Abstract
The content of the cyanogenic glucoside dhurrin in sorghum (Sorghum bicolor L. Moench) varies depending on plant age and growth conditions. The cyanide potential is highest shortly after onset of germination. At this stage, nitrogen application has no effect on dhurrin content, whereas in older plants, nitrogen application induces an increase. At all stages, the content of dhurrin correlates well with the activity of the two biosynthetic enzymes, CYP79A1 and CYP71E1, and with the protein and mRNA level for the two enzymes. During development, the activity of CYP79A1 is lower than the activity of CYP71E1, suggesting that CYP79A1 catalyzes the rate-limiting step in dhurrin synthesis as has previously been shown using etiolated seedlings. The site of dhurrin synthesis shifts from leaves to stem during plant development. In combination, the results demonstrate that dhurrin content in sorghum is largely determined by transcriptional regulation of the biosynthetic enzymes CYP79A1 and CYP71E1.
More than 2,650 plant species from more than 550 genera and 130 families are known to be cyanogenic, and this phenomenon is typically based on the presence of cyanogenic glucosides (Hegnauer, 1986; Seigler, 1991). The biological function of cyanogenic glucosides has been difficult to assess (Jones, 1998; Selmar, 1999; Jones et al., 2000). Cyanogenic glucosides are constitutively produced in healthy plant tissues and belong to the class of natural products referred to as “phytoanticipins” (Osbourn, 1996). Mechanical disruption of plant tissue containing cyanogenic glucosides results in their degradation by the sequential action of β-glucosidases and α-hydroxynitrilases (Cicek and Esen, 1998; Lechtenberg and Nahrstedt, 1999; Selmar, 1999; Jones et al., 2000) and release of hydrogen cyanide. The toxicity of hydrogen cyanide renders it obvious to assume that cyanogenic glucosides repel herbivores (Jones, 1998). However, many trials do not support this hypothesis (Hruska, 1988) and effectiveness may be strongly influenced by the feeding strategy of the animals (Compton and Jones, 1985). With regard to the interaction between plants and microorganisms, the release of hydrogen cyanide from cyanogenic glucosides may be more damaging to the plant than to the microorganism because of inhibition of phytoalexin production (Lieberei et al., 1989). In accordance, highly cyanogenic plants are preferred by some fungi and insects compared with plants with lower cyanogenic potential (Nahrstedt, 1996; Møller and Seigler, 1999). Aglycones released from cyanogenic glucosides formed from Phe or Tyr may give rise to the formation of compounds with antifungal activities (Siebert et al., 1996). Other possible roles of cyanogenic glucosides are as nitrogen storage compounds (Clegg et al., 1979; Selmar et al., 1988; Forslund and Jonsson, 1997) or as osmoprotectants. The latter functions are likely to require the presence of high amounts of cyanogenic glucosides in a particular cell type or tissue. This is often the case as in sorghum (Sorghum bicolor), where the cyanogenic glucoside content in the tip of young seedlings reaches 6% of the dry weight (Akazawa et al., 1960; Halkier and Møller, 1989). When cyanogenic glucosides are present in high amounts in the edible parts of crop plants, the cyanogenic potential constitutes a health risk for humans and domestic animals (Maxwell, 1903; Nelson, 1953; Olūwole et al., 2000). To increase food and feed safety, it is of interest to be able to lower the content of cyanogenic compounds in these plants through classical breeding or molecular genetics. It is also of great interest to know the effect of growth conditions on cyanogenic glucoside accumulation to avoid incidences of cyanide intoxication due to occasionally unexpected high concentrations that cannot be handled using normal precautionary measures.
Sorghum synthesizes the cyanogenic glucoside dhurrin from Tyr. Dhurrin accumulation is highest in seedlings (Akazawa et al., 1960; Halkier and Møller, 1989), which because of their cyanide potential can be lethal to domestic animals (Boyd et al., 1938). The cyanide content in both seedlings and older plants depends highly on growth conditions and genetic background (Boyd et al., 1938; Nelson, 1953; Gillingham et al., 1969; Gorz et al., 1987). Furthermore, there is a significant turnover of dhurrin even in seedlings (Bough and Gander, 1971; Adewusi, 1990), suggesting that the dhurrin content could be regulated both by changes in synthesis and in breakdown. The in vivo mechanisms for turnover of cyanogenic glucosides remain elusive and do not necessarily involve degradation by the action of β-glucosidases and α-hydroxynitrilases as seen after disruption of the subcellular structure (Jones et al., 2000).
