Abstract
An antisense oligodeoxynucleotide (As-ODN) to the 3′ untranslated region of the mRNA sequence expressing the intracellular adhesion molecule-1 (ICAM-1) was employed to determine ICAM-1's role in renal ischaemia–reperfusion injury in the rat. Wistar-Kyoto rats receiving i.v. either lipofectin–As-ODN (As-ODN group), lipofectin–reverse ODN (Rv-ODN group) or lipofectin (ischaemia control group) 8 h prior to study were anaesthetized and subjected to 30 min of renal artery occlusion. Renal haemodynamic and excretory parameters were monitored before and after renal ischaemia. On termination of the study renal tissue was subjected to histological and Western blot analysis. Renal blood flow decreased in the 3 h post-ischaemia period in the ischaemia control and Rv-ODN groups, but was maintained in the As-ODN group. Glomerular filtration rate was depressed initially but gradually increased to 10% above basal levels in the ischaemia control and Rv-ODN groups, but was below basal levels (20%) in the As-ODN group. There was a three- to fourfold increase in sodium and water excretion following ischaemia in the ischaemia control and reverse-ODN groups but not in the As-ODN treated group. The As-ODN ameliorated the histological evidence of ischaemic damage and reduced ICAM-1 protein levels to a greater extent in the medulla than cortex. These observations suggested that in the post-ischaemic period afferent and efferent arteriolar tone was increased with a loss of reabsorptive capacity which was in part due to ICAM-1. The possibility arises that the action of ICAM-1 at vascular and tubular sites in the deeper regions of the kidney contributes to the ischaemia–reperfusion injury.
Renal ischaemia followed by reperfusion may initiate a cascade of events leading to the development of renal ischaemia–reperfusion injury. In hospitalized patients, renal ischaemia and hypotension are prone to develop during renal transplantation, aortic cross-clamping, extensive trauma and resuscitation following systemic hypotension (Weight et al. 1996). At present no effective treatment is available for the renal injury following ischaemia (Weight et al. 1996), which may progress into acute renal failure giving a high mortality rate in these patients (Zarnado et al. 1994). Thus, understanding the mechanisms underlying this injury may allow novel therapeutic approaches to be investigated.
The development and progression of the renalischaemic injury involves the renal vascular endothelium and components involved with local inflammatory responses (Cattell, 1994). In a renal ischaemia–reperfusion event, ischaemia and toxic and mechanical injuries inflicted on the kidney may initiate diverse renal inflammatory responses and eventually leucocyte infiltration. Under such circumstances, the production and release of various local inflammatory mediators, vasoconstrictors and chemo-attractants will be up-regulated. As a result, prolonged renal hypoxia will occur and at a later stage, lymphocyte and macrophage infiltration into the renal tissue will take place under the influence of the released chemo-attractants. The leucocyte infiltration is capable of causing extensive damage to renal tissues via physical plugging of the renal capillaries, proteolytic enzyme secretion and cytokine/vasoconstrictor production (Cattell, 1994). One approach to reducing the severity of the renal ischaemic injury would be to suppress the occurrence of the renal inflammatory response and leucocyte infiltration during the ischaemia–reperfusion event.
It is now recognized that the intracellular adhesion molecule-1 (ICAM-1) protein, which is expressed on the renal vascular endothelial surface, plays an important role in the initial development of the renal inflammatory response to ischaemic injury by facilitating renal leucocyte infiltration (Bonventre, 1993). It is also evident that the ICAM-1 proteins contribute to the inflammatory response through other unknown mechanisms. Earlier reports have shown that suppression of the ICAM-1 protein expression could reduce the renal inflammatory response and leucocyte infiltration, thereby decreasing the severity of renal ischaemic injury (Bonventre, 1993). In 1996, Haller et al. demonstrated that the suppression of the renal ICAM-1 protein expression using antisense oligodeoxynucleotides (As-ODN), which inhibited the gene allowing ICAM-1 protein translation (Chiang et al. 1991), was effective in ameliorating histological manifestations of renal ischaemic injury in a rat model. However, the functional significance of the As-ODN in preventing the renal ischaemia–reperfusion injury during the initial stages of reperfusion was not investigated and the actual role of the ICAM-1 proteins in the initial phase of the renal ischaemic damage is only now being evaluated.
