Abstract
The hypocretin system is involved in the integration of hypothalamic functions with sleep and wake. Hypocretin-1 release peaks at the end of the active period in both diurnal and nocturnal species. A role for hypocretin-1 in the generation of locomotor activity has been suggested by electrophysiological and neurochemical studies in rodents, dogs and cats. These species, however, do not consolidate wake into a single, daily bout and manipulations of locomotion elicit changes in wakefulness, making it difficult to parse the relative contribution of these two factors. We have examined the relationship between locomotion and hypocretin-1 in a wake-consolidating animal, the squirrel monkey (Saimiri sciureus). Strikingly, we found that restricting locomotion to 17% of usual activity had no significant effect on the normal diurnal rise in cerebrospinal fluid (CSF) hypocretin-1, despite an associated increase in CSF cortisol. Increasing locomotion to greater than baseline activity did not significantly increase CSF hypocretin-1 concentrations, but did appear to have a positive modulatory effect on CSF hypocretin-1. In this wake-consolidating animal, locomotion is not necessary for CSF hypocretin-1 to increase throughout the daytime, but high levels of locomotion are likely to provide a small positive feedback onto the hypocretin system.
Hypocretin-1 is a neuropeptide whose production is primarily localized to a group of neurones in the lateral hypothalamus. Hypocretin-containing axons project widely to cortical, subcortical and spinal regions (de Lecea et al. 1998; Sakurai et al. 1998). Since a dysfunction or a loss of hypocretin results in the sleep disorder narcolepsy (Nishino et al. 2000; Peyron et al. 2000; Thannickal et al. 2000), hypocretin is believed to be involved in the regulation of wakefulness. This contention is supported by anatomical projections of hypocretin-containing axons to monoaminergic and cholinergic areas (Peyron et al. 1998; Date et al. 1999; Horvath et al. 1999; Moore et al. 2001). In addition, physiological data indicate that hypocretin is able to stimulate wake-promoting areas such as the tuberomammillary nucleus (Eriksson et al. 2002) and the locus coeruleus (Bourgin et al. 2000).
The hypocretinergic system is difficult to study in humans. Hypocretin-1 is only consistently found in brain tissue and cerebrospinal fluid (CSF). Squirrel monkeys (Saimiri sciureus) are small, diurnal (day-active), New World primates that, like humans, exhibit long, consolidated wake and sleep bouts (Richter, 1968; Wexler & Moore-Ede, 1985; Edgar et al. 1993). Based on studies in humans who lack hypocretin (narcoleptics; Dantz et al. 1994; Broughton et al. 1998) and our own examination of hypocretin in squirrel monkeys (Zeitzer et al. 2003), we have hypothesized that hypocretin is critically involved in consolidating wakefulness into a single extended period in humans and squirrel monkeys. Other mammals, including cats, dogs, mice and rats, do not consolidate wake (polyphasic, having relatively short periods of interspersed wake and sleep) and are therefore imperfect models of human sleep–wake physiology. Another advantage of the squirrel monkey lies in the fact that CSF is readily obtainable from the cerebellomedullary cistern (cisterna magna). There is good temporal concordance between concentrations of cisternal hypocretin-1 and dialysate hypocretin-1 from both the hypothalamus and medial thalamus (Fujiki et al. 2001; Yoshida et al. 2001). In contrast, collection of CSF in humans is typically limited to lumbar sampling. The relationship between brain release of hypocretin-1 and lumbar CSF concentrations of hypocretin-1 is confounded by an extensive temporal delay (Di Chiro et al. 1976), dampening of diurnal fluctuations (Salomon et al. 2003) and the presence of hypocretin-containing nerve endings in the spinal cord (van den Pol, 1999).
