Abstract
Asphyxia in utero in pre-term fetuses is associated with evolving hypoperfusion of the gut after the insult. We examined the role of the sympathetic nervous system (SNS) in mediating this secondary hypoperfusion. Gut blood flow changes were also assessed during postasphyxial seizures. Preterm fetal sheep at 70% of gestation (103–104 days, term is 147 days) underwent sham asphyxia or asphyxia induced by 25 min of complete cord occlusion and fetuses were studied for 3 days afterwards. Phentolamine (10 mg bolus plus 10 mg h−1 i.v.) or saline was infused for 8 h starting 15 min after the end of asphyxia or sham asphyxia. Phentolamine blocked the fall in superior mesenteric artery blood flow (SMABF) after asphyxia and there was a significant decrease in MAP for the first 3 h of infusion (33 ± 1.6 mmHg versus vehicle 36.7 ± 0.8 mmHg, P < 0.005). During seizures SMABF fell significantly (8.3 ± 2.3 versus 11.4 ± 2.7 ml min−1, P < 0.005), and SMABF was more than 10% below baseline for 13.0 ± 1.7 min per seizure (versus seizure duration of 78.1 ± 7.2 s). Phentolamine was associated with earlier onset of seizures (5.0 ± 0.4 versus 7.1 ± 0.7 h, P < 0.05), but no change in amplitude or duration, and prevented the fall in SMABF. In conclusion, the present study confirms the hypothesis that postasphyxial hypoperfusion of the gut is strongly mediated by the SNS. The data highlight the importance of sympathetic activity in the initial elevation of blood pressure after asphyxia and are consistent with a role for the mesenteric system as a key resistance bed that helps to maintain perfusion in other, more vulnerable systems.
In the pre-term infant, pre- and postnatal haemodynamic disturbances are associated with a high rate of impaired postnatal intestinal adaptation during the first days of life; for example, delayed meconium passage, abdominal distension, bilious vomiting and a delay in tolerating enteral feeding (Robel-Tillig et al. 2002). Early establishment of enteral feeding is important, promoting the normal development of the gut as a physical, mechanical, physiological and immunological barrier (Strodtbeck, 2003). The key link between the variety of disparate clinical problems and early gastrointestinal dysfunction, as well as increased risk of severe complications such as necrotizing enterocolitis (NEC), appears to be impaired perfusion of the mesenteric bed (Coombs et al. 1990; Malcolm et al. 1991; Coombs et al. 1992; Akinbi et al. 1994). Currently, however, there is little information on the specific mechanisms that control gut perfusion in the perinatal period.
We have recently reported that exposure of the premature fetus to asphyxia in utero is associated with development of delayed hypoperfusion of the gut during the early phase of recovery (Bennet et al. 2000). This phenomenon is not specific to fetal life or the gut (Conger & Weil, 1995), but its mechanisms and significance remain controversial. Some data in the adult suggest that both central and peripheral secondary hypoperfusion is related to impaired endothelial control of blood flow (Hossmann, 1997; Karimova & Pinsky, 2001; Reber et al. 2002; Ten & Pinsky, 2002), while others suggest that it is actively mediated, reflecting reduced metabolic requirement (Michenfelder & Milde, 1990; Gold & Lauritzen, 2002). Although there is no specific information in the fetus, several lines of evidence suggest the hypothesis that α-adrenergic sympathetic nervous system (SNS) activity may play a key role in regulating gut perfusion during recovery after an asphyxial insult. The fetal gut is innervated by the autonomic nervous system from very early in development (Read & Burnstock, 1970), and the autonomic nervous system is an important regulator of intestinal blood flow under a variety of physiological and pathological conditions (Nuwayhid et al. 1975; Jensen et al. 1987b; Paulick et al. 1991; Jensen & Lang, 1992; Rouwet et al. 2000; Mulder et al. 2002). Redistribution of blood flow away from the peripheral organs such as the gut during hypoxia and asphyxia in utero is, for example, largely mediated by α-adrenergic receptors (Iwamoto et al. 1983; Jensen & Lang, 1992; Giussani et al. 1993), and sympathectomy in the sheep fetus results in increased meconium passage (Westgate et al. 2002).
A potential further complication of exposure to asphyxia is the development of seizures, which may occur both pre- and postnatally (Osiovich & Barrington, 1996; Ingemarsson & Spencer, 1998; Westgate et al. 1999; Keogh et al. 2000; Patane & Ghidini, 2001). Seizures may cause further circulatory instability, although most studies of seizures have largely focused on their impact on cerebral circulation, with little attention given to other vascular beds. In the adult, however, data show that the marked increase in blood pressure typically observed during seizures is facilitated primarily by increased peripheral vascular resistance, in particular in the mesenteric bed, rather than by changes in cardiac output (Doba et al. 1975; Kreisman et al. 1993). In the adult, there is marked activation of the SNS during seizures, which is likely to mediate this vasoconstriction (Baumgartner et al. 2001). It is known that seizures in pre-term infants also cause marked increases in blood pressure (Perlman & Volpe, 1983), but the effects on peripheral blood flow have not been evaluated.
Therefore it was the aim of the present study to test the hypothesis that the postasphyxial secondary hypoperfusion of the pre-term fetal sheep gut is mediated by α-adrenergic receptor activation. Further, we characterized the impact of postasphyxial seizures on gut blood flow and examined the role of α-adrenergic receptors in mediating changes in gut blood flow during seizures.
Methods
Surgical procedures
All procedures were approved by the Animal Ethics Committee of The University of Auckland. Twenty-eight time-mated singleton Romney–Suffolk cross sheep were operated on at 98–99 days of gestation (term is 147 days). Food, but not water, was withdrawn 18 h before surgery. Ewes were given 5 ml of Streptocin (procaine penicillin, 250 000 IU, and dihydrostreptomycin, 250 mg ml−1, Stockguard Laboratories Ltd, Hamilton, New Zealand) intramuscularly for prophylaxis 30 min before the start of surgery. Anaesthesia was induced by i.v. injection of Alfaxan (alphaxalone, 3 mg kg−1, Jurox, Rutherford, NSW, Australia), and general anaesthesia maintained using 2–3% halothane in O2. Ewes were intubated but not ventilated and the depth of anaesthesia, maternal heart rate and respiration were constantly monitored by trained anaesthetic staff. Under anaesthesia a 20 gauge i.v. catheter was placed in a maternal front leg vein, and the ewes were placed on a constant infusion isotonic saline drip (at an infusion rate of approximately 250 ml h−1) to maintain maternal fluid balance.
