Abstract
Obstructive sleep apnoea (OSA), which is characterized by periodic inspiratory obstruction, is associated with hypertension and possibly with changes in the baroreceptor reflex. In this investigation we induced changes in inspiratory resistance and in inspiratory oxygen and carbon dioxide content, which simulate some of the changes in OSA, to determine whether this caused changes in the gain or setting of the carotid baroreflex. In eight healthy subjects (aged 21–62 years) we changed the stimulus to carotid baroreceptors, using neck chambers and graded pressures of −40 to +60 mmHg, and assessed vascular resistance responses in the brachial artery from changes in blood pressure (Finapres) divided by brachial artery blood flow velocity (Doppler ultrasound). Stimulus–response curves were defined during (a) sham (no additional stimulus), (b) addition of an inspiratory resistance (inspiratory pressure −10 mmHg), (c) breathing asphyxic gas (12% O2, 5% CO2), and (d) combined resistance and asphyxia. Sigmoid or polynomial functions were applied to the curves and maximum differentials (equivalent to peak gain) and the corresponding carotid pressures (equivalent to ‘set point’) were determined. The sham test had no effect on either gain or ‘set point’. Inspiratory resistance alone had no effect on blood pressure and did not displace the curve. However, it reduced gain from −3.0 ± 0.6 to −2.1 ± 0.4 units (P < 0.05). Asphyxia alone did increase blood pressure (+7.0 ± 1.1 mmHg, P < 0.0005) and displaced the curve to higher pressures by +16.8 ± 2.1 mmHg (P < 0.0005). However, it did not affect gain. The combination of resistance and asphyxia both reduced gain and displaced the curve to higher pressures. These results suggest that inspiratory resistance and asphyxia cause changes in the baroreceptor reflex which could lead to an increase in blood pressure. These changes, if sustained, could provide a mechanism linking hypertension to obstructive sleep apnoea.
Obstructive sleep apnoea (OSA) is a common clinical problem which affects approximately 2% of middle aged women and 4% of middle aged men (Young et al. 1993). The apnoeic events are characterized by inspiratory flow obstruction associated with hypoxia and hypercapnia. They terminate when the subject arouses and makes strong inspiratory efforts. These apnoeic episodes are associated with large changes in blood pressure and in sympathetic activity (Somers et al. 1995; Smith et al. 1996; Morgan, 2001). A particular complication of obstructive sleep apnoea is the increased risk of developing hypertension and this occurs in 40–60% of patients (Partinen & Telakivi, 1992; Parati et al. 1997; Bixler et al. 2000; Grote et al. 2000; Lavie et al. 2000; Nieto et al. 2000; Peppard et al. 2000). The reason for development of hypertension is unknown, but one possibility is that it could be related to a change in baroreceptor sensitivity or due to baroreceptor resetting occurring as the result of frequent repeated episodes of nocturnal hypertension.
This study was undertaken, using healthy control subjects, to attempt to elucidate some of the mechanisms which might link the obstructive apnoeic events to increases in blood pressure. In these experiments we induced mild asphyxia, inspiratory gas flow obstruction, and both, and examined the effects on carotid baroreflex sensitivity and ‘set point’. It was hypothesized that these conditions would alter carotid baroreflex sensitivity and ‘set point’ in the direction that would promote higher blood pressure. We were particularly interested in examining responses of vascular resistance since we consider these to be more important than cardiac responses in blood pressure control (Cooper & Hainsworth, 2002); all previous studies have concentrated only on the cardiac arm of the reflex (Ziegler et al. 1995; Carlson et al. 1996; Parati et al. 1997).
Methods
Subjects
We studied eight volunteer subjects (4 male) aged between 21 and 62 years (35.5 ± 5.3 years, mean ± s.e.m.). All subjects were apparently healthy with no symptoms of cardiovascular or respiratory disease and no history of snoring or excessive daytime sleepiness. None was hypertensive. The range of arterial pressures for all subjects was 105–132 mmHg systolic and 64–87 mmHg diastolic. All subjects gave informed written consent and the study was approved by the Leeds Teaching Hospitals Research Ethics Committee. All procedures conformed with the Declaration of Helsinki.
Measurements
Subjects sat comfortably and breathed through a mouthpiece connected to a three-way valve. ECG was recorded using three leads and a Hewlett Packard 78325C (Boebringen, Germany) recorder. Brachial arterial pressure was determined using a standard sphygmomanometer taking diastolic pressure as Korotkof phase 5. Brachial pressures were used to calibrate the finger estimates. Finger arterial pressure was recorded continuously during each experiment using a finger photoplethysmographic device (Finapres, Ohmeda 2300, WI, USA). Brachial artery blood velocity was determined using a pulse wave Doppler system (T2-Dop,DWL Elektronische System GmbH, Sipplingen, Germany) with a 4 mHz probe positioned over the brachial artery at or near the antecubital fossa. The probe was adjusted to provide the strongest signal and was then held firmly in position using a clamp. Although we could not ensure that the position of the Doppler probe was the same in each experiment we took extreme care to ensure that the angle with the brachial artery remained constant during each experiment. End tidal CO2 and O2 were recorded continuously through expiratory ports on the mouthpiece connected to a CO2 analyser (Instrumentation Laboratories, IL200, Lexington, MA, USA) and an O2 analyser (Servomex 570A, Crowborough, UK). Mouth pressure was recorded through a catheter connected to a mouthpiece and a pressure transducer (Statham P23Gb). All variables were recorded on a direct writing electrostatic recorder (Gould, Ballainvilliers, France, model ES1000) and on a personal computer via a data acquisition program (Windaq, Dataq Instruments, Akron, OH, USA).