We have reported that cisternal CSF concentrations of hypocretin-1 in squirrel monkeys have a robust daily oscillation, with a peak late in the waking day and a nadir just around typical waketime, as theorized for a circadian alertness signal (Zeitzer et al. 2003). In animals in which wake has been extended past usual bedtime, hypocretin-1 concentrations stay elevated (Yoshida et al. 2001; Zeitzer et al. 2003; Pedrazzoli et al. 2004). This implies that hypocretin-1 secretion can be driven or modulated by homeostatic mechanisms (i.e. those dependent on the immediate history of sleep and wake). An alternative explanation is that the elevated hypocretin-1 concentrations observed during wake extension are due to a difference in activity (high locomotion during wake versus low locomotion during sleep).
Anatomical evidence supports a role of endogenous hypocretin in regulating motor activity (Peyron et al. 1998; Moore et al. 2001; Krout et al. 2003; Baldo et al. 2003). Intracerebroventricular injection of exogenous hypocretin-1 in rats increases locomotor activity (Ida et al. 1999; Hagan et al. 1999; Piper et al. 2000), though it is not well understood whether this is a direct induction of locomotion or if it is secondary to increased body temperature (Yoshimichi et al. 2001) or wakefulness (España et al. 2002). Wakefulness in association with spontaneous movements causes a greater induction of c-fos in cat hypocretin-containing neurones than quiet wakefulness (Torterolo et al. 2003). Forced locomotion also increases CSF hypocretin in both dogs (Wu et al. 2002) and rats (Martins et al. 2002), though this manipulation causes sleep deprivation in these species, an intervention that increases CSF hypocretin (Fujiki et al. 2001; Zeitzer et al. 2003). In this study, we have used squirrel monkeys to study the effects of increased and decreased locomotion throughout the day, independent of sleep deprivation.
Methods
Husbandry
Squirrel monkeys (Saimiri sciureus sciureus) were housed indoors in groups of six (cage dimensions: 2.1 × 2.4 × 2.0 m) and exposed to a 12 h light : 12 h dark cycle (lights on at 07.00 h, supplemented with artificial lighting 07.00–19.00 h to ensure 12 h of light per day). Animals had ad libitum access to food (New World Primate Diet 5040; PMI Nutrition International Inc., Brentwood MO, USA) and water; food was replenished daily at 10.00 h except on experimental days, when it was done at 07.00 h. All husbandry and experimental procedures were reviewed and approved by the Stanford University Administrative Panel on Laboratory Animal Care (IACUC).
General protocol
Using a percutaneous cisternal tap, we collected CSF samples from a group of 10 adult female squirrel monkeys (9–10 years old, body weight 686–902 g). Samples were collected at 10.00, 13.00, 16.00 and 19.00 h, both under baseline conditions (no intervention) and after restricting activity for 3, 6, 9 or 12 h (i.e. 07.00–10.00 h, 07.00–13.00 h, 07.00–16.00 h or 07.00–19.00 h, respectively). Samples were also collected twice at 10.00 h after an extended period of environmental enrichment from 07.00 to 10.00 h, and once at 10.00 h after an extended period of forced locomotion from 07.00 to 10.00 h. There were at least 2 weeks between each CSF collection. Animals maintained weight during the study period and exhibited no negative indications following CSF collection. Post-procedure analgesia was available and would have been prescribed by the veterinary staff had they or we deemed it necessary. All samples were assayed in duplicate for hypocretin-1 and cortisol.
