Abstract
We have explored the mechanisms involved in the facilitation of glutamate release mediated by the activation of kainate receptors in the rat hippocampus using isolated nerve terminal (synaptosome) and slice preparations. In hippocampal nerve terminals, kainate (KA) produced an increase of glutamate release at concentrations of agonist ranging from 10 to 1000 μm. In hippocampal slices, KA at low nanomolar concentrations (20–50 nm) also produced an increase of evoked excitatory postsynaptic currents (eEPSCs) at mossy fibre–CA3 synapses. In both, synaptosomes and slices, the effect of KA was antagonized by CNQX, and persisted after pretreatment with a cocktail of antagonists for other receptors whose activation could potentially have produced facilitation of release. These data indicate that the facilitation of glutamate release observed is mediated by the activation of presynaptic glutamate receptors of the kainate type. Mechanistically, the observed effects of KA appear to be the same in synaptosomal and slice preparations. Thus, the effect of KA on glutamate release and mossy fibre–CA3 synaptic transmission was occluded by the stimulation of adenylyl cyclase by forskolin and suppressed by the inhibition of protein kinase A by H-89 or Rp-Br-cAMP. We conclude that kainate receptors present at presynaptic terminals in the rat hippocampus mediate the facilitation of glutamate release through a mechanism involving the activation of an adenylyl cyclase–second messenger cAMP–protein kinase A signalling cascade.
Kainate receptors are a family of glutamate receptors that can postsynaptically mediate excitatory synaptic transmission at some synapses, but also presynaptically modulate neurotransmitter release at others. In the latter context, kainate receptors have been implicated in the modulation of both glutamate and GABA release in the hippocampus (see Kullmann, 2001; Lerma, 2003; Huettner, 2003 for reviews).
Studies on the effect of the activation of kainate receptors on evoked excitatory postsynaptic currents (eEPSCs) in the hippocampus have produced diverse results. While some authors describe kainate receptor activation as producing a decrease in the amplitude of eEPSCs in CA1 (Chittajallu et al. 1996; Vignes et al. 1998; Kamiya & Ozawa, 1998; Frerking et al. 2001) and in the mossy fibre–CA3 region of the hippocampus (Kamiya & Ozawa, 2000; Schmitz et al. 2000; Contractor et al. 2000), others report that the activation of kainate receptors produces an increase of the amplitude of eEPSCs (Contractor et al. 2000; Schmitz et al. 2001; Lauri et al. 2001a, b). Interestingly, with regard to the bidirectionality of the reported effects of kainate (KA), some studies indicate that kainate receptor activation has a biphasic effect, such that low (20–50 nm) KA concentrations produce an increase in glutamatergic transmission, whereas higher concentrations produce a decrease in eEPSCs (see Kullmann, 2001; Lerma, 2003; Huettner, 2003 for reviews). With respect to the potentiating effects, kainate receptors have been suggested to contribute to the strong facilitation observed at mossy fibre synapses when the presynaptic axons are stimulated at intermediate frequencies (Schmitz et al. 2001). Contractor et al. (2000) verified the involvement of kainate receptors by demonstrating that the deletion of glutamate GluR6 receptor subunits reduced this frequency-dependent facilitation. Further evidence for a presynaptic facilitatory role for kainate receptors has come from reports implicating these receptors in mossy fibre long-term potentiation (LTP) (Bortolotto et al. 1999; Contractor et al. 2001; Lauri et al. 2001b; Schmitz et al. 2001), a form of LTP defined as being independent of postsynaptic NMDA receptors (Nicoll & Malenka, 1995).
The exact mechanism by which kainate receptors produce an increase in glutamate release remains unclear. Indeed, the precise localization of receptors that are responsible for this facilitation remains to be demonstrated. In the present work, we have examined the effect of KA on glutamate release from isolated hippocampal nerve terminals (synaptosomes), a preparation with which any confounding postsynaptic effects of KA on glutamate release are obviated by the minimal presence of functional postsynaptic elements. Additionally, we have used a more intact preparation, in the form of hippocampal slices, to study the effects of low concentrations of KA on glutamate release at the mossy fibre–CA3 synapse. Finally, we determined the mechanism involved in the effects of KA observed with the two complementary preparations. We found that the facilitation of glutamate release shows a major sensitivity to the stimulation of adenylyl cyclase (AC) activity leading to cAMP-mediated activation of protein kinase A (PKA). This indicates that kainate receptors are coupled to a cAMP cascade in some parts of the hippocampus, including, at least, mossy fibre terminals.
Methods
Preparation of synaptosomes
Synaptosomes were prepared from the cerebral hippocampi of 2-month-old male rats (N = 35) essentially as previously described (Sihra, 1997). Animals were killed by stunning followed by decapitation under procedures covered by a project licence issued by the Home Office, under the Animals (Scientific Procedures) Act 1986. The final synaptosomal fraction was resuspended in Hepes-buffered incubation medium (HBM) containing (mm): 140 NaCl, 5 KCl, 5 NaHCO3, 1 MgCl2·6H2O, 1.2 Na2HPO4, 10 glucose, 20 Hepes (pH 7.4). Protein concentration was then determined using the Bradford assay. Synaptosomes were centrifuged in the final wash to obtain synaptosomal pellets with 0.5 mg protein. Synaptosomal pellets were stored on ice and used within 1–2 h.
Glutamate release assay
Glutamate release was assayed by on-line fluorometry (Nicholls & Sihra, 1986). Pelleted synaptosomes were resuspended at a protein concentration of 0.5 mg ml−1 in HBM containing 16 μm bovine serum albumin (BSA) and incubated in a stirred and thermostatted cuvette at 37°C in a Perkin-Elmer LS-3B spectrofluorimeter. NADP+ (1 mm), glutamate dehydrogenase (50 units ml−1) and CaCl2 (1 mm) were added after 3 min. After a further 10 min of incubation, 1 mm 4-aminopyridine (4-AP) was added to stimulate glutamate release. The oxidative deamination of released glutamate, leading to the reduction of NADP+, was monitored by measuring NADPH fluorescence at excitation and emission wavelengths of 340 and 460 nm, respectively. Data were accumulated at 2 s intervals. A standard of exogenous glutamate (5 nmol) was added at the end of each experiment and the fluorescence change produced by the standard addition was used to calculate the released glutamate as nanomoles glutamate per milligram synaptosomal protein. Release traces are shifted vertically to align the point of depolarization as zero release. Release values quoted in the text are levels attained at ‘steady-state’ after 4 min of depolarization (nmol (mg protein)−1 (4 min)−1). Cumulative data were analysed using Lotus 1-2-3 spreadsheets and MicroCal Origin. Statistical analysis was performed by Student's t test (* denotes P < 0.05; different from control).