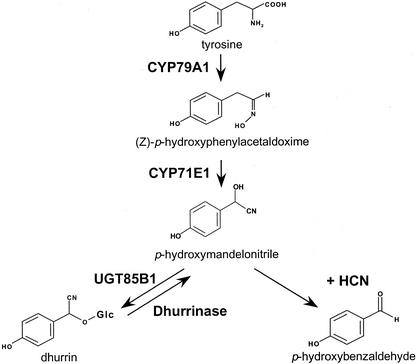
To understand the regulation of dhurrin content in sorghum, we have chosen to study the biosynthetic enzymes (Fig. 1). The two first enzymes in the pathway for dhurrin synthesis, CYP79A1 and CYP71E1, are membrane-bound P450 enzymes that catalyze the conversion of Tyr to p-hydroxymandelonitrile, the aglycone of dhurrin (Sibbesen et al., 1994; Koch et al., 1995; Kahn et al., 1997; Bak et al., 1998a). CYP79A1 catalyzes the conversion of Tyr to Z-p-hydroxyphenylacetaldehyde oxime, which was previously shown to be the rate-limiting step in dhurrin synthesis in etiolated sorghum seedlings (Møller and Conn, 1979; Sibbesen et al., 1995). Because this is the first committed step in dhurrin synthesis, it is thought to be a key regulatory point. Not surprisingly, CYP79A1 exhibits high substrate specificity (Kahn et al., 1999). CYP71E1 catalyzes the subsequent conversion of Z-p-hydroxyphenylacetaldehyde oxime to p-hydroxymandelonitrile. This enzyme has lower substrate specificity (Kahn et al., 1999). Finally, a soluble glycosyltransferase UDP-Glc p-hydroxymandelonitrile glycosyltransferase UGT85B1 (previously pHMNGT) converts p-hydroxymandelonitrile into dhurrin (Jones et al., 1999). Studies with microsomal membranes (conversion of Tyr to p-hydroxymandelonitrile) have indicated that the biosynthetic activity increases the first 2 d after germination and that the activity of the enzymes involved and the dhurrin produced are found in the upper part of the shoot (Halkier and Møller, 1989).
Figure 1.
The biosynthetic pathway for the cyanogenic glucoside dhurrin in sorghum.
In the present study, we demonstrate a close relationship between dhurrin content and CYP79A1 and CYP71E1 activity in young light-grown sorghum seedlings. Application of nitrogen fertilizer to older sorghum plants increases the activity of CYP79A1 and CYP71E1 and is accompanied by an increase in dhurrin content. At the experimental conditions tested, the activity of both enzymes correlates with their mRNA levels, demonstrating that transcriptional regulation of the biosynthetic enzymes is a major determinant of dhurrin synthesis.