The present study set out to investigate the impact of ischaemia on the physiological function of kidneys which had been subjected to short periods of blockade of ICAM-1 generation. This was done by pretreating rats with the ICAM As-ODN and assessing the renal haemodynamic and excretory changes which occurred in the first 3 h following 30 min of renal ischaemia.
Methods
Preparation
All experiments were approved by the local Animal Care Committee and conformed to National guidelines. Four groups of male Wistar-Kyoto rats (250–300 g) were used. All rats were fasted overnight before the experiment. At 8 h prior to the acute study, each group was given i.v. 1.5 ml of either lipofectin (as vehicle, 1.6 mg kg−1, ischaemia control group, n = 6), the As-ODN (2 mg kg−1 As-ODN + 1.6 mg kg−1 lipofectin, As-ODN treated ischaemia group, n = 6), or reverse ODN to ICAM-1 (2 mg kg−1 As-ODN + 1.6 mg kg−1 lipofectin, Rv-ODN treated ischaemia group, n = 6). The structure of the As-ODN to ICAM-1 was: 5′-ACC GGA TAT CAC ACC TTC CT-3′, which was directed against the 3′ untranslated region of the ICAM mRNA sequence and has been shown to effectively reduce protein expression (Chaing et al. 1991). The reverse-ODN's sequence was used as the ODN control. All ODNs were manufactured by Alta Bioscience (University of Birmingham, UK), and lipofectin was obtained from Gibco-Invitrogen (CA, USA).
Animal surgery and acute study
Approximately 5 h after the i.v. injections for each of the above treatments, the rats were anaesthetized with sodium pentobarbital (60 mg kg−1, i.p.). The left carotid artery and jugular vein were cannulated for blood pressure measurement and infusion of saline (140 mm NaCl), at 3 ml h−1 and anaesthetic at 12.5 mg kg−1 h−1 while the left femoral artery was cannulated for blood sampling. The left kidney was exposed via a flank incision, its ureter cannulated and a flow probe placed on the renal artery. Inulin (10 mg ml−1) was included in the infusate. Thereafter, a 2 ml priming dose solution (containing 140 mm NaCl and 10 mg ml inulin−1) was given to the rats via the jugular vein. After a 1 h equilibration period, two basal 15 min clearances were taken. The renal artery was then clamped for 30 min to induce ischaemia (the time control, TM, group was not subjected to ischaemia); thereafter, 2 × 15 min, 3 × 30 min and 1 × 60 min clearances were taken. In each clearance period, urine and blood samples were collected while arterial blood pressure (Grass model 7D, MA, USA and Statham pressure transducers, CT, USA) and renal blood flow (Carolina Square-wave Electromagnetic flowmeter, Carolina Medical, NC, USA) were continuously recorded. At the end of the study, the rat was killed by a rapid injection of 1 ml sodium pentobarbital and the ischaemic kidney harvested for histological evaluation and ICAM-1 protein analysis via Western blot study.
Assays
Urine and plasma electrolyte sodium concentrations were analysed using a flame photometer (Sherwood, model 420, UK). The urine and plasma samples were deproteinized according to the method proposed by Bojesen (1952) and thereafter the inulin content was measured according to the method of Somogyi (1930). Glomerular filtration rate (GFR) was then calculated from the inulin clearance and was expressed as ml min−1 (kg body weight)−1.
Kidney tissue processing
Ischaemic kidneys were harvested at the end of the acute study and were divided into two halves using a central transverse section. One section was fixed overnight in 10% buffered formalin for tissue slide production purposes, and the other section was cryo-frozen immediately for Western blotting purposes.
Histology
After formalin fixation, the renal tissue samples were processed into paraffin wax tissue blocks, and thin tissue sections (5 μm) were produced using a microtome and placed onto glass slides. Thereafter, the sections were stained with haematoxylin and eosin (H&E) and were assessed by a certified pathologist who was unaware of the experimental protocol. Attention was focused on the level of renal tissue damage/morphological changes, presence of renal interstitial congestion and leucocyte (neutrophil) infiltration.