Hippocampal slices
Hippocampal slices were prepared from 21- to 24-day-old rats (N = 20), as described in detail previously (Rodríguez-Moreno et al. 1997; Rodríguez-Moreno & Lerma, 1998). The whole brain containing the two hippocampi was positioned on the stage of a vibratome slicer and cut to obtain 350 μm thick transverse brain slices, which were maintained continously oxygenated for at least 1 h before use.
Electrophysiological recordings
Electrophysiological recordings were performed from neurones visually identified by IR-DIC microscopy using a 40 × water immersion objective. All experiments were carried out at room temperature (23–26°C). Slices were continously perfused with a solution consisting of (mm): 124 NaCl, 2.69 KCl, 1.25 KH2PO4, 2 MgSO4,1.8 CaCl2, 26 NaHCO3 and 10 glucose (pH 7.3, 300 mosmol l−1), supplemented with antagonists as required. Drugs were applied by gravity, switching between four perfusion lines. To evoke mossy fibre EPSCs, electrical pulses were applied by a bipolar electrode made from a glass pipette, placed in stratum lucidum just adjacent to the limit of the dentate gyrus and always within 100–200 μm from the recording site. Tight-seal (> 1 GΩ) whole-cell recordings were obtained from the cell body of neurones situated in the CA3 pyramidal layer. Patch electrodes were fabricated from borosilicate glass and had a resistance of 5–10 MΩ when filled with (mm): 120 CsCl, 8 NaCl, 1 MgCl2, 0.2 CaCl2, 10 Hepes, 2 EGTA (pH 7.3, 287 mosmol l−1)). In all experiments, 20 mm QX-314 was included in the pipette solution to avoid firing of unclamped cell compartments. Neurones were voltage clamped using an Axopatch 200B amplifier (Axon Instruments). Access resistance (8–30 MΩ) was regularly monitored during recordings and cells were rejected if it changed more than 15% during the experiment. Data were filtered at 2 kHz, digitized and stored on a computer using pCLAMP (Axon Instruments).
Compounds
Bicuculline methobromide, kainate, naloxone, and salts were purchased from Sigma; 2-hydroxy-saclofen, SYM2206, GYKI52466, DPCPX, d-2-phosphonovaleric acid (D-APV), MPPG, MCPG and L-CCG-1 were obtained from Tocris. Forskolin, dideoxyforskolin, IBMX, H-89 and Rp-Br-cAMP were purchased from Calbiochem.
Results
Kainate receptors activation increases glutamate release in synaptosomes
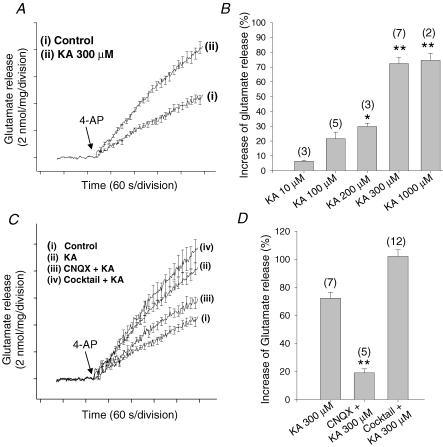
Using an on-line enzymatic assay for measuring glutamate, we observed KA-mediated facilitation of glutamate release from hippocampal synaptosomes (Fig. 1A). We applied KA at different concentrations to hippocampal synaptosomes in the presence of the non-competitive AMPA receptor antagonists GYKI52466 or SYM2206 (100 μm) to avoid the activation of AMPA receptors by KA. The application of 10 and 100 μm KA produced a slight (6 ± 0.7 and 21 ± 4.5%, respectively) and statistically insignificant facilitation of glutamate release evoked by 1 mm 4-AP (nmol (mg protein)−1 (4 min)−1: Control, 4.7 ± 0.2, n = 20; 10 μm KA, 5.0 ± 0.4; 100 μm KA, 5.7 ± 0.5). The application of 200, 300 and 1000 μm KA, all produced a clear (30 ± 2.3, 72 ± 4.2 and 74 ± 4.9%, respectively) and statistically significant facilitation of glutamate release (nmol (mg protein)−1 (4 min)−1: 200 μm KA, 6.1 ± 0.2, n = 3; 300 μm KA, 8.1 ± 0.4, n = 7; 1000 μm KA, 8.2 ± 0.6, n = 2) (Fig. 1B). We never observed a decrease in glutamate release at any of the KA concentrations used. Given that in our hands 300 and 1000 μm KA produced similar extents of potentiation of glutamate release (72 and 74%), to minimize any effects of KA on glutamate transporters at high concentrations, we use 300 μm KA forthwith in our study.
Figure 1. Kainate-induced facilitation of 4-AP-evoked glutamate release in hippocampal synaptosomes: pharmacological properties.
A, glutamate release in the absence (i) and presence (ii) of 300 μm KA (added 1 min before the addition of 4-AP). B, concentration dependency of effect of KA, in the presence of 100 μm of the non-competitive AMPA antagonist SYM2206, on glutamate release expressed as a percentage increase from control. C, effect of KA on glutamate release in control conditions (i) or following addition of 300 μm KA (ii), 100 μm CNQX + 300 μm KA (iii), and inhibitor cocktail (1.5 mm MCPG, 1.5 mm MPPG, 25 μm bicuculline, 150 μm 2-OH-saclofen, 50 μm atropine sulphate, 0.1 μm DPCPX, and 100 μm naloxone) + 300 μm KA (iv). D, quantification of modulation using release levels achieved 4 min post 4-AP. The numbers in parentheses indicate the number of experiments using independent synaptosomal preparations. Results are the mean ± s.e.m. (*P < 0.05, **P < 0.01, Student's unpaired t test).