Carotid baroreceptor tests
Changes in the stimulus to carotid sinus baroreceptors were effected by changing the extramural carotid pressures by applying suction or pressure to the neck overlying the sinuses. Negative pressures were applied using a lead chamber similar to that described by Eckberg et al. (1975). The chamber was moulded to fit to the subject from the lower border of the mandible to the upper border of the chest and to the posterior neck muscles. The edges of the chamber were fitted with neoprene to facilitate an airtight seal without causing discomfort to the subject. To apply positive pressures we used paired chambers as described by Kelly et al. (1993, 1996). These were smaller chambers made from thermoplastic and were of a range of sizes to fit various shapes of neck. The open ends of the devices were lined with a thin latex membrane to transmit the pressure to the carotid bifurcation regions, without air leaking. Pressure in the chamber(s) was recorded using a catheter and a Statham P23Gb transducer. The chamber(s) were connected via a solenoid valve to a 10 litre reservoir, the pressure of which was controlled by a vacuum/pressure source (Henry NV300, Numatic, Beaminster, UK). Tests of baroreceptor responses were performed by setting the pressure in the reservoir to the required level and opening the solenoid valve while the neck chamber(s) were held in place. Baroreceptor stimulus–response relationships were determined by changing the pressure in the neck chamber(s) in the following sequence: −40, −20, −10, +10, +20, +40, +60 mmHg and then in reverse order. Each pressure was maintained for 20 s. Pressure was restored to atmospheric between each step.
Inspiratory resistance and asphyxia
Inspiratory resistance was applied by restricting the inspiratory flow until the peak inspiratory pressure recorded in the mouthpiece was −10 mmHg. The resistance valve was continuously adjusted manually to maintain the required pressure. Mild asphyxia was applied, either with or without the inspiratory resistance, by connecting the inspiratory port to a Douglas bag containing 12% O2, 5% CO2 in N2.
Experimental procedure
Subjects were instructed to abstain from caffeine containing beverages from the evening before the tests. Tests were carried out on four separate occasions and in random order, but always at the same time of day. The subjects were seated and the various monitoring devices attached. After the baseline assessments one of the following interventions was applied: (a) sham: no further intervention was applied; (b) inspiratory resistance; (c) asphyxic gas; or (d) a combination of inspiratory resistance and asphyxia.
Carotid baroreceptor stimulus–response relationships were determined as described, firstly, 10 min after breathing room air at normal pressure, then 10 min after one of the above interventions, and finally, 10 min after again breathing room air normally.
Data analysis
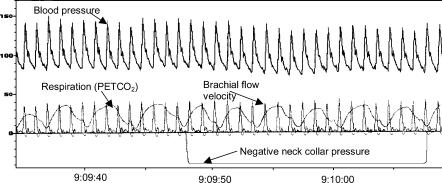
Vascular resistance was calculated as mean arterial pressure divided by mean blood flow velocity. We analysed the average values of vascular resistance taken over each respiratory cycle (from the CO2 trace). Each value of vascular resistance during stimulation was calculated as the maximum percentage changes averaged over a complete respiratory cycle from the average values from the two respiratory cycles before the onset of stimulation. Values obtained during each intervention were compared with the averages of the two control tests undertaken before and after each intervention. Mean arterial pressure responses were also calculated from the same beats used to calculate vascular resistance. A sample tracing of the stimulus applied and the responses measured is given in Fig. 1. Maximum change in RR interval from the pre-stimulus three-beat average was also assessed. Transmission of pressure from the collar to the carotid sinus was assumed to be 100% and carotid sinus pressure was calculated as mean arterial pressure minus collar pressure.
Figure 1. A sample recording showing the application of negative neck chamber pressure (mmHg) and the responses of arterial blood pressure (mmHg) and brachial flow velocity (cm s−1).
Respiratory cycles were taken from the PET,CO2 curve (mmHg). Control measurements were taken from the mean blood pressure and blood flow velocity during the two respiratory cycles preceding the onset of stimulation and vascular resistance was calculated as pressure/flow. Values of vascular resistance were calculated using values of mean blood pressure and blood flow velocity for each respiratory cycle during stimulation and calculated as a percentage change from control. The maximum change was taken as the response.
Pressure–response curves were plotted and fitted with either a sigmoid function or a third-order polynomial, depending on which curve best fitted the data (GraphPad Prism v3.0, GraphPad Software Inc., San Diego, CA, USA). The differentials of these curves were then calculated. The maximal differentials, which correspond to the maximum slopes of the pressure–response curves, were taken as the measures of baroreflex sensitivity. The carotid sinus pressures corresponding to the maximal differentials were termed ‘set points’ and these values were used to establish whether or not the baroreflex had reset. Each subject served as his or her own control on each experimental day, since conditions such as probe positioning may not have been constant between days, but was kept constant during each experimental day. Thus, values of control and stimulus were compared using Student's paired t test and all values, unless otherwise stated, are presented as means ± s.e.m.
Results
Baseline Results
Effects on PO2 and PCO2. The effects of the different interventions on the end-tidal partial pressures of oxygen and carbon dioxide are displayed in Table 1. Sham had no effect. Breathing with an inspiratory resistance caused a small but significant decrease in PO2 (P < 0.05) and increase in PCO2 (P < 0.05). Asphyxia both alone and in combination with the inspiratory resistance caused expected decreases in PO2 and increases in PCO2.