Western blot analysis (cryo-frozen sections)
In the current study, densitometric based Western blot analysis was performed to determine the ICAM-1 protein content in the renal cortex and medulla. The renal tissue sample was homogenized in buffer (10 mm Tris-HCl pH 7.4, 100 mm NaCl, 300 mm sucrose, 1 mm EDTA, 1 μg ml−1 leupeptin, 0.5% Triton X-100) in a ratio 1 part of tissue (g) to 8 parts of buffer (ml), sonicated for 30 s, incubated for 2 h at 4°C with shaking and then spun at 13000 g (refrigerated centrifuge) for 10 min. The total protein content in the supernatant was then determined using a BCA protein assay kit. A 12 μl aliquot containing 25 μg tissue protein was mixed with 6 μl of loading buffer (containing 5 × DTT, 10% SDS and ddH2O in ratio of 2: 2: 1), boiled for 5 min and loaded onto a Gradipore (New South Wales, Australia) precast SDS-PAGE gel (8–16%), and electrophoresis was performed using the Bio-Rad (CA, USA) Mini-protein 3 electrophoresis module. At the end of the electrophoresis, the gel was removed from the electrophoresis module and protein in the gel was transblotted onto a nitrocellulose membrane. Immunodetection of ICAM-1 protein on the blot was performed using the Bio-Rad Opti-4 CN detection kit, according to the manufacturer's prepared protocol (Bio-Rad) with modifications. The primary antibody employed was mouse anti-rat ICAM-1 antibody and the secondary antibody used was HRP-labelled goat anti-mouse antibody. Upon completion of the immunostaining, the ICAM-1 protein expression on the blot was quantified by a densitometric method (using a high resolution scanner and Bio-Rad Quantity 1 program).
Statistical analysis
All the data obtained in the current study were expressed as means ± standard error of the mean (s.e.m.) and subjected to analysis of variance (Super ANOVA, Abacus, CA, USA) and the significance of the difference between groups was further examined using a Bonferroni/Dunn (all means) test. Experimental differences were considered statistically significant if P < 0.05.
Results
The basal levels of arterial blood pressure and renal haemodynamic and excretory variables of various groups in the current study are listed in Table 1. There were no significant differences between groups for any of the variables measured. Throughout the study, all physiological and renal functional parameters of the time control group remained stable over the duration of the period of experimental observation (data not shown).
Table 1.
Baseline values for all assessed physiological variables
| Ischaemia control group | Rv-ODN treated ischaemia group | Time control group | As-ODN treated ischaemia group | |
|---|---|---|---|---|
| Arterial blood pressure (mmHg) | 122 ± 2 | 122 ± 3 | 121 ± 2 | 122 ± 4 |