We next re-examined the observed KA-induced facilitation in the presence of the AMPA/kainate receptor antagonist CNQX (100 μm). In our conditions, CNQX is effectively a kainate receptor antagonist, given that we routinely blocked AMPA receptors with GYKI52466 or SYM2206 (100 μm). In the presence of CNQX, the release produced by the application of KA (300 μm) was 5.6 ± 0.39 nmol mg−1(n = 5) (versus 8.1 ± 1.1 in the absence of CNQX), indicating that the facilitatory effect of KA is blocked by CNQX. These results suggest that the facilitation of glutamate release that we observe is mediated by the activation of a presynaptic kainate receptor (Fig. 1C and D). However, this effect of KA on glutamate release from hippocampal synaptosomes may be indirect, given that a number of other presynaptic receptors may control the release of glutamate, with the neurotransmitter ligands for these receptors being released secondarily in response to KA application. To minimize the potential participation of the most prominent of these presynaptic receptors, in the present experiments, we treated the synaptosomes with an inhibitor cocktail which included the mGluR antagonists MCPG and MPPG (1.5 mm each), as well as naloxone (100 μm), bicuculline (25 μm), 2-OH-saclofen (150 μm), atropine sulphate (50 μm) and DPCPX (0.1 μm), to block metabotropic glutamate receptors, and opioid, GABAA, GABAB, muscarinic and adenosine receptors, respectively. Under these conditions, KA was equally effective; thus in the presence of the cocktail, KA produced an 86% increase in 4AP-evoked release (up to 9.5 ± 0.8 nmol (mg protein)−1 (4 min)−1, n = 8) versus 8.1 ± 0.4 nmol (mg protein)−1 (4 min)−1 KA-facilitated release in the absence of cocktail; Fig. 1C and D. The release in the presence of the cocktail alone was slightly increased, by 8 ± 3% (5.1 ± 0.9 nmol (mg protein)−1 (4 min)−1, n = 12; control, 4.7 ± 0.2 nmol (mg protein)−1 (4 min)−1), but this effect was statistically insignificant. These data therefore argue against the possibility that the action of KA on glutamate release was due to transmitters secondarily activating one of the aforementioned receptors and suggest that the selective activation of a presynaptic glutamate receptor of the kainate type produces a facilitation of glutamate release in hippocampal nerve terminals.
The kainate-induced increase in glutamate release involves the cAMP cascade in hippocampal synaptosomes
The mechanism underlying the facilitation of glutamate release by KA remains to be elucidated. Having confirmed the selectivity of the action of KA on glutamate release, we further explored the mechanism underlying the effect. We looked at the possibility that a second messenger system acts as a mediator of this effect. Lauri et al. (2001b), in studies with mossy fibre–CA3 synapses, have suggested that a signalling cascade leading to protein kinase C (PKC) activation is not involved because the facilitatory effects of KA are still present in the presence of the selective PKC inhibitor calphostin C. Given that the cAMP cascade is one of the major second messenger systems regulating glutamate release at several hippocampal synapses, we analysed the effects of upregulating and inhibiting this pathway on KA-mediated facilitation of glutamate release from hippocampal synaptosomes.
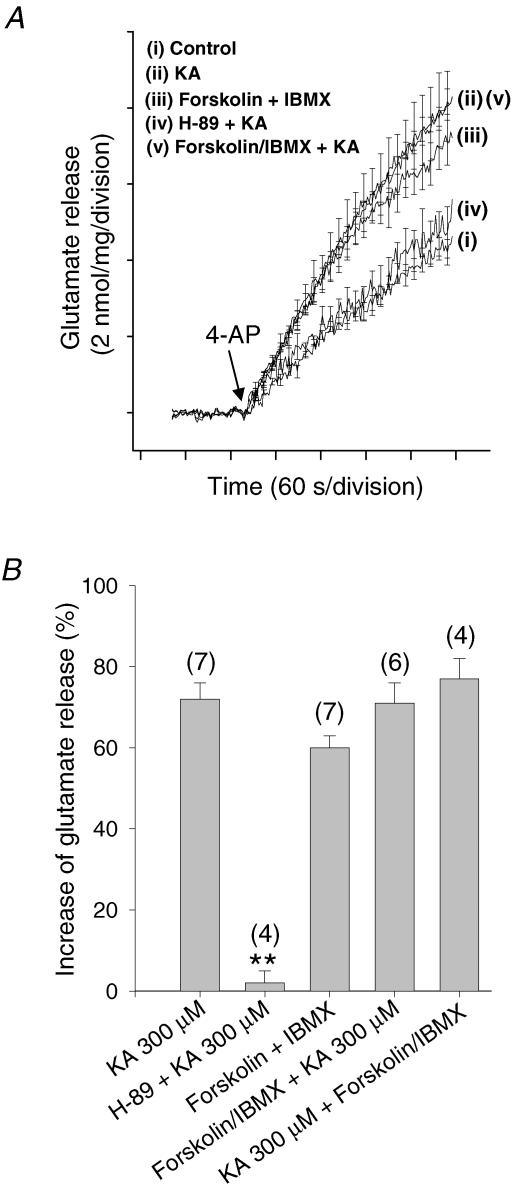
We first analysed the effect of the inhibition of the cAMP-dependent protein kinase (PKA) on glutamate release by using the cell-permeable and selective inhibitor H-89 (100 μm). H-89 itself produced an inhibition of glutamate release of 30% (data not shown), but subsequent application of 300 μm KA increased glutamate release by 30% (i.e. to a level of release comparable to control) versus the 72% facilitation of glutamate release obtained without H-89. These results indicate that inhibition of the activation of PKA somewhat prevents the action of KA (Fig. 2A and B).
Figure 2. Activation of adenylyl cyclase and downstream protein kinase A underlies the kainate-mediated facilitation of 4-AP-evoked glutamate release in hippocampal synaptosomes.
A, glutamate release under control conditions (i) and in the presence of H-89 + KA (iv), forskolin/IBMX (iii), KA (ii) and forskolin + IBMX + KA (v). B, quantification of modulation using release levels achieved 4 min post 4-AP. The numbers in parentheses indicate the number of experiments using independent synaptosomal preparations. Results are the mean ± s.e.m. (**P < 0.01, Student's unpaired t test). Note that the reverse experiment, with the addition of KA being prior to forskolin, produced a facilitation of glutamate essentially indistinguishable from the above.
To further test the hypothesis that the PKA pathway mediates the action of KA on glutamate release, we decided to increase PKA activity by enhancing cAMP production with forskolin. The application of forskolin (100 μm) + IBMX (50 μm) caused a 60 ± 3.4% increase in glutamate release (7.5 ± 0.4 nmol (mg protein)−1 (4 min)−1, n = 7; control, 4.7 ± 0.2 nmol (mg protein)−1 (4 min)−1). In the presence of forskolin and IBMX, KA (300 μm) induced an 11% increase in glutamate release (up to 8.3 ± 0.4 nmol (mg protein)−1 (4 min)−1, n = 6), indicating that the activation of adenylyl cyclase by forskolin occludes the action mediated by the activation of kainate receptors (Fig. 2A and B). Forskolin stimulates cAMP production through adenylyl cyclase activation, but it may also have a pharmacological effect through cAMP-independent mechanisms. The isomer of forskolin, 1,9-dideoxyforskolin, on the other hand, does not activate adenylyl cyclase, but mimics some of the cAMP-independent actions of forskolin (Hoshi et al. 1988). In cortical synaptosomes (including the hippocampus), previous studies have confirmed, however, that dideoxyforskolin has no effects on 4-AP-evoked glutamate release (Wang & Sihra, 2003). This indicates that the actions of forskolin are related to increases in cAMP levels in synaptosomes and are not confounded by non-specific effects of the compound.