All surgical procedures were performed using aseptic techniques (Bennet et al. 1999, 2000). Catheters were placed in the left fetal femoral artery and vein, right brachial artery and vein, and the amniotic sac. A 2S-type ultrasonic blood flow probe (Transonic Systems Inc., Ithaca, NY, USA) was placed around the superior mesenteric artery (SMA) to measure gastrointestinal blood flow. Two pairs of electrodes (AS633–5SSF, Cooner Wire Co., Chatsworth, CA, USA) were placed on the dura over the parasagittal parietal cortex (5 and 10 mm anterior to bregma and 5 mm lateral) and secured with cyanoacrylate glue to measure electrocortical activity (EEG). A reference electrode was sewn over the occiput. Electromyographic electrodes were placed in the nuchal muscle to measure electromyogram (EMG) activity, and electrocardiogram (ECG) electrodes were sewn across the chest to record fetal heart rate (FHR). An inflatable silicone occluder was placed around the umbilical cord of all fetuses (In Vivo Metric, Healdsburg, CA, USA). All fetal leads were exteriorized through the maternal flank and a maternal long saphenous vein was catheterized to provide access for postoperative care and euthanasia. Antibiotics (80 mg gentamicin, Pharmacia and Upjohn, Rydalmere, NSW, Australia) were administered into the amniotic sac before closure of the uterus.
Postoperatively, all sheep were housed together in separate metabolic cages with access to water and food ad libitum. They were kept in a temperature-controlled room (16 ± 1°C, humidity 50 ± 10%), in a 12 h light–dark cycle. A period of 4–5 days postoperative recovery was allowed before experiments commenced, during which time antibiotics were administered daily for 4 days i.v. to the ewe (600 mg benzylpencillin sodium, Novartis Ltd, Auckland, New Zealand, and 80 mg gentamicin). Fetal catheters were maintained patent by continuous infusion of heparinized saline (20 IU ml−1 at 0.15 ml h−1) and the maternal catheter maintained by daily flushing.
Experimental design
Experiments were conducted at 103–104 days gestation. Mean arterial pressure (MAP) measured from the femoral artery, mean venous pressure measured from the femoral vein (both corrected for maternal movement by subtraction of amniotic fluid pressure), FHR, SMABF, EEG and nuchal EMG activity were recorded continuously from 12 h before occlusion until 72 h after occlusion. Data were stored to disk by custom software for offline analysis (Labview for Windows, National Instruments Ltd, Austin, TX, USA).
Fetuses were randomly assigned to one of four groups: sham occlusion plus vehicle infusion (sham vehicle group, n = 7); sham occlusion plus infusion of the α-adrenergic antagonist phentolamine (sham phentolamine, n = 5); occlusion plus vehicle infusion (asphyxia vehicle, n = 8); and occlusion plus infusion of phentolamine (asphyxia phentolamine, n = 8). Phentolamine (Regitine, Novartis Pharma AG, Basel, Switzerland) was administered i.v., via the brachial vein, to the fetus as a 10 mg in 1 ml bolus given over 5 min, followed by a continuous infusion at 10 mg h−1 at 1 ml h−1, starting 15 min after the end of occlusion or sham occlusion and continued for 8 h. In the vehicle groups, isotonic saline was infused at the same volume and rate over the same time period. The period of 8 h was chosen because this was the mean duration of gastrointestinal hypoperfusion postasphyxia observed in previous studies (Bennet et al. 2000).
In the occlusion groups, asphyxia was induced by rapid inflation of the umbilical cord occluder, for 25 min, with sterile saline of a defined volume known to completely inflate the occluder as determined by previous pilot experiments using flow probes placed on an umbilical vein. Successful occlusion was confirmed by a rapid rise in MAP and bradycardia (Bennet et al. 2000). Arterial blood was taken from the brachial artery 15 min before asphyxia, at 20 min from commencement of asphyxia, and 2, 4, 6, 10, 24, 48 and 72 h postasphyxia for preductal pH and blood gas determination (Ciba-Corning Diagnostics 845 blood gas analyser and co-oximeter, MA, USA) and for glucose and lactate measurements (YSI model 2300, Yellow Springs, OH, USA).
On completion of the experiment at 72 h, ewes and fetuses were killed by an overdose of pentobarbitone sodium (9 g i.v. to the ewe; Pentobarb 300, Chemstock International, Christchurch, New Zealand).
Data analysis and statistics
The nuchal EMG signal was bandpass filtered between 100 Hz and 1 kHz; the signal was then integrated using a time constant of 1 s. The EEG signal was low-pass filtered with a sixth order low-pass Butterworth filter, with a cut-off frequency of 50 Hz. A power spectrum was then calculated from this 256 Hz sampled signal. Data from left and right EEG electrodes were averaged to give mean total EEG activity. For clarity of data display the EEG intensity was log transformed (dB, 20 × log(intensity); Williams et al. 1992). Additionally the raw EEG signal was processed through a digital FIR low-pass filter with a cut-off frequency of 30 Hz and stored at a sampling rate of 64 Hz for analysis of seizures. Seizures were identified visually and defined as the concurrent appearance of sudden, repetitive, evolving stereotyped waveforms in the EEG signal lasting more than 10 s and of an amplitude greater than 20 μV (Scher et al. 1993). Superior mesenteric artery vascular resistance was calculated using the formula (mean arterial pressure – mean venous pressure)/blood flow (mmHg (ml min−1)−1).
Statistical analysis was performed using SPSS for Windows (SPSS, Chicago, IL, USA). For the analysis of the long-term recovery data (1–72 h postocclusion) the control pre-occlusion baseline period was taken as the mean of the 12 h before occlusion. For between group comparisons two-way analysis of variance for repeated measures was performed. When statistical significance was found between groups, or between group and time, analysis of covariance (ANCOVA) was used to compare selected time points, using the baseline control periods before occlusion as a covariate. For seizure analysis, the preseizure baseline period was taken as the 2 min before the onset of a seizure. Maximum changes observed during seizures were compared to the preseizure baseline using a paired Student's t test. Percentage changes in nuchal EMG are presented as median (25th, 75th percentile). Data are presented as means ± s.e.m. Significance was accepted when P < 0.05.
Results
Blood composition measurements
Values and statistical comparisons for arterial pH, blood gases, glucose and lactate for the control and treatment groups before occlusion, at 20 min of sham occlusion or occlusion, and 2, 4, 6, 8, 24, 48 and 72 h after sham occlusion or occlusion, are presented in Table 1.
Table 1.