Table 1.
Partial pressures of oxygen and carbon dioxide during the four procedures
| PO2 (mmHg) | PCO2 (mmHg) | |||||
|---|---|---|---|---|---|---|
| Control | Test | P | Control | Test | P | |
| Sham | 110.3 ± 1.2 | 109.7 ± 1.1 | n.s | 33.3 ± 1.2 | 33.1 ± 1.3 | n.s |
| Inspiratory resistance | 109.2 ± 1.1 | 106.8 ± 1.5 | < 0.05 | 36.2 ± 1.5 | 37.5 ± 1.2 | < 0.05 |
| Asphyxia | 113.2 ± 1.3 | 75.2 ± 2.8 | < 0.0001 | 34.9 ± 1.4 | 45.3 ± 1.6 | < 0.0001 |
| Combination | 112.7 ± 1.1 | 74.3 ± 1.3 | < 0.0001 | 34.6 ± 0.8 | 45.0 ± 0.7 | < 0.0001 |
Effects on resting values of vascular resistance, blood pressure and heart rate
The baseline values of blood pressure and heart rate on each experimental day and in the absence of any intervention were not different (Table 2). The effects of the different test conditions on vascular resistance mean blood pressure and heart rate are given in Table 3. Vascular resistance changes were calculated as the percentage change from control value breathing room air without inspiratory resistance, before and after the intervention. Sham had no effect on vascular resistance, blood pressure or heart rate. Breathing with an inspiratory resistance also did not significantly change vascular resistance, mean blood pressure or heart rate. However, asphyxia both alone and in combination with inspiratory resistance caused significant increases in mean blood pressure (from 87.0 ± 3.0 mmHg to 94.0 ± 3.2 mmHg, P < 0.0005 (asphyxia alone) and from 89.3 ± 3.0 mmHg to 96.8 ± 4.8 mmHg, P < 0.02 (combination)). Asphyxia alone caused a significant increase in vascular resistance (+49.5 ± 16.9%, P < 0.05). Asphyxia in combination with inspiratory resistance tended to increases vascular resistance but this change was not quite statistically significant (+26.3 ± 11.4%). Heart rate was increased by 5.6 ± 1.9 b.p.m. (P < 0.05) during asphyxia and 6.4 ± 1.6 b.p.m. (P < 0.01) during the combination of asphyxia and inspiratory resistance.
Table 2.
Mean baseline values of arterial pressure and heart rate
| Sham | Inspiratory resistance | Asphyxia | Combination | |
|---|---|---|---|---|
| MAP (mmHg) | 87.5 ± 4.6 | 85.4 ± 3.6 | 85.5 ± 3.1 | 86.8 ± 3.0 |
| HR (b.p.m.) | 67.4 ± 2.5 | 66.9 ± 3.6 | 67.6 ± 2.9 | 66.5 ± 3.3 |
Table 3.
Vascular resistance, mean arterial pressure and heart rate responses to the different test procedures. Values are in the absence of pressure change applied to the neck chamber
| Sham | Inspiratory resistance | Asphyxia | Combination | |
|---|---|---|---|---|
| Vascular resistance (%) | +1.8 ± 7.8 | +0.6 ± 0.2 | +49.5 ± 16.9* | +26.3 ± 11.4 |
| Mean arterial pressure (mmHg) | −0.4 ± 1.0 | −0.2 ± 1.4 | +7.0 ± 1.1** | +7.6 ± 2.5* |
| Heart rate (b.p.m.) | −1.9 ± 1.1 | −0.8 ± 1.5 | +5.6 ± 1.9* | +6.4 ± 1.6* |
Student's paired t test
P < 0.05
P < 0.005 difference from baseline.
Effects on baroreceptor function
Baroreflex responses were calculated as the changes in vascular resistance from the preceding control values. That is, during normal air breathing, inspiratory resistance, asphyxia or combination. The effects of the interventions on the control values are given in Table 3. Responses of arterial pressure were calculated as absolute changes from the control period.
Blood pressure and cardiac interval responses. There was no significant effect of sham, inspiratory resistance or combined resistance and asphyxia on baroreceptor sensitivity or ‘set point’ of the relationship between carotid pressure and mean arterial blood pressure (Table 4). However, asphyxia alone did increase ‘set point’. The sensitivity and ‘set point’ of the responses of cardiac interval were not significantly altered by any of the interventions (Table 4).
Table 4.
Mean arterial pressure and carotid baroreflex sensitivity and ‘set point’
| Arterial Pressure | Sham | Inspiratory resistance | Asphyxia | Combination |
|---|---|---|---|---|
| Control (gain) | −0.803 ± 0.194 | −0.524 ± 0.006 | −0.617 ± 0.173 | −0.649 ± 0.005 |
| Intervention (gain) | −0.704 ± 0.134 | −0.482 ± 0.004 | −0.622 ± 0.077 | −0.915 ± 0.129 |
| Control (set point) | 87.7 ± 3.9 | 93.1 ± 5.6 | 86.6 ± 4.0 | 91.3 ± 3.7 |
| Intervention (set point) | 90.2 ± 4.9 | 93.1 ± 5.2 | 93.4 ± 3.4* | 99.5 ± 4.9 |
| Pulse interval | ||||
| Control (gain) | 9.6 ± 2.3 | 9.4 ± 2.1 | 15.8 ± 5.0 | 8.5 ± 1.3 |
| Intervention (gain) | 12.1 ± 2.8 | 13.3 ± 3.5 | 12.4 ± 2.4 | 8.8 ± 2.3 |
| Control (set point) | 94.1 ± 5.0 | 99.6 ± 7.0 | 98.5 ± 3.4 | 95.0 ± 3.0 |
| Intervention (set point) | 101.0 ± 4.7 | 91.0 ± 4.2 | 96.2 ± 7.7 | 98.1 ± 5.3 |
P < 0.01.
