Abstract
C-terminal fragments of the sulphonylurea receptor SUR2A can alter the functional expression of cloned ATP-sensitive K+ channels (KATP). To investigate the protective role of KATP channels during metabolic stress we transfected SUR2A fragments into adult rat cardiac myocytes. A fragment comprising residues 1294–1358, the A-fragment, reduced sarcolemmal KATP currents by over 85% after 2 days (pinacidil-activated current densities were: vector alone 7.04 ± 1.22; and A-fragment 0.94 ± 0.07 pA pF−1, n = 6,6, P < 0.001). An inactive fragment (1358–1545, current density 6.30 ± 0.85 pA pF−1, n = 6) was used as a control. During metabolic inhibition (CN and iodoacetate) of isolated myocytes stimulated at 1 Hz, the A-fragment delayed action potential shortening and contractile failure, but accelerated rigor contraction and increased Ca2+ loading. On reperfusion, A-fragment-transfected cells also showed increased intracellular Ca2+ and the proportion of cells recovering contractile function was reduced from 40.0 to 9.5% (P < 0.01). The protective effect of pretreatment with 2,4-dinitrophenol, measured from increased functional recovery and reduced Ca2+ loading, was abolished by the A-fragment. Our data are consistent with a role for KATP channels in causing action potential failure and reduced Ca2+ loading during metabolic stress, and with a major role in protection by preconditioning. The effects of the A-fragment may arise entirely from reduced expression of the sarcolemmal KATP channel, but we also discuss the possibility of mitochondrial effects.
ATP-sensitive K+ (KATP) channels were first identified in the sarcolemma of cardiac muscle by Noma (1983), who proposed that they might serve to shorten the action potential in ischaemia and so protect the heart from calcium overload. Following the discovery of ischaemic preconditioning (IPC), in which brief periods of ischaemia protect the heart against damage by subsequent prolonged ischaemia, several studies showed that IPC could be mimicked and blocked, respectively, by pharmacological activators and blockers of KATP channels (Gross & Auchampach, 1992; Auchampach et al. 1992; reviewed by Yellon & Downey, 2003; Gross & Peart, 2003). Subsequently, however, it was proposed that mitochondrial KATP channels (mitoKATP), rather than sarcolemmal KATP channels (sarcoKATP), underlie the cardioprotective effect of IPC (Garlid et al. 1997; Liu et al. 1999; Grover & Garlid, 2000). The issue remains controversial, however, because the molecular structure of mitoKATP channels is not yet known and so the evidence for their involvement comes from pharmacological experiments, and the selectivity of the agents used has been questioned recently (see Discussion).
An alternative approach is to use molecular biological methods to manipulate known constituents of KATP channels. Cardiac sarcoKATP channels form as hetero-octamers of four Kir6.2 channel subunits and four SUR2A sulphonylurea receptor subunits (Aguilar-Bryan et al. 1998). Recent experiments using Kir6.2 knockout mice show that their hearts lack functional sarcoKATP channels and cannot be protected by IPC (Suzuki et al. 2002). These experiments suggest a key role for sarcoKATP in cardioprotection by IPC. The authors caution, though, that the importance of sarcoKATP may be exaggerated in mice because of their very high heart rate (Suzuki et al. 2002). These considerations, and possible adaptive developmental changes in transgenic animals, make it important to assess the effects of acute down-regulation of KATP activity in adult cardiac muscle, and in other species. We have therefore developed molecular constructs that will down-regulate channel activity in adult rat cells.
We have shown recently that a fragment of SUR2A that comprises the entire C-terminus (residues 1254–1545) coimmunoprecipitates with Kir6.2, and also acts as a dominant negative construct when transfected into HEK 293 cells stably expressing Kir6.2 and SUR2A, reducing the number of functional KATP channels at the cell surface (Rainbow et al. 2004). The critical C-terminal region for coimmunoprecipitation and dominant negative effects lies within the 65 amino acid region encompassing residues 1294–1358. In contrast, the distal C-terminus, residues 1358–1545, does not immunoprecipitate with Kir6.2 and does not reduce the number of functional KATP channels (Rainbow et al. 2004). In the present study, we have investigated the effects of these SUR2A C-terminal fragments on native cardiac muscle cells by transfecting them into adult rat ventricular myocytes, which were then maintained in primary culture for 48 h. We show that fragment 1294–1358 (hereafter referred to as the A-fragment to indicate activity) reduced sarcolemmal KATP currents by over 85%, whereas fragment 1358–1545 (the inactive I-fragment) was without effect. Using cells transfected with the I-fragment as controls, we show that the A-fragment delayed action potential failure and contractile failure on metabolic inhibition, but accelerated rigor contraction. It increased Ca2+ loading during metabolic inhibition and reperfusion, reduced the proportion of cells that recovered contractile function after metabolic inhibition, and abolished the protective effect of pretreatment with 2,4-dinitrophenol.