Fetal arterial pH, blood gases, glucose and lactate values for all groups 15 min before (control), during (20 min) and after (2, 4, 6, 8, 24, 48 and 72 h), either sham umbilical occlusion or occlusion
| Control | 20 min | 2 h | 4 h | 6 h | 8 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|---|---|---|
| pHa | |||||||||
| Sham veh. | 7.39 ± 0.0 | 7.38 ± 0.0 | 7.39 ± 0.0 | 7.39 ± 0.0 | 7.39 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | 7.37 ± 0.0 | 7.37 ± 0.0 |
| Sham phent. | 7.38 ± 0.0 | 7.39 ± 0.0 | 7.40 ± 0.0 | 7.39 ± 0.0 | 7.39 ± 0.0 | 7.39 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 |
| Asphyxia veh. | 7.37 ± 0.0 | 6.83 ± 0.0§ | 7.34 ± 0.0* | 7.40 ± 0.0 | 7.42 ± 0.0* | 7.39 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | 7.37 ± 0.0 |
| Asphyxia phent. | 7.36 ± 0.0 | 6.88 ± 0.0§ | 7.36 ± 0.0 | 7.41 ± 0.0 | 7.40 ± 0.0 | 7.36 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | 7.37 ± 0.0 |
| PaCO2 (mmHg) | |||||||||
| Sham veh. | 48.0 ± 1.8 | 48.6 ± 1.7 | 47.6 ± 1.8 | 48.9 ± 2.3 | 48.8 ± 2.0 | 49.7 ± 1.6 | 47.5 ± 1.9 | 48.7 ± 1.8 | 48.3 ± 2.8 |
| Sham phent. | 45.2 ± 3.1 | 45.0 ± 3.0 | 47.2 ± 1.2 | 46.2 ± 3.4 | 47.1 ± 3.7 | 46.2 ± 2.6 | 48.0 ± 2.3 | 49.0 ± 3.2 | 45.7 ± 2.0 |
| Asphyxia veh. | 47.2 ± 0.9 | 137.7 ± 7.7§ | 44.1 ± 1.4 | 45.3 ± 0.8 | 43.0 ± 1.0* | 48.3 ± 1.1 | 44.8 ± 1.8 | 45.3 ± 0.8 | 44.3 ± 1.2 |
| Asphyxia phent. | 48.9 ± 3.6 | 132.0 ± 3.4* | 46.1 ± 3.6 | 46.4 ± 2.0 | 47.5 ± 2.0 | 46.7 ± 1.8 | 46.1 ± 0.7 | 46.1 ± 0.7 | 44.9 ± 0.7 |
| PaO2 (mmHg) | |||||||||
| Sham veh. | 23.5 ± 0.8 | 23.2 ± 0.7 | 23.1 ± 0.9 | 23.2 ± 1.0 | 23.4 ± 0.7 | 23.3 ± 0.9 | 24.9 ± 0.9 | 24.9 ± 1.4 | 25.0 ± 1.1 |
| Sham phent. | 25.6 ± 1.2 | 25.6 ± 1.3 | 26.9 ± 1.3 | 24.9 ± 0.5 | 25.0 ± 0.7 | 25.3 ± 0.8 | 26.0 ± 1.2 | 27.8 ± 2.7 | 25.4 ± 1.0 |
| Asphyxia veh. | 24.4 ± 0.7 | 8.2 ± 1.1* | 26.8 ± 1.2* | 24.2 ± 1.4 | 24.8 ± 1.0 | 26.8 ± 0.9* | 28.4 ± 0.9* | 28.7 ± 0.8* | 29.1 ± 1.2 |
| Asphyxia phent. | 23.6 ± 1.6 | 9.0 ± 1.2§ | 23.8 ± 1.9 | 24.5 ± 1.2 | 25.8 ± 2.3 | 27.4 ± 2.2 | 28.0 ± 1.2 | 28.0 ± 1.2 | 28.1 ± 1.6 |
| Lactate (mmol) | |||||||||
| Sham veh. | 0.82 ± 0.1 | 0.81 ± 0.1 | 0.87 ± 0.2 | 0.85 ± 0.1 | 0.86 ± 0.1 | 1.15 ± 0.2 | 0.66 ± 0.2 | 0.75 ± 0.1 | 0.85 ± 0.1 |
| Sham phent. | 0.73 ± 0.0 | 0.75 ± 0.2 | 0.72 ± 0.1 | 0.76 ± 0.1 | 0.75 ± 0.0 | 0.82 ± 0.1 | 0.65 ± 0.1 | 0.74 ± 0.1 | 0.78 ± 0.0 |
| Asphyxia veh. | 0.74 ± 0.1 | 6.11 ± 0.2§ | 3.71 ± 0.7‡ | 2.56 ± 0.5† | 1.83 ± 0.3* | 2.03 ± 0.5 | 0.95 ± 0.1 | 0.73 ± 0.1 | 0.83 ± 0.1 |
| Asphyxia phent. | 0.88 ± 0.1 | 6.02 ± 0.2§ | 1.90 ± 0.4* | 1.15 ± 0.5 | 1.25 ± 0.4 | 0.67 ± 0.1 | 0.64 ± 0.0 | 0.64 ± 0.0 | 0.72 ± 0.1 |
| Glucose (mmol) | |||||||||
| Sham veh. | 1.03 ± 0.1 | 1.02 ± 0.1 | 1.09 ± 0.1 | 1.03 ± 0.1 | 1.07 ± 0.1 | 1.19 ± 0.2 | 1.15 ± 0.1 | 1.16 ± 0.1 | 1.16 ± 0.1 |
| Sham phent. | 0.91 ± 0.1 | 0.93 ± 0.1 | 0.80 ± 0.1 | 0.64 ± 0.1* | 0.88 ± 0.1* | 0.90 ± 0.1 | 0.83 ± 0.1 | 0.94 ± 0.1 | 0.93 ± 0.1 |
| Asphyxia veh. | 1.07 ± 0.1 | 0.59 ± 0.1† | 1.25 ± 0.1 | 1.28 ± 0.1 | 1.36 ± 0.1 | 1.52 ± 0.2 | 1.43 ± 0.1 | 1.24 ± 0.1 | 1.18 ± 0.1 |
| Asphyxia phent. | 0.90 ± 0.1 | 0.40 ± 0.1‡ | 1.02 ± 0.1 | 0.96 ± 0.1 | 0.97 ± 0.1 | 1.09 ± 0.1 | 1.07 ± 0.1 | 1.07 ± 0.1 | 1.09 ± 0.1 |
pHa is the arterial pH, PaCO2 is the arterial partial pressure of CO2 and PaCO2 is the arterial partial pressure of O2. Data are means ± s.e.m.
P < 0.05,
P < 0.01,
P < 0.005,
P < 0.001 compared to sham vehicle group values.