| Renal blood flow (ml min−1 kg−1) | 8.79 ± 0.47 | 9.82 ± 0.77 | 10.24 ± 1.16 | 8.09 ± 0.47 |
| Glomerular filtration rate (ml min−1 kg−1) | 3.16 ± 0.26 | 2.12 ± 0.24 | 2.30 ± 0.24 | 2.28 ± 0.29 |
| Urine flow rate (μl min−1 kg−1) | 23.89 ± 2.03 | 21.12 ± 4.94 | 23.62 ± 2.57 | 19.71 ± 4.62 |
| Plasma sodium (mmol l−1) | 159.6 ± 2.0 | 166.0 ± 1.9 | 165.0 ± 4.6 | 155.9 ± 7.9 |
| Absolute sodium excretion (μmol min−1 kg−1) | 3.63 ± 0.65 | 2.94 ± 1.35 | 3.15 ± 0.94 | 3.51 ± 1.97 |
| Fractional sodium excretion (%) | 0.76 ± 0.09 | 0.75 ± 0.20 | 0.94 ± 0.26 | 0.68 ± 0.44 |
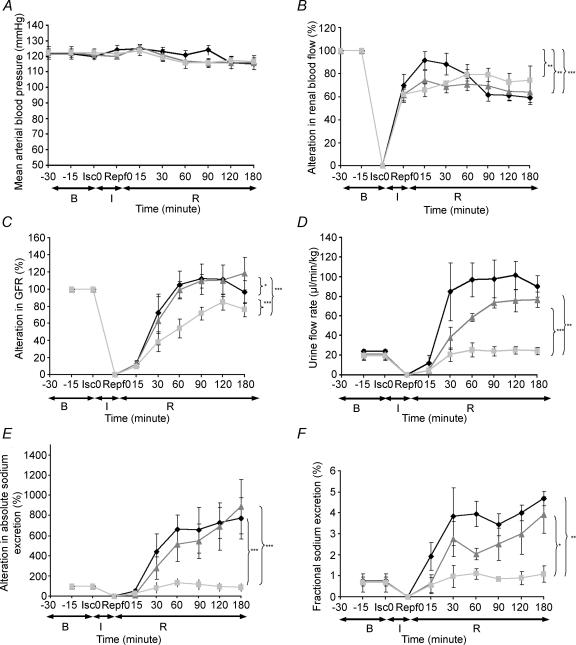
It was observed that the arterial blood pressure was not altered by the ischaemic challenge and remained stable within the physiological range during the 3 h of reperfusion in all groups (Fig. 1A). Meanwhile, there was a gradual reduction in renal blood flow (RBF, from 80 to 60% and 70 to 60% of baseline, respectively, Fig. 1B) in the ischaemia control and Rv-ODN treated groups in the reperfusion period (P < 0.01). In the As-ODN treated ischaemia group (Fig. 1B), there was a tendency for renal blood flow to gradually recover (from 60 to 80% of baseline) during the reperfusion period.
Figure 1. Haemodynamic and renal excretory function changes of the rats (with renal blood flow, GFR and absolute sodium excretion presented as a percentage of basal values) during and following the ischaemic challenge.
B, baseline; I, ischaemia period; R, reperfusion period; Isc 0, initiation of ischaemia; Repf 0, initiation of reperfusion. Black lozenges, ischaemia control group; grey squares, As-ODN treated ischaemia group; grey triangles, Rv-ODN treated ischaemia group.
Glomerular filtration rate (GFR, Fig. 1C) in the As-ODN treated ischaemia, Rv-ODN treated ischaemia and ischaemia control groups was decreased by 90% over the first 15 min of reperfusion, but in the subsequent 45 min, GFR of the Rv-ODN treated ischaemia and ischaemia control groups rose above basal levels by some 20%(P < 0.05). In the As-ODN treated ischaemia group, GFR recovered more gradually reaching basal levels at the end of the study. Urine flow rate (UFR, Fig. 1D) was raised some fourfold in the ischaemia control and threefold in Rv-ODN treated ischaemia groups during reperfusion, which was accompanied by a concurrent increase in absolute sodium excretion (Fig. 1E) of some fourfold and fractional sodium excretion (Fig. 1F) of some threefold in both groups after ischaemia. By contrast, there were no significant changes in the UFR, absolute sodium excretion or fractional excretion of sodium (FENa) in the As-ODN treated ischaemia group compared to basal level, which were responses significantly different from the ischaemia control and Rev-ODN groups (all P < 0.01–0.001).