Methods
Isolation, transfection and culture of single ventricular myocytes
Adult male Wistar rats (around 300 g) were killed by cervical dislocation. The care and killing of animals conformed to the requirements of the UK Animals (Scientific Procedures) Act 1986. Single ventricular myocytes were isolated by enzymatic digestion of hearts using collagenase and protease, as we have previously described (Rodrigo et al. 2002). For transfection, the active (1294–1358) and inactive (1358–1545) SUR2A fragments were cloned in the pIRES2-EGFP-F vector (Rainbow et al. 2004), which encodes farnesylated enhanced green fluorescent protein that is localized to the inner face of the plasma membrane, or the pIRES2-DS-Red2 vector (BD Biosciences, Oxford, UK). Myocytes were transfected using LipofectAMINE 2000 (Invitrogen, Paisley, UK) applied for 40 min in Hepes-buffered minimal Eagle's medium with Earl's salts (MEM) immediately after removal of the isolation enzymes. This procedure gave a transfection efficiency up to 20%. After transfection, myocytes were cultured at 37°C in MEM containing 1% nonessential amino acids, 5%l-glutamine and penicillin and streptomycin (final concentrations 100 U ml−1 and 100 μg ml−1). To minimize changes in the myocytes induced by cell culture, fetal calf serum was omitted from the medium (Mitcheson et al. 1998). After 48 h the culture medium was replaced with Tyrode solution and myocytes were transferred to an experimental chamber for electrophysiological or functional experiments. Transfected cells were identified by membrane-localized green fluorescence under mercury lamp illumination (Fig. 1), except in experiments where Ca2+ was measured, where fluorescence was excited by a xenon light source and transfected cells were identified by DS-Red fluorescence.
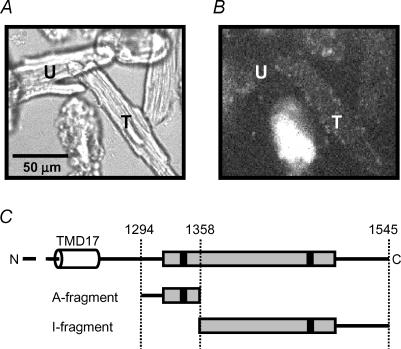
Figure 1. Transfection with SUR2A fragments A and B.
Confocal images of the same field of rat ventricular myocytes transfected with pIRES2-EGFP-F and viewed with transmitted light (A) or fluorescence excited at 488 nm and measured at >580 nm (B). T indicates a healthy myocyte that has been successfully transfected and shows fluorescence close to the membrane, while U indicates an untransfected myocyte. The severely damaged myocyte at the bottom centre of the field has rounded morphology with membrane blebbing and shows strong autofluorescence that is not localized to the membrane. C, location of the fragments within the C-terminal region of SUR2A. The entire C-terminal region is shown above. TMD17 is the 17th putative transmembrane domain, the shaded box indicates nucleotide binding domain 2 and the filled boxes Walker binding motifs A and B. The A- and I-fragments are shown below.
Electrophysiology
Conventional patch pipettes were used in the whole-cell configuration in either voltage-clamp or current-clamp mode to record membrane current or action potentials, respectively, and in the cell-attached configuration to record single channel activity, using an Axopatch 200B patch clamp amplifier (Axon Instruments, Foster City, CA, USA). Patch pipettes were filled with a solution containing (mm): KCl, 140; ATP, 0.1; ADP, 0.1; MgCl2, 1; 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid Hepes, 10; and BAPTA, 10; titrated to pH 7.2 with KOH, and had resistances of 3–6 MΩ when filled with this solution. For experiments in which action potentials were recorded, 5 mm EGTA was used instead of BAPTA. The nucleotide concentrations were chosen to minimize the possibility that delivery of ATP or washout of ADP by the pipette might delay the intracellular change in these nucleotides consequent on metabolic inhibition (Rodrigo et al. 2004). In cell-attached patch experiments, the pipette was filled with intracellular solution and the pipette voltage was held at +60 mV. Under these conditions the equilibrium potential for K+ is close to 0 mV, and the voltage across the membrane patch is around −130 mV (the sum of the pipette potential and the resting potential of the cell) so that single KATP channel openings lead to inward currents (Fig. 2C).
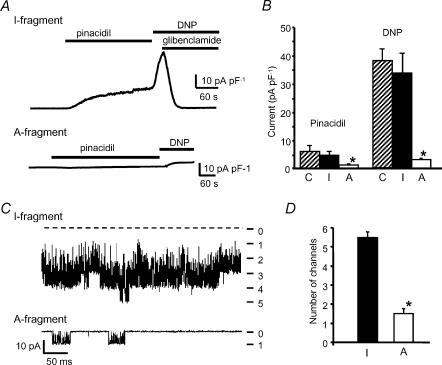
Figure 2. Effect of SUR2A fragments on sarcolemmal KATP currents.
A, recordings of whole-cell current from rat ventricular myocytes transfected with the SUR2A I-fragment (above) and the A-fragment (below). The holding potential was 0 mV and pinacidil (200 μm), DNP (200 μm) and glibenclamide (10 μm) were added as indicated. B, mean (± s.e.m.) KATP current density activated by pinacidil or DNP in control cells transfected with pIRES2-EGFP-F vector alone (C), and in cells transfected with the I-fragment or A-fragment of SUR2A; n = 6 cells in each case. *P < 0.001 versus control. C, KATP channel activity recorded in cell-attached patches on myocytes transfected with SUR2A fragments and exposed to metabolic inhibition with cyanide and iodoacetate. The records were made after 4 min metabolic inhibition. The dashed line in the upper record shows the zero current level and the numbers at the right indicate the number of open channels. D, maximum number of channels seen in cell-attached patches during metabolic inhibition in myocytes transfected with SUR2A I- or A-fragments (n = 4 patches in each case, *P < 0.01).