Activity manipulation
Four conditions were used: baseline, restricted, enriched or forced. Baseline refers to normal activity, a situation in their standard home cage without investigator contact before CSF collection. Under restricted activity conditions, monkeys were placed in a small transport cage (46 × 46 × 46 cm) that limited movement (10 monkeys in two cages, 5 in each). The monkeys were monitored remotely using a video camera. Before the start of this experiment, monkeys had been exposed many times to the small activity restriction cages, since they were used to hold the monkeys for short periods of time for routine management procedures (e.g. transport between colony rooms). Investigator contact was limited to few, brief interventions to check on the monkeys, adjust monitoring equipment, or to ensure reduced movement. The monkeys typically clung to the sides of the cage or sat on the cage floor. No sleep was evident. Food and water were available ad libitum. Under enriched activity conditions, monkeys lived in their standard home cage and every 20–40 min, an investigator would add or subtract an enrichment variable comprised of the following: opening/closing the barn doors (exposure to outdoors), adding extra horizontal or vertical perching, hanging wire rope or rubber bungees from the cage top, hanging a circular wire rope with sliding plastic pieces attached, hanging a reflective mirror sheet outside the cage, shaking a metal board (loud noise), or giving a board with food boxes attached and openings in the board to access the food (standard chow). In preliminary experiments, monkeys were exposed to these enriching variables one at a time and the manipulations were all found to increase activity in some, but not all, monkeys at a given time. Monkeys were remotely monitored with a video camera such that when a decline in their activity was observed, an environmental variable was changed. The final activity condition was forced locomotion. This condition is similar to a procedure we have used previously to extend wakefulness in this species (Zeitzer et al. 2003), although more intensive. In brief, between 07.00 and 10.00 h, a single investigator (J.M.Z.) remained in the cage and would rattle the perch upon which the monkeys were resting. When the monkeys came to rest on a second perch, that perch would be rattled, and so on. In this way, the investigator forced locomotion for approximately 15 out of every 30 min.
Activity monitoring
In order to objectively quantify locomotor activity, all monkeys wore an actigraph (Actiwatch-64, MiniMitter, Bend, OR, USA) attached to their collar. An actigraph is a small (17 g) device capable of detecting movement through the use of an accelerometer. Actigraphy data were analysed using Sleepwatch software (v. 2.82, Cambridge Neurotechnology, Cambridge, UK). Activity measurement was unsuccessful in 11 of 110 collection intervals (one baseline collection {at 13.00 h}, eight transport cage collections {one at 13.00 h, three at 16.00 h, and four at 19.00 h}, two forced locomotion collections). Data from all other collections (n = 99) were used in subsequent analyses.
CSF collection
Cisternal CSF was collected from anaesthetized monkeys using a siliconized syringe (Lyons et al. 1999; Zeitzer et al. 2003). Anaesthesia was induced by intrasaphenous injection of 10.0 mg kg−1 ketamine hydrochloride with 0.5 mg kg−1 diazepam, and supplemented as needed with an intramuscular injection of 5.0 mg kg−1 ketamine hydrochloride. CSF (200 μl) was collected and placed in a siliconized tube on ice. Within 1 h of collection, CSF samples were placed in a −80°C freezer. CSF was successfully obtained in all but one instance (1 monkey in the forced locomotion condition).
Assays
We used 25 μl of squirrel monkey CSF, diluted with 75 μl of RIA buffer, to measure hypocretin-1 using a commercially available radioimmunoassay and a custom hypocretin-1 antibody provided by Dr Shahrad Taheri and Dr Ling Lin (detection limit = 100 pg ml−1, 5% intra-assay variability, Phoenix Pharmaceuticals, Belmont, CA, USA). CsFn (10 μl) was diluted with 50 μl of RIA buffer and assayed for cortisol using a commercially available radioimmunoassay (Diagnostic Products Inc., Los Angeles, CA, USA). All samples were assayed in duplicate.
Statistics
Data were analysed using Microsoft Excel (v. 10.2614.2625) using Student's paired t test or analysis of variance (ANOVA), as appropriate. Correlated (dependent) ANOVA were done with the java script available at http://faculty.vassar.edu/lowry/anova1u.html. Data from the two enriched activity conditions were combined within monkey before statistical analysis. Percent data were log transformed before averaging. Unless otherwise noted, all data are presented as means ± s.e.m.
