Abstract
Root systems of 28-d-old cowpea (Vigna unguiculata L. Walp cv Vita 3: Bradyrhizobium sp. strain CB756) plants bearing nitrogen-fixing nodules in sand culture were exposed to an atmosphere of Ar:O2 (80:20, v/v) for 48 h and then returned to air. Root systems of control plants were maintained in air throughout. Nodules were harvested at the same times in control and Ar:O2-treated root systems. Activities of two enzymes of de novo purine synthesis, glycinamide ribonucleotide transformylase (GART; EC 2.1.2.2), aminoimidazole ribonucleotide synthetase (AIRS; EC 6.3.3.1), uricase (EC 1.7.3.3), and phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) were measured together with the protein level of each using immune-specific polyclonal antibodies. AIRS activity and protein both declined to very low levels within 6 h in Ar:O2 together with a decline in transcript level of pur5, the encoding gene. GART activity, protein, and transcript (pur3) levels were relatively stable. Uricase activity declined in Ar:O2 as rapidly as AIRS activity but the protein was stable. PEPC activity showed evidence of increased sensitivity to inhibition by malate but the protein level was stable. The data indicate that the flux of fixed N from bacteroids (N2-fixing nodule bacteria) is in some way associated with transcriptional control over pur5 and possibly also catabolism of AIRS protein. In contrast, there is limited posttranslational control over GART and PEPC and close posttranslational control over uricase activity. The significance of these different levels of regulation is discussed in relation to the overall control of enhanced expression of plant enzymes in the cowpea symbiosis.
Activities of metabolic pathways that use fixed N in legume root nodules are significantly greater than in supporting root tissue. In temperate legumes, the principal translocated product of fixation is Asn and activity of Asn synthetase is enhanced in nodules of these plants (Atkins et al., 1984a). In legumes of tropical origin, the major solutes are the ureides, allantoin and allantoic acid, and in nodules of these species, activity of the de novo purine pathway and enzymes of purine oxidation is exceptionally high (Atkins, 1991). In both types of legume, Asn and purine biosynthesis are expressed in most tissues, and this has led to speculation as to why in a particular species one pathway and not the other is used for N-assimilation in nodules (Atkins and Smith, 2000). Equally important is the mechanism(s) that causes enhanced activity of one or other pathway for NH3 assimilation. Although enzymes required for these pathways appear not to be nodule-specific, their level of activity is related to effective symbioses, and it has been speculated that in some way, their enhancement is linked to the flux of fixed N (Atkins, 1991; Kim et al., 1995).
Growing nodulated plants with their root systems in an atmosphere where the substrate for nitrogenase, N2, is absent has provided a way to test the idea that NH3 or some product of its metabolism is required for enhanced expression of enzymes associated with N-assimilation. Nodules developing on cowpeas (Vigna unguiculata [L.] Walp.) grown with their root systems in Ar:O2 (80:20, v/v; Atkins et al., 1984b, 1984c) are comparable with those grown in air for up to 16 d after sowing. There was no difference between the two types of nodule in the basal level of activity of enzymes of NH3 assimilation, amino acid metabolism, and purine oxidation, and although the level of de novo purine synthesis was much lower in Ar:O2-grown nodules, the pathway was nevertheless active. Enhanced expression of these pathways only occurred in air-grown nodules, as N2 fixation commenced. However, Ar-grown nodules began to senesce after 16 d and were no longer comparable with nodules that had developed in air (Atkins et al., 1984b).
To overcome this problem, root systems of intact cowpea and lupin (Lupinus albus) plants with mature nodules were transiently exposed to Ar:O2 (Atkins et al., 1984a). The results indicated that the levels of activity of a number of plant enzymes in the nodule appeared to be linked to the flux of fixed N. That is, their activity declined rapidly in the absence of N2 and was at least partially restored when Ar was replaced by N2. This was by no means uniform; some activities were stable, whereas others declined slowly and some very rapidly. In both symbioses, activity of the initial ammonia-assimilating enzyme, Gln synthetase was stable in nodules even after 6 d exposure to Ar:O2, whereas enzymes of pathways using Gln rapidly lost activity after 3 d in Ar. Thus, Gln or some other product of the assimilatory pathways, rather than NH3 itself, may influence expression of the N-assimilating enzymes in nodules. These studies provided no information as to the mechanisms underlying changes in enzyme activities, but the time course for restoration of activity after transient N2 deficiency (Atkins et al., 1984a) was generally consistent with synthesis of new proteins.