Measurement of contractile activity and intracellular Ca2+
Myocytes were placed in a 500 μl Perspex chamber mounted on the stage of an inverted microscope, continuously superfused with Tyrode solution at a rate of 5 ml min−1, and stimulated at 1 Hz by electrical field stimulation. Contractile activity was determined from video observation of a field of view containing four to eight cells using a 20 × objective. In some experiments, contraction of individual myocytes was determined from changes in cell length using a video-edge detection system (Model VED 103, Crescent Electronics, Sandy, UT, USA). To measure [Ca2+]i, myocytes were loaded with fura-2 by placing them in Tyrode containing 5 μm fura-2 AM for 10 min and excited alternately at 340 and 380 nm using a monochromator. Emitted light was collected at >520 nm and fluorescence intensity was measured using a video imaging system (PTI, Lawrenceville, NJ, USA).
Drugs and experimental solutions
Tyrode solution contained (mm): NaCl, 135; KCl, 5; NaH2PO4, 0.33; Na-pyruvate, 5; glucose, 10; MgCl2, 1; CaCl2, 2; and Hepes, 10; titrated to pH 7.4 with NaOH. MI Tyrode, which was used to cause metabolic inhibition, contained 2 mm NaCN to inhibit oxidative phosphorylation and 1 mm iodoacetic acid (IAA) to inhibit glycolysis, in substrate-free Tyrode solution (without glucose or pyruvate). Pinacidil, 2,4-dinitrophenol and glibenclamide (Sigma) were dissolved in dimethyl sulphoxide (DMSO) at 200 mm. The final DMSO concentration did not exceed 0.2%, which had no measurable effect on any of the cellular parameters measured. Fura-2 was purchased from Molecular Probes and dissolved in DMSO containing 5% pluronic acid.
Data acquisition and statistics
Electrophysiological data were recorded directly to hard disk using pClamp8 software. Statistical significance was calculated using ANOVA followed by Student–Neuman–Keuls test, Student's t test or χ2 as appropriate and P < 0.05 was considered significant. Data are presented as means ± s.e.m. throughout. All experiments were done at 32 ± 2°C.
Results
The A-fragment of SUR2A reduces expression of functional sarcolemmal KATP channels
To assess KATP channel function in ventricular myocytes transfected with the active or inactive fragments of SUR2A, we measured whole-cell KATP currents at 0 mV activated by the KATP channel opener pinacidil (200 μm) and by metabolic inhibition with 2,4-dinitrophenol (DNP, 200 μm). Figure 2A shows patch clamp records of whole-cell current from myocytes transfected with the inactive I-fragment or the active A-fragment of SUR2A, and mean results for current density are shown in Fig. 2B. Current density in cells transfected with the I-fragment (pinacidil current: 6.30 ± 0.85 pA pF−1, n = 6) was not different from that in cells transfected with the pIRES2-EGFP-F vector alone (7.04 ± 1.22 pA pF−1, n = 6). The A-fragment reduced currents activated by either pinacidil or DNP to around 15% of their value in cells transfected with the I-fragment (pinacidil current: 0.94 ± 0.07 pA pF−1, n = 6, P < 0.001). We also estimated the number of active sarcoKATP channels by measuring channel activity in cell-attached patches on myocytes exposed to metabolic inhibition with MI Tyrode solution. Under these conditions, sarcoKATP channels opened after around 3 min of metabolic inhibition and rapidly reached a peak of activity. Figure 2C shows recordings from patches on cells transfected with SUR2A fragments. In the patch on a cell transfected with the I-fragment (top) up to five channels were open simultaneously after 4 min of metabolic inhibition. In the cell transfected with the A-fragment (bottom), only one channel can be seen to be active. Similar recordings from cells transfected with either the I- or A-fragment show that the A-fragment did not affect the time to sarcoKATP channel opening during metabolic inhibition (A-fragment 3.10 ± 0.38 min; I-fragment 2.89 ± 0.33 min, n = 4), but reduced the maximum number of channels seen in the patch (Fig. 2D). These findings are consistent with the reduction in whole-cell current in response to pinacidil or DNP shown in Fig. 2A and B.