Superior mesenteric artery blood flow and vascular resistance
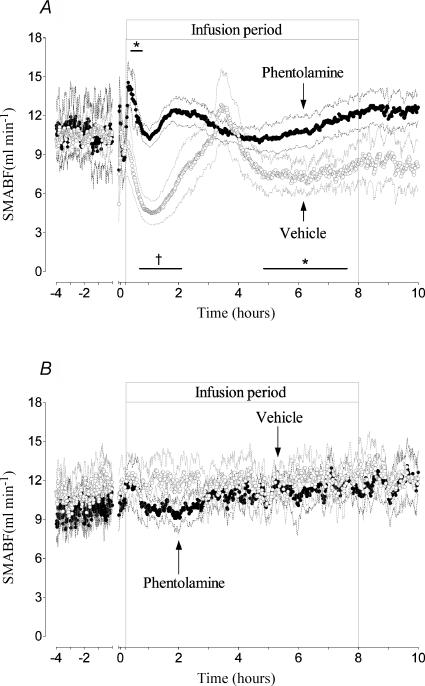
There were no significant differences in SMA blood flow or resistance between groups before occlusion and between the asphyxia groups during occlusion. SMABF rapidly returned to sham vehicle values immediately postocclusion in both occlusion groups (Fig. 1A). In the asphyxia vehicle group SMABF then changed in a biphasic hypoperfusion pattern similar to that previously described (Bennet et al. 2000), with an initial hypoperfusion between 35 min and 2 h after the start of infusion (nadir at 73 min postocclusion of 4.5 ± 0.9 versus 11.5 ± 0.8 ml min−1 in the sham vehicle group, P < 0.01). Hypoperfusion was associated with a significant increase in vascular resistance (8.6 ± 2.6 versus 3.3 ± 0.4 mmHg (ml min−1)−1, compared to sham vehicle, P < 0.005). This first period of hypoperfusion was followed by a transient return to basal values, maximal at 3.5 h, before falling significantly again between 4.5 and 7.5 h (P < 0.05) and then returning again to baseline before seizures started. This secondary decrease in SMABF was also associated with a small but significant rise in vascular resistance (5.6 ± 0.4 mmHg (ml min−1)−1, P < 0.05).
Figure 1. Time sequence of changes in SMABF during the 4 h before occlusion and for 10 h postocclusion.
A shows the responses of the asphyxia vehicle group (○) and the asphyxia phentolamine group (•). The groups are further denoted by arrows. Note the biphasic fall in SMABF in the vehicle group, interrupted by a transient period of vasodilatation. Phentolamine causes an initial vasodilatation, but the secondary hypoperfusion phases are prevented. The same transient vasodilatation seen in the asphyxia vehicle group also occurs in the phentolamine group, but the response occurs earlier. B shows the SMABF responses of the sham vehicle group (◯) and sham phentolamine group (•), showing no differences between groups. Data are 1 min averages, means ± s.e.m., *P < 0.05, †P < 0.01 compared to sham vehicle group. The axis break denotes the period of occlusion, not shown.
In the asphyxia phentolamine group there was a transient rise in SMABF, maximal at 20 min after the start of infusion (14.6 ± 1.7 versus 11.0 ± 2.6 ml min−1 in the sham vehicle group, P < 0.05, Fig. 1A). This was associated with a small but significant drop in vascular resistance (1.9 ± 0.4 versus 3.4 ± 0.4 mmHg (ml min−1)−1, compared to the sham vehicle group, P < 0.05). Thereafter SMABF was not significantly different to sham vehicle group values, despite a tendency to rise between 1.5 and 2.5 h; this rise was similar to that seen in the asphyxia vehicle group, but occurred earlier (maximal at 2 versus 3.5 h). In the sham phentolamine group, phentolamine infusion produced a transient but modest rise during the first 15 min of infusion, followed by a slight reduction in flow between 1 and 3 h (coincidental with the transient fall in FHR seen in this group after initial tachycardia, Fig. 4B), but there were no overall significant differences between the sham vehicle and sham phentolamine groups (Fig. 1B).
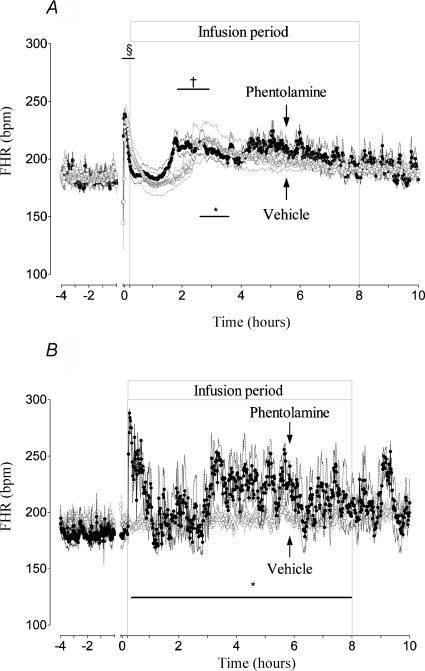
Figure 4. Time sequence of changes in FHR during the 4 h before occlusion and for 10 h postocclusion.
A shows the responses of the asphyxia vehicle group (○) and the asphyxia phentolamine group (•). The groups are further denoted by arrows. Both groups had a similar initial response, but note the earlier onset of tachycardia in the phentolamine group at around 2 h postocclusion, corresponding with the transient period of increased SMABF shown in Fig. 1. Note also the increase in FHR after 4 h in this group, corresponding with the appearance of seizures. B shows the FHR responses of the sham vehicle group (○) and sham phentolamine group (•). Note the tachycardia observed with phentolamine infusion alone, which is not seen in the asphyxia phentolamine group in A. Data are means ± s.e.m., *P < 0.05, †P < 0.01, §P < 0.001 compared to sham vehicle group. The axis break denotes the period of umbilical cord occlusion, not shown.
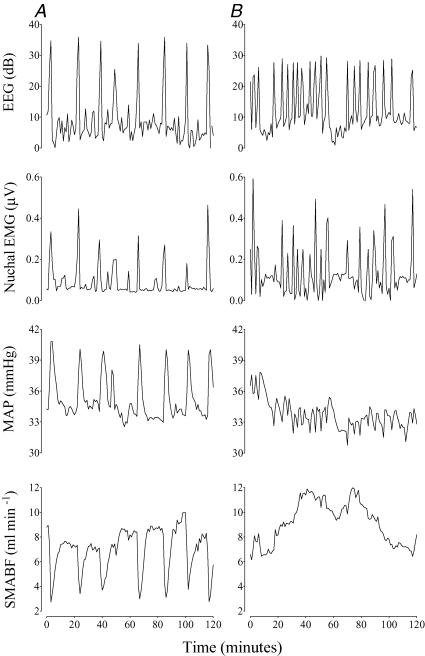
During seizures SMABF fell in the asphyxia vehicle group from 11.4 ± 2.7 ml min−1 to a nadir of 8.3 ± 2.3 ml min−1 (P < 0.005, Fig. 2A), regardless of whether electrographic seizures were present. SMABF was more than 10% below baseline for 13.0 ± 1.7 min per seizure (versus seizure duration of 78.1 ± 7.2 s). The fall in SMABF was associated with a significant increase in SMA vascular resistance (from 4.3 ± 0.7 mmHg (ml min−1)−1 preseizure to a peak of 8.6 ± 2.3 mmHg (ml min−1)−1, P < 0.05). In contrast, there was no change in SMABF in the asphyxia phentolamine group during any seizures (9.0 ± 1.4 versus 8.6 ± 1.4 min ml−1, Fig. 2B) and no significant change in resistance (5.2 ± 1.1 versus 5.4 ± 1.3 mmHg (ml min−1)−1. No seizures occurred in the sham occlusion groups.
Figure 2. Examples of changes in EEG, nuchal EMG, MAP and SMABF during seizures.