The impact of N2 deficiency on activity of the ureide pathway in cowpea nodules indicated that levels of both de novo purine synthesis and oxidation were linked to N-flux (Atkins et al., 1984a). However, the effect was not uniform on the component enzymes of the pathway. Although inosine monophosphate (IMP) synthesis from [14C]Gly by extracts from nodules exposed to Ar:O2 was very low, nevertheless some Gly was used, and labeled formyl glycinamide ribonucleotide (FGAR) accumulated. Thus, enzymes of the latter one-half of the de novo purine pathway were apparently more sensitive to N-flux than enzymes up to and including glycinamide ribonucleotide transformylase (GART). Furthermore, the activity of these enzymes may regulate the flow of N through the overall pathway. The present study tests this idea by examining the impact of transient N2 deficiency on the level of expression of GART and an enzyme catalyzing a reaction two steps further on in the pathway, aminoimidazole ribonucleotide synthetase (AIRS). A third pathway enzyme, uricase, which catalyzes the ultimate step in purine oxidation, and phosphoenolpyruvate carboxylase (PEPC), a key enzyme in nodule C metabolism, were also studied. PEPC was chosen because it has been identified as a possible regulatory site for the flux of oxidizable substrates to N2-fixing bacteroids (Laing et al., 1979; Coker and Schubert, 1981; Vance et al., 1994; Zhang et al., 1995; Wadham et al., 1996).
RESULTS
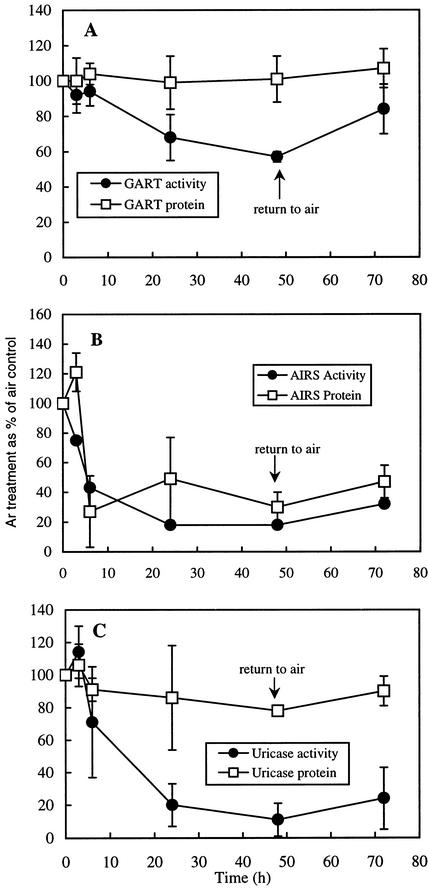
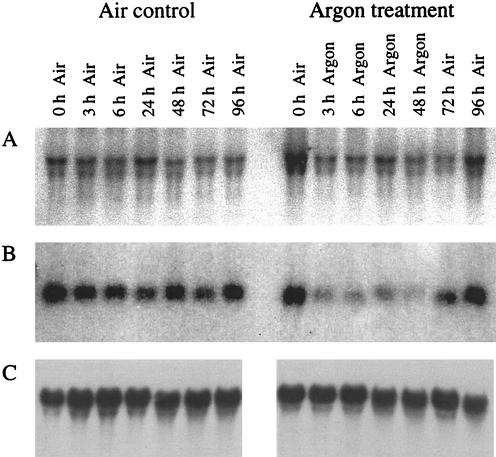
There was a rapid decline in AIRS activity in the nodules of Ar:O2-treated plants within the first 3 to 6 h of the switch from air to Ar:O2 (Fig. 1A) and by 24 h in Ar:O2 activity had declined by almost 80% compared with nodules maintained in air. The decline in AIRS activity was roughly paralleled by a decline in AIRS protein (Fig. 1A) and pur5 (encoding AIRS) mRNA (Fig. 2B). There was significant recovery in AIRS activity but no increase in AIRS protein within 24 h of returning the Ar:O2-treated plants to air. However, in a subsequent experiment, assays for AIRS activity and protein 48 h after return of Ar-treated root systems to air showed that both returned to 80% of controls maintained in air throughout (data not shown). The pur5 mRNA level 48 h after return to air was equivalent to that at the start of the experiment (Fig. 2B).