To confirm the occlusion of the effects of KA by cAMP production, we also performed the reverse experiment with KA and forskolin, in which KA was added prior to forskolin. The application of 300 μm KA produced an increase in glutamate release of 62 ± 6% (n = 4). The subsequent addition of forskolin + IBMX (100 and 50 μm, respectively) induced a facilitation of glutamate release of 15 ± 3%– indicating that KA occludes the action of the forskolin (Fig. 2B). The results indicate that the actions of forskolin and those of KA reciprocally occlude each other, suggesting their dependent use of a common intracellular mechanism to produce a facilitation of glutamate release.
The activation of kainate receptors by low concentrations of KA produces an increase in the amplitude of NMDA- and AMPA-evoked postsynaptic currents in transverse hippocampal slices
While the results from studies with hippocampal slices looking at the effect of the activation of kainate receptors on eEPSCs have been diverse (Chittajallu et al. 1996; Vignes et al. 1998; Kamiya & Ozawa, 1998, 2000; Schmitz et al. 2000, 2001; Contractor et al. 2000; Frerking et al. 2001; Lauri et al. 2001a, b), in all cases a presynaptic locus of the action for KA was proposed, postulating that both facilitatory and inhibitory effects are mediated by the activation of presynaptic kainate receptors. We reproduced these results and determined whether the mechanisms of action of KA that we observed in synaptosomes are similar to those responsible for the increases in eEPSC amplitude that we observed in slice recordings from CA3 pyramidal neurones receiving mossy fibre inputs (Fig. 3A).
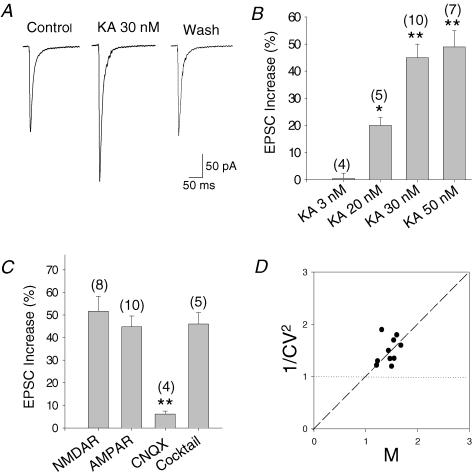
Figure 3. Low concentrations of kainate increase the eEPSC amplitude in hippocampal slices.
A, effect of 30 nm KA on AMPA receptor-mediated EPSC recorded from CA3 neurones in the presence of D-APV and bicuculline. B, concentration dependency of effect of KA on eEPSC amplitude, expressed as a percentage increase from control. C, 30 nm KA produces a similar increase in NMDA- and AMPA-mediated eEPSCs. The effect is antagonized by CNQX in NMDA-mediated currents. In the presence of a cocktail that contains metabotropic glutamate receptors and GABAA, GABAB, opioid, muscarinic, β-adrenergic and adenosine receptor antagonists, KA produces a similar increase of the amplitude of AMPA receptor-mediated currents. The numbers in parentheses indicate the number of experiments. Results are the mean ± s.e.m. (**P < 0.01, Student's t test). D, mean EPSC amplitudes and their CVs were measured during KA application and normalized by the respective control values in each cell. Mversus the fractional variation in 1/CV2 in all neurones studied is plotted. Experimental data follow the predicted relation for a purely presynaptic (diagonal line) rather than postsynaptic (horizontal line) site of action.
We performed some experiments in transverse hippocampal slices from 3- to 4-week-old rats. Whole-cell patch clamp recordings were obtained by stimulating mossy fibres and recording from CA3 cells. We first recorded NMDA-mediated excitatory postsynaptic currents in the presence of SYM2206 (100 μm) (to selectively block AMPA receptors), bicuculline (25 μm) and glycine (10 μm). We recorded the synaptic responses mediated by these receptors at a membrane potential of −30 mV to relieve their blockade by Mg2+. To be sure that our stimulating electrode was activating mostly mossy fibres, we introduced the group II mGluR agonist L-CCG-1, which has been shown to block synaptic transmission at mossy fibre terminals, but not from commissural associational connections. More that 90% of the evoked EPSC was blocked by 30 μm L-CCG-1 (91 ± 4.6%, n = 8), indicating that most of the synaptic current that we were recording originated from mossy fibre activation. In agreement with previous reports, KA (30 nm) produced an increase in the amplitude of the eEPSCs of the 52 ± 7%(n = 8) (Fig. 3C). The effect of 30 nm KA was antagonized almost completely by the non-selective AMPA/kainate receptor antagonist CNQX (100 μm), such that in the presence of the antagonist, KA increased the eEPSCs by just 6%(n = 4) (Fig. 3C). These data therefore indicated that the effect is mediated by the activation of kainate receptors. Next, we recorded AMPA-mediated currents (eEPSCs) in the presence of D-APV (50 μm) and bicuculline (25 μm). The addition of KA (30 nm) caused an increase in the amplitude of eEPSCs of 45 ± 5%(n = 10), thus indicating a similar degree of facilitation by KA of both NMDA- and AMPA-mediated EPSC (Fig. 3A and C). As the dose–response curves for facilitation indicated that 20, 30 and 50 nm KA produced a significant increase in AMPA-mediated eEPSC amplitude (Fig. 3B), we subsequently used 30 nm KA in this study.
As noted for synaptosomal experiments, the effect of KA on mossy fibre terminals could be indirect and occurring in response to the activation of presynaptic receptors by neurotransmitter(s) released secondarily to KA application. To minimize the participation of putative presynaptic receptors, with the current experiments we treated the slices with a cocktail which included the mGluR antagonists MCPG and MPPG (1.5 mm each), as well as naloxone (100 μm), bicuculline (25 μm), 2-OH-saclofen (150 μm), atropine sulphate (50 μm), propanolol (100 μm) and DPCPX (0.1 μm), to block metabotropic glutamate receptors, and opioid, GABAA, GABAB, muscarinic, β-adrenergic and adenosine receptors, respectively. Under these blocking conditions, KA was equally effective, with −30 nm KA producing a 46 ± 5%(n = 5) increase in the EPSCs. This effectively eliminated the possibility of an indirect action of KA on glutamate release through, at the least, the aforementioned receptors as presynaptic regulators (Fig. 3C).