Data are 1 min averages from single fetuses, recorded over 2 h, 3 h after seizures had begun. A shows the response of an asphyxia vehicle group fetus, demonstrating marked vasoconstriction and increased blood pressure during seizures. B shows the response of an asphyxia phentolamine group fetus, showing abolition of both the blood flow and blood pressure responses to each seizure.
Blood pressure
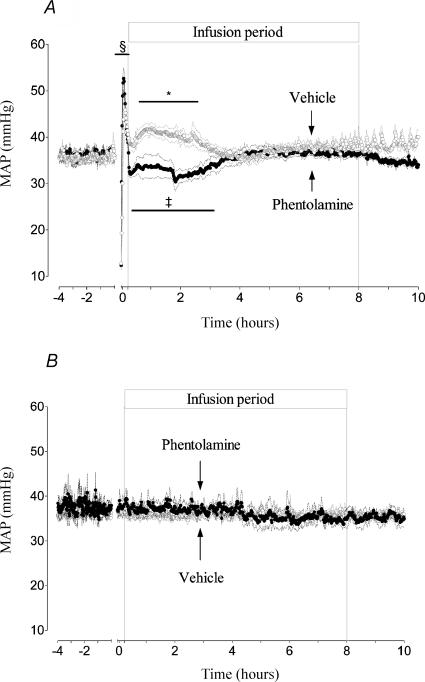
There was no significant difference in MAP between groups before occlusion and between the asphyxia groups during occlusion. In the asphyxia vehicle group an initial transient period of hypertension immediately postocclusion (P < 0.001, Fig. 3A) was followed by a period of sustained elevation of pressure between 30 min and 3 h after the start of infusion (41.0 ± 1.6 versus 36.0 ± 0.8 mmHg in the sham vehicle group, P < 0.05). Thereafter MAP returned to basal values until seizures started. During seizures MAP increased during individual seizure episodes from 36.3 ± 0.4 to 42.0 ± 0.9 mmHg (P < 0.005, Fig. 2A).
Figure 3. Time sequence of changes in MAP during the 4 h before occlusion and for 10 h postocclusion.
A shows the responses of the asphyxia vehicle group (○) and the asphyxia phentolamine group (•). The groups are further denoted by arrows. Note the lower blood pressure in the phentolamine group compared to vehicle infusion, and the subsequent further fall in blood pressure corresponding with the transient vasodilatation seen in the phentolamine group in Fig. 1. Similarly, MAP falls in the vehicle group during the same period of vasodilatation, but at a slightly later time. B shows the MAP responses of the sham vehicle group (○) and sham phentolamine group (•), showing no differences between groups. Data are 1 min averages, means ±s.e.m., *P < 0.05, ‡P < 0.005, §P < 0.001 compared to sham vehicle group. The axis break denotes the period of occlusion, not shown.
In the asphyxia phentolamine group MAP was also significantly increased immediately postocclusion (P < 0.001, Fig. 3A), but pressure fell rapidly at the onset of infusion and was reduced for 3 h after the start of infusion (33 ± 1.6 versus 36.7 ± 0.8 mmHg in the sham vehicle group, P < 0.005). There was a notable secondary fall in pressure starting at 1.8 h postocclusion for 54 min (nadir of 30.4 ± 2.1 mmHg, P < 0.001); thereafter MAP progressively returned to baseline. During seizures there were no significant changes in MAP during phentolamine infusion (Fig. 2B). There was no difference in MAP between sham occlusion groups (Fig. 3B).
Fetal heart rate
In the asphyxia vehicle group, after a transient period of rebound tachycardia during the first 10 min postocclusion (P < 0.001, Fig. 4A), FHR returned to sham vehicle group values until 1.6 h, when FHR slowly began to rise again, increasing abruptly at 2.2 h for 54 min (220.0 ± 15 versus 183.4 ± 4.4 beats min−1 in the sham vehicle group, P < 0.05). FHR then gradually returned to baseline values until seizures started. There were no significant changes in FHR during seizures.
In the asphyxia phentolamine group FHR followed a similar but time-shifted pattern to the asphyxia vehicle group. Rebound tachycardia (P < 0.001, Fig. 4A) was followed by a transient return to baseline for 1.45 h, then after a gradual rise FHR rose abruptly for 67 min (209.9 ± 7.0 versus 183.6 ± 5.6 beats min−1 in the sham vehicle group, P < 0.005); thereafter FHR was variably increased as a function of seizures, rising significantly to 219.7 ± 8.5 beats min−1 versus an interseizure baseline of 189.0 ± 3.2 beats min−1, P < 0.05). Phentolamine infusion alone significantly increased FHR during the first hour (maximal at 5 min of infusion; 282.4 ± 10.6 beats min−1 versus sham vehicle group values of 189.4 ± 5.5 beats min−1, P < 0.001, Fig. 4B); thereafter the changes in FHR were variable, but there was a significant effect of treatment over time compared to the sham vehicle group (P < 0.05).
EEG, EMG and seizure activity
EEG amplitude was rapidly suppressed at the onset of occlusion in both groups, and remained significantly suppressed (5.3 ± 0.9 versus 45.6 ± 5.8 μV in the sham vehicle group and 5.4 ± 0.9 versus 43.6 ± 6.7 μV in the sham phentolamine group, P < 0.001 both groups), except between 9 and 18 h in the asphyxia vehicle group and 6 and 14 h in the asphyxia phentolamine group, when EEG amplitude increased due to seizures.
The onset of seizures occurred significantly earlier in the asphyxia phentolamine group compared to the asphyxia vehicle group (5.01 ± 0.4 versus 7.1 ± 0.7 h, respectively, P < 0.05). There were no significant differences, however, in the total seizure duration (15.5 ± 3.5 versus 23.2 ± 3.7 h, asphyxia vehicle versus asphyxia phentolamine), total number of seizures (56.0 ± 10.0 versus 73.2 ± 28.0), number of seizures per hour (3.1 ± 0.7 versus 4.2 ± 1.3), intensity of individual seizures (118.2 ± 20.8 versus 172.0 ± 25.3 μV) or duration of individual seizures (78.1 ± 7.2 versus 76.8 ± 12.0 s). All fetuses in the asphyxia groups had electrographic evidence of seizures (Fig. 2A and B), except one fetus in the asphyxia vehicle group, which showed only cardiovascular and nuchal EMG evidence of seizure activity. There were no seizures in the sham groups. Nuchal EMG activity was present during 75.0% (73,81) of seizures in the asphyxia vehicle group, and 80.0% (79,88) of seizures in the asphyxia phentolamine group (Fig. 2A and B).