Figure 1.
Effect of Ar:O2 treatment on the levels of AIRS (A), GART (B), and uricase (C) activity and protein in cowpea nodule extracts. Nodules were harvested at the times indicated. All data were calculated per milligram of soluble protein, and then the values for the Ar:O2-treated plants were expressed as a percentage of the corresponding values for the control plants assayed at the same times. The pots were sealed so that the nodulated root systems could be flushed with air (control) or 80% Ar:20% O2. The treatments were initiated at 0 h and after 48 h in Ar:O2 the nodulated root systems were returned to air. The AIRS, GART, and uricase activity data are the means of two, four, and three separate experiments, respectively, and in each there were four plants per pot. The levels of AIRS, GART, and uricase protein were determined by ELISA, and in each case, the data are means of three separate experiments. Vertical bars represent se of the mean and where they are not shown are within the dimensions of the symbol.
Figure 2.
Effect of Ar:O2 treatment on the mRNA level of Vupur3 (A; encoding GART) and Vupur5 (B; encoding AIRS), and 18S ribosomal RNA (C). Plants were treated as described in the legend to Figure 1. Nodules were harvested at the times indicated. Total RNA (20 μg) isolated from nodules collected at each time point was separated on a formaldehyde gel, transferred to a nylon membrane, and hybridized to a [α-32P]dCTP-labeled pur5 or pur3 probe. As a control for even loading of RNA, a replicate blot was probed with an 18S rRNA probe.
Levels of GART activity, protein, and pur3 mRNA were much less affected by Ar:O2 than those of AIRS. For the first 6 h after the switch from air to Ar, activity remained at the control level and by 48 h was still 60% of control (Fig. 1B). The level of GART protein was unaffected by Ar:O2 treatment (Fig. 1B). Returning the Ar:O2 treated nodules to air for 24 h resulted in a significant recovery in GART activity. Within 3 h of exposure to Ar, there was a decline in the level of pur3 transcripts, but this level remained stable for the rest of the 48-h period in Ar. Returning the root systems to air for 48 h resulted in a return to the level of transcript present at zero time (Fig. 2A).
The response of uricase to Ar:O2 treatment was similar to that of AIRS, and by 48 h, activity had fallen by 80% compared with the level of the air control (Fig. 1C). However, the uricase protein did not decline in Ar (Fig. 1C). There was a small, but statistically insignificant, recovery of uricase activity 24 h after Ar:O2-treated nodulated roots systems were returned to air.
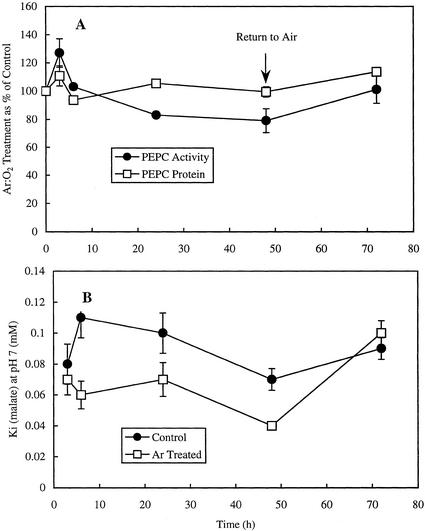
The effect of Ar:O2 treatment on PEPC activity and protein (Fig. 3A) was similar to the effect on GART (Fig. 1B). After 48 h in Ar:O2, the maximum extractable activity of PEPC (i.e. the activity assayed under optimal conditions—at pH 8 in the presence of saturating PEP and 15% [v/v] glycerol) had declined by only 20%, and there was no significant effect on the level of PEPC protein. The Ki (malate) for PEPC, assayed at pH 7, was consistently lower for the Ar:O2-treated plants than for the controls except at the 3-h time point in Ar:O2 and 24 h after the Ar:O2-treated plants had been returned to air (Fig. 3B). Although some effects of Ar:O2 treatment on Km (PEP) for PEPC were recorded, they showed no obvious pattern and were not consistent throughout the time course of the experiment (data not shown).