The A-fragment delays action potential failure during metabolic inhibition
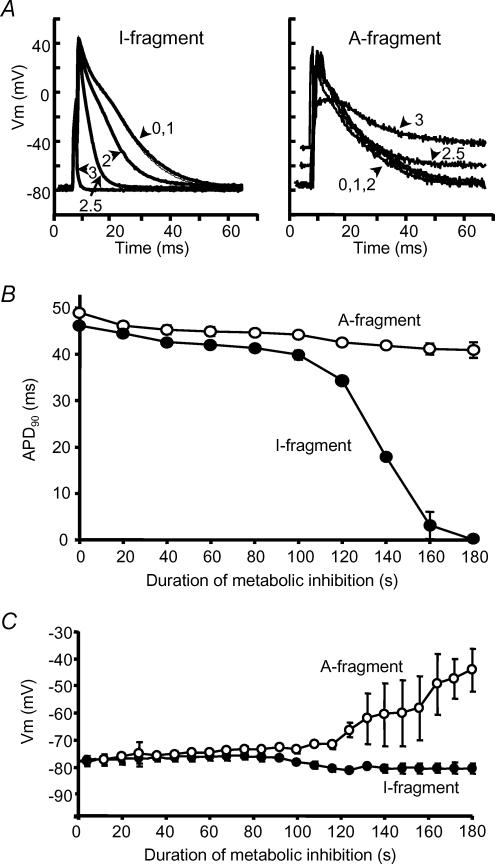
During metabolic inhibition or hypoxia in adult cardiac myocytes the action potential shortens and then fails as a result of an increase in K+ conductance as sarcoKATP channels open (Stern et al. 1988; Lederer et al. 1989). We used whole-cell patch clamp in current-clamp mode to record the behaviour of the action potential during metabolic inhibition in cells transfected with inactive or active SUR2A fragments. Cells were stimulated at 1 Hz using a 5 ms current pulse at 130% threshold. Figure 3 shows selected action potentials recorded in such an experiment. In cells transfected with the I-fragment, action potentials had shortened substantially after 2 min metabolic inhibition and had failed by 3 min (Fig. 3A and B), while cells transfected with the A-fragment showed no significant shortening of the action potential up to 3 min. Figure 3C shows that the resting potential also behaved differently during metabolic inhibition in cells transfected with different SUR2A fragments. In I-fragment-transfected cells action potential failure was associated with hyperpolarization of the resting potential at around 120 s of metabolic inhibition. In contrast, A-fragment-transfected cells began to depolarize at this time, leading to substantial depolarization to around −45 mV after 3 min metabolic inhibition.
Figure 3. Action potential shortening during metabolic inhibition.
A, recordings of action potentials from rat ventricular myocytes transfected with the SUR2A I-fragment (left) and the A-fragment (right). Traces show action potentials recorded at the start of metabolic inhibition (0), and after 1, 2, 2.5 and 3 min as indicated. B, mean (± s.e.m.) action potential duration at 90% repolarization (APD90) and C, mean resting membrane potential during metabolic inhibition in cells transfected with the I-fragment (•) and A-fragment (○) of SUR2A. n = 4 cells in each case.
The A-fragment delays contractile failure, but accelerates rigor
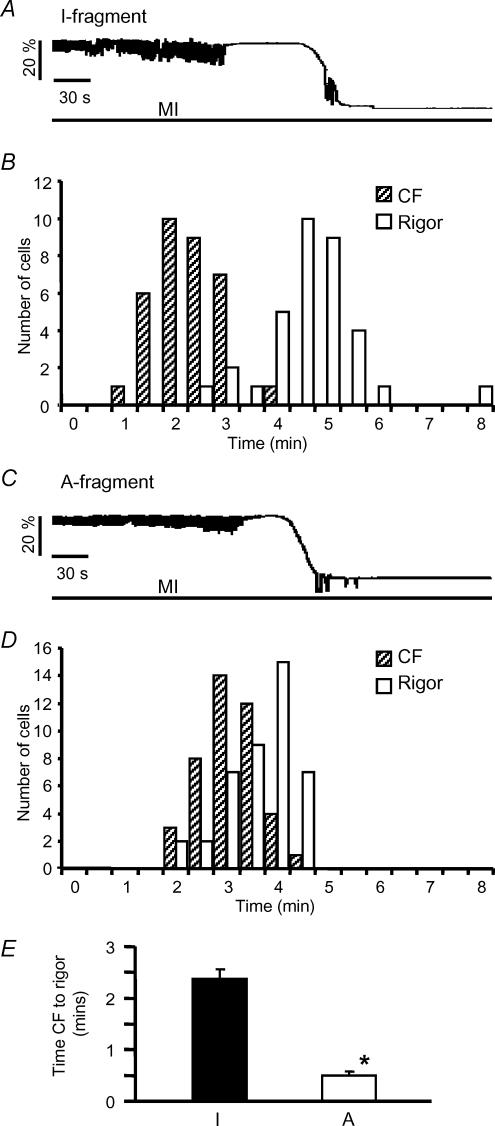
In freshly isolated rat myocytes, metabolic inhibition leads to failure of contraction which is consequent on action potential failure, and this is followed after a delay by rigor contraction, indicating the exhaustion of ATP around the myofilaments (Eisner et al. 1989; Silverman & Stern, 1994). We investigated the time to contractile failure (CF) and rigor in field-stimulated myocytes transfected with SUR2A fragments. Figure 4A shows a recording of cell length indicating that cells transfected with the SUR2A I-fragment behave essentially as has been previously described for freshly isolated myocytes (Eisner et al. 1989). The histogram of Fig. 4B shows the distribution of times to CF and rigor from 34 such cells; the mean times to CF and rigor were 2.55 ± 0.10 and 4.92 ± 0.16 min, respectively. In contrast, in cells transfected with the A fragment (Fig. 4C and D), contractile failure occurred later, corresponding to the delayed action potential failure described above, but was followed very shortly by rigor; times to CF and rigor were 3.36 ± 0.10 and 3.82 ± 0.10 min, respectively (n = 42), and the delay between CF and rigor is shown in Fig. 4E. Thus, transfection with the A-fragment caused contraction to fail later during metabolic inhibition, but rigor to occur earlier.