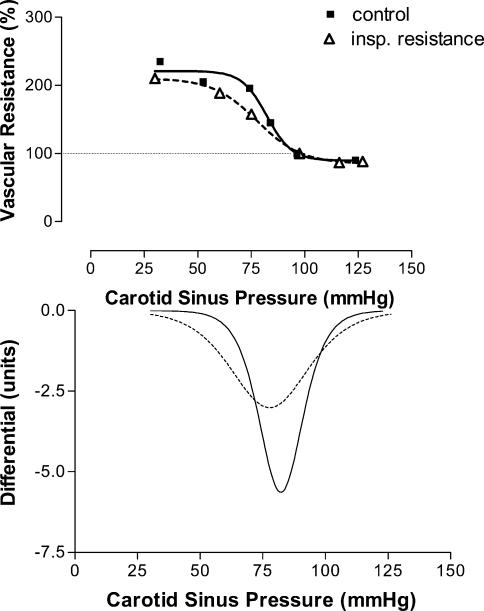
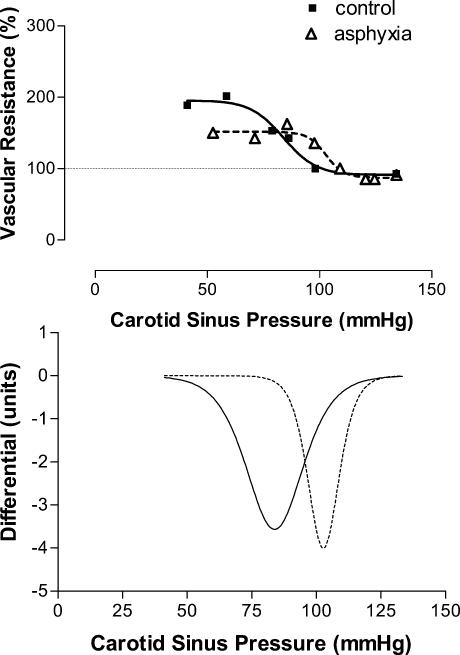
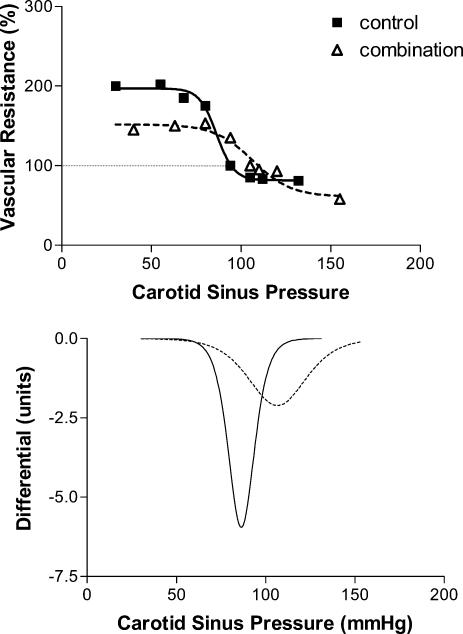
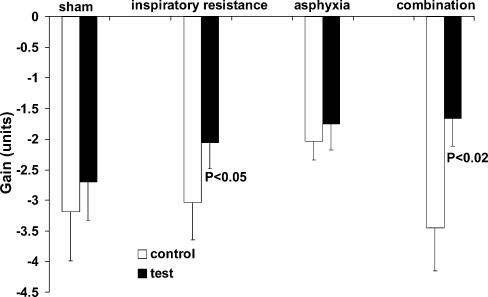
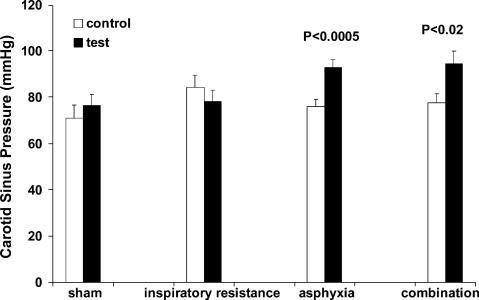
Vascular responses. Sham had no effect on either the baroreflex sensitivity (peak gain) or ‘set point’. Example slopes for the different conditions are shown in Figs 2 (inspiratory resistance), 3 (asphyxia) and 4 (combination). Figures 5 and 6 show the group responses of baroreflex sensitivity and ‘set point’ during the various conditions. Breathing with an inspiratory resistance significantly decreased the baroreflex sensitivity (from −3.0 ± 0.6 to −2.1 ± 0.4 cm s−1 mmHg−1; P < 0.05; example in Fig. 2), but there was no change in the ‘set point’; that is the curve was not displaced. Asphyxia alone had no effect on baroreflex sensitivity but displaced the curve to higher pressures by +16.8 ± 2.1 mmHg (P < 0.0005; example in Fig. 3). The combination of inspiratory resistance and asphyxia essentially resulted in a combination of the effects in that it both reduced baroreflex sensitivity (from −3.4 ± 0.7 to −1.7 ± 0.4 cms−1 mmHg−1; P < 0.02) and increased the ‘set point’ by +16.8 ± 4.9 mmHg (P < 0.02; example in Fig. 4).
