+5/0). After the inactivation was shifted by +11 mV, the model again generated tonic firing (1/+5/+11). In the model with both activation and inactivation shifted to positive potentials a stronger reduction in gNa to 0.29 was needed for the induction of adaptation in the whole stimulation range (0.29/+5/+11). In this case the maximum depolarization rate of the first spike became 147 V s−1 (+ 30 pA stimulation). Thus, both a reduction in gNa and a positive shift in activation characteristics promote spike frequency adaptation, while a positive shift in inactivation has an opposite effect.
Figure 7. Computer simulation of firing adaptation in SG neurone.
A basic model of TFN from Melnick et al. (2004). Repetitive firing evoked by 500 ms current pulses of increasing intensity in a basic model (1/0/0). Reduction of Na+ conductance (gNa) in the axon initial segment to 0.41 induced firing adaptation (0.41/0/0). An activation shift by +5 mV, without lowering gNa, resulted in a strong firing adaptation (1/+5/0). A +11 mV shift in inactivation characteristics restored tonic firing in a model with control gNa (1/+5/+11). Reduction in gNa to 0.29 induced spike frequency adaptation in a model with shifted activation and inactivation characteristics (0.29/+5/+11). The resting potential was −70 mV in all simulations.
Discussion
This study has shown that reduced expression of Na+ channels is a critical factor determining the appearance of spike frequency adaptation in SG neurones. Therefore, regulation of the intrinsic firing pattern in a SG neurone through modulation of Na+ channels may represent an important element of spinal sensory encoding.
The degree of spike frequency adaptation is a criterion most frequently used for electrophysiological classification of superficial dorsal horn neurones (Thomson et al. 1989; Lopez-Garcia & King, 1994; Grudt & Perl, 2002; Prescott & De Koninck, 2002; Ruscheweyh & Sandkuhler, 2002; Hu & Gereau, 2003). Adapting-firing neurones (AFNs) which generate a short burst of spikes at the beginning of depolarization were also called phasic-, burst- or transient-type neurones. Most were physiologically classified as nociceptive-specific neurones (Lopez-Garcia & King, 1994). AFNs from lamina II (SG) receive monosynaptic input from slowly conducting primary C-type afferents (Grudt & Perl, 2002). Our data support a number of reports showing that adapting firing patterns are generated by only a subpopulation of neurones (Thomson et al. 1989; Lopez-Garcia & King, 1994; Grudt & Perl, 2002; Hu & Gereau, 2003; Melnick et al. 2004) rather than by all neurones in SG (lamina II) as proposed by Ruscheweyh & Sandkuhler (2002).
The present study shows that Ca2+-dependent conductances do not contribute to the discharge pattern in AFNs. In this respect AFNs differ from tonic-firing neurones (TFNs), where Ca2+-dependent slow after-hyperpolarization (AHP) regulated discharge rate and stabilized the basic form of tonic firing generated by voltage-gated Na+ and delayed-rectifier KDR currents (Melnick et al. 2004). The present finding can also explain a difference in the shape of the AHP seen between TFNs (prolonged and polyphasic) and AFNs (short and monophasic) (Thomson et al. 1989; Prescott & De Koninck, 2002).
Voltage-gated K+ channels are similar in both AFNs and TFNs. Fast inactivating KA current was found to be very small and strongly inactivated at resting potential and it was also not activated by a subthreshold depolarization. The lack of KA current appears to be important for the generation of typical discharge patterns in AFNs as well as TFNs, since the expression of large KA currents was shown to result in delayed-onset firing or irregular bust-like discharges (Yoshimura & Jessell, 1989; Grudt & Perl, 2002; Ruscheweyh & Sandkuhler, 2002). KDR current formed a major K+ conductance in both AFNs and TFNs and had similar kinetics and activation ranges. Although KDR channels are necessary for the maintenance of sustained firing in TFNs (Melnick et al. 2004), their partial block by TEA did not result in the appearance of the typical pattern of spike frequency adaptation seen in AFNs. Thus, KA and KDR currents are unlikely to be responsible for the firing adaptation in SG neurones studied here.