Figure 4. Effect of SUR2A fragments on contractile behaviour during metabolic inhibition.
A, recording showing the change in cell length during metabolic inhibition from a myocyte transfected with the SUR2A I-fragment. B, the distribution of times to contractile failure and rigor in 34 such cells. C and D, length recording and times as in A and B, but from myocytes transfected with the SUR2A A-fragment (n = 42). E, mean (± s.e.m.) interval between contractile failure and rigor in cells transfected with I- or A-fragments, *P < 0.001.
Effects of SUR2A fragments on intracellular calcium during metabolic inhibition
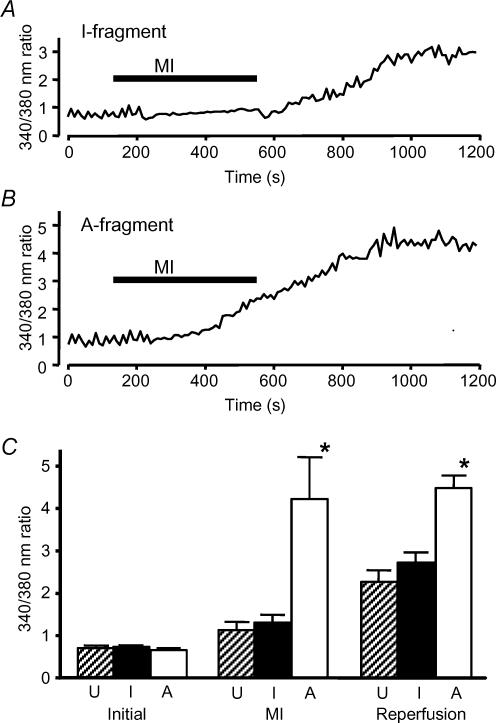
The ability of cardiac myocytes to maintain Ca2+ homeostasis is a critical determinant of their ability to survive ischaemia and reperfusion (Silverman & Stern, 1994). We therefore assessed the effects of SUR2A fragments on the pattern of changes in intracellular Ca2+ in response to metabolic inhibition and reperfusion. Neither fragment affected the mean resting [Ca2+]i (Fig. 5C). Freshly isolated rat cardiac myocytes show a modest increase in [Ca2+]i during 7 min metabolic inhibition, followed by a much more substantial increase on reperfusion (Rodrigo et al. 2004). Essentially the same pattern of changes in [Ca2+]i was seen in untransfected myocytes cultured for 2 days, and in myocytes transfected with the I-fragment of SUR2A (Fig. 5A). Figure 5B shows an example recording from a myocyte transfected with the A-fragment. It can be seen that [Ca2+]i increased substantially during metabolic inhibition, and rose to a higher levels during reperfusion. The mean values for [Ca2+]i (as fura-2 340/380 nm ratio) from similar experiments are shown in Fig. 5C. Neither fragment affected the mean initial [Ca2+]i, and [Ca2+]i at the end of 7 min metabolic inhibition and after 10 min reperfusion did not differ in cells transfected with the I-fragment of SUR2A from those in untransfected cells. However, in A-fragment-transfected cells, [Ca2+]i was substantially higher at the end of metabolic inhibition and after 10 min reperfusion (Fig. 5C). Thus Ca2+ loading during metabolic inhibition and reperfusion is increased in cells transfected with the A-fragment of SUR2A.
Figure 5. Effects of SUR2A fragments on intracellular calcium during metabolic inhibition.
A, example recording showing the change in intracellular Ca2+ from a cell transfected with the I-fragment of SUR2A and exposed to MI Tyrode solution as indicated. Ca2+ was indicated by the ratio of the fura-2 signals with 340 and 380 nm excitation, respectively, from pairs of images collected at 0.1Hz. B, recording of intracellular Ca2+ as in A, but from a cell transfected with the A-fragment of SUR2A. The peaks in the early parts of the recordings of A and B arise from aliasing of the Ca2+ transients associated with contraction as sampling and stimulation change their phase relationship with time (Rodrigo et al. 2004). It can be seen that these peaks were lost at the time when contraction fails during metabolic inhibition. C, mean (± s.e.m.) intracellular Ca2+ (measured as fura-2 fluorescence ratio) in untransfected myocytes (U) and in myocytes transfected with I or A SUR2A fragments, measured before metabolic inhibition, after 7 min MI and after 10 min reperfusion. (n = 40,20,14, respectively; *P < 0.001 versus untransfected cells).