Figure 3.
Effect of Ar:O2 treatment on the activity of PEPC assayed under optimal conditions (i.e. at pH 8, in the presence of saturating PEP and 15% [v/v] glycerol) and the level of PEPC protein assayed by ELISA (A) and the Ki (malate) at pH 7.0 for PEPC in cowpea nodule extracts (B). Data are the means and se from three separate experiments. Other details were as described in the legends to Figures 1 and 2.
Nitrogenase activity of whole nodulated root systems of plants, measured by H2 evolution into the gas stream passing through the pots, did not change in those maintained in air throughout (data not shown). In Ar, after an initial increase in H2 evolution, the rate from 3 to 24 h was not significantly different; and although the rate of evolution had declined by 50% at 48 h, after return to air, the rates for plants that had been in Ar and those maintained in throughout were the same. The soluble protein content of nodules maintained in air did not change over the time course of the experiments (14–15.5 mg g−1 fresh weight of nodule). In nodules transferred to Ar:O2, protein levels declined by 10% after 24 h but remained at this level throughout the rest of the time course.
DISCUSSION
The primary aim of this study was to test the idea that the level of expression of selected plant enzymes of N and C metabolism in nodules is linked to the flux of fixed N from N2-fixing bacteroids. Transient exposure to Ar:O2 is a useful means of interrupting this flux without significantly affecting nitrogenase activity, which continues to function as a high rate of H2 production. As a consequence, a substantial portion of the incoming phloem-delivered sugar is likely to be used and the flux of C to bacteroids maintained at close to that when N2 is available. Measurements of the pool sizes of NH3 and other nitrogenous solutes in soybean (Glycine max) nodules after transfer of nodulated root systems of intact plants to Ar:O2 indicated that they all declined with a half-time of about 2 h (Walsh et al., 1989). In cowpea, the time course of decline in Gln content of nodules and export of ureides after transfer to Ar:O2 (Atkins et al., 1984a) was similar. Thus, if currently produced NH3 and/or products of its assimilation were involved in regulating purine/ureide synthesis, after 6 h in Ar:O2 their effects on expression of the pathway would have been greatly diminished. Earlier studies established that N2 deficiency imposed in this way for similar or even longer periods (up to 6 d) did not result in losses of some nodule enzyme activities (e.g. nitrogenase, GS, Ala dehydrogenase, and allantoinase), whereas others declined slowly and others lost activity rapidly (Atkins et al., 1984a).
The data indicate that the activities of individual enzymes of the purine/ureide pathway are regulated in relation to the flux of reduced N. The rapid losses of VUpur5 transcripts together with AIRS protein and activity are consistent with regulation of pur5 transcription. However, rates of loss of transcript and protein are not closely coupled, possibly because both transcription and AIRS protein catabolism are responsive to the dramatic change in N flux. On the other hand, GART and uricase proteins are relatively stable but show evidence of posttranslational regulation. Although other enzymes of purine/ureide synthesis could also have been down-regulated when the flux of reduced N to the pathway was suddenly eliminated, loss of AIRS alone would have prevented the formation of purines. Close dependence of AIRS expression, but not GART expression, on N-flux is consistent with earlier studies with cowpea nodules (Atkins et al., 1984a) that showed activity of the purine pathway up to FGAR synthesis to be less tightly coupled to N-flux than the latter one-half of the pathway beyond GART.