RESULTS
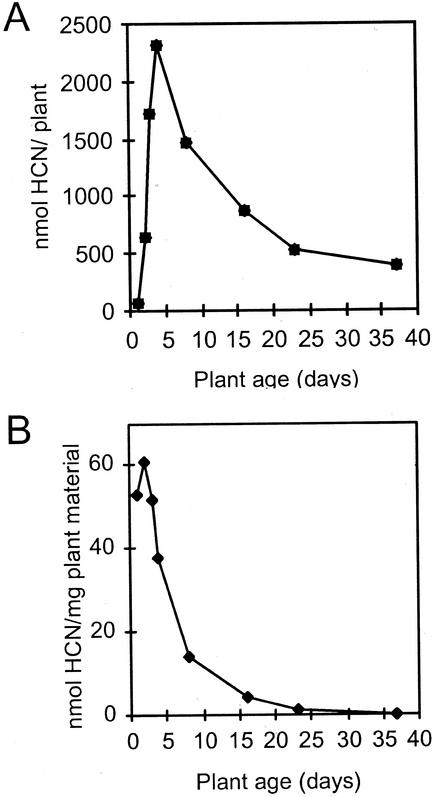
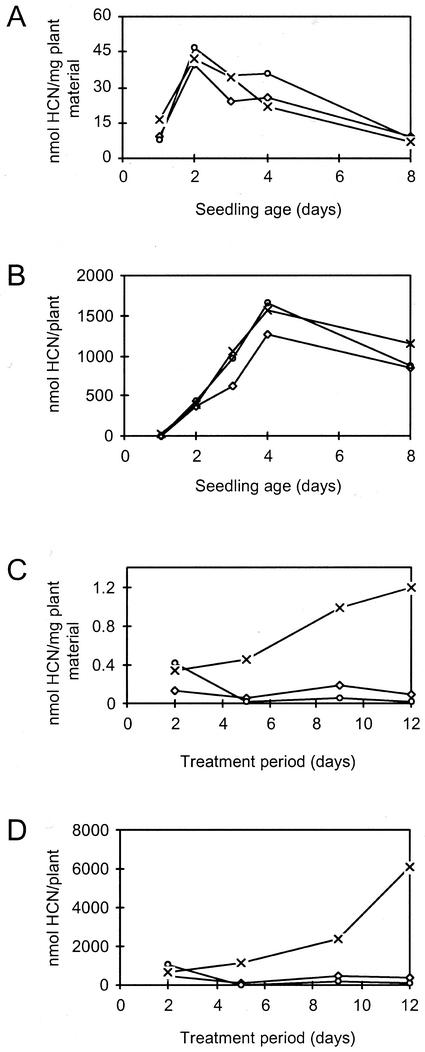
The cyanide potential of sorghum increases rapidly during germination and early seedling formation, after which it declines with plant age (Fig. 2). The cyanide potential per plant peaks 4 d after germination (Fig. 2A). At this time point, the rate of dhurrin synthesis and breakdown are equal. When measured per milligram of fresh plant material, the cyanide potential peaks already 2 d after germination (Fig. 2B).
Figure 2.
The effect of seedling age on cyanide potential in sorghum. The cyanide potential is shown per plant (A) and per milligram of plant material (B; fresh weight). Seeds were imbibed in water for 3 h, and seedlings were grown for the time periods indicated. Cyanide potential in the whole plant except roots was measured as described in “Materials and Methods.”
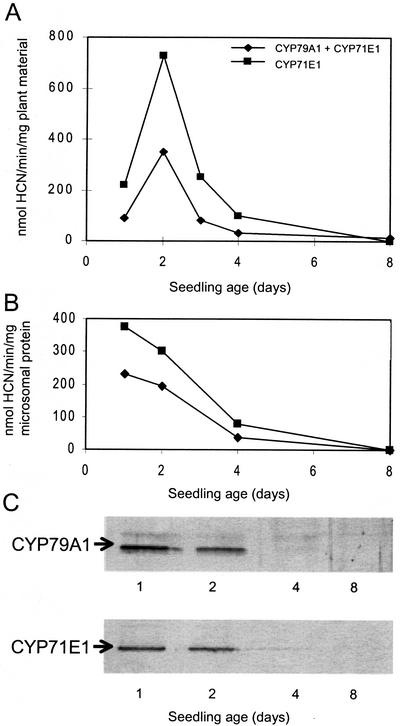
To determine whether the enzyme activities associated with dhurrin synthesis are major determinants of cyanide potential, microsomes were isolated from sorghum seedlings at different developmental stages, and their biosynthetic activity was assessed by administration of precursors. The microsomes convert Tyr into p-hydroxymandelonitrile, which dissociates into p-hydroxybenzaldehyde and hydrogen cyanide (Fig. 1; Halkier and Møller, 1989). Cyanide production from Tyr reflects the combined catalytic activity of the multifunctional cytochromes P450, CYP79A1 and CYP71E1. The activity of CYP71E1 was measured separately by administration of its substrate p-hydroxyphenylacetaldoxime to microsomes (Kahn et al., 1997). In all cases tested, the activity of CYP71E1 was higher than the combined activity of the enzymes (Fig. 3A). The biosynthetic pathway is highly channeled with no accumulation of the aldoxime (Møller and Conn, 1980). Therefore, cyanide production from Tyr reflects the activity of CYP79A1. The activity of both enzymes as measured per milligram of plant material increased until d 2 after germination, after which it declined (Fig. 3A). Thus, the enzyme activities of CYP79A1 and CYP71E1 both peak the same day as the cyanide potential per milligram of plant material (Fig. 2B). CYP79A1 and CYP71E1 are membrane-bound proteins. In accordance, their activity as a function of seedling age is also presented per milligram of microsomal protein (Fig. 3B). The content of membrane protein per milligram of fresh plant material is lowest immediately after germination, and this shifts the enzyme activity maximum to 1 d after germination when calculated based on the same amount of microsomal protein.