The A-fragment reduces ischaemic tolerance and protection by dinitrophenol pretreatment
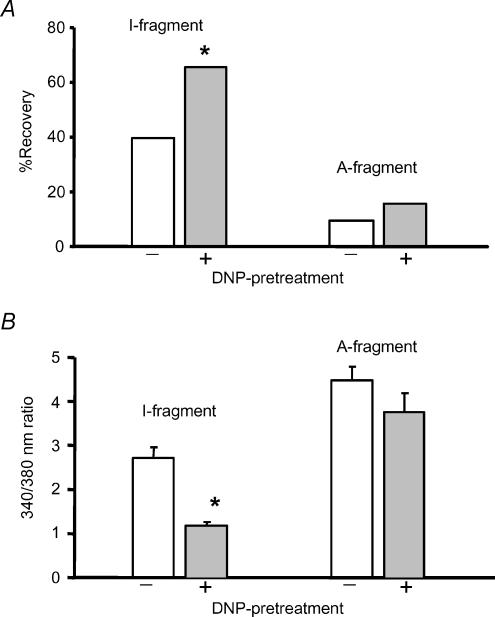
In addition to reducing infarct size, sarcoKATP channels have been proposed to contribute to recovery of contractile function after ischaemia and reperfusion (Toyoda et al. 2000; Suzuki et al. 2002). We assessed recovery of isolated ventricular cells after metabolic inhibition (7 min) and reperfusion by measuring the proportion of cells that had recovered the ability to contract in response to 1 Hz field stimulation 10 min after the removal of metabolic inhibition. Down-regulation of KATP with the SUR2A A-fragment reduced the proportion of cells that recovered contractile function. Only 9.5% of cells transfected with the A-fragment recovered, compared to 40.0% of I-fragment-transfected cells (Fig. 6A). We also investigated the effect of KATP down-regulation on protection by pretreatment with 2,4-dinitrophenol (DNP), which we have shown previously to be protective against subsequent damage by metabolic inhibition and reperfusion in freshly isolated rat myocytes (Rodrigo et al. 2002). To induce protection, DNP (50 μm) was applied for 5 min followed by 5 min wash with substrate-free Tyrode solution before metabolic inhibition with MI Tyrode solution for 7 min followed by reperfusion with normal Tyrode solution. In I-fragment-transfected myocytes, DNP pretreatment increased the proportion of cells that had recovered contractile function 10 min after reperfusion to 65.3%% (P < 0.02 versus no DNP pretreatment, Fig. 6A). However, in A-fragment-transfected cells DNP pretreatment did not significantly increase recovery of contractile function (Fig. 6A).
Figure 6. The A-fragment reduces recovery from MI and abolishes protection by DNP.
A, mean (± s.e.m.) percentage of cells transfected with I- or A-fragments, respectively, that had recovered contractile function in response to 1 Hz field stimulation 10 min after reperfusion with normal Tyrode solution following 7 min metabolic inhibition. In each case the left-hand bar shows effects of metabolic inhibition and reperfusion alone and that on the right results from cells protected by pretreatment with 50 μm DNP before metabolic inhibition (n = 30,23,21,25 cells, respectively, *P < 0.02 versus absence of DNP pretreatment, χ2 test). B, Ca2+ levels, measured as 340/380 nm fura-2 ratio, after 10 min reperfusion following 7 min metabolic inhibition. The bars show results from I- and A-fragment-transfected cells without or with DNP pretreatment (n = 20,14,14,11, respectively; *P < 0.001).
In freshly isolated myocytes, the protective effect of DNP pretreatment is also indicated by an improvement in Ca2+ homeostasis, limiting the rise in [Ca2+]i that results from subsequent metabolic inhibition and reperfusion (Rodrigo et al. 2002). Therefore, we examined the effect of DNP pretreatment on the metabolic inhibition/reperfusion-induced rise in Ca2+ in cells transfected with SUR2A fragments. Figure 6B shows that in I-fragment-transfected cells DNP pretreatment substantially reduced Ca2+ loading, so that Ca2+ after 10 min reperfusion had returned near to the resting level (cf. Fig. 5C). In cells transfected with the A-fragment, DNP pretreatment did not significantly reduce the Ca2+ level measured after 10 min reperfusion (Fig. 6B). Thus, transfection with the A-fragment of SUR2A abolished the protective effect of DNP measured either from improved contractile recovery or reduced Ca2+ loading.
Discussion
We have shown that it is possible to transfect freshly isolated adult rat cardiac myocytes with DNA encoding fragments of SUR2A, and to examine their functional effects after 2 days in culture. The SUR2A C-terminal fragment 1294–1358 (the A-fragment) reduced the surface expression of functional sarcoKATP channels in ventricular myocytes, measured 2 days after transfection, by around 85% compared to cells transfected with pIRES2-EGFP-F vector alone. In contrast, the distal region of the C-terminus, fragment 1358–1545, was without effect. Functional channel expression was estimated from the maximal whole-cell KATP current activated by 200 μm pinacidil or 200 μm DNP, and by measuring the maximum number of KATP channels observed in on-cell patches during metabolic inhibition; each measure gave consistent estimates for the effect of the A-fragment. Our previous findings in HEK 293 cells showed that the A-fragment can associate with Kir6.2, thereby preventing its association with native SUR2A and trafficking to the surface membrane (Rainbow et al. 2004).