Figure 2. Example of the effects of breathing with an inspiratory resistance on the carotid baroreceptor pressure–response curve.
Baseline values in absence of applied neck chamber pressure are taken as 100%. Note the reduction in slope with little displacement of the curve.
Figure 3. Example of the effects of asphyxia on the carotid baroreceptor pressure–response curve.
Baseline values in absence of applied neck chamber pressure are taken as 100%. Note there is no change in slope of the curve, but the curve is displaced to the right.
Figure 4. Example of the effects of a combination of asphyxia and inspiratory resistance on the carotid baroreceptor pressure–response curve.
Baseline values in absence of applied neck chamber pressure are taken as 100%. Note the reduction in slope of the curve and the curve is displaced to the right.
Figure 5. Carotid baroreflex sensitivity during control and test conditions.
Asphyxia alone had no significant effect but inspiratory resistance either alone or in combination with asphyxia significantly reduced sensitivity.
Figure 6. Baroreflex ‘set point’ during control and test conditions.
Inspiratory resistance had no significant effect on ‘set point’ but asphyxia, either alone or in combination with inspiratory resistance significantly increased ‘set point’.
Discussion
The important novel findings of the present study are: (1) breathing with an inspiratory resistance reduces the sensitivity of the baroreceptor control of the vascular resistance; (2) asphyxia results in an increased set point of this baroreceptor–vascular resistance reflex; and (3) the combination of inspiratory resistance and asphyxia results in a combination of the effects of the two stimuli applied separately.
Methodological considerations
Before interpreting the significance of our findings it is necessary to consider the suitability and possible limitations of the various techniques employed. In particular, we need to consider the method used for stimulation of carotid baroreceptors and that for assessing responses of peripheral vascular resistance.
The neck chamber technique for changing carotid transmural pressure was originally pioneered by Ernsting & Parry (1957) and developed by Eckberg et al. (1975). Our device for applying negative pressures was similar to that of Eckberg, but to apply positive pressure, that is to unload the carotid baroreceptors, we changed to paired devices moulded from thermoplastic. This was because it is impossible to obtain a reliable and airtight seal with positive pressures using the single chamber. We consider this approach to be valid as in a previous investigation we had shown that the paired chambers induced quantitatively similar responses to those obtained using a single chamber when applying positive pressures, although the responses to negative pressure were smaller (Kelly et al. 1996). Furthermore, the rate of onset of the stimulus was similar (A. P. Kelly & R. Hainsworth, unpublished observations).
One problem with the neck chamber technique is that the carotid reflex is studied in a closed loop situation and any responses obtained would feed back to both carotid and other baroreceptors to ‘buffer’ the net effect. This problem is unavoidable in human experimentation, but we attempted to minimize any effect by recording the responses at the time at which they were maximal, thereby assuming that any buffering effect would be minimal. The consequence of this is that cardiac responses were recorded almost immediately after application of the stimulus due to the short latency of vagal responses, and vascular responses were taken at their maxima, which were 10–15 s after stimulus application. The effect of the ‘buffering’, as well as possible adaptation of the receptors, inevitably implies that the responses would be underestimated. However, the same constraints would apply to all conditions and should not invalidate any comparisons of the responses. In assessing the sensitivity of the reflex and its ‘set point’ we plotted the responses against calculated carotid sinus pressure and fitted either a sigmoid function or a third-order polynomial, whichever gave the best fit. The calculation of carotid pressure was made assuming that all the applied positive or negative pressures were transmitted to the sinus. This is probably not entirely true. Previous studies have indicated that although almost all the negative pressure is transmitted (Eckberg, 1976), about 86% of the positive pressure (Ludbrook et al. 1977) is transmitted. Our assumption of 100% transmission is unlikely to result in major errors and, in any case should be similar in all conditions studied.
The use of the Doppler ultrasound technique to assess flow velocity in the brachial artery has the major advantage over plethysmography in that it allows continuous estimates to be made. Microneurography provides fairly similar information but has the disadvantage of being invasive. Also, we felt that repeated recordings from the same subjects would be unacceptable. The ultrasound method involves the assumption that flow is proportional to velocity and that the vessel diameter does not change. Although information on vessel diameter is lacking, there have been several comparisons with venous occlusion plethysmography which have shown good agreement over a wide range of conditions (Levy et al. 1979; Tschakousky et al. 1995; Shoemaker et al. 1998). Not only does the ultrasound method provide continuous estimates of velocity, but it avoids the necessity to occlude the veins which, by itself, may change flow (Henriksen & Sejrsen, 1976). Probably the main limitation of the method is that it does not provide a measure of the absolute flow and, although with care we can be confident that the velocity signal is directly related to flow, because of uncertainty regarding the precise position and angle of the probe, measurements on different occasions cannot be directly compared. Thus we believe that the ultrasound method would provide reliable estimates of changes in blood flow during a single study although absolute values are unknown, and values are likely to differ on different experimental days.
Effects of inspiratory resistance and/or asphyxia on baroreceptor reflex
Inspiratory resistance. Breathing against a moderate inspiratory resistance had no significant effect on resting blood pressure or vascular resistance. However, it did result in a decrease in the sensitivity of the carotid baroreceptor–vascular resistance relationship. Obstruction to inspiration is similar to the Mueller manoeuvre (Sharpey-Schafer, 1965) and there are several consequences of this, including effects on cardiac filling, effects on intrathoracic mechanoreceptors and effects on inspiratory drive.