To examine whether the observed effects of KA on EPSCs were presynaptic, we plotted the change in the coefficient of variation (CV) of synaptic responses versus the change in their averaged amplitude. Consistent with a presynaptic effect of KA, the increase in the mean EPSC (AMPA receptor-mediated) amplitude was paralleled by an increase in 1/CV2, a parameter known to vary as a function of quantal content rather than of quantal size (Forsythe & Clements, 1990; reviewed by Thomson & Deuchars, 1995). Indeed, the change in 1/CV2 was proportional to the change in mean EPSC amplitude, implying that a change in release probability accounts for most of the observed change in amplitude (Fig. 3D). The same result was obtained from NMDA-mediated currents (data not shown). These results indicate that the selective activation of a presynaptic rather than postsynaptic kainate receptor at mossy fibre synapses is instrumental in the facilitation of glutamate release, seen here as an increase in EPSC amplitude.
In hippocampal slices, as in synaptosomes, kainate receptor-mediated facilitation of glutamate release involves a cAMP-dependent cascade
Having confirmed the selectivity of the action of KA, we further explored the possibility that the same second messenger system that acts to mediate effects in synaptosomes also operates in the facilitation of EPSCs in slices.
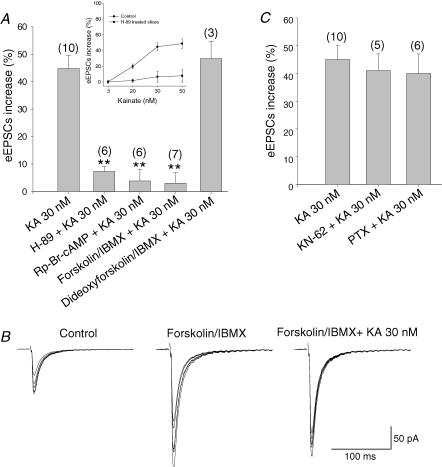
Firstly, we studied the effect of the PKA inhibitor H-89 on glutamate release. H-89 (100 μm) itself produces an inhibition of a glutamate release of 16 ± 3% in slices; the subsequent application of KA increased glutamate release by 7% (versus the 45 ± 5% effect of KA (30 nm) without H-89) (Fig. 4A). The effect of H89 was apparent across the full range of KA concentrations producing facilitation (Fig. 4A, inset). Thus, the inhibition of PKA prevents the action of KA. We also used the PKA inhibitor Rp-Br-cAMP (100 μm). In the presence of Rp-Br-cAMP, 30 nm KA produced a small and statistically insignificant increase of a 3.8 ± 4% increase, indicating a complete abolition of the effect of KA on eEPSC amplitudes (Fig. 4A).
Figure 4. Activation of adenylyl cyclase and downstream protein kinase A underlies the kainate-mediated facilitation of glutamate release in hippocampal slices.
A, in slices pretreated (incubated) with H-89, Rp-Br-cAMP or with forskolin + IBMX, KA does not produce an increase of the eEPSC amplitude. The effect is specific since in the presence of the inactive analogue of forskolin, dideoxyforskolin, KA produces an increase of 49 ± 7% of the mean eEPSC amplitude. Inset shows the inhibitory effect of H89 across the full range KA concentrations that produce facilitation. B, a representative experiment showing the increase that is produced by bath-applied forskolin and IBMX. After a steady potentiation forskolin was washed out and KA was introduced, KA (30 nm) does not affect the amplitude of the AMPA-mediated eEPSCs in this situation. C, the KA-induced increase in glutamate release was unaltered by treating the slices with the inhibitor of CaMKII KN62 (5 μm) and with the G-protein inhibitor pertussis toxin (PTX; 5 μg ml−1, 3–4 h 37°C). Results are the mean ± s.e.m. (**P < 0.01, Student's t test).
Next, we looked at the effect of adenylyl cyclase activation on the KA-mediated increase in EPSCs by using forskolin. Forskolin-induced potentiation of synaptic transmission in CA3 is long lasting (Tong et al. 1996). For this reason, most experiments were done after incubating slices with forskolin for at least 1 h. In a few instances, forskolin was applied to the bath whilst measuring the synaptic transmission and removed just before application of KA (see Fig. 4B). The application of forskolin (30 μm) + IBMX (5 μm) caused a 3-fold potentiation of mossy fibre responses (312 ± 47% with respect the control) (n = 4). However, under these same conditions, KA (30 nm) failed to affect the eEPSC amplitude (3 ± 5%, n = 4versus the 45% increase in the absence of forskolin + IBMX). This therefore indicated that the activation of adenylyl cyclase by forskolin and consequent cAMP production occludes the action mediated by the activation of kainate receptors (Fig. 4A). When the experimental protocol was reversed with respect to KA and forskolin, i.e we applied 30 nm KA first followed by forskolin 5 min later, while KA produced the expected increase of 49 ± 7%(n = 4) in EPSC amplitudes, subsequent addition of forskolin only produced a modest increase of 39 ± 7%(n = 4) (i.e, to 207% with respect the control versus 312% with forskolin alone), confirming that KA reciprocally occludes the action of forskolin.
As confirmed for synaptosomes previously (Wang & Sihra, 2003), to ensure in slices that the observed forskolin effects were due to cAMP production rather than any non-specific effect of the diterpene, we performed control experiments using 1,9-dideoxyforskolin, the inactive analogue of forskolin. In the presence of dideoxyforskolin (100 μm), KA (30 nm) produced an increase of 48 ± 6%(n = 4) in the amplitude of the eEPSCs, thus displaying no significant effect on eEPSCs compared to KA alone (Fig. 4A). This points to a large part of the forskolin effect on KA-mediated modulation being attributable to increases in cAMP levels produced by adenylyl cyclase activation.
Once again, we confirmed that the downstream effects of forskolin were mediated through PKA by measuring the effect of Rp-Br-cAMP on forskolin-mediated enhancement of EPSCs. Slices pre-incubated with 100 μm Rp-Br-cAMP for 30 min (the cyclic nucleotide was retained in the experimental buffer during forskolin treatment) showed a virtually complete abrogation of the forskolin-mediated enhancement of EPSCs. Together, these results indicate that forskolin, through an adenylyl cyclase (AC)–cAMP–PKA pathway, occludes the action of KA and vice versa, and that the modulation seen with these two regulators may involve a common intracellular mechanism to produce a facilitation of glutamate release.