Results
Effect of restricted locomotion on the diurnal rhythm of CSF hypocretin-1
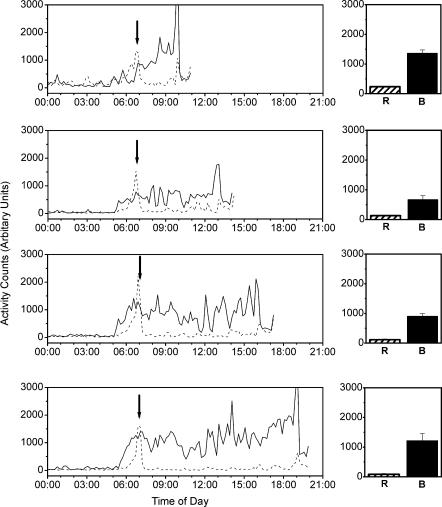
The restricted activity condition robustly diminished locomotor activity as compared to baseline at all time points examined (Fig. 1). In comparing the restricted activity condition to baseline, there was an 81.0 ± 2.58% decrease in locomotor activity between 07.00 and 10.00 h before the 10.00 h CSF collection, a 75.3 ± 6.65% decrease in locomotor activity between 07.00 and 13.00 h before the 13.00 h CSF collection, an 86.8 ± 4.81% decrease in locomotor activity between 07.00 and 16.00 h before the 16.00 h CSF collection, and a 92.4 ± 2.81% decrease in locomotor activity between 07.00 and 19.00 h before the 19.00 h CSF collection. Despite the large difference in locomotor activity that we were able to induce, there was no difference in cisternal CSF hypocretin-1 concentrations after the restricted activity conditions as compared to time-matched baseline values (Fig. 2, left panel).
Figure 1. Locomotor activity during restricted (R, dashed line) and baseline conditions (B, continuous line).
Restriction of locomotion was achieved by transferring the monkeys to smaller cages (see Methods). Data in the right panel are the average (± s.e.m.) activity levels in the 10 monkeys between 07.00 h and the time of CSF collection. Data in the left panel are the average activity patterns of the 10 monkeys in the two conditions and four time points. Arrows indicate the time at which monkeys were moved into the transport cages, at which time a transient investigator-induced increase in locomotor activity occurred. Data were collected in 1 min intervals and are shown as 10 min averages.
Figure 2. Mean (± s.e.m.) cisternal CSF hypocretin-1 (left) and cortisol concentrations (right) obtained during baseline (continuous line) or restricted (dashed line) activity conditions at four times of day.
There were no statistically significant differences in hypocretin-1 concentrations at any time of day (Student's paired t tests, see text). At all four sampling times, cortisol was significantly (P < 0.05, Student's paired t tests) elevated in the restricted activity condition.
Effect of restricted locomotion on HPA axis activation
We examined whether a long duration stay in a transportation cage caused an activation of the hypothalamic–pituitary–adrenal (HPA) axis, as measured by changes in CSF cortisol. Under baseline conditions, CSF cortisol concentrations exhibited the expected daily variation, being highest near wake time and declining throughout the day. In all CSF samplings that were preceded by an extended stay in the transport cage, CSF cortisol concentrations were significantly increased by 112 ± 12% (Fig. 2, right panel). The magnitude of the increase in cortisol observed was similar across the four time points and durations (single-factor ANOVA, d.f. = 39, P = 0.76).