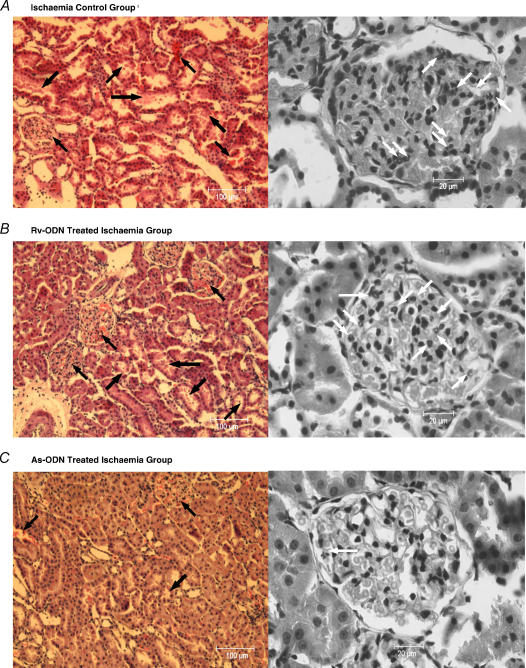
Histological examination of renal tissue showed the presence of congestion in the renal interstitium of the ischaemia control and Rv-ODN treated ischaemia groups, particularly in the glomerular capillaries. Focal degenerative changes could also be seen in some of the renal tubules (Fig. 2A and B). There was a significant focal neutrophil accumulation in most glomeruli (>5 neutrophils per glomerulus, Fig. 2A and B). Moreover, cellular casts/debris and exfoliated renal tubular epithelial cells were also found in the renal tubules (Fig. 2A and B). In the As-ODN treated ischaemia group there was mild congestion in the renal interstitium with some partially degenerated cells in the tubules (Fig. 2C), but generally, the renal tissue structure remained intact and few neutrophils (0–2 neutrophils per glomerulus) were found in most glomeruli (Fig. 2C).
Figure 2. Low and high power histological sections of the kidneys.
A and B, general renal medulla tissue histology (H&E) and renal glomerulus histology (H&E) for ischaemia control and Rv-ODN treated ischaemia group. The renal interstitium (particularly glomerulus) of the ischaemia control and Rv-ODN treated ischaemia group were found to be congested and degenerative changes can be seen in some of the renal tubules with cellular casts/debris and exfoliated renal tubular epithelial cells could also be found in the renal tubules (black arrows). Meanwhile, significant focal neutrophil accumulation (> 5 neutrophils per glomerulus) could be seen in most glomerulus (neutrophils: white arrows). C, general renal medulla tissue histology (H&E) and renal glomerulus histology (H&E) for As-ODN treated ischaemia groups (H&E). Mild congestion of the renal interstitium and some partially degenerated cells in the tubules (black arrows) was observed in As-ODN treated ischaemia group's tissue sections. Generally, the renal tissue structure remained intact and few/no neutrophils (0–2 neutrophils per glomerulus) were found in the most glomerulus (neutrophils: white arrows).
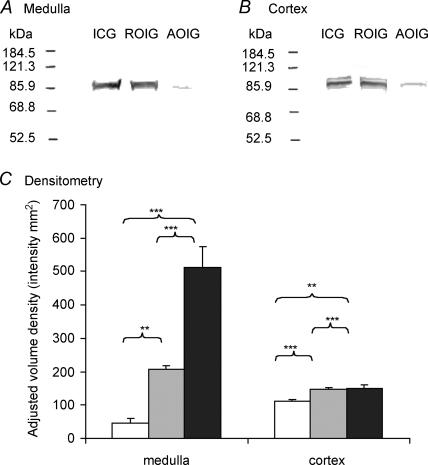
The immunostained blot membranes (Fig. 3A and B) were analysed densitometrically for ICAM-1 expression, which was reported in terms of adjusted volume (AV, intensity mm2 or int. mm2, Fig. 3C). The medullary expression of ICAM-1 in the ischaemia control and Rv-ODN treated ischaemia groups was found to be increased significantly compared to the As-ODN treated ischaemia group (about 20- and eightfold, respectively, both P < 0.01, Fig. 3C). The medullary ICAM-1 expression of the Rv-ODN treated ischaemia group was significantly lower than the ischaemia control group (P < 0.001). The cortical expression of ICAM-1 in the As-ODN treated ischaemia group was found to be significantly lower compared to the Rv-ODN treated ischaemia and ischaemia control groups (both P < 0.01). Nevertheless, the cortical expression of ICAM-1 in the As-ODN treated ischaemia group was significantly higher when compared to its medullary ICAM-1 expression (P < 0.05, Fig. 3C).
Figure 3. Immuno-blots for the medulla and cortex.