Adenylyl cyclase I (AC I) is specifically expressed in large amounts in mossy fibres (Xia et al. 1991). The activation of the AC I to produce an increase in glutamate release can be mediated by the activation of the calcium–calmodulin-dependent protein kinase II (CaMKII), as proposed by Weisskopf et al. (1994) to explain increases in glutamate release and LTP at mossy fibre-CA3 synapses. We tested for this possibility in slices treated with the membrane-permeable inhibitor of CaMKII, KN-62. In the presence of 5 μm KN-62, KA was, however, found to be equally effective at increasing the eEPSC amplitude (41 ± 6%versus 45 ± 5% in control) (Fig. 4C). This therefore indicates that the proposed signalling pathway activated by CaMKII does not converge with the pathway triggered by kainate receptors at mossy fibre terminals.
Finally, given that slices also displayed an inhibitory effect of KA, albeit at higher concentrations of the agonist, we carried out one final control to ensure that this effect in no way impinged on the facilitation we observed. The inhibitory effects of KA have been attributed to a metabotropic mechanism involving the inhibitory G-proteins Gi/o. However, at the concentrations of KA producing facilitating effects, we observed no effect of pertussis toxin (PTX) on the response (Fig. 4C).
Discussion
Using biochemical and electrophysiological studies in hippocampal nerve terminals and slices, this paper examines the mechanism of kainate receptor-mediated modulation of synaptic glutamate release. The activation of kainate receptors produced an increase in glutamate release. This action is compatible with the well-known convulsant and excitotoxic properties of this compound (Coyle, 1983). Thus, we performed experiments to demonstrate that the facilitatory effect of KA was not only present in hippocampal synaptosomes but also in hippocampal slices. Subsequently, we also showed that the KA-mediated increase of glutamate release that we observe from hippocampal synaptosomes and slices involves the activation of adenylyl cyclase, the production of second messenger cAMP and the downstream stimulation of PKA. Taken together, our results indicate that the activation of presynaptic kainate receptors in the hippocampus produces facilitation of glutamate release that is functionally linked to the activation of the adenylyl cyclase and the subsequent activation of the protein kinase A.
Presynaptic kainate receptors
From our results, it is apparent that the activation of kainate receptors produces an increase in glutamate release (at all concentrations of KA that we used in synaptosomes and at nanomolar concentrations in slices). Considering that the other glutamate receptors that can be activated by KA are blocked by SYM2206 (or GYKI52466 in some cases), and that the block of other metabotropic and ionotropic receptors potentially present did not prevent the action of KA, we can conclude that the effect that we observe is mediated by the activation of glutamate receptors of the kainate type. Additionally, CNQX, which in our experimental conditions (in synaptosomes and in slices when recording NMDA-mediated currents) was used as a kainate receptor antagonist (with AMPA receptors being blocked by SYM2206), prevented the action of KA, thus again indicating that the modulation was due to kainate receptors. Given that, in the synaptosome preparation, the presence of postsynaptic membranes is minimal and that, in slices, the change in mean current amplitude was proportional to the change in the coefficient of variation, a presynaptic mode of action for KA was indicated.
The facilitatory effects of KA on glutamate release from synaptosomes and the eEPSCs in slices qualitatively display remarkable similarity with respect to the involvement of cAMP–PKA. However, the key detractor from the unequivocal proposal of a commonality of mechanism underlying the facilitation in the two models, is the different dose dependencies observed. This discrepancy is perhaps not surprising given the undeniable difference in nature of the two preparations; however, several specific reasons can be posited for the exquisite sensitivity to KA of slices compared to synaptosomes. Firstly, axonal localization of kainate receptors has been indicated by GluR6/7 immunolabelling studies of the mossy fibre–CA3 synapse, for instance (Petralia et al. 1994). While these receptors would obviously be activated by KA in slice preparations, in isolated nerve terminals devoid of an axonal compartment, the contributions of these receptors would be severely attenuated, if not completely removed. Also likely contributing to the relative sensitivity to KA of the slices compared to synaptosomes is the potential enhancement of response in the former by heterosynaptic interactions of synapses through presynaptic, juxtasynaptic kainate receptors (Schmitz et al. 2000). This latter phenomena, thought to contribute to frequency facilitation of glutamate responses mediated through presynaptic kainate receptors (Schmitz et al. 2001), would clearly not occur in the dissociated nerve terminal situation, where indeed the very nature of the release assay used here obviates any potential effects due to endogenously released glutamate. Secondly, the parsimonious possibility remains that the higher concentrations of KA required to obtain facilitation in the synaptosomal model reflect an uncoupling/inactivation of functional receptors due to the preparative procedures. Indeed, the requirement for the relatively higher concentrations of agonist in the synaptosomal preparation compared to slices is a feature of most studies that have examined KA-mediated presynaptic modulation using biochemical methodology. Notwithstanding this, even in slice preparations, KA sensitivity can be seen to vary broadly depending on the synapse (autoreceptor activation in CA1 synapses requires 10 times higher KA than elsewhere: Kamiya & Ozawa, 1998) and subunit constitution (GluR7 subunit-containing receptors show 10-fold lower affinity that other non-NMDA receptors: Schiffer et al. 1997). These observations together reason against the rejection of a commonality of mechanism between the two models used here, purely on the basis of differing concentration dependencies of modulation.
Several previous reports have indicated that kainate receptor activation has a biphasic effect on synaptic transmission, whereby low KA concentrations (20–50 nm) produced an increase in glutamate release, but higher concentrations effected a decrease in eEPSC. While the latter inhibition was also apparent in our hands (data not shown), the hippocampal synaptosomes studied herein never displayed a decrease in glutamate release, regardless of the KA concentration used. This observation, our previous observations using cortical synaptosomes (Perkinton & Sihra, 1999), and other synaptosomal studies, including those with hippocampal synaptosomes (Malva et al. 1996; Poli et al. 1985; Zhou et al. 1995), have all supported the ‘classical studies’ with slices from a variety of brain areas (Coyle, 1983) showing facilitation of glutamate release in response to KA. In contrast to this body of literature is a prominently cited report of KA-mediated inhibition of [3H]-glutamate release from hippocampal synaptosomes (Chittajallu et al. 1996). Notwithstanding the notable fact that, while the former studies mentioned measured endogenous neurotransmitter, the latter study utilized radiolabelled tracer to assay release (with the inherent ambiguities of the methodology discussed in Perkinton & Sihra, 1999 and Nicholls & Sihra, 1986), numerous electrophysiological observations have indicated a clear inhibitory effect of KA on excitatory synaptic transmission (see Lerma, 2003; Huettner, 2003 for reviews). The question of how these studies can be reconciled with the large majority of aforementioned biochemical studies showing KA-mediated increases in neurotransmitter release therefore remains unanswered. The notion that the inhibitory kainate receptors/effects are in fact localized exclusively postsynaptically is certainly not supportable from the available evidence. However, another rather facile explanation for the lack of inhibition of glutamate release, at least in synaptosomes, could be that the ‘inhibitory kainate receptor’ is somehow lost or inactivated during the synaptosomal preparation. Yet another possibility is that presynaptic terminals need a postsynaptically released messenger to produce inhibition, and such a retrograde messenger is lost in the preparation and purification of synaptosomes. Perhaps the most tenable explanation of all arises from considering the bimodal effects of KA in slice preparations. Thus, while facilitation might occur at low concentrations of agonist through potential mechanisms discussed below, at higher concentrations, the increased ionotropic effects of KA may reasonably be suggested to cause the inactivation of voltage-dependent ion channels supporting nerve terminal excitability and thereby inhibit glutamate release (MacDermott et al. 1999; Miller, 1998; Chittajallu et al. 1996; Kamiya & Osawa, 1998). Isolated nerve terminals would not be subject to such inactivation effects to same degree, given their lack of axonal input and consequent dependence on the use of chemical activators/secretagogues for stimulation.