Although the basic properties of Na+ channels in AFNs were similar to those in TFNs (Melnick et al. 2004), their expression was lower and voltage dependency was slightly shifted to more positive potentials. Experiments with TTX and computer simulations have shown that a reduction in Na+ conductance is critical for the appearance of adaptation. Simulations have also revealed a dual effect of a positive shift in voltage dependency of Na+ channels on firing behaviour. Although the activation shift promoted spike frequency adaptation, a stronger shift in inactivation characteristics had an opposite effect. Thus, a lower Na+ conductance seems to be a major factor introducing adaptation in SG neurones.
It is possible that Na+ channels in AFNs and TFNs are formed by different combinations of principal (α) and auxiliary (β1 and β2) subunits (Black et al. 1994; Waxman et al. 1999; Blackburn-Munro & Fleetwood-Walker, 1999). In this case, the change in subunit expression as well as the up-regulation of Na+ channels associated with neuropathic pain (Waxman et al. 1999; Blackburn-Munro et al. 1999; Hains et al. 2003) can lead to hyperexcitability of dorsal horn neurones via the induction of long-term plasticity of their intrinsic firing properties. Besides, a regulation of Na+ channels through an integration of cell-specific G-protein-coupled synaptic inputs (Ma et al. 1994; Carr et al. 2003) may introduce a short-term modulation of intrinsic firing and thus dynamic modification of spinal sensory encoding.
The smaller Na+ conductance in AFNs is unlikely to result from axonal injury during the slicing procedure, as all AFNs labelled with biocytin possessed extensively branching axons crossing the borders with neighbouring laminae I and III. Labelled parts of axons were considerably longer than the 25 μm needed for spike generation in SG neurones (Safronov, 1999; Safronov et al. 1999). AFNs studied here belonged to one morphological class with its predominant localization in the lateral part of SG. The dendritic tree of AFNs has probably a rostro-caudal orientation within SG. Morphologically, AFNs were similar to islet cells (Gobel, 1978; Gobel et al. 1980; Todd, 1988; Eckert et al. 2003), although a strict conclusion could not be drawn based on neuronal images in transverse sections.
In conclusion, we suggest that different expression of Na+ channels may be responsible for variation in the degree of spike frequency adaptation in SG neurones. The present results also imply that a modulation of Na+ channels can represent an effective mechanism altering the firing behaviour of SG neurones. In addition to metabolic regulation of inwardly rectifying and A-type K+ channels (Derjean et al. 2003; Hu & Gereau, 2003), a cell-specific modulation of Na+ conductance provides one more mechanism of sensory, i.e. nociceptive, encoding and processing in the spinal cord.
Acknowledgments
We would like to thank Ms Helena Pereira for technical assistance. The work was supported by a grant from the Portuguese Foundation for Science and Technology (FCT).
References
- Alessandri-Haber N, Paillart C, Arsac C, Gola M, Couraud F, Crest M. Specific distribution of sodium channels in axons of rat embryo spinal motoneurones. J Physiol. 1999;518:203–214. doi: 10.1111/j.1469-7793.1999.0203r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley GN, Gent JP. Electrophysiological properties of substantia gelatinosa neurones in a novel adult spinal slice preparation. J Neurosci Meth. 1994;53:157–162. doi: 10.1016/0165-0270(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Black JA, Yokoyama S, Higashida H, Ransom BR, Waxman SG. Sodium channel mRNAs I, II and III in the CNS: cell-specific expression. Brain Res Mol Brain Res. 1994;22:275–289. doi: 10.1016/0169-328x(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Fleetwood-Walker SM. The sodium channel auxiliary subunits beta 1 and beta 2 are differentially expressed in the spinal cord of neuropathic rats. Neuroscience. 1999;90:153–164. doi: 10.1016/s0306-4522(98)00415-1. [DOI] [PubMed] [Google Scholar]
- Brown AG. Organization in the Spinal Cord. Berlin, Heidelberg: Springer-Verlag; 1981. [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Cervero F. Dorsal horn neurones and their sensory inputs. In: Yaksh TL, editor. Spinal Afferent Processing. New York: Plenum Press; 1987. pp. 197–216. [Google Scholar]