Discussion
This study demonstrates for the first time that the sympathetic nervous system is a key mediator of delayed gastrointestinal hypoperfusion during recovery from an asphyxial insult in pre-term fetal sheep. Prevention of this delayed hypoperfusion by α-adrenergic blockade significantly reduced fetal blood pressure during the early recovery phase after asphyxia. Further, it confirms that sympathetic activity mediates the fall in gut blood flow during postasphyxial seizures in the immature fetus. Interestingly, gut blood flow remained markedly suppressed for several minutes after resolution of each seizure, indicating that sympathetic activation was not simply a nonspecific effect of the epileptic discharge. Thus, while postasphyxial seizures were brief and infrequent compared to those seen at term, their impact on gut blood flow was marked.
Secondary hypoperfusion is known to develop in many vascular beds after ischaemic insults both pre- and postnatally, typically in the first few days after an insult (Conger & Weil, 1995). The duration and speed of onset, and to a lesser extent the degree of the hypoperfusion, are reported to be related to the severity of the insult, at least in the brain, where the phenomenon has mostly been assessed (Karlsson et al. 1994; Huang et al. 1999). The underlying factors which mediate this phenomenon remain the subject of research. Proposed hypotheses include endothelial dysfunction, disturbed blood–vessel wall interactions and reduced metabolic demand (Hossmann, 1997; Reber et al. 2002; Ten & Pinsky, 2002). The present study shows that for the pre-term fetal sheep gut after asphyxia, hypoperfusion is not related to hypotension, but rather is primarily related to a sympathetically mediated increase in vascular resistance. Both the relative rapidity of the changes in flow and its variable nature, with a transient reduction in vasoconstriction between 2 and 4 h postasphyxia in both asphyxia groups, argues against the reduction in blood flow being due to passive vascular dysfunction.
The physiological significance of this apparently active and relatively prolonged period of hypoperfusion of the gut during the latent phase of recovery after asphyxia is unknown; however, we may hypothesize that its purpose is to maintain systemic perfusion pressure. In the adult, under conditions of decreased cardiac output caused by cardiogenic or hypovolaemic shock, selective vasoconstriction of the afferent mesenteric arterioles is reported to be crucial in sustaining total systemic vascular resistance, thereby maintaining systemic arterial pressure (Reilly et al. 2001; Ceppa et al. 2003). Under these conditions, while there is some degree of vasoconstriction in other peripheral systems, it is disproportionately greater within the mesenteric circulation, and thus perfusion of non-mesenteric organs is maintained at the expense of the gut (Reilly et al. 2001; Ceppa et al. 2003). Similarly, in the fetal sheep marked constriction of the mesenteric bed occurs during acute asphyxia to facilitate redistribution of combined ventricular output in favour of central organs such as the heart, adrenals and brain (Jensen et al. 1987a; Yaffe et al. 1987; Bennet et al. 2000), but its role during postasphyxial recovery has not been evaluated.
Although it was not possible to evaluate myocardial histology and function directly in the present study, there is considerable clinical and experimental evidence to show that reversible myocardial injury and associated cardiac dysfunction are common during recovery from exposure to perinatal asphyxia (Gunn et al. 2000; Lumbers et al. 2001; Hunt & Osborn, 2002). Consistent with the hypothesis of early myocardial dysfunction, in the present study phentolamine infusion led to a reduction in blood pressure for 3–4 h despite normalization of SMA blood flow for most of this period. This is in contrast with the significant elevation seen in the asphyxia vehicle group, which was associated with an increase in SMA vascular resistance. This hypothesis is further supported by the fall in blood pressure observed in the asphyxia vehicle group during the period of spontaneous gastrointestinal vasodilatation around 4 h postasphyxia, which was followed by a modest increase in pressure during the second period of hypoperfusion between 5 and 7 h. Currently it is unclear what is mediating this transient rise in blood flow. It may be related to a reduction in sympathetic tone; however, other factors are likely to play a role in mediating both the vasoconstriction and vasodilatation. Nitric oxide (NO), for example, is known to be an important modulator of perfusion in the pre-term gut (Fan et al. 1998; Reber et al. 2002) and is also known to modulate sympathetic activity (Prast & Philippu, 2001). In addition, NO can alter vascular reactivity at the sympathetic neuroeffector junction in the rat mesenteric bed by deactivation of noradrenaline (Kolo et al. 2004). There may also be similar roles for other neurotransmitters and peptides such as neuropeptide Y (Sanhueza et al. 2003; Kolo et al. 2004).
Finally, it is striking that in the sham phentolamine group, phentolamine infusion was associated with a marked immediate tachycardia, but no change in blood pressure. Subsequently FHR varied, but on average was elevated for the remainder of the infusion period. Since, in the fetus, stroke volume is relatively fixed and therefore changes in combined ventricular output are primarily dependent on changes in FHR, this finding strongly implies that under normal conditions the fetus responds to the fall in SMA resistance during α-adrenergic blockade with an increase in combined ventricular output to prevent hypotension. Conversely, the transient return of FHR to baseline values between 2 and 3 h in this group was associated with an increase in resistance and a fall in SMA blood flow. In marked contrast, there was no increase in FHR in the asphyxia phentolamine group in response to the start of phentolamine, and when FHR was elevated at around 2–3 h, at the same time as SMA vasodilatation, pressure fell abruptly. These data suggest a limited ability to increase combined ventricular output after asphyxia, consistent with myocardial dysfunction, and further highlight the requirement for peripheral vasoconstriction for pressure support.
The other major findings of the present study are that postasphyxial seizures in the pre-term sheep fetus are also associated with mesenteric vasoconstriction, and that the SNS plays a key role in mediating this phenomenon. In the human newborn, postasphyxial seizures generally occur within a few hours of birth (Scher, 1997); however, under some circumstances seizures may also start in utero (Osiovich & Barrington, 1996; Ingemarsson & Spencer, 1998; Westgate et al. 1999; Patane & Ghidini, 2001), and then continue after delivery (Keogh et al. 2000). The present study is the first to report the appearance of stereotypically evolving seizures in the pre-term fetal brain. We have previously reported that such seizures do not develop at 60% of gestation (Bennet et al. 1999) and it is likely that the appearance of seizures at 70% of gestation reflects changes in neuronal development, such as the onset of cortical myelination (Barlow, 1969).
Neonatal seizures in pre-term infants are classically described as being brief and subtle, sometimes composed of unusual behaviours which are difficult recognize and classify and thus typically EEG analysis is required to confirm their presence (Scher et al. 2003). In part, because such EEG monitoring has not been routine during the early recovery period (Hellstrom-Westas et al. 1995; Caravale et al. 2003; Scher et al. 2003) there have been limited studies of the impact of seizures on cerebral circulation in pre-term infants (Perlman & Volpe, 1983; Boylan et al. 2000), and none which have evaluated peripheral blood flow. The present study demonstrates that seizures were brief and relatively infrequent events which caused marked vasoconstriction of the gut. This vasoconstriction was mediated by sympathetic activity, with complete abolition of the reduction in SMA blood flow during each seizure by α-adrenergic blockade. However, while the seizure duration was short it was notable that the impact on the gut was longer lasting, with blood flow to the gut being reduced for longer than the seizure duration itself, with a corresponding elevation in MAP. Speculatively, this prolonged effect may help support perfusion of critical organs, such as the brain, until cerebral metabolism fully recovers after each seizure. Further, our data support a role for the SNS in modulating seizure activity in the pre-term sheep fetus. Postnatally, the SNS is well known to play a role in modulating the threshold for seizures. Endogenous noradrenaline is an anticonvulsant neurotransmitter, and blockade of α- and β-adrenoreceptors potentiates seizures (Weinshenker & Szot, 2002). Consistent with this, in the present study, the mixed α-receptor blocker, phentolamine, was associated with significantly earlier onset of stereotypic evolving seizures but no change in their amplitude, frequency or total duration.