Mechanism of facilitatory actions of kainate on excitatory synaptic transmission
If the observed facilitation in the synaptosome and slice models studied herein does have commonality as we contend, what then are the potential mechanism(s) that might underlie facilitatory modulation? In elucidating this question, we demonstrate that the activation of the adenylyl cyclase occludes the effect of KA facilitating glutamate release. Moreover our observations that: (i) the reverse experiment (i.e. forskolin applied after KA) did not produced any additional increase in glutamate release (current study) (ii) the inactive analog of forskolin (dideoxyforskolin) did not affect glutamate release (Wang & Sihra, 2003) and (iii) the effect of KA in the presence of dideoxyforskolin in slices is unchanged (current study) taken together, suggest that the activation of the adenylyl cyclase is linked to the action of KA. Our demonstration that, following the inhibition of PKA in synaptosomes or slices, using H-89 or Rp-Br-cAMP, forskolin and KA fail to affect glutamate release, invokes an occlusion and implies that the effects of these agents are mediated by a common intracellular cascade involving the activation of PKA.
From the foregoing results discussed, the clear possibility arising is that the activation of kainate receptors somehow leads to the stimulation of the adenylyl cyclase or vice versa. What then might be the basis of such an interaction or dependence? Invoking the established ionotropic properties of kainate receptors, one immediate possibility is that a rise in intracellular Ca2+, either through a depolarization-dependent activation of Ca2+ channels or by direct kainate receptor-mediated conduction, effects a Ca2+-dependent activation of adenylate cyclase (Weisskopf et al. 1994; Cooper, 2003). The evidence regarding the entry of Ca2+ following kainate receptor activation is varied in both synaptosomes and slices, with some studies showing measurable increases in intracellular Ca2+ (Malva et al. 1995; Lauri et al. 2003) and others no effect (Perkinton & Sihra, 1999; Kamiya et al. 2002), or even decreases (Kamiya & Ozawa, 1998, 2000). One reason for the disparity may be that determination of changes in cytosolic Ca2+ in some cases is confounded by the relative inability of Ca2+-probes to detect plasma membrane localized changes in the concentration of the cation. Nevertheless, localized Ca2+ entry may certainly be sufficient or even necessary to activate a plasma membrane resident enzymes such as, for instance, Ca2+/calmodulin-sensitive isotypes of adenylate cyclase including AC1 and AC8 (Cooper, 2003).
In a recent paper, Lauri et al. (2003) have proposed that kainate receptors that are permeable to Ca2+ may be responsible for the facilitation of synaptic transmission and LTP seen at the mossy fibre-CA3 synapses, putatively through the release of intracellular Ca2+ stores, although, notably, other authors do not observe any role of Ca2+ during LTP induction at this type of synapse (Kamiya et al. 2002). While it remains to be established whether the facilitatory mechanism that we describe here is in any way involved in the induction of LTP by kainate receptors described in the aforementioned preparation, one clear indication from our data is the that facilitation is at least not sensitive to the Ca2+/calmodulin-dependent kinase II (Ca2+/CAM KII) inhibitor, KN62. Indeed the latter inhibitor has previously been shown to be ineffective in mossy fibre LTP (Huang et al. 1994). The lack of involvement of downstream activation of Ca2+/CAM KII in the KA-mediated presynaptic facilitation seen here would therefore delineate this type of modulation from the synaptic enhancement underlying hippocampal LTP in the proposed models strongly invoking a role for the kinase (Weisskopf et al. 1994). Moreover, this observation obviates the involvement of Ca2+/CAM KII-mediated phosphorylation of kainate receptor subunits (Ghetti & Heinemann, 2000; Yakel et al. 1995) in the regulation reported here.
Other than a mechanism based on the ionotropic properties of KA, in principle, the facilitatory effect described herein may be mediated by the coupling of kainate receptors through a heterotrimeric G-protein, as has been described for the modulation of GABA release by KA application (Rodríguez-Moreno & Lerma, 1998; Rodríguez-Moreno et al. 2000). A metabotropic and G-protein dependent mechanism for the action of KA might in some ways more easily rationalize the observed interactions with the AC/PKA cascade. However, while the inhibitory modulation of GABA release by KA could be confirmed on the basis of sensitivity to the Gi/o inhibitor pertussis toxin (PTX), the same type of approach is not possible with a stimulatory G-protein as might be posited for a facilitatory response. Certainly in our hands, the stimulatory effect of KA on eEPSCs persisted with the Gs stimulator, cholera toxin (data not shown), and perhaps unsurprisingly, it was also insensitive to PTX. Although this might lead one to speculate on the involvement of an ‘atypical G-protein’, at this stage as discussed above, a G-protein independent mechanism, operating under the auspices of the classical ionotropic remit of kainate receptors, remains manifestly tenable.