Effect of elevated locomotor activity on CSF hypocretin-1 and cortisol concentrations
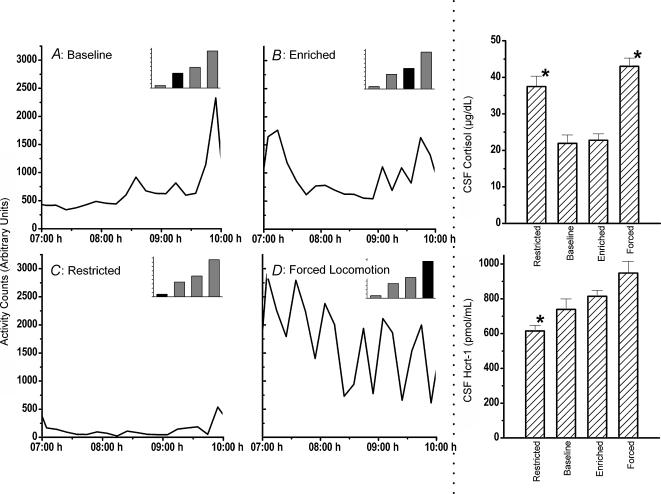
We next examined the effects of enhancing motor activity on CSF hypocretin-1 and cortisol concentrations at 10.00 h. Results are illustrated in Fig. 3. The restricted, enriched, forced and baseline conditions produced both different absolute amounts and overall patterns of locomotion between 07.00 and 10.00 h. Under baseline conditions, locomotor activity gradually increased between 07.00 and 10.00 h (685 ± 56.6 arbitrary units {a.u.}), as previously reported (Fig. 1, top panel). In contrast, monkeys in the restricted activity condition showed relatively little locomotion between 07.00 and 10.00 h (118 ± 11.8 a.u., 81.2 ± 2.54% less than baseline). Enriched conditions (962 ± 42.3 a.u., 45.2 ± 9.68% above baseline) evoked a pattern of activity similar to baseline with the addition of an initial burst of locomotion. Finally, the forced locomotion condition increased activity significantly (1721 ± 201 a.u., 161 ± 40.1% above baseline), with especially elevated bursts during the time of investigator interaction. Overall, average locomotor activity in each condition was significantly different from each other (ANOVA, d.f. = 47, P < 0.001; post hoc student's paired t tests, P < 0.005).
Figure 3. Locomotor activity between 07.00 and 10.00 h under baseline (A), enriched (B), restricted (C) and forced conditions locomotion (D).
For detailed experimental conditions, see Methods. Data were averaged across monkeys within each condition and are shown as a 10 min average. Average locomotion for each condition is shown in the histogram in the upper right corner of each of the activity plots; the filled bar represents the activity in the condition being displayed below. All monkeys had cisternal CSF collected at 10.00 h and the corresponding concentrations of hypocretin-1 (right, bottom panel) and cortisol (right, top panel) are shown. Cisternal CSF cortisol concentrations were significantly (*P < 0.02) higher in the restricted and forced locomotion conditions than in either baseline or enriched conditions. CSF hypocretin-1 concentrations were significantly (*P < 0.01) lower in the restricted activity condition as compared with the enriched and forced locomotion conditions.
At the single 10.00 h time point, hypocretin-1 concentrations increased progressively from restricted (615 ± 33.4 pg ml−1) to baseline (739 ± 59.9 pg ml−1) to enriched (814 ± 40.2 pg ml−1) to forced locomotion (948 ± 66.8 pg ml−1). ANOVA indicated a significant effect of condition on hypocretin-1 concentrations (d.f. = 35, P < 0.001). Despite the difference in activity between all conditions, post hoc student's paired t tests (two-tailed) indicated that there was a significant difference in hypocretin-1 concentrations only between the restricted activity conditions and both the enriched (P < 0.001) and forced locomotion conditions (P < 0.001). Note that there was no significant difference between baseline hypocretin-1 concentrations and those observed after a 161% increase in locomotion (forced condition). There was a trend towards significance in the comparison of the enriched and forced conditions (P = 0.04), but this difference was not significant after Bonferroni correction for multiple comparisons.
As is evident in Fig. 3, the different activity conditions also evoked different cortisol responses. Cortisol concentrations at 10.00 h were 37.46 ± 3.029 μg dl−1 (restricted), 21.93 ± 2.155 μg dl−1 (baseline), 22.78 ± 1.740 μg dl−1 (enhanced) and 43.02 ± 2.147 μg·dl−1 (forced). Statistical analysis indicated a significant effect of condition (ANOVA, d.f. = 48, P < 0.001) with post hoc analysis indicating that cortisol concentrations observed in the restricted and forced conditions were each significantly higher than those observed in the baseline and enriched conditions (Student's paired t tests, P < 0.02). Cortisol concentrations were not significantly different in restricted versus forced locomotion conditions (Student's paired t tests, P > 0.18). There was no significant linear correlation between CSF cortisol and hypocretin-1 concentrations (r =–0.07, P = 0.68, Pearson moment coefficient).