Figure 3.
The effect of seedling age on activity and amount of CYP79A1 and CYP71E1 in sorghum microsomes. A, Enzyme activity per milligram of fresh plant material; B, enzyme activity per milligram of microsomal protein; and C, western-blot analysis of the content of CYP79A1 and CYP71E1 with equal amounts of microsomal protein applied to each lane. Microsomes were prepared from whole seedlings except roots. The combined activity of CYP79A1 and CYP71E1 was measured with Tyr as substrate, and the activity of CYP71E1 was measured with (Z)-p-hydroxyphenylacetaldoxime as substrate. The cyanohydrin formed as the final product of the enzymatic reactions was hydrolyzed in alkali and cyanide release measured as described in “Materials and Methods.”
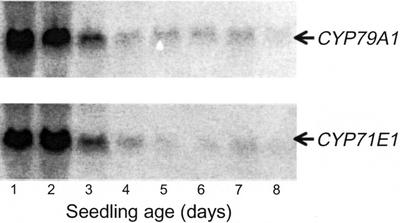
The protein level of CYP79A1 and CYP71E1 per milligram of microsomal protein as a function of seedling growth was investigated using western blotting (Fig. 3C). The protein level of CYP79A1 and of CYP71E1 correlates well with the corresponding enzyme activities (Fig. 3B). Thus, no obvious evidence for regulation of enzymatic activity by post-translational modification or by the presence of inhibitors is evident. CYP79A1 and CYP71E1 mRNA levels during seedling growth were measured by northern blotting with equal amounts of total RNA applied to each lane (Fig. 4). Their expression levels showed the same overall pattern and matched the pattern of the corresponding enzyme activities and protein levels (Fig. 3). The timing of the mRNA levels points to transcriptional regulation as a major determinant of enzyme activity and suggests that dhurrin synthesis in sorghum seedlings is regulated by the rate of de novo synthesis of the biosynthetic enzymes.
Figure 4.
The effect of seedling age on mRNAs encoding CYP79A1 and CYP71E1 in sorghum. Total RNA was isolated and analyzed by northern blotting. Equal amounts of RNA were applied to all lanes as measured by ethidium bromide staining. The blots were hybridized with the CYP79A1 and the CYP71E1 probes as indicated.
The effect of mineral salts on dhurrin synthesis in sorghum was studied using sorghum plants at different developmental stages (Fig. 5). In seedlings up to 8 d of age, growth in the presence of different mineral salts did not cause an increase in cyanide potential when calculated per milligram of fresh plant material (Fig. 5A) or when calculated per plant (Fig. 5B). The CYP79A1 and CYP71E1 genes may already be transcribed at maximal rate in seedlings, thereby preventing further induction. As an alternative, limitations in the pool of free Tyr or other effectors may prevent increased synthesis. In contrast to seedlings, application of nitrogen fertilizer in the form of potassium nitrate to 5-week-old (60-cm-tall) sorghum plants resulted in a pronounced increase in cyanide potential (Fig. 5, C and D). The cyanide potential of the whole plant was increased by a factor of 7. The increase in cyanide potential was not a simple salt stress response, because neither potassium chloride (Fig. 5) nor potassium phosphate (data not shown) exerted any measurable changes in cyanide potential in comparison with untreated plants. The salt concentration necessary to induce the cyanide potential is difficult to assess in soils. Therefore, the experiment was repeated using plants that were grown as above but removed from the soil, and their roots gently were washed before placing them in water, 25 mm potassium chloride, or 25 mm potassium nitrate. This experiment resulted in the same increase in cyanide potential upon nitrate administration as observed with plants grown in soil (data not shown). The increase in cyanide potential was the same when the plants were placed in 10 to 50 mm nitrate, showing that full induction of dhurrin synthesis is achieved already at 10 mm nitrate (data not shown). Despite the observed increase in cyanide potential in 5-week-old plants upon nitrate administration, the cyanide potential per milligram of plant material only reached about 2% to 3% of the maximum concentration in seedlings (Figs. 2A and 5).