The Mueller manoeuvre is, in some ways, the opposite of the Valsalva. Typically, it involves decreases in intrathoracic pressures of up to −50 mmHg which are sustained for 10–15 s (Sharpey-Schafer, 1965). The negative pressure is generated by activity in inspiratory muscles, particularly the diaphragm, with an obstructed airway. This results in an increase in the abdominal–thoracic pressure gradient and enhanced venous return and cardiac output. However, unlike the Valsalva, which compresses both chest and abdomen, the cardiovascular effects of the Mueller manoeuvre are relatively modest with a small increase in cardiac output but little or no change in mean blood pressure. This would imply that there is a small decrease in total peripheral resistance. In our study we did not see any effects on blood pressure or in brachial artery vascular resistance to negative pressure breathing. However, the pressure changes were relatively small and, furthermore, they were intermittent.
The increased negativity of the intrathoracic pressure would increase the transmural pressure across the intrathoracic mechanoreceptors thereby increasing their afferent activity. The reflex effects of these are very complex due to their diversity (Hainsworth, 1991). Stimulation of aortic (Hainsworth et al. 1970) and coronary (McMahon et al. 1996) baroreceptors would be expected to decrease blood pressure and vascular resistance. Effects may also result from low pressure receptors in the atria and pulmonary arteries. Atrial receptors have little effect on blood pressure or vascular resistance (Linden & Kappagoda, 1982), but stimulation of pulmonary receptors causes peripheral vasoconstriction (Ledsome & Kan, 1977). It has recently been shown that the responses to stimulation of pulmonary baroreceptors are indeed enhanced by application of negative intrathoracic pressures (McMahon et al. 2000; Moore et al. 2002). It might be expected therefore that this mechanism could lead to vasoconstriction and increased blood pressure in our subjects during negative pressure breathing.
The other possible way in which negative pressure breathing could influence the cardiovascular system is through a direct effect of the increased respiratory drive. The baroreceptor reflex is effectively ‘gated’ by activity in central inspiratory neurones (Eckberg, 2003). This effect can be seen clearly during normal respiration where baroreceptors only induce responses during the expiratory phase (Eckberg et al. 1980). Thus the central effect could explain the reduced baroreflex sensitivity seen in our experiment.
It seems conceivable that, in our experiment, the decreased baroreflex sensitivity without changes in blood pressure could be explained by the combination of the various effects. Thus the increased cardiac output due to mechanical effects and possible pressor effects due to pulmonary baroreceptors could be countered by an increase in stimulation of coronary and aortic baroreceptors. Central effects could well explain the reduced carotid baroreflex sensitivity.
Asphyxia
The combination of hypoxia and hypercapnia is well known to result in increases in blood pressure and vascular resistance (Marshall, 1999). The mechanisms, however, are quite complex. Stimulation of peripheral chemoreceptors by hypoxia causes vasoconstriction and hypertension (Hainsworth et al. 1983a, b) although the effects of chemoreceptors may be reduced when respiration increases (Daly et al. 1967). This effect of increased ventilatory efforts could explain the tendency for the vascular resistance to increase less when inspiratory obstruction was added to the asphyxic stimulus.
Central hypercapnia also results in increases in vascular resistance (Soladoye et al. 1985). The combination therefore of hypoxia and hypercapnia, even when relatively mild as in our experiment, would provide a particularly effective pressor stimulus. The effects of asphyxia did not appear to interact with the baroreflex in that, although there was a shift in the position of the stimulus–response curve, there was no change in the gain of the reflex as assessed from the maximum slope of the curve. These findings are compatible with those of Halliwill & Minson (2002) who reported that hypoxia also shifted the relationship between muscle sympathetic activity and arterial blood pressure to higher levels without altering slope.
We found that the combined stimulus resulted in both effects of the individual stimuli, i.e. decreased baroreflex sensitivity with increased vascular resistance and blood pressure.
Relevance of findings to patients with OSA
Patients with OSA have episodes of apnoea followed by hyperpnoea and these vary in frequency during sleep from 5 to more than 30 events per hour. A proportion of these patients proceed to develop hypertension and one possible link between this and OSA could be a change in baroreceptor function. The model that we have used to investigate this, inspiratory resistance and breathing an asphyxic gas mixture, does simulate the changes of OSA in some ways, but there are differences. Patients do not just inspire against a resistance, they have periods of apnoea which can last up to 90 s. They also have periods of hypoventilation and hyperventilation and during these events the changes in inspiratory pressure and blood gases would be qualitatively similar to those tested here, but quantitatively they would be much greater. A further factor to consider is that measurements were carried out on healthy control subjects with no historic evidence of OSA. There is evidence that chemoreflex responses to hypoxia are potentiated in patients with OSA (Narkiewicz et al. 1998, 1999) and it is therefore possible that the results of the present study may have underestimated the effects of inspiratory resistance and particularly asphyxia in patients with OSA. Also, measurements were made during daytime wakefulness, yet the effects of apnoeic events on sympathetic activity is different during REM and non-REM sleep stages (Somers et al. 1995). Nevertheless, despite these limitations, the model does simulate the increased inspiratory pressures and the asphyxial changes which occur in OSA and should be of help in interpreting the changes seen in that condition.