- Chery N, Yu XH, De Konink Y. Visualization of lamina I of the dorsal horn in live adult rat spinal cord slices. J Neurosci Meth. 2000;96:133–142. doi: 10.1016/s0165-0270(99)00195-8. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, LeMasson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nature Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Eckert WA, McNaughton KK, Light AR. Morphology and axonal arborization of rat spinal inner lamina II neurons hyperpolarized by μ-opioid-selective agonists. J Comp Neurol. 2003;458:240–256. doi: 10.1002/cne.10587. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis) J Comp Neurol. 1978;180:395–414. doi: 10.1002/cne.901800213. [DOI] [PubMed] [Google Scholar]
- Gobel S, Falls WM, Bennet GJ, Abdelmoumene M, Hayashi H, Humphrey E. An EM analysis of the synaptic connections of horseradish peroxidase-filled stalked cells and islet cells in the substantia gelatinosa of adult cat spinal cord. J Comp Neurol. 1980;194:781–807. doi: 10.1002/cne.901940406. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvment in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML. NEURON – a program for simulation of nerve equation. In: Eeckman FH, editor. Neural Systems: Analysis and Modeling. Boston: Kluwer Academic Publishers; 1993. pp. 127–136. [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Hu H-J, Gereau RW. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- LaMotte C. Distribution of the tract of Lissauer and the dorsal root fibers in the primate spinal cord. J Comp Neurol. 1977;172:529–561. doi: 10.1002/cne.901720308. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Differential termination of large-diameter and small-diameter primary afferent fibers in the spinal dorsal gray matter as indicated by labelling with horse-radish peroxidase. Neurosci Lett. 1977;6:59–63. doi: 10.1016/0304-3940(77)90065-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. Eur J Neurosci. 1994;6:998–1007. doi: 10.1111/j.1460-9568.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Ma JY, Li M, Catterall WA, Scheuer T. Modulation of brain Na+ channels by a G-protein-coupled pathway. Proc Natl Acad Sci U S A. 1994;91:12351–12355. doi: 10.1073/pnas.91.25.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M, de Curtis M, Wanke E. Modalities of distortion of physiological voltage signals by patch-clamp amplifiers: a modeling study. Biophys J. 1998;74:831–842. doi: 10.1016/S0006-3495(98)74007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M, Guatteo E, Wanke E. Action potentials recorded with patch-clamp amplifiers: are they genuine. Trends Neurosci. 1996;19:530–534. doi: 10.1016/s0166-2236(96)40004-2. [DOI] [PubMed] [Google Scholar]
- Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol. 1997;505:593–603. doi: 10.1111/j.1469-7793.1997.593ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick IV, Santos SFA, Szokol K, Szucs P, Safronov BV. Ionic basics of tonic firing in spinal substantia gelatinosa neurons of rat. J Neurophysiol. 2004;91:646–655. doi: 10.1152/jn.00883.2003. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol. 2002;539:817–836. doi: 10.1113/jphysiol.2001.013437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethelyi M. Preterminal and terminal axon arborizations in the substantia gelatinosa of cat's spinal cord. J Comp Neurol. 1977;172:511–521. doi: 10.1002/cne.901720307. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkuhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV. Spatial distribution of Na+ and K+ channels in spinal dorsal horn neurones: role of the soma, axon and dendrites in spike generation. Prog Neurobiol. 1999;59:217–241. doi: 10.1016/s0301-0082(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. J Physiol. 1995;487:91–106. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Wolff M, Vogel W. Functional distribution of three types of Na+ channel on soma and processes of dorsal horn neurones of rat spinal cord. J Physiol. 1997;503:371–385. doi: 10.1111/j.1469-7793.1997.371bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Wolff M, Vogel W. Axonal expression of sodium channels in rat spinal neurones during postnatal development. J Physiol. 1999;514:729–734. doi: 10.1111/j.1469-7793.1999.729ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W, Dieudonné S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-d-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Headley PM. Membrane characteristics and synaptic responsiveness of superficial dorsal horn neurons in a slice preparation of adult rat spinal cord. Eur J Neurosci. 1989;1:479–488. doi: 10.1111/j.1460-9568.1989.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Electron microscope study of Golgi-stained cells in lamina II of the rat spinal dorsal horn. J Comp Neurol. 1988;275:145–157. doi: 10.1002/cne.902750112. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and pain. Proc Natl Acad Sci U S A. 1999;96:7635–7639. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991. [Google Scholar]
- Yoshimura M, Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]