The only report (Reynolds et al., 1984) of potential regulatory features of an enzyme of purine synthesis in legume nodules is for phosphoribosyl pyrophosphate amidotransferase (PRPP-AT), the first committed step of the pathway. The initial nucleotide product, IMP, inhibited PRPP-AT activity of a partially purified extract from soybean nodules. The purified enzyme from avian and mammalian tissues shows a similar response to purine nucleotide, and feedback control of the whole pathway has been inferred. Kim et al. (1995) have more recently provided some evidence for enhanced expression of pur1 transcripts in soybean roots after exogenous application of Gln but not of NH3. Whether Gln is also involved in regulating the transcription of AIRS or expression of other pathway enzymes has yet to be determined. Given the result in Atkins et al. (1984a) that there was a build up of FGAR after nodules were exposed in Ar:O2, it seems unlikely that transcription of pur1 or PRPP-AT activity are being affected by this treatment.
This study clearly shows that when N supply is reduced, urate oxidation potential is almost eliminated by what appears to be a posttranslational form of control. Perhaps when normal fluxes of reduced N to the purine oxidation pathway are established in the nodule, an effector or protein modification reflecting this supply favorably alters the kinetic properties of uricase. The cowpea enzyme has an apparent Km (O2) that is extremely unfavorable (30 μm; Rainbird and Atkins, 1981), so that even small changes in catalytic features of the protein would have a significant impact on its potential activity in vivo (Thumfort et al., 1999). Such a regulatory mechanism is consistent with data showing that, although uricase protein could be detected using immune serum 6 to 7 d before the onset of N2-fixation, enzymic activity could not be detected until after nitrogenase activity was established (P.J. Mark, P.J. Storer, and C.A. Atkins, unpublished data). In vitro studies of the purified protein from cowpea nodules found NH3, Gln, and purines, especially the bases (adenine, guanine, and xanthine), to be inhibitory (Rainbird and Atkins, 1981). The only positive effects on activity in vitro were recorded for Asn and Fe3+, but it is difficult to see how these could be effectors in vivo.
In soybean and alfalfa (Medicago sativa) nodules, PEPC is regulated at the posttranslational level by protein phosphorylation (Pathirana et al., 1992; Schuller and Werner, 1993; Vance et al., 1994; Zhang et al., 1995). In soybean nodules (Schuller and Werner, 1993; Zhang et al., 1995) and in the leaves of C4 and CAM plants (for review, see Chollet et al., 1996), the phosphorylation status of PEPC is negatively correlated with its sensitivity to inhibition by malate at pH 7. Thus, malate sensitivity at pH 7 has been used as an indirect indicator for phosphorylation status of PEPC in plants. The small difference between control and Ar-treated nodules (Fig. 3) is consistent with some level of control under these conditions. Previous studies with soybean nodule PEPC found that a marked increase in sensitivity to inhibition by malate was associated with treatments that inhibited nitrogenase activity (Zhang et al., 1995; Wadham et al., 1996); including shoot removal, phloem-girdling, and prolonged darkness. The results of the present study indicate that Ar:O2 treatment could be added to the list, but in this case, there was not a sharp inhibition of nitrogenase activity. On the contrary, high rates of nitrogenase-catalyzed H2 evolution were maintained in Ar. Thus, unlike all of the earlier treatments that limit sugar supply to the nodule, exposure to Ar:O2 is likely to reduce the use of C substrates associated with NH3 assimilation and ureide export but maintain a relatively high rate of sugar import. Certainly there would have been significant alterations to the pool sizes and C flux through the ammonia-assimilating pathways, but these would have been much less significant than in treatments that “starve” the nodule for currently delivered C. Changes in C metabolism that accompany a sharply reduced requirement for NH3 assimilation in the nodule could conceivably also be involved in regulation of purine pathway enzymes.