We further refined our analysis by examining the relationship between hypocretin-1 and the specific activity patterns observed within each condition in each monkey. By studying only the 10.00 h time point, we avoid a ceiling or basement effect in the hypocretin-1 data (Zeitzer et al. 2003). Activity data in the restricted, enhanced and forced locomotion conditions were changed into percentage change from baseline in order to account for intermonkey variability in basal locomotor rate. Hypocretin-1 concentrations were similarly normalized. Average locomotor activity between 07.00 and 10.00 h was highly correlated with the relative hypocretin-1 concentrations obtained at 10.00 h (Pearson moment correlation, r = 0.58, P < 0.001). The variation in the pattern of locomotion observed in the enriched activity condition (Fig. 3B) also enabled us to examine which part of the locomotor activity best reflected the change in hypocretin-1 concentrations (i.e. are hypocretin-1 concentrations reflecting proximal or distal bursting in activity, or the middle nadir; see Fig. 3B). We examined the relationship between the relative change in hypocretin-1 concentration obtained in the 20 cisternal CSF samples and the average locomotor activity before the CSF collection in the enriched condition. Using a dependent, sequential correlation analysis, we examined all correlations between hypocretin-1 concentrations and up to 11 h of prior locomotor activity data. We repeated this analysis after excluding up to 3 h of locomotor data, in 15 min increments, proximal to the CSF collection. These analyses indicated that the best correlation between the hypocretin-1 and locomotor data occurred when locomotor data from 45 to 77 min before obtaining CSF were integrated. Significant (P < 0.05) data were also obtained when locomotor data were integrated between 45 and up to 83 min. In other words, excluding the locomotion occurring 45 min before obtaining the CSF sample and integrating the next 30 min of locomotor data yielded the best correlation. When this was extended to data from all conditions, and locomotor data were normalized to baseline conditions, the Pearson moment correlation was similarly significant (r > 0.54, P < 0.001). We did a χ-square analysis of these data, comparing the observed distribution of hypocretin-1 values with the assumption of a random distribution of hypocretin-1 values. Such analysis indicated that hypocretin-1 was non-randomly distributed (χ2= 8.58, d.f. = 3, P < 0.04) with respect to locomotion. This indicates a significant impact of locomotion on hypocretin-1.
Discussion
Given that an 83% reduction in locomotor activity does not significantly change hypocretin-1 concentrations, we have demonstrated that locomotion is not necessary for the normal daily rise in CSF hypocretin-1 in the wake-consolidating squirrel monkey. Reduced locomotor activity independent of sleep does not therefore provide a significant negative feedback signal onto hypocretin-containing neurones. While it is possible that the restricted movement caused a decrease that was offset by an increase owing to elevated cortisol, neither our present data nor previous investigations in squirrel monkeys (Zeitzer et al. 2003) indicate that cortisol has such an effect. In fact, in the present study, cortisol was increased in both the restricted and forced activity conditions, two situations with different hypocretin-1 concentrations (Fig. 3). This is not to say that the stress of the restricted and forced activity conditions was identical, physiologically or psychologically, but that the changes in cortisol, the major endocrine output of the stress-activated HPA-axis in the squirrel monkey, are indistinguishable in the two conditions. A low activity condition in the absence of an increase in cortisol, akin to the ‘constant routine’ protocol used to examine human circadian rhythms, would be required to fully address this issue. This condition, however, may not be achievable in the squirrel monkey without the use of extended sedation.