In conclusion, the present study confirms the hypothesis that delayed postasphyxial hypoperfusion of the gut is mediated by α-adrenergic activity. The data highlight the importance of endogenous sympathetic activity in maintaining the initial elevation of blood pressure after asphyxia and are consistent with the hypothesis that the mesenteric system is a key resistance bed that helps to support perfusion in other, more vulnerable systems. Further, this study has shown that seizures develop after asphyxia in the premature fetus at 70% of gestation and that these seizures are associated with a significant impact on blood flow to the fetal gut. Postnatally, perfusion of the mesenteric circulation is tightly linked with gut function. Therefore, although the hypoperfusion seems to be responsive rather than a consequence of local injury, we may predict that it will be associated with gastrointestinal disturbances and delayed adaptation to postnatal life (Coombs et al. 1990, 1992; Malcolm et al. 1991; Akinbi et al. 1994).
Acknowledgments
We acknowledge the support of the Auckland Medical Research Foundation, Lottery Grants Board of New Zealand, The National Institutes of Health, USA (HD32752) and the Health Research Council of New Zealand. J.S.Q. was supported by the Foundation ‘De Drie Lichten’ in the Netherlands.
References
- Akinbi H, Abbasi S, Hilpert PL, Bhutani VK. Gastrointestinal and renal blood flow velocity profile in neonates with birth asphyxia. J Pediatr. 1994;125:625–627. doi: 10.1016/s0022-3476(94)70024-9. [DOI] [PubMed] [Google Scholar]
- Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- Baumgartner C, Lurger S, Leutmezer F. Autonomic symptoms during epileptic seizures. Epileptic Disord. 2001;3:103–116. [PubMed] [Google Scholar]
- Bennet L, Quaedackers JS, Gunn AJ, Rossenrode S, Heineman E. The effect of asphyxia on superior mesenteric artery blood flow in the premature sheep fetus. J Pediatr Surg. 2000;35:34–40. doi: 10.1016/s0022-3468(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Bennet L, Rossenrode S, Gunning MI, Gluckman PD, Gunn AJ. The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion. J Physiol. 1999;517:247–257. doi: 10.1111/j.1469-7793.1999.0247z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000;48:12–17. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Caravale B, Allemand F, Libenson MH. Factors predictive of seizures and neurologic outcome in perinatal depression. Pediatr Neurol. 2003;29:18–25. doi: 10.1016/s0887-8994(03)00046-8. [DOI] [PubMed] [Google Scholar]
- Ceppa EP, Fuh KC, Bulkley GB. Mesenteric hemodynamic response to circulatory shock. Curr Opin Crit Care. 2003;9:127–132. doi: 10.1097/00075198-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Conger JD, Weil JV. Abnormal vascular function following ischemia-reperfusion injury. J Invest Med. 1995;43:431–442. [PubMed] [Google Scholar]
- Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS. Gut blood flow velocities in the newborn: effects of patent ductus arteriosus and parenteral indomethacin. Arch Dis Child. 1990;65:1067–1071. doi: 10.1136/adc.65.10_spec_no.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS. Abnormal gut blood flow velocities in neonates at risk of necrotising enterocolitis. J Pediatr Gastroenterol Nutr. 1992;15:13–19. doi: 10.1097/00005176-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Doba N, Beresford HR, Reis DJ. Changes in regional blood flow and cardiodynamics associated with electrically and chemically induced epilepsy in cat. Brain Res. 1975;90:115–132. doi: 10.1016/0006-8993(75)90686-1. [DOI] [PubMed] [Google Scholar]
- Fan WQ, Smolich JJ, Wild J, Yu VY, Walker AM. Major vasodilator role for nitric oxide in the gastrointestinal circulation of the mid-gestation fetal lamb. Pediatr Res. 1998;44:344–350. doi: 10.1203/00006450-199809000-00013. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99:7699–7704. doi: 10.1073/pnas.112012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Maxwell L, de Haan HH, Bennet L, Williams CE, Gluckman PD, et al. Delayed hypotension and subendocardial injury after repeated umbilical cord occlusion in near-term fetal lambs. Am J Obstet Gynecol. 2000;183:1564–1572. doi: 10.1067/mob.2000.108084. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:F34–F38. doi: 10.1136/fn.72.1.f34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock. 1997;8:95–101. 102–103. doi: 10.1097/00024382-199708000-00004. discussion. [DOI] [PubMed] [Google Scholar]
- Huang J, Kim LJ, Poisik A, Pinsky DJ, Connolly ES., Jr Titration of postischemic cerebral hypoperfusion by variation of ischemic severity in a murine model of stroke. Neurosurgery. 1999;45:328–333. doi: 10.1097/00006123-199908000-00027. [DOI] [PubMed] [Google Scholar]
- Hunt R, Osborn D. Dopamine for prevention of morbidity and mortality in term newborn infants with suspected perinatal asphyxia. Cochrane Database Syst Rev. 2002:CD003484. doi: 10.1002/14651858.CD003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingemarsson I, Spencer JA. Fetal seizure activity associated with lethal cerebral damage at birth: two cases. Acta Obstet Gynecol Scand. 1998;77:127–129. doi: 10.1080/00016349808565828. [DOI] [PubMed] [Google Scholar]
- Iwamoto HS, Rudolph AM, Mirkin BL, Keil LC. Circulatory and humoral responses of sympathectomized fetal sheep to hypoxemia. Am J Physiol. 1983;245:H767–H772. doi: 10.1152/ajpheart.1983.245.5.H767. [DOI] [PubMed] [Google Scholar]
- Jensen A, Hohmann M, Kunzel W. Dynamic changes in organ blood flow and oxygen consumption during acute asphyxia in fetal sheep. J Dev Physiol. 1987a;9:543–559. [PubMed] [Google Scholar]
- Jensen A, Kunzel W, Kastendieck E. Fetal sympathetic activity, transcutaneous PO2, and skin blood flow during repeated asphyxia in sheep. J Dev Physiol. 1987b;9:337–346. [PubMed] [Google Scholar]
- Jensen A, Lang U. Foetal circulatory responses to arrest of uterine blood flow in sheep: effects of chemical sympathectomy. J Dev Physiol. 1992;17:75–86. [PubMed] [Google Scholar]
- Karimova A, Pinsky DJ. The endothelial response to oxygen deprivation: biology and clinical implications. Intensive Care Med. 2001;27:19–31. doi: 10.1007/s001340000790. [DOI] [PubMed] [Google Scholar]