There is also evidence from an earlier study (Atkins et al., 1992) that activity of the purine pathway may in turn regulate nitrogenase activity. Inhibition of the flux of reduced N through the purine/ureide pathway by blocking xanthine dehydrogenase with allopurinol in vivo causes inhibition of nitrogenase. Increasing the pO2 around nodules overcomes the inhibition, consistent with their decreased permeability to O2. Whether or not the increased diffusive resistance is the cause or the result of decreased nitrogenase activity, a link between the purine pathway in the plant fraction and the regulation of bacteroid metabolism seems possible. The potential level of nitrogenase activity by bacteroids isolated from nodules of plants exposed to allopurinol was unaffected, and the accumulated products (purine bases) from the blocked pathway had no effect on their activity (Atkins et al., 1988). It is conceivable that in addition to the slight down-regulation of PEPC in Ar, C4 acid synthesis is also limited by a lack of reduced pyridine nucleotide in the cytosol of the infected plant cell when the IMP dehydrogenase- and xanthine dehydrogenase-catalyzed reactions of purine oxidation cease in Ar (Atkins et al., 1992).
Clearly, products of the assimilation of fixed N either participate directly or are in some indirect way involved in a variety of regulatory mechanisms, which together control the level of C and N metabolism in the infected plant cell. Although this study has identified some of the likely sites of both transcriptional and posttranslational regulation, the nature of the effector(s) involved is yet to be determined. Regulation of AIRS transcription indicates that detailed analysis of the promoter of the pur5 gene should yield information about the nature of the regulatory mechanism and possibly allow identification of the effector(s).
MATERIALS AND METHODS
Plant Material
Nodulated cowpea (Vigna unguiculata L. Walp. cv Vita 3) plants were grown in sand culture in a naturally illuminated greenhouse from seed inoculated with a peat suspension of Bradyrhizobium sp. strain CB756. Seeds were allowed to germinate until the emerging roots were 2 to 3 cm long. Four seedlings were sown per 2-L pot, and the developing plants were watered with a nutrient solution free of combined N (Hewitt, 1966) twice each day to field capacity. Four weeks after sowing, the pots were sealed so that the root systems of the plants could be exposed to compressed air (control plants) or 80% Ar: 20% O2 (Ar:O2-treated plants). Both gas mixtures (air and Ar:O2) were humidified at the ambient temperature of the greenhouse and passed through the pots at a flow rate of 100 mL min−1. However, to ensure that the plants did not suffer water deficits during treatments, an extra 100 mL of nutrient solution was added to the pots before sealing. Enzymes were assayed in crude desalted extracts prepared from nodules harvested 3, 6, 24, 48, 72, and in some cases, 96 h after exposing the root systems to either air or Ar:O2. All four plants from one pot were harvested and combined as one of three or four replicates. Nodules were removed from the roots of these plants as quickly as possible and stored in liquid N2 before extraction.
Enzyme Assays
The activities of AIRS, GART, and uricase in desalted crude nodule extracts were assayed as described previously (Atkins et al., 1997). Rates were calculated on a gram fresh weight of nodule or milligram soluble protein basis, and those for nodules in Ar:O2 were expressed as percentage of the control values at each time. PEPC activity was assayed as described by Schuller et al. (1990). The maximum extractable activity of PEPC was determined at pH 8.0 (optimum pH) in the presence of 2 mm PEP (saturating substrate concentration) and 15% (v/v) glycerol. The Ki (malate) for PEPC was determined at pH 7.0 (physiological pH) in the absence of glycerol. The sensitivity of soybean (Glycine max) nodule PEPC to inhibition by malate at physiological pH is negatively correlated with the phosphorylation status of the enzyme (Schuller and Werner, 1993; Zhang et al., 1995). Thus, the Ki (malate) can be used as an indirect indicator of the phosphorylation status of PEPC with the more highly phosphorylated form of the enzyme having a higher Ki (malate) value.
Protein Determinations
AIRS, GART, uricase, and PEPC protein contents of the nodules were determined by ELISA using immunospecific polyclonal antibodies raised in rabbits. The antigens used to produce the AIRS and GART antibodies were purified recombinant proteins expressed in Escherichia coli cells transformed with AIRS and GART cDNAs isolated from a cowpea nodule cDNA library (Smith et al., 1998; D. Hall, P.M.C. Smith, and C.A. Atkins, unpublished data). The antigen for uricase was the 35-kD subunit of the native enzyme purified from cowpea nodules (Atkins et al., 1991). The rabbit anti-PEPC immune serum was raised against the alfalfa (Medicago sativa) nodule enzyme (a gift from C.P. Vance, University of Minnesota). The ELISA assays used goat anti-rabbit IgG coupled to alkaline phosphatase as secondary antisera and were quantified by A405 due to p-nitrophenyl phosphate hydrolysis. Changes in absorbance were calculated on the basis of milligrams soluble protein, and those for Ar:O2-treated nodules were expressed as percentage of controls at each sampling time. Total soluble protein in nodule extracts was determined according to Lowry et al. (1951) using crystalline bovine serum albumin as standard.