The A-fragment of SUR2A had substantial effects on the responses of cardiac myocytes to metabolic stress. In control cells, metabolic inhibition led to action potential shortening after about 100 s that was associated with hyperpolarization of the resting membrane potential and culminated shortly afterwards in action potential failure (Fig. 3). In contrast, action potential shortening did not occur in A-fragment-transfected cells. These results are consistent with previous findings in freshly isolated myocytes that KATP channel opening shortens or abolishes the action potential in hypoxia and metabolic inhibition (Noma, 1983; Lederer et al. 1989; Rodrigo et al. 2004). In control cells we observed action potential shortening that was associated with resting membrane hyperpolarization, as expected if substantial numbers of KATP channels open. However, in A-fragment-transfected cells the membrane potential depolarized during metabolic inhibition. This suggests that KATP channel opening may also defend the resting membrane potential during metabolic stress. In A-fragment-transfected cells, it is likely that the action potential finally failed because of resting membrane depolarization. As expected, the delayed action potential failure in A-fragment-transfected cells was associated with a delay in contractile failure during metabolic inhibition (Fig. 4). Noma (1983) suggested that action potential shortening in response to KATP channel opening would reduce Ca2+ entry and so cardiac energy consumption. In agreement with such an energy-sparing affect of KATP, we found that A-fragment-transfected cells, which have substantially fewer KATP channels, showed increased Ca2+ loading during metabolic inhibition and went into rigor sooner than control cells, suggesting an earlier fall in ATP around the contractile machinery in these cells. These results are consistent with findings in hearts from Kir6.2 knockout mice, where contractile failure during ischaemia is also delayed and the contracture that corresponds to rigor is accelerated (Suzuki et al. 2002).
We also examined the effects of SUR2A fragments on functional recovery of cardiac myocytes after metabolic inhibition and reperfusion and on the protective effect of pretreatment with DNP. The proportion of cells that had recovered the ability to respond to electrical stimulation after 10 min reperfusion was substantially less in A-fragment-transfected cells than in controls transfected with the I-fragment (Fig. 6). We also found that the protective effect of pretreatment with DNP, measured either as increased functional recovery or reduced Ca2+ overload, was abolished in cells transfected with the A-fragment (Fig. 6). These results are also in agreement with the findings of Suzuki et al. (2002) in Kir6.2 knockout mice where the damaging effects of ischaemia and reperfusion were exacerbated and protection by ischaemic preconditioning was abolished.
Our results are consistent with a major role for sarcoKATP in protecting cardiac muscle during metabolic stress, as originally proposed by Noma (1983) and supported by recent studies in Kir6.2 knockout mice (Suzuki et al. 2002; Zingman et al. 2002). It has been suggested that the role of sarcoKATP might be exaggerated in mice relative to other species because of their high heart rate (Suzuki et al. 2002), but our results suggest that their importance may be extended to the rat at least. In terms of the extra protection afforded by ischaemic preconditioning (IPC), much recent work has suggested that a mitochondrial KATP channel rather than sarcoKATP underlies the effects of IPC (Garlid et al. 1997; Liu et al. 1998; Sato et al. 2000; Ghosh et al. 2000). This evidence, however, currently rests on the selectivity of the opener diazoxide and the blocker 5-hydroxydecanoate for mitoKATP and that of the sulphonylurea HMR 1883 for sarcoKATP. Both diazoxide and 5-hydroxydecanoate have been shown recently to have channel-independent effects on mitochondria so that the role and even the existence of mitoKATP has been questioned (Hanley et al. 2002; Hanley et al. 2003; Das et al. 2003). Sulphonylureas are known to become less effective blockers under conditions of metabolic stress, possibly because their block is reduced by increased intracellular ADP (Findlay, 1993; Lawrence et al. 2002; Reimann et al. 2003), and our unpublished findings suggest this is also true for HMR 1883. Thus, assessment of the role of sarcoKATP channels during ischaemia from the sensitivity of protective effects to HMR 1883 may underestimate their importance.
Is it possible that a mitochondrial KATP channel or other mitochondrial mechanism might contribute to some of the effects of the active SUR2A A-fragment on ischaemic tolerance? This question is difficult to answer while the molecular constituents of mitoKATP remain undetermined. Both the pore-forming KATP channel subunits Kir6.2 and Kir6.1 are expressed in cardiac myocytes, but it has been suggested that neither contributes to mitoKATP since flavoprotein fluorescence, used as an assay of mitoKATP activity, is unaffected by knockout of either subunit (Suzuki et al. 2001; Miki et al. 2002). However, other recent work has questioned the validity of flavoprotein fluorescence as an assay of mitoKATP activity (Lawrence et al. 2001; Hanley et al. 2002), since correlation of flavoprotein fluorescence with mitoKATP activity is based on their common sensitivity to diazoxide and 5-hydroxydecanoate. Both these agents can have channel-independent effects, as discussed above. Immunocytochemical studies indicate that Kir6.2 shows strong localization to the surface membrane of cardiac muscle, but that it also occurs in mitochondria (Singh et al. 2003; Lacza et al. 2003). Kir6.1 has been reported to occur in mitochondria and colocalize with mitochondrial markers in some studies (Suzuki et al. 1997; Singh et al. 2003; Lacza et al. 2003) but not in another (Seharaseyon et al. 2000), possibly reflecting the different antibodies used. Further, immunocytochemical studies suggest that SUR2A may also occur in mitochondria, although at a lower level than in the sarcolemma (Singh et al. 2003). In HEK 293 cells, the A-fragment of SUR2A can reduce currents resulting from coexpression of Kir6.1 and SUR2B (Rainbow et al. 2004), although this subunit combination has not been suggested to occur in cardiac muscle. It remains possible, then, that if Kir6.1, Kir6.2 or SUR2A were to contribute to a mitochondrial channel the A-fragment of SUR2A might also have mitochondrial effects, as indeed might knockouts of these subunits. These considerations highlight the future need for information on the molecular structure of the mitochondrial KATP channel.