Figure 5.
The effect of nitrate on cyanide potential in sorghum plants at different developmental stages. A and B, Seeds were imbibed in water for 3 h, and seedlings were grown for the time periods indicated in water (⋄—⋄), 25 mm KCl (○—○), or 25 mm KNO3 (×—×). C and D, Plants were grown for 35 d in soil. Watering was then continued with H2O (⋄—⋄) or changed to 25 mm KCl (○—○) or 25 mm KNO3 (×—×) for the time period indicated. The cyanide potential in the whole plant except roots was measured as described in “Materials and Methods.”
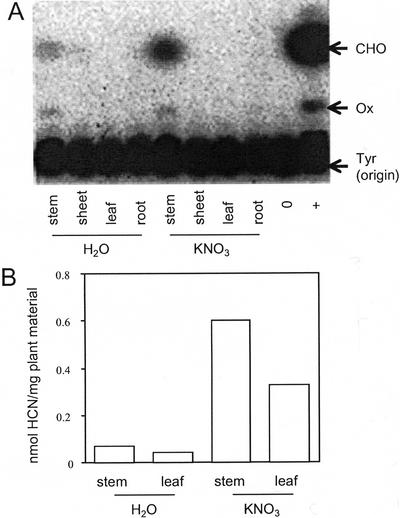
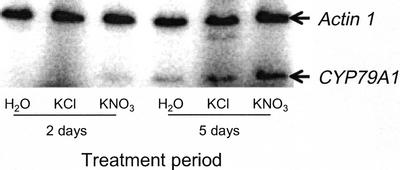
Not surprisingly, the activity of CYP79A1 and CYP71E1 is lower in 5-week-old plants than in seedlings. The colorimetric assay for determination of cyanide was not sensitive enough to detect biosynthetic activity in microsomes isolated from these older plants. Instead, the biosynthetic activity was measured by administration of [UL-C14]-labeled Tyr to the microsomes and by detection of the intermediates formed by radio thin-layer chromatography (TLC). Using this more sensitive method, CYP79A1 and CYP71E1 activity was found in microsomes isolated from the stem of the plants but not in microsomes isolated from leaves, leaf sheets, or roots (Fig. 6A). In 5-week-old plants, the cyanide potential per milligram of fresh plant material is 0.08 nmol in stems, whereas it is 0.05 nmol in leaves (Fig. 6B). Overall, about one-third of the cyanide potential of the plant resides in the leaves. The presence of biosynthetic activity in the stem indicates that dhurrin may be transported from stem to leaves. Upon administration of nitrate to the plants, the enzymatic conversion of Tyr into p-hydroxymandelonitrile by the microsomal system isolated from stems was strongly increased (Fig. 6A), suggesting that the increase in dhurrin content is the result of increased biosynthetic capacity. This was clearly reflected by increases in the cyanide potential of stems and leaves to 0.6 and 0.4 nmol mg−1 plant material, respectively. To investigate whether the increase in biosynthetic activity was achieved by up-regulation of the mRNA level of the biosynthetic enzymes, the amount of CYP79A1 mRNA was measured by quantitative reverse transcription PCR. In each PCR reaction, the actin gene was also amplified as an internal control. In control experiments, the relative amplification of the actin and CYP79A1 cDNA was found to be constant over two decades of cDNA concentrations. Both primer sets were placed over introns to amplify the cDNA without interference from contaminating genomic DNA in the RNA preparation. The amount of CYP79A1 mRNA increased in response to nitrate (Fig. 7), suggesting that also during nitrate induction, the synthesis of dhurrin is regulated at the transcriptional level and, thereby, linked to de novo synthesis of the biosynthetic enzymes.