The immuno-blots for the medulla and cortex of the ischaemia control group (ICG), Rv-ODN treated ischaemia group (ROIG) and As-ODN treated ischaemia group (AOIG) are presented in A and B, respectively, and the densitometric evaluations are given in C. **P < 0.01, ***P < 0.001. Filled bars, ischaemia control group; grey bars, reversed ODN treated ischaemia group; open bars, antisense ODN treated ischaemia group.
Discussion
The objective of the current investigation was to elucidate the contribution of ICAM-1 to the initial phase of renal ischaemia–reperfusion injury. This was done by evaluating the changes in renal haemodynamic and excretory function and correlating these with tissue histology and ICAM-1 expression in groups subjected to ischaemia alone and compared to those treated with antisense ODN to ICAM-1 or the non-functional Rv-ODN.
In both the As-and Rv-ODN treated ischaemia groups, blood pressure did not change meaningfully either during ischaemia or in the subsequent time of reperfusion. It was evident in the ischaemia control group that following the ischaemic challenge, there was a rebound in the renal blood flow to within 80% of basal values, but thereafter flow gradually declined. This reduction in renal blood flow would suggest an increase in renal arteriolar vascular resistance contributed by both the efferent and afferent arterioles (Vander, 1995). Interestingly, the pattern of the post-ischaemia reductions in renal blood flow were similar in both the As- and Rv-ODN treated ischaemia groups suggesting that ICAM-1 was unlikely to be a factor generating these major changes in renal vascular resistance. This pattern of renal haemodynamic response to ischaemia was similar to that reported previously in this model of ischaemia–reperfusion injury. Indeed, there is good evidence that the ischaemic manoeuvre causes the release of a number of autocrine and local factors, particularly superoxide anions (Weight et al. 1996) and endothelin (Liberthal, 1997), which may contribute to the observed reductions in renal blood flow.
The initial marked reductions in glomerular filtration rate are most likely attributable to a marked affererent arteriolar vasoconstriction. However, in the subsequent stages of reperfusion, glomerular filtration rate was restored towards basal levels, or slightly above in the ischaemia group. A similar pattern of change in glomerular filtration rate was observed in an earlier study using this model (Hestin & Johns, 1999). This relative maintenance of glomerular filtration rate would suggest that although afferent arteriolar resistance was raised, as reflected by the reduction in renal blood flow, efferent arteriolar resistance was also increased, to a degree whereby filtration pressure, and hence filtration rate, was normalized by the end of the 3-h period. What was of significance was that the pattern of change in the As-ODN group was somewhat different in that glomerular filtration recovered at a much slower rate. One interpretation of this observation would be that there was relatively less efferent arteriolar constriction due to a blunted generation of vasoconstrictor agents at this site. Indeed, because the Rv-ODN treated ischaemia group was similar to the ischaemia control group and different from the As-ODN treated ischaemia group, it would seem that the production of the vasoconstrictor agents was related to ICAM-1 production.
An important role for ICAM-1 in the renal vasculature was supported by the observation that there was a significant renal interstitial congestion in the outer medulla, as was evident from the histological assessment in the ischaemia control group, which would be consistent with an increased efferent arteriolar resistance. The increased resistance could be due, in part, to the raised local generation of ICAM-1 as shown by the raised expression of medullary ICAM-1 in the ischaemia control group. A consequence of the elevated ICAM-1 expression could be enhanced accumulation, adhesion and infiltration of circulating leucocytes into the renal vasculature including the glomerular capillaries, which when activated could result in the release of leukotiene B4, thromboxane A2 and platelet activating factors leading to plugging of the renal capillaries (Kelly & Bonventre, 1995). However, neutrophil infiltration was observed primarily in the glomerular capillaries and it was less evident in the peritubular capillaries or the vasa recta of the ischaemia control and Rv-ODN treated ischaemia group. Perhaps more importantly, this neutrophil infiltration was not observed in the AS-ODN treated ischaemia group. Thus, although there is good evidence implicating ICAM-1 in the later phases of the renal ischaemic insult, some 8–24 h (Haller et al. 1996), this is one of the first studies to demonstrate a potential role for the ICAM-1 in the initial phases as the reperfusion injury.