While it was not the purpose of this study to assign facilitatory or inhibitory kainate receptor function on the basis of subunit composition of the receptors, it is of interest to note that, of the most prevalent transcripts of kainate receptor subunits in the hippocampus (GluR5 and GluR6), neurones projecting facilitatory KA inputs (principal and dentate granule cells to CA3 neurones), express GluR6 mRNA most abundantly (Paternain et al. 2000). The notion arising from this, that GluR6 subunits may subserve a key role in the facilitatory effects of kainate receptors, is supported by the sensitivity of synaptosomal glutamate release facilitation to NS-102, a selective GluR6 subunit antagonist (Perkinton & Sihra, 1999). Moreover, studies showing an impairment in the KA-mediated frequency facilitation of synaptic transmission and LTP at mossy fibre-CA3 synapses in GluR6 knockout mice (Contractor et al. 2001) also indicate as much. Taken together with observations that the facilitatory aspect of the bimodal modulation by KA is lost in KA2 subunit knockout mice (Contractor et al. 2003), a tentative assignment can me made of a facilitatory kainate receptor composed of oligomers of GluR6 and KA2 subunits. Intriguingly, with respect to the link of facilitatory kainate receptors to AC/PKA activation reported herein, GluR6 subunits are known to be PKA substrates (Raymond et al. 1993) and phosphorylation of this subunit-type leads to up-regulation of channels activity (Traynelis & Wahl, 1997; Wang et al. 1993). Be it at this locus or another, clearly future experiments are warranted to better understand the precise link between kainate receptors and the PKA pathway alluded to here, and thereby allow a more complete elucidation of the intracellular signalling cascade leading to the facilitation of glutamate release.
In summary, we provide support for the functional interaction of facilitatory kainate receptors with a second messenger cAMP-dependent signalling cascade leading to PKA activation.
Acknowledgments
This work was supported Wellcome Trust Grant support to T.S.S. A.R.-M. was a recipient of a Short-Term fellowships awarded by EMBO and by Human Frontier Science Program. We thank Professor P. Tatham and Dr G. Thomas for the loan of the spectrofluorimeter for part of this study.
References
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Odgen A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O'Gorman S, Heinemann SF. Identification of the kainate receptors subunit underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Neurotoxic actions of kainic acid. J Neurochem. 1983;41:1–11. doi: 10.1111/j.1471-4159.1983.tb11808.x. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Clements JD. Glutamate autoreceptors depress excitatory monosynaptic transmission between mouse hippocampal neurons. J Physiol. 1990;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Kainate receptors depress excitatory synaptic transmission at CA3-CA1 synapses in the hipoccampus via a direct presynaptic action. J Neurosci. 2001;21:2958–2966. doi: 10.1523/JNEUROSCI.21-09-02958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti A, Heinemann SF. NMDA-Dependent modulation of hippocampal kainate receptors by calcineurin and Ca(2+)/calmodulin-dependent protein kinase. J Neurosci. 2000;20:2766–2773. doi: 10.1523/JNEUROSCI.20-08-02766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Gaber SS, Aldrich RW. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988;240:1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel E. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol. 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol. 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Umeda K, Ozawa S, Manabe T. Presynaptic Ca2+ entry is unchanged during hippocampal mossy fiber long-term potentiation. J Neurosci. 2002;22:10524–10528. doi: 10.1523/JNEUROSCI.22-24-10524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM. Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy-fiber LTP. Neuron. 2001b;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Delany C, Clarke VEJ, Bortolotto ZA, Ornstein PI, Isaac JT, Collingridge GL. Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptor-mediated synaptic transmission at hippocampal mossy fibre synapses. Neuropharmacology. 2001a;41:907–915. doi: 10.1016/s0028-3908(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Malva JO, Ambrosio AF, Cunha RA, Ribeiro JA, Carvalho AP, Carvalho CM. A functionally active presynaptic high-affinity kainate receptor in the rat hippocampal CA3 subregion. Neurosci Lett. 1995;185:83–86. doi: 10.1016/0304-3940(94)11230-g. [DOI] [PubMed] [Google Scholar]
- Malva JO, Carvalho AP, Carvalho CM. Domoic acid induces the release of glutamate in the rat hippocampal CA3 subregion. Neuroreport. 1996;7:1330–1334. doi: 10.1097/00001756-199605170-00023. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Sihra TS. Synaptosomes possess and exocytotic pool of glutamate. Nature. 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties ot two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassembly to form functional receptors. J Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkinton MS, Sihra TS. A high-affinity presynaptic kainate-type glutamate receptor facilitates glutamate exocytosis from cerebral cortex nerve terminals (synaptosomes) Neuroscience. 1999;90:1281–1292. doi: 10.1016/s0306-4522(98)00573-9. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Poli A, Contestabile A, Migani P, Rossi L, Rondelli C, Virgili M, Bissoli R, Barnabei O. Kainic acid differentially affects the synaptosomal release of endogenous and exogenous amino acidic neurotransmitters. J Neurochem. 1985;45:1677–1686. doi: 10.1111/j.1471-4159.1985.tb10522.x. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Blackstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993;361:637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, López-García JC, Lerma J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc Natl Acad Sci USA. 2000;97:1293–1298. doi: 10.1073/pnas.97.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Sihra TS. Protein phosphorylation and dephosphorylation in isolated nerve terminals (synaptosomes) In: Hemmings HC Jr, editor. Regulatory Protein Modification. Techniques and Protocols. Totowa, NJ, USA: Humana; 1997. pp. 67–119. [Google Scholar]
- Thomson AM, Deuchars J. Diverse pre- and postsynaptic properties of fast excitatory synapses. In: Wheal H, Thomson A, editors. Excitatory Amino Acids and Synaptic Transmission. London: Academic Press; 1995. pp. 145–172. [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal granule cells: a presynaptic form of plasticity. Neuron. 1996;16:1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Clarke VRJ, Parry MJ, Bleakman D, Lodge D, Ornstein PL, Collingridge GL. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology. 1998;37:1269–1277. doi: 10.1016/s0028-3908(98)00148-8. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Sihra TS. Opposing facilitatory and inhibitory modulation of glutamate release elicited by cAMP production in cerebrocortical nerve terminals (synaptosomes) Neuropharmacology. 2003;44:686–697. doi: 10.1016/s0028-3908(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Wang LY, Taverna FA, Huang XP, MacDonald JF, Hampson DR. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993;259:1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potantiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Xia ZG, Refsdal CD, Merchant KM, Dorsa DM, Storm DR. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron. 1991;6:431–443. doi: 10.1016/0896-6273(91)90251-t. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Vissavajjhala P, Derkach VA, Brickey DA, Soderling TR. Identification of a Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in non-N-methyl-D-aspartate glutamate receptors. Proc Natl Acad Sci USA. 1995;92:1376–1380. doi: 10.1073/pnas.92.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Peterson CL, Lu YB, Nadler JV. Release of glutamate and aspartate from CA1 synaptosomes: selective modulation of aspartate release by ionotropic glutamate receptor ligands. J Neurochem. 1995;64:1556–1566. doi: 10.1046/j.1471-4159.1995.64041556.x. [DOI] [PubMed] [Google Scholar]