Figure 6.
The effect of nitrate on induction of CYP79A1 and CYP71E1 catalytic activity and cyanide potential in different parts of 5-week-old sorghum plants. A, Microsomes were prepared from different plant parts, and their ability to convert 14C-labeled l-Tyr to p-hydroxymandelonitrile was analyzed by phosphor image-TLC of enzymatic reaction mixtures as described in “Materials and Methods.” ○, No microsomes added; +, positive control using microsomes prepared from 2-d-old sorghum seedlings; CHO, p-hydroxybenzaldehyde (degradation product of p-hydroxymandelonitrile); OX, Z-p-hydroxyphenylacetaldehyde oxime; and TYR, l-Tyr (which stays at the origin of the TLC plate). B, The cyanide potential of leafs and stems measured after growth in the absence and presence of KNO3 as described in “Materials and Methods.” In all experiments shown, the induction period used was 12 d.
Figure 7.
Nitrate induction of CYP79A1 mRNA in 35-d-old plants. The plants were grown for 35 d in soil where after they were watered with 25 mm KNO3 or 25 mm KCl for 2 or 5 d. Total RNA was extracted from leaf and stems and used for reverse transcription PCR with CYP79A1 and ac1 actin-specific primers. The primers were placed over introns to exclude amplification from contaminating genomic DNA. Equal amounts of each cDNA was used for PCR.
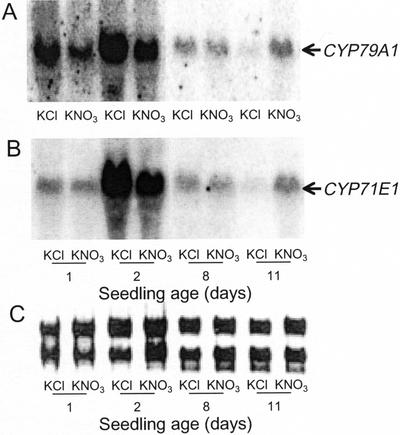
Germination of sorghum seeds in a nitrate-containing medium did not result in an increase in the cyanide potential of 8-d-old seedlings (Fig. 5). In contrast, treatment of 8-d-old seedlings with nitrate for 1 week did result in an increased dhurrin content in 15-d-old plants (data not shown). To elucidate at which point during seedling development CYP79A1 and CYP71E1 synthesis become responsive to nitrate, sorghum seedlings at different ages were treated with nitrate for 24 h, followed by measurements of the level of CYP79A1 and CYP71E1 mRNA. As previously shown (Fig. 4), the mRNA level was strongly induced during early germination, but no further up-regulation by nitrate was observed (d 1 and 2). In seedlings more than 8 d old, nitrate treatment resulted in a marked increase in the mRNA levels of CYP79A1 as well as CYP71E1 (Fig. 8).
Figure 8.
The effect of nitrate on CYP79A1 and CYP71E1 mRNA levels in sorghum seedlings of different ages. Seeds were imbibed and germinated in water for the time periods indicated and then incubated in 25 mm KCl or 25 mm KNO3 for 24 h. Total RNA was isolated and analyzed by northern blotting. A, Hybridization with a probe for CYP79A1. B, Hybridization with a probe for CYP71E1. C, Ethidium bromide staining of the RNA (only ribosomal RNA is visible) to confirm that the same amount of RNA was applied to each lane.
DISCUSSION
The content of the cyanogenic glucoside dhurrin in light-grown sorghum seedlings shows a transient increase during germination (Figs. 2 and 3). When measured per plant, the cyanide potential peaks at d 4 after germination. When measured per milligram of fresh plant material and per milligram of microsomal protein, the cyanide potential peaks at d 2 and 1, respectively. A transient increase has previously been reported by Akazawa et al. (1960). In the present study, we have investigated the two multifunctional cytochrome P450 enzymes that convert Tyr to p-hydroxymandelonitrile, the aglycone of dhurrin. We demonstrate that the transient increase in cyanide potential correlates with the activity of the biosynthetic enzymes (Fig. 3). In older sorghum plants, the enzymes are induced by nitrate application that also results in an increased dhurrin level (Figs. 5 and 6). These results point to the biosynthetic enzymes as the major determinant of cyanide potential in sorghum compared with the rate of cyanogenic glucoside degradation.