It was also evident that the natriuresis and diuresis in the post-ischaemic period were markedly blunted in the AS-ODN treated ischaemia group compared to the Rv-ODN treated ischaemia group suggesting that generation of ICAM-1 was a contributory factor. The diuresis and natriuresis in the reperfusion period have been reported in previous studies utilizing this model of ischaemia–reperfusion injury. The underlying mechanism was most likely due to tubular damage and impaired reabsorption by the epithelial cells and it is recognized that the most susceptible areas are the proximal straight tubule and the thick ascending limb of the loop of Henle (Hays, 1992). Indeed, it was evident from the histological study that in both the ischaemia control and Rv-ODN treated ischaemia group there was tubular degeneration, cellular casts and exfoliated epithelial cells within the tubules of the cortex. Of greater significance was that the post-ischaemic natriuresis and diuresis were not evident in the As-ODN treated ischaemia group suggesting that some protection was conferred on the epithelial cells which was mediated by an action of ICAM-1. The question arises as to mechanisms underlying the blunted excretory responses in the As-ODN treated ischaemia group and the role played by ICAM-1. It may well be that because of the prolonged and exaggerated efferent arteriolar constriction in the ischaemia control and Rv-ODN treated ischaemia groups there could have been a greater degree of medullary hypoxia and hence tissue damage, as reflected in the histology, leading to the depressed reabsorption in these deeper areas. By contrast, the lesser degree of post-glomerular constriction in the As-ODN treated ischaemia group could well have resulted in a better perfusion in the medullary areas, to some extent minimizing epithelial cell damage and the reabsorption processes. It was of interest that in a somewhat different scenario of renal transplantation, Dragun et al. (1998) demonstrated enhanced success of renal isografts following suppression of ICAM-1 expression with the As-ODN.
A number of preliminary tests were undertaken to assess the optimal timing and dosing of the ODNs and lipofectin vehicle which were effective in terms of reducing ICAM-1 protein content of the kidney and functional responses to the ischaemic challenge. It became evident that it was necessary to administer the ODN plus lipofectin vehicle no earlier than 8 h before the acute study, as if given prior to this time the excretory responses were comparable to those of the lipofectin (ischaemia) control. Importantly, the Western blot data indicated that in the medulla ischaemia caused a marked rise in ICAM-1 protein content although, interestingly, even the Rv-ODN data appeared to depress the response compared to the lipofectin (ischaemia) control. Nonetheless, it was apparent that medullary ICAM-1 levels in the As-ODN treated ischaemia group were not significantly different from the time control group. These observations clearly indicate that the As-ODN was effective in suppressing ICAM-1 protein production. Moreover, these data provide further support for the report of De Greef et al. (2003) using renal ischaemia–reperfusion in the rat, who found 2 h after ischaemia, the earliest time point studied, that inner medullary ICAM-1 expression was raised.
The situation with the ICAM-1 protein expression in the cortex was somewhat different in that in the As-ODN treated ischaemia group the cortex ICAM-levels were greater than the medullary ICAM-1 level. In spite of this, cortical ICAM-1 protein levels were significantly decreased in the As-ODN treated ischaemia group compared with the Rv-ODN treated ischaemia and ischaemia control groups. Thus, this has been taken to reflect the fact that the As-ODN was exerting an action suppressing protein production although the observations suggest that at this time point the rundown in ICAM-1 protein in the cortex was relatively less than that which occurred in the medulla. Indeed, why the medullary ICAM-1 protein expression should be more responsive to the As-ODN than the cortex is unclear, but it may point to differential mechanisms present in these areas regulating the turnover of the protein.
In summary, this study has shown in anaesthetized rats that following a 30-min period of renal ischaemia, in the subsequent 3 h there was a raised renal vascular resistance associated with a marked natriuresis and diuresis. Histological evaluation was consistent with glomerular leucocyte infiltration and tubular damage. Administration of ICAM-1 As-ODN, but not Rv-ODN, partly attenuated the renal haemodynamic but completely prevented the natriuretic and diuretic responses to the ischaemic challenge. The As-ODN partly offset the histological changes induced by the ischaemic period and decreased renal ICAM-1 protein content, to a much greater extent in the medulla than cortex. These findings point to an important role for ICAM-1 in contributing to the renal damage in the initial few hours of reperfusion.