It has previously been reported that patients with OSA have a reduction in baroreflex sensitivity (Carlson et al. 1996; Parati et al. 1997). However, these studies examined only the cardiac responses and not the vascular responses which we believe to be of greater importance (Cooper & Hainsworth, 2002). There have been a number of studies in which peripheral vascular responses have been assessed from the effects on muscle sympathetic activity. Episodes of OSA result in increases in muscle sympathetic nerve activity (MSNA) which persist during wakefulness (Carlson et al. 1993; Somers et al. 1995; Morgan, 2001). Hedner et al. (1988) suggested that the high and fluctuating levels of MSNA due to widely varying baroreceptor stimuli in patients may result in decreases in baroreflex sensitivity. The repeated chemoreceptor stimulation also increases MSNA (Narkiewicz et al. 1998, 1999) and there is likely to be a synergistic effect of the combined hypoxia and hypercapnia (Fletcher et al. 1992).
Our study is the first to simulate both effects of OSA –inspiratory obstruction and asphyxia – and to examine the effects separately and together on the full carotid baroreceptor–vascular resistance stimulus–response curve. The results certainly provide a mechanism linking OSA with hypertension. However, they simulated only the effects of a relatively mild episode of OSA and did not examine whether the responses persisted following the stimulus. There is evidence from previous reports of patients with OSA that blood pressure does not immediately return to the pre-apnoeic level and indeed with each apnoeic event there is a progressive increase in blood pressure (Tilkian et al. 1976). Xie et al. (2001) noted that sympathetic activation in humans in response to hypoxia persisted for at least 20 min after cessation of the stimulus. Thus repeated episodes of inspiratory obstruction and asphyxia would be likely to increase sympathetic drive and blood pressure, in some patients, for much of the night. This would be likely to result in changes in baroreceptor function. Even brief periods of hypertension can result in some resetting of the baroreceptor curve (McMahon et al. 1996). This is likely to be due partly to central effects and partly to alterations in the function of the baroreceptors themselves (Snitsarev et al. 2002).
Whether these effects on baroreceptor function would persist during the following day is uncertain. However, the persisting hypertension and increased sympathetic tone would be likely to be associated with baroreceptor changes, although whether these are the cause of the hypertension or the effect is uncertain.
It is also possible that repeated exposure to hypoxia may lead to chemoreceptor resetting resulting in increases in sympathetic tone (García-Río et al. 2000) and catecholamine levels (Somers et al. 1995). Thus it is possible that changes in function of baroreceptors and chemoreceptors may both contribute to the hypertensive effect of repeated episodes of OSA.
Conclusion
The results of the present study demonstrate the potential role of the arterial baroreceptors in the causative effect of OSA on hypertension. A reduction in baroreflex sensitivity and thus a reduction in the tight regulation of blood pressure control induced by inspiratory resistance and the rightward resetting of the baroreceptor curve induced by asphyxia would both promote hypertension.
Acknowledgments
We would like to acknowledge the technical assistance provided by the late Suzanne Davis before her untimely death in November 2002.
References
- Bixler EO, Vgontzas AN, Lin H-M, Have TT, Leiby BE, Vela-Bueno A, Kales A. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin G. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner JA, Sellegrem J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:1490–1496. doi: 10.1164/ajrccm.154.5.8912770. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Hainsworth R. Effect of head-up tilting on baroreceptor control in subjects with different tolerances to orthostatic stress. Clin Sci. 2002;103:221–226. doi: 10.1042/cs1030221. [DOI] [PubMed] [Google Scholar]
- Daly M de B, Hazzeldine JL, Ungar A. The reflex effects of alterations in lung volume on systemic vascular resistance in the dog. J Physiol. 1967;188:331–351. doi: 10.1113/jphysiol.1967.sp008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. Temporal response patterns of the human sinus node to brief carotid baroreceptor stimuli. J Physiol. 1976;258:769–782. doi: 10.1113/jphysiol.1976.sp011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. J Physiol. 2003;248:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Laboratory Clin Med. 1975;85:167–173. [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsting J, Parry DJ. Some observations on the effects of stimulating stretch receptors in the carotid artery of man. J Physiol. 1957;137:45 P–46 P. [Google Scholar]
- Fletcher EC, Lesseke J, Behm R, Miller CC, III, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- García-Río F, Racionero MA, Pino J, Martinez I, Ortuno F, Villasante C, Villamor J. Sleep apnea and hypertension: the role of peripheral chemoreceptors and the sympathetic system. Chest. 2000;117:1417–1425. doi: 10.1378/chest.117.5.1417. [DOI] [PubMed] [Google Scholar]