A closer analysis shows that, although the activity of both CYP79A1 and CYP71E1 changes during germination (Fig. 3), the activity of the first enzyme in the pathway is always rate limiting. This is an efficient way of avoiding accumulation of toxic intermediates in dhurrin synthesis (Fig. 6). It requires a tight coregulation of the two enzyme activities and is further secured by metabolic channeling (Møller and Conn, 1980). In agreement with this, only minute amounts of the intermediates are found when radiolabeled Tyr is administered to sorghum seedlings (Conn, 1973). The UDP-Glc:p-hydroxymandelonitrile-O-glucosyltransferase (UGT 85B1) that catalyzes the final glucosylation step has been cloned and shown to have higher activity than the two P450 enzymes (Jones et al., 1999). The routinely observed lack of accumulation of p-hydroxymandelonitrile in vivo supports the notion that this enzyme is always present in excess (Conn, 1973). In vivo, the glucosyl transferase may attach to the membrane-bound cytochrome P450s to create a metabolon as observed in flavonoid synthesis (Burbulis and Winkel-Shirley, 1999). The entire pathway for dhurrin synthesis has recently been transferred from sorghum to Arabidopsis using genetic engineering (Tattersall et al., 2001). Dhurrin synthesis in the transgenic Arabidopsis plants also showed strong metabolic channeling.
During sorghum seedling development (Fig. 3) and upon the application of nitrate (Fig. 5), the measured enzyme activities of CYP79A1 and CYP71E1 were found to correlate well with the corresponding mRNA levels (Figs. 4 and 8), indicating that their activity in planta is controlled at the transcriptional level and most likely by common transcriptional control mechanisms. Precedences for such type of regulation in the biosynthesis of aromatic compounds are the prechorismate pathway, where the mRNA levels of all but the first enzyme (3-deoxy-d-arabino-heptulosonate 7-phosphate synthase) appear to be regulated in concert (Görlach et al., 1994; Herrmann, 1995). In carrot (Daucus carota), the enzyme activities necessary for anthocyanin synthesis are all up-regulated when anthocyanin synthesis is induced, but it is not known whether this reflects regulation at the transcriptional level (Glassgen et al., 1998). It would be interesting to determine in more detail how the regulation of the dhurrin synthesizing enzymes at the transcriptional level is achieved. An obvious target for regulation at the enzyme level would be feedback inhibition of the first committed and also rate-limiting enzyme CYP79A1 by its product p-hydroxyphenylacetaldehyde oxime. Feedback inhibition of CYP79A1 is not observed with the sorghum in vitro system (Møller and Conn, 1979) but is observed with the corresponding enzymes CYP79E1 and CYP79E2 in seaside arrow grass (Triglochin maritima; Cutler et al., 1981; Nielsen and Møller, 1999). The CYP79E1 and CYP79E2 enzymes responsible for Tyr metabolism in seaside arrow grass show 49% sequence identity to sorghum CYP79A1 (Nielsen and Møller, 2000).
Similar to cyanogenic glucoside biosynthesis, the membrane-bound steps in synthesis of glucosinolates are catalyzed by two cytochrome P450 enzymes (Halkier and Du, 1997; Bak et al., 1998b, 2001; Hansen et al., 2001a). In Arabidopsis, cytochrome P450 enzymes belonging to different CYP79 subfamilies (http://www.biobase.dk/P450) have been shown to catalyze the conversion of different parent amino acids including chain-elongated forms into the corresponding oximes (Mikkelsen et al., 2000; Wittstock and Halkier, 2000; Hansen et al., 2001b). The second cytochrome P450 involved in glucosinolate synthesis is thought to convert the oxime to a nitrile oxide that is nonenzymatically converted into an alkyl thiohydroximate. For the Trp-derived indol glucosinolates, CYP83B1 has recently been identified as the enzyme catalyzing this process in Arabidopsis (Bak and Feyereisen, 2001; Bak et al., 2001; Hansen et al., 2001a). The flux of indole-3-acetaldoxime through CYP83B1 apparently serves to regulate the in vivo level of the indole-3-acetaldoxime-derived plant hormone indole-3-acetic acid (Bak and Feyereisen, 2001; Feyereisen et al., 2001). Transgenic Arabidopsis plants expressing sorghum CYP79A1 efficiently convert Tyr into p-hydroxyphenylacetaldehyde oxime, which is then converted to p-hydroxybenzyl glucosinolate with a resulting 3- to 4-fold increase in the total glucosinolate content of the plant (Bak et al., 1999).