Acknowledgments
The authors are grateful to the Ministry of Science, Technology and Environment of Malaysia for supporting this work under IRPA grant no. 06-02-03-0541 and NSF for financial support.
References
- Bojesen E. A method for the determination of inulin in plasma and urine. Acta Med Scand. 1952;142(Suppl. 266):275–282. doi: 10.1111/j.0954-6820.1952.tb13376.x. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Mechanisms of ischaemic acute renal failure. Kidney Int. 1993;43:1160. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- Cattell V. Macrophages in acute glomerular inflammation. Kidney Int. 1994;45:945–952. doi: 10.1038/ki.1994.128. [DOI] [PubMed] [Google Scholar]
- Chiang MY, Chan H, Zounes MA, Freier SM, Lima WF, Bennett CF. Antisense oligonucleotides inhibit intracellular adhesion molecule 1 expression by two distinct mechanisms. J Biol Chem. 1991;266:18162–19171. [PubMed] [Google Scholar]
- De Greef KE, Ysebaert DK, Persey V, Vercauteren SR, De Bros ME. ICAM-1 expression and leucocyte accumulation in inner stripe of outer medulla in early phase ischemic compared to HgCl2-induced ARF. Kidney Int. 2003;63:1697–1707. doi: 10.1046/j.1523-1755.2003.00909.x. [DOI] [PubMed] [Google Scholar]
- Dragun D, Lutitsch I, Tullis SG, Qun Y, Park JK, Schneider W, Luft FC, Haller H. Inhibition of intercellular adhesion molecule-1 with antisense deoxynucleotides prolongs renal isograft survival in the rat. Kidney Int. 1998;54:2113–2122. doi: 10.1046/j.1523-1755.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Haller H, Dragun D, Miethke A, Park JK, Weis A, Lippoldt A, Grob V, Luft FC. Antisense oligonucleotides for ICAM-1 attenuate reperfusion injury and renal failure in the rat. Kidney Int. 1996;50:473–480. doi: 10.1038/ki.1996.338. [DOI] [PubMed] [Google Scholar]
- Hays SR. Southwestern Internal Medicine Conference: Ischaemic acute renal failure. Am J Med Sci. 1992;302:93–108. doi: 10.1097/00000441-199208000-00005. [DOI] [PubMed] [Google Scholar]
- Hestin D, Johns E. The influence of allopurinol on kidney haemodynamic and excretory responses to renal ischaemia in anaesthetized rats. Br J Pharmac. 1999;128:255–261. doi: 10.1038/sj.bjp.0702789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ, Bonventre JV, Goligorsky MS. Acute Renal Failure. New Concepts and Therapeutic Strategies. New York: Churchill Livingstone Inc.; 1995. Protection against ischaemic renal injury with blockade of intracellular adhesion molecule-1; pp. 401–423. [Google Scholar]
- Kelly KJ, Williams WWJ, Colvin RB, Bonventre JV. Antibody to intracellular adhesion molecule 1 protects the kidney against ischaemic injury. Proc Natl Acad Sci U S A. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberthal W. Biology of acute renal failure: Therapeutic implications. Kidney Int. 1997;52:1102–1115. doi: 10.1038/ki.1997.435. [DOI] [PubMed] [Google Scholar]
- Somogyi M. A method for the preparation of blood filtrates for the determination of sugar. J Biol Chem. 1930;86:655–663. [Google Scholar]
- Vander AJ. Renal Physiology. 5. New York: McGraw-Hill, Inc.; 1995. pp. 24–50. [Google Scholar]
- Weight SC, Bell PRF, Nicolson ML. Renal ischaemia reperfusion injury. Br J Surg. 1996;83:162–170. [PubMed] [Google Scholar]
- Zarnado G, Michielon P, Paccagnella A, et al. Acute renal failure in the patient undergoing cardiac operation: prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg. 1994;107:1489–1495. [PubMed] [Google Scholar]