Nitrogenase Assay
Nitrogenase activity of intact plants was assayed in situ by passing the gas streams exiting the pots through an in-line H2 detector (Qubit Systems Inc., Kingston, Ontario, Canada). The output from the H2 detector was fed to a Universal Lab Interface and analyzed using Logger Pro software (Vernier Software, Portland, OR). The H2 detector was calibrated separately for measurement in air and Ar:O2 (Qubit operating manual).
Northern Analysis
RNA was isolated from plant tissues as described by Smith et al. (1998). Total RNA was separated on a 1.2% (w/v) agarose formaldehyde gel and transferred to Hybond-N+ membrane (Amersham, Buckinghamshire, UK) according to Sambrook et al. (1989). Hybridizations were performed at 42°C, as described by Sambrook et al. (1989). The hybridization buffer was 50% (v/v) deionized formamide, 2× sodium chloride/sodium phosphate/EDTA (0.36 m NaCl, 20 mm sodium phosphate, and 2 mm EDTA, pH 7.7), 7% (w/v) SDS, 0.5% (w/v) milk powder, 1% (w/v) PEG 20000, and 0.5 mg mL−1 salmon sperm DNA. Stringency washes were 0.2× SSC (20× SSC: 3 m NaCl and 0.3 m Na3 citrate, pH 7.0)/0.1% (w/v) SDS at 60°C for 30 min.
ACKNOWLEDGMENTS
We thank Carroll Vance (University of Minnesota) for the gift of immune sera to PEPC and S. Mole for technical assistance with plant culture.
Footnotes
This work was supported by grants from the Australian Research Council (to C.A.A., P.M.C.S., and K.A.S.) and by a Feodor Lynen Fellowship (to H.W.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010714.
LITERATURE CITED
- Atkins CA. Ammonia assimilation and export of nitrogen from the legume nodule. In: Dilworth MJ, Glenn AR, editors. Biology and Biochemistry of Nitrogen Fixation. Amsterdam: Elsevier Science Publishers; 1991. pp. 293–319. [Google Scholar]
- Atkins CA, Fernando M, Hunt S, Layzell DB. A metabolic connection between nitrogenase activity and the synthesis of ureides in nodulated soybean. Physiol Plant. 1992;84:441–447. [Google Scholar]
- Atkins CA, Pate JS, Shelp BJ. Effects of short-term N2 deficiency on N metabolism in legume nodules. Plant Physiol. 1984a;76:705–710. doi: 10.1104/pp.76.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CA, Sanford PJ, Storer PJ, Pate JS. Inhibition of nodule functioning in cowpea by a xanthine oxidoreductase inhibitor, allopurinol. Plant Physiol. 1988;88:1229–1234. doi: 10.1104/pp.88.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CA, Shelp BJ, Kuo J, Peoples MB, Pate JS. Nitrogen nutrition and the development and senescence of nodules on cowpea seedlings. Planta. 1984b;162:316–326. doi: 10.1007/BF00396743. [DOI] [PubMed] [Google Scholar]
- Atkins CA, Shelp BJ, Storer PJ, Pate JS. Nitrogen nutrition and the development of biochemical functions associated with nitrogen fixation and ammonia assimilation of nodules on cowpea seedlings. Planta. 1984c;162:327–333. doi: 10.1007/BF00396744. [DOI] [PubMed] [Google Scholar]
- Atkins CA, Smith PMC. Ureide synthesis in legume nodules. In: Triplett EJ, editor. Prokaryotic Nitrogen Fixation. A Model System for the Analysis of a Biological Process. Wymondham, Norfolk, UK: Horizon Scientific Press; 2000. pp. 559–587. [Google Scholar]