Further evidence for lack of a robust interaction of activity with hypocretin was demonstrated using conditions aimed at stimulating locomotion in squirrel monkeys. Despite robust increases in locomotion after enriched and forced activity, hypocretin-1 did not increase significantly. A trend toward increasing hypocretin-1 concentrations was, however, noticed when comparing reduced, baseline, enriched and forced locomotion conditions, suggesting a modulating effect. Hypocretin-1 concentrations were significantly lower in restricted versus elevated activity conditions, substantiating this observation. When locomotion increased above baseline, hypocretin-1 concentrations were significantly above baseline. In contrast, however, when locomotion was lower than baseline, hypocretin-1 concentrations were distributed randomly, being both higher and lower than baseline. This suggests that increased locomotion may enhance hypocretin secretion while reduced activity has less effect. In addition, the magnitude of the change induced by locomotion was small in comparison to the diurnal change that occurs independent of locomotion. In the restricted activity condition, concentrations increased by 711 ± 69.6 pmol l−1 between 10.00 and 19.00 h (Fig. 2). This can be attributed mostly, if not solely, to a diurnal mechanism, probably controlled by a suprachiasmatic nucleus-dependent mechanism (Zhang et al. 2004). In contrast, we observed a change of only 221 ± 109 pmol l−1 in response to a 161% increase in locomotion in the forced activity condition. The diurnal change is therefore approximately three times larger than that induced by almost constant activity. Thus, while locomotion does not appear necessary for the expression of the daytime fluctuation in CSF hypocretin-1, increased locomotion probably modulates hypocretin-1 concentrations independent of sleep.
The consolidation of wakefulness into a single episode in the absence of external stimulation is unique to humans and some New World monkeys. In rodents, and to a lesser degree cats and dogs, sleep and wake are normally fragmented throughout the 24 h day, with a strong nocturnal or diurnal preference. It has been hypothesized that the hypocretin system is important for the consolidation of wakefulness into a single daily episode (Broughton et al. 1998; Dantz et al. 1994; Zeitzer et al. 2003), owing to its ability to increase wakefulness late in the day. In this model, hypocretin would represent the basis of the proposed circadian alertness signal (Edgar et al. 1993; Dijk & Czeisler, 1994). The ‘circadian alertness signal’ rises late in the waking day to offset the increasing homeostatic pressure for sleep, thereby aiding in the consolidation of wake into a single, elongated segment. Given the difference in consolidation patterns and the proposed role of hypocretin, however, one would anticipate a large difference in hypocretin regulation between wake-consolidating and polyphasic species. In fact, the daily pattern of CSF hypocretin-1 concentrations is surprisingly similar in rats and monkeys (cf. Fujiki et al. 2001 and Zeitzer et al. 2003). Others have found that hypocretin-1 is significantly affected by the concomitants of wake, such as feeding, locomotion and stress (Sakurai et al. 1998; Zhu et al. 2002; Stricker-Krongrad & Beck, 2002; Wu et al. 2002; Martins et al. 2002; España et al. 2002; Baldo et al. 2003; Diano et al. 2003; Yamanaka et al. 2003). We have not found a robust effect of these factors on hypocretin-1 in the squirrel monkey (present study; Zeitzer et al. 2003; J. M. Zeitzer & E. Mignot, unpublished observations). For example, in the squirrel monkey the change in hypocretin-1 observed during sleep deprivation is not dependent on a change in cortisol (Zeitzer et al. 2003). A possible explanation may be that in all polyphasic species it is impossible to study these factors without disturbing sleep. Differences in the methods used to measure ‘hypocretin activity’ (e.g. c-fos staining, RNA versus protein measurements) may also play a role (e.g. hypocretin release may be increased locally but not globally by a stressful stimulus). Alternatively, the daily rhythm of hypocretin-1 is more strongly modified by exogenous and endogenous factors that require wakefulness in polyphasic animals. This may possibly lead to a sleep–wake structure that is more sensitive to external stimuli in polyphasic animals (i.e. the stronger the presence of these factors, the greater the length of the wake bout). In wake-consolidating squirrel monkeys, and by extension humans, there is also an underlying daily variation of cisternal CSF hypocretin-1 (Salomon et al. 2003). However, in the squirrel monkey, unlike the situation in polyphasic mammals, CSF hypocretin-1 is not strongly influenced by stress and only slightly influenced by locomotion. Wake-consolidating mammals may not need an exogenous or endogenous ‘reason’ to be remain awake continuously for extended periods of time (up to 16 h). Given additional evidence from humans who have dysfunctional hypocretin systems (i.e. narcoleptics), and the data accrued from our experiments in squirrel monkeys, hypocretin-1 probably provides a wake-promoting signal that peaks in the evening hours and is lowest at wake time, enabling hypocretin-1 to help offset the homeostatic drive for sleep that builds up throughout the day. It may be that the evolutionary leap that has occurred in species able to consolidate wakefulness into a single, continuous daily bout is that the hypocretin systems of these species are no longer dependent upon exogenous factors or that the hypocretin system is sufficient in and of itself to allow wakefulness.