- Grote L, Hedner J, Peter JH. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens. 2000;18:679–685. doi: 10.1097/00004872-200018060-00004. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Hainsworth R, Karim F, McGregor KH, Rankin AJ. Effect of stimulation of aortic chemoreceptors on abdominal vascular resistance and capacitance in anaesthetised dogs. J Physiol. 1983b;334:421–431. doi: 10.1113/jphysiol.1983.sp014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R, Karim F, McGregor KH, Wood LM. Responses of abdominal vascular resistances and capacitances to stimulation of carotid chemoreceptors in anaesthetised dogs. J Physiol. 1983a;334:409–419. doi: 10.1113/jphysiol.1983.sp014502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R, Ledsome JR, Carswell F. Reflex responses from carotid baroreceptors. Am J Physiol. 1970;218:423–429. doi: 10.1152/ajplegacy.1970.218.2.423. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol. 2002;93:857–864. doi: 10.1152/japplphysiol.01103.2001. [DOI] [PubMed] [Google Scholar]
- Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens. 1988;6(Suppl. 4):S529–S531. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- Henriksen L, Sejrsen P. Local reflex in microcirculation in human cutaneous tissue. Acta Physiol Scand. 1976;98:227–231. doi: 10.1111/j.1748-1716.1976.tb10299.x. [DOI] [PubMed] [Google Scholar]
- Kelly AP, Croft JS, Hainsworth R. A paired neck chamber for applying localized stimuli to the carotid baroreceptors in humans. J Physiol. 1996;497.P:5P. [Google Scholar]
- Kelly AP, El-Bedawi KM, Hainsworth R. An improved neck chamber for the study of carotid baroreceptors in humans. J Physiol. 1993;467:142P. [Google Scholar]
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledsome JR, Kan K. Reflex changes in hind limb and renal vascular resistance in response to distension of the isolated pulmonary arteries of the dog. Circ Res. 1977;40:64–72. doi: 10.1161/01.res.40.1.64. [DOI] [PubMed] [Google Scholar]
- Levy BI, Vallaclares WR, Ghaem A, Martinaud JP. Comparison of plethysmographic methods with pulsed Doppler blood flowmetry. Am J Physiol. 1979;236:H899–H903. doi: 10.1152/ajpheart.1979.236.6.H899. [DOI] [PubMed] [Google Scholar]
- Linden RJ, Kappagoda CT. Atrial Receptors. Cambridge, UK: Cambridge University Press; 1982. Monographs of the Physiological Society 39; pp. 1–363. [PubMed] [Google Scholar]
- Ludbrook J, Mancia G, Ferrari A, Zanchetti A. The variable pressure neck-chamber method for studying the carotid baroreflex in man. Clin Sci Mol Medical. 1977;53:165–171. doi: 10.1042/cs0530165. [DOI] [PubMed] [Google Scholar]
- McMahon NC, Drinkhill MJ, Hainsworth R. Vascular responses to stimulation of carotid, aortic and coronary baroreceptors with pulsatile and non-pulsatile pressures in anaesthetised dogs. Exp Physiol. 1996;81:397–408. doi: 10.1113/expphysiol.1996.sp003997. [DOI] [PubMed] [Google Scholar]
- McMahon NC, Drinkhill MJ, Myers DS, Hainsworth R. Reflex responses from the main pulmonary artery and bifurcation in anaesthetised dogs. Exp Physiol. 2000;85:411–420. [PubMed] [Google Scholar]
- Marshall JM. The integrated response to hypoxia: from circulation to cells. Exp Physiol. 1999;84:449–470. [PubMed] [Google Scholar]
- Moore JP, Hainsworth R, Drinkhill MJ. Distension of the main pulmonary artery stimulates vagal activity in anaesthetised dogs. J Physiol. 2002;544.P:28 P. [Google Scholar]
- Morgan BJ. Neurocirculatory consequences of sleep apnoea. Proceedings 34th International Congress of Physiological Sciences; Christchurch, New Zealand. 2001. [Google Scholar]
- Narkiewicz K, Van de Borne PJH, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Van de Borne PJH, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- Nieto FL, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, Bonsignore G, Mancia G. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex during sleep. J Hypertens. 1997;15:1621–1626. doi: 10.1097/00004872-199715120-00063. [DOI] [PubMed] [Google Scholar]
- Partinen M, Telakivi T. Epidemiology of obstructive sleep apnea syndrome. Sleep. 1992;69:2143–2148. doi: 10.1093/sleep/15.suppl_6.s1. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Sharpey-Schafer EP. Effect of respiratory acts on the circulation. In: Hamilton WF & Dow P, editor. Handbook of Physiology. III. Washington, DC: American Physiological Society; 1965. pp. 1875–1886. section 2, Circulation. [Google Scholar]
- Shoemaker JK, Hogeman CS, Silber DH, Gray K, Herr M, Sinoway LL. Head-down tilt alters forearm vasodilator and vasoconstrictor responses. J Appl Physiol. 1998;84:1756–1762. doi: 10.1152/jappl.1998.84.5.1756. [DOI] [PubMed] [Google Scholar]
- Smith ML, Neidermaier ONW, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Sys. 1996;56:184–190. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Snitsarev V, Whiteis SC, Abboud F, Chapleau M. Differential activity-dependent ‘resetting’ of subtypes of isolated baroreceptor neurons in culture. Clin Auton Res. 2002;12:305. [Google Scholar]
- Soladoye AO, Rankin AJ, Hainsworth R. Influence of carbon dioxide tension in the cephalic circulation on hind-limb vascular resistance in anaesthetised dogs. Quart J Exp Physiol. 1985;70:527–538. doi: 10.1113/expphysiol.1985.sp002939. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep induced apnea. Studies during wakefulness and sleep. Ann Intern Med. 1976;85:714–719. doi: 10.7326/0003-4819-85-6-714. [DOI] [PubMed] [Google Scholar]
- Tschakousky ME, Shoemaker JK, Hughson RL. Beat-by-beat forearm blood flow with Doppler ultrasound and strain-gauge plethysmography. J Appl Physiol. 1995;79:713–719. doi: 10.1152/jappl.1995.79.3.713. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Ziegler MG, Nelesen RA, Mill PJ, Ancoli-Israel S, Clausen JL, Watkins L, Dimsdale JE. The effect of hypoxia on baroreflexes and pressor sensitivity in sleep apnea and hypertension. Sleep. 1995;18:859–865. [PubMed] [Google Scholar]