- Karlsson BR, Grogaard B, Gerdin B, Steen PA. The severity of postischemic hypoperfusion increases with duration of cerebral ischemia in rats. Acta Anaesthesiol Scand. 1994;38:248–253. doi: 10.1111/j.1399-6576.1994.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Keogh JM, Badawi N, Kurinczuk JJ, Dixon G, Jongeling B, Stanley FJ. Maternal awareness of fetal seizures in pregnancies resulting in newborn encephalopathy. Acta Obstet Gynecol Scand. 2000;79:787–789. [PubMed] [Google Scholar]
- Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol. 2004;286:H296–H303. doi: 10.1152/ajpheart.00668.2003. [DOI] [PubMed] [Google Scholar]
- Kreisman NR, Gauthier-Lewis ML, Conklin SG, Voss NF, Barbee RW. Cardiac output and regional hemodynamics during recurrent seizures in rats. Brain Res. 1993;626:295–302. doi: 10.1016/0006-8993(93)90590-j. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Gunn AJ, Zhang DY, Wu JJ, Maxwell L, Bennet L. Nonimmune hydrops fetalis and activation of the renin-angiotensin system after asphyxia in preterm fetal sheep. Am J Physiol. 2001;280:R1045–R1051. doi: 10.1152/ajpregu.2001.280.4.R1045. [DOI] [PubMed] [Google Scholar]
- Malcolm G, Ellwood D, Devonald K, Beilby R, Henderson-Smart D. Absent or reversed end diastolic flow velocity in the umbilical artery and necrotising enterocolitis. Arch Dis Child. 1991;66:805–807. doi: 10.1136/adc.66.7_spec_no.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michenfelder JD, Milde JH. Postischemic canine cerebral blood flow appears to be determined by cerebral metabolic needs. J Cereb Blood Flow Metab. 1990;10:71–76. doi: 10.1038/jcbfm.1990.9. [DOI] [PubMed] [Google Scholar]
- Mulder AL, Miedema A, De Mey JG, Giussani DA, Blanco CE. Sympathetic control of the cardiovascular response to acute hypoxemia in the chick embryo. Am J Physiol. 2002;282:R1156–R1163. doi: 10.1152/ajpregu.00634.2001. [DOI] [PubMed] [Google Scholar]
- Nuwayhid B, Brinkman CR, Su C, Bevan JA, Assali NS. Development of autonomic control of fetal circulation. Am J Physiol. 1975;228:337–344. doi: 10.1152/ajplegacy.1975.228.2.337. [DOI] [PubMed] [Google Scholar]
- Osiovich H, Barrington K. Prenatal ultrasound diagnosis of seizures. Am J Perinatol. 1996;13:499–501. doi: 10.1055/s-2007-994436. [DOI] [PubMed] [Google Scholar]
- Patane L, Ghidini A. Fetal seizures: case report and literature review. J Matern Fetal Med. 2001;10:287–289. doi: 10.1080/714052746. [DOI] [PubMed] [Google Scholar]
- Paulick RP, Meyers RL, Rudolph CD, Rudolph AM. Hemodynamic responses to alpha-adrenergic blockade during hypoxemia in the fetal lamb. J Dev Physiol. 1991;16:63–69. [PubMed] [Google Scholar]
- Perlman JM, Volpe JJ. Seizures in the preterm infant: effects on cerebral blood flow velocity, intracranial pressure, and arterial blood pressure. J Pediatr. 1983;102:288–293. doi: 10.1016/s0022-3476(83)80545-9. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Read JB, Burnstock G. Developement of the adrenergic innervation and chromaffin cells in the human fetal gut. Dev Biol. 1970;22:513–534. doi: 10.1016/0012-1606(70)90166-1. [DOI] [PubMed] [Google Scholar]
- Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol. 2002;29:23–39. doi: 10.1016/s0095-5108(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- Robel-Tillig E, Vogtmann C, Bennek J. Prenatal hemodynamic disturbances — pathophysiological background of intestinal motility disturbances in small for gestational age infants. Eur J Pediatr Surg. 2002;12:175–179. doi: 10.1055/s-2002-32723. [DOI] [PubMed] [Google Scholar]
- Rouwet EV, De Mey JG, Slaaf DW, Heineman E, Ramsay G, Le Noble FA. Development of vasomotor responses in fetal mesenteric arteries. Am J Physiol. 2000;279:H1097–H1105. doi: 10.1152/ajpheart.2000.279.3.H1097. [DOI] [PubMed] [Google Scholar]
- Sanhueza EM, Johansen-Bibby AA, Fletcher AJ, Riquelme RA, Daniels AJ, Seron-Ferre M, et al. The role of neuropeptide Y in the ovine fetal cardiovascular response to reduced oxygenation. J Physiol. 2003;546:891–901. doi: 10.1113/jphysiol.2002.034488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher MS. Seizures in the newborn infant. Diagnosis, treatment, and outcome. Clin Perinatol. 1997;24:735–772. [PubMed] [Google Scholar]
- Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG — clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–280. doi: 10.1016/s0887-8994(02)00621-5. [DOI] [PubMed] [Google Scholar]
- Scher MS, Hamid MY, Steppe DA, Beggarly ME, Painter MJ. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia. 1993;34:284–288. doi: 10.1111/j.1528-1157.1993.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Strodtbeck F. The role of early enteral nutrition in protecting premature infants from sepsis. Crit Care Nurs Clin North Am. 2003;15:79–87. doi: 10.1016/s0899-5885(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care. 2002;8:242–250. doi: 10.1097/00075198-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Szot P. The role of catecholamines in seizure susceptibility: new results using genetically engineered mice. Pharmacol Ther. 2002;94:213–233. doi: 10.1016/s0163-7258(02)00218-8. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Bennet L, Gunn AJ. Fetal seizures causing increased heart rate variability during terminal fetal hypoxia. Am J Obstet Gynecol. 1999;181:765–766. doi: 10.1016/s0002-9378(99)70532-6. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Bennet L, Gunn AJ. Meconium and fetal hypoxia: some experimental observations and clinical relevance. Br J Obstet Gynaecol. 2002;109:1171–1174. doi: 10.1111/j.1471-0528.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- Williams CE, Gunn AJ, Mallard C, Gluckman PD. Outcome after ischemia in the developing sheep brain: an electroencephalographic and histological study. Ann Neurol. 1992;31:14–21. doi: 10.1002/ana.410310104. [DOI] [PubMed] [Google Scholar]
- Yaffe H, Parer JT, Block BS, Llanos AJ. Cardiorespiratory responses to graded reductions of uterine blood flow in the sheep fetus. J Dev Physiol. 1987;9:325–336. [PubMed] [Google Scholar]