Acknowledgments
The authors wish to thank Drs Shahrad Taheri and Ling Lin for generous donation of the hypocretin-1 antibody. J.M.Z. was supported by a National Sleep Foundation Pickwick Fellowship and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award. This work was supported by grants MH47573 and NS23724.
References
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitrón-Reséndiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton R, Krupa S, Boucher B, Rivers M, Mullington J. Impaired circadian waking arousal in narcolepsy-cataplexy. Sleep Res Online. 1998;1:159–165. [PubMed] [Google Scholar]
- Dantz B, Edgar DM, Dement WC. Circadian rhythms in narcolepsy: studies on a 90 minute day. Electroencephalogr Clin Neurophysiol. 1994;90:24–35. doi: 10.1016/0013-4694(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiro G, Hammock MK, Bleyer WA. Spinal descent of cerebrospinal fluid in man. Neurol. 1976;26:1–8. doi: 10.1212/wnl.26.1.1. [DOI] [PubMed] [Google Scholar]
- Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the non-human primate hypocretin (orexin) system and its postsynaptic targets. Endocrinol. 2003;144:3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2002;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, Nishino S. Changes in CSF hypocretin-1 (orexin-A) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12:993–997. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol A. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neurosci. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Wang OJ, Lindley SE, Levine G, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic-pituitary-adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinol. 1999;24:131–140. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- Martins PJF, D'Almeida V, Pedrazzoli M, Lin L, Nishino S, Mignot E, Tufik S. Increased hypocretin-1 (orexin-A) levels in cerebrospinal fluid of rats after forced activity. J Sleep Res. 2002;11(S1):147. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Moore RY, Abrahamson EA, van den Pol A. The hypocretin neuron system: an arousal system in the human brain. Arch Italiennes Biologie. 2001;139:195–205. [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli M, D'Almeida V, Martins JFM, Machado RB, Ling L, Nishino S, et al. Increased hypocretin-1 levels in cerebrospinal fluid after REM sleep deprivation. Brain Res. 2004;995:1–6. doi: 10.1016/j.brainres.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol A, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. Inherent twenty-four hour and lunar clocks of a primate – the squirrel monkey. Comm Behav Biol. 1968;1:305–332. [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Zeitzer JM, Nishino S, Mignot E. Diurnal variation of CSF hypocretin-1 (orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Beck B. Modulation of hypothalamic hypocretin/orexin mRNA epxression by glucocorticoids. Biochem Biophys Res Comm. 2002;296:129–133. doi: 10.1016/s0006-291x(02)00848-3. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;26:25–28. [PubMed] [Google Scholar]
- Wexler DB, Moore-Ede MC. Circadian sleep-wake cycle organization in squirrel monkeys. Am J Physiol. 1985;248:R353–R362. doi: 10.1152/ajpregu.1985.248.3.R353. [DOI] [PubMed] [Google Scholar]
- Wu M-F, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol. 2002;283:R1079–R1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, et al. Fluctuation of extracellular hypocretin-1 (orexin-A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Yoshimichi G, Yoshimatsu H, Masaki T, Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp Biol Med. 2001;226:468–476. doi: 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM, Mignot E. Effect of suprachiasmatic nucleus lesions on the regulation of hypocretin-1 release. Sleep. 2004;27 doi: 10.1093/sleep/27.4.619. in press. [DOI] [PubMed] [Google Scholar]
- Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]