In summary, our active SUR2A fragment clearly reduces functional expression of sarcoKATP and has effects that are consistent with this, such as the abolition of action potential shortening, delay of contractile failure and acceleration of rigor. It also reduces the tolerance of myocytes to metabolic stress and blocks the protective effect of pretreatment with DNP. These effects on metabolic tolerance may also be a consequence of reduced sarcoKATP expression, but it is conceivable that actions on another channel, possibly mitochondrial, that involves Kir subunits could contribute to these effects.
Acknowledgments
We thank Mrs D. Everitt for skilled technical help, and the British Heart Foundation and MRC for support.
References
- Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992;26:1054–1062. doi: 10.1093/cvr/26.11.1054. [DOI] [PubMed] [Google Scholar]
- Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Nichols CG, O'Neill SC, Smith GL, Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. Sulphonylurea drugs no longer inhibit ATP-sensitive K+ channels during metabolic stress in cardiac muscle. J Pharmacol Exp Ther. 1993;266:456–467. [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Standen NB, Galinanes M. Evidence for mitochondrial KATP channels as effectors of human myocardial preconditioning. Cardiovasc Res. 2000;45:934–940. doi: 10.1016/s0008-6363(99)00407-1. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Garlid KD. ATP-Sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Gopalan KV, Lareau RA, Srivastava DK, von Meltzer M, Daut J. Beta-oxidation of 5-hydroxydecanoate, a putative blocker of mitochondrial ATP-sensitive potassium channels. J Physiol. 2003;547:387–393. doi: 10.1113/jphysiol.2002.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacza Z, Snipes JA, Miller AW, Szabo C, Grover G, Busija DW. Heart mitochondria contain functional ATP-dependent K+ channels. J Mol Cell Cardiol. 2003;35:1339–1347. doi: 10.1016/s0022-2828(03)00249-9. [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Billups B, Rodrigo GC, Standen NB. The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation. Br J Pharmacol. 2001;134:535–542. doi: 10.1038/sj.bjp.0704289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CL, Rainbow RD, Davies NW, Standen NB. Effect of metabolic inhibition on glimepiride block of native and cloned cardiac sarcolemmal KATP channels. Br J Pharmacol. 2002;136:746–752. doi: 10.1038/sj.bjp.0704770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ, Nichols CG, Smith GL. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sato T, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sato T, Seharaseyon J, Szewczyk A, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels. Viable candidate effectors of ischemic preconditioning. Ann NY Acad Sci. 1999;874:27–37. doi: 10.1111/j.1749-6632.1999.tb09222.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Rainbow RD, James M, Hudman D, Al Johi M, Singh H, Watson PJ, Davies NW, Lodwick D, Norman RI. Proximal C-terminal domain of sulphonylurea receptor 2A interacts with the pore-forming Kir6 subunits in KATP channels. Biochem J. 2004;379:173–181. doi: 10.1042/BJ20031087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Dabrowski M, Jones P, Gribble FM, Ashcroft FM. Analysis of the differential modulation of sulphonylurea block of β-cell and cardiac ATP-sensitive K+ KATP channels by Mg-nucleotides. J Physiol. 2003;547:159–168. doi: 10.1113/jphysiol.2002.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo GC, Davies NW, Standen NB. Diazoxide causes early activation of cardiac sarcolemmal KATP channels during metabolic inhibition by an indirect mechanism. Cardiovasc Res. 2004;61:570–579. doi: 10.1016/j.cardiores.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Lawrence CL, Standen NB. Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. J Mol Cell Cardiol. 2002;34:555–569. doi: 10.1006/jmcc.2002.1536. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasaki N, Seharaseyon J, O'Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal KATP channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- Seharaseyon J, Ohler A, Sasaki N, Fraser H, Sato T, Johns DC, O''vRourke B, Marban E. Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral Kir gene transfer. J Mol Cell Cardiol. 2000;32:1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- Silverman HS, Stern MD. Ionic basis of ischaemic cardiac injury: insights from cellular studies. Cardiovasc Res. 1994;28:581–597. doi: 10.1093/cvr/28.5.581. [DOI] [PubMed] [Google Scholar]
- Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J Mol Cell Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Stern MD, Silverman HS, Houser SR, Josephson RA, Capogrossi MC, Nichols CG, Lederer WJ, Lakatta EG. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci USA. 1988;85:6954–6958. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, Takata K. Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochem Biophys Res Comm. 1997;241:693–697. doi: 10.1006/bbrc.1997.7891. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Friehs I, Parker RA, Levitsky S, McCully JD. Differential role of sarcolemmal and mitochondrial KATP channels in adenosine-enhanced ischemic preconditioning. Am J Physiol. 2000;279:H2694–H2703. doi: 10.1152/ajpheart.2000.279.6.H2694. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]