- Atkins CA, Smith PMC, Storer PJ. Reexamination of the intracellular localization of de novo purine synthesis in cowpea nodules. Plant Physiol. 1997;113:127–135. doi: 10.1104/pp.113.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CA, Storer PJ, Young EB. Translocation of nitrogen and expression of nodule-specific uricase (nodulin-35) in Robinia pseudoacacia. Physiol Plant. 1991;83:483–491. [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Coker GT, Schubert KR. Carbon dioxide fixation in soybean roots and nodules: I. Characterization and comparison with N2 fixation and composition of xylem exudate during early nodule development. Plant Physiol. 1981;67:691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt EJ. Sand and Water Culture Methods Used in the Study of Plant Nutrition. Wallingford, UK: Commonwealth Agricultural Bureau; 1966. pp. 431–446. [Google Scholar]
- Kim JH, Delauney AJ, Verma DPS. Control of de novo purine biosynthesis genes in ureide-producing legumes: induction of glutamine phosphoribosylpyrophosphate amidotransferase gene and characterization of its cDNA from soybean and Vigna. Plant J. 1995;7:77–86. doi: 10.1046/j.1365-313x.1995.07010077.x. [DOI] [PubMed] [Google Scholar]
- Laing WA, Christeller JT, Sutton WD. Carbon dioxide fixation by lupin root nodules: II. Studies with 14C-labeled glucose, the pathway of glucose catabolism, and the effects of some treatments that inhibit nitrogen fixation. Plant Physiol. 1979;63:450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Pathirana SM, Vance CP, Miller SS, Gantt JS. Alfalfa root nodule phosphoenolpyruvate carboxylase: characterization of the cDNA and expression in effective and plant-controlled ineffective nodules. Plant Mol Biol. 1992;20:437–450. doi: 10.1007/BF00040603. [DOI] [PubMed] [Google Scholar]
- Rainbird RM, Atkins CA. Purification and some properties of urate oxidase from nitrogen-fixing nodules of cowpea. Biochim Biophys Acta. 1981;659:132–140. doi: 10.1016/0005-2744(81)90277-1. [DOI] [PubMed] [Google Scholar]
- Reynolds PHS, Blevins DG, Randall DD. 5-Phosphoribosylpyrophosphate amidotransferase from soybean root nodules: kinetic and regulatory properties. Arch Biochem Biophys. 1984;229:623–631. doi: 10.1016/0003-9861(84)90195-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schuller KA, Turpin DH, Plaxton WC. Metabolite regulation of partially purified soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1990;94:1429–1435. doi: 10.1104/pp.94.3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller KA, Werner D. Phosphorylation of soybean (Glycine max L.) nodule phosphoenolpyruvate carboxylase in vitro decreases sensitivity to inhibition by l-malate. Plant Physiol. 1993;101:1267–1273. doi: 10.1104/pp.101.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PMC, Mann AJ, Goggin DE, Atkins CA. AIR synthetase in cowpea nodules: a single gene product targeted to two organelles? Plant Mol Biol. 1998;36:811–820. doi: 10.1023/a:1005969830314. [DOI] [PubMed] [Google Scholar]
- Thumfort PP, Layzell DB, Atkins CA. Diffusion and reaction of oxygen in the central tissue of ureide-producing legume nodules. Plant Cell Environ. 1999;22:1351–1363. [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS. Primary assimilation of nitrogen in alfalfa nodules: molecular features of the enzyme involved. Plant Sci. 1994;101:51–64. [Google Scholar]
- Wadham C, Winter H, Schuller KA. Regulation of soybean nodule phosphoenolpyruvate carboxylase in vivo. Physiol Plant. 1996;97:531–535. [Google Scholar]
- Walsh KB, Canny MJ, Layzell DB. Vascular transport and soybean nodule function: II. A role for phloem supply in product export. Plant Cell Environ. 1989;12:713–723. [Google Scholar]
- Zhang X-Q, Li B, Chollet R. In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1995;108:1561–1568. doi: 10.1104/pp.108.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]