p-Hydroxybenzylglucosinolate is not produced in wild-type Arabidopsis plants, and the production of high amounts of this new glucosinolate is not followed by a concomitant reduction in the level of endogenous glucosinolates. This indicates that glucosinolates do not exert feedback inhibition on the CYP79 enzymes. The most likely explanation for the dramatic increase in glucosinolate content in the transgenic Arabidopsis plants expressing CYP79A1 is that the enzymes converting p-hydroxyphenylacetaldehyde oxime to the glucosinolate show broad substrate specificity and are present in a large excess. As an alternative, the overexpression of CYP79A1 leads to an up-regulation of the subsequent enzymes. In contrast to the demonstrated coupling between Trp-derived glucosinolates and indole-3-acetic acid, no evidence is currently available to suggest that the enzymes involved in cyanogenic glucoside production serve to regulate the synthesis of other important compounds, which would require an even more complex overall fine tuning of their activity.
It has long been known that the dhurrin content of sorghum depends on the amount of nitrate available (Maxwell, 1903; Pinckney, 1924; Boyd et al., 1938; Nelson, 1953; Gillingham et al., 1969). Here, we show that the biosynthetic enzymes for dhurrin are induced by nitrate (Fig. 5) and are important determinants of the elevated dhurrin level in the plants. The CYP79A1 and the CYP71E1 genes were not nitrate-inducible until d 8 after germination (Fig. 8), indicating age-dependent regulation of the genes. The sorghum caryopsis contains only low amounts of dhurrin (Erb et al., 1981). In accordance, the rate of dhurrin synthesis during early seedling development is very high. It is likely that the genes are already transcribed at such a high rate during the first days after germination that no further induction is feasible. However, nitrate-induction may also depend on an age-specific factor. The latter possibility is supported by the finding that the site of dhurrin synthesis changes with age. In sorghum seedlings, dhurrin is synthesized in the shoot tip (Halkier and Møller, 1989), whereas the present study has shown that synthesis in 5-week-old plants take place in the stem (Fig. 6). The induction of dhurrin synthesis by nitrate raises the question of whether dhurrin serves as a nitrate sink. Ample turnover of dhurrin with breakdown rates reaching up to 34% of the synthesis rate has been reported in sorghum seedlings (Bough and Gander, 1971; Adewusi, 1990). In the present study, we found that in the period from a few days after seed germination to 5-week-old plants (Figs. 2 and 3), dhurrin catabolism exceeds de novo synthesis. Degradation products of dhurrin may serve as precursors for Asn (Jones, 1979; Piotrowski et al., 2001) or ubiquinone synthesis (Møller and Conn, 1979).
There is a great agronomical interest in lowering the dhurrin content of sorghum to avoid cyanide poisoning of domestic animals feeding on sorghum and to increase fungal resistance. It was previously shown that at least five genes condition dhurrin content of sorghum (Gorz et al., 1987). Our findings point to the CYP79A1 gene as an important target for manipulation of dhurrin synthesis. This could be done by using CYP79A1 as a molecular marker in traditional breeding for selecting sorghum with lower dhurrin synthesis. This would imply finding sorghum plants with lower expression of CYP79A1 or with a mutation that renders the gene inactive. Another possibility is to reduce dhurrin synthesis in transgenic plants (Casas et al., 1993; Zhao et al., 2000) using RNA interference technology (Smith et al., 2000) with CYP79A1 as target. A new interesting way to inactivate CYP79A1 is by chimeratherapy (Zhu et al., 1999). If desired, the nitrate-responsive promoter element(s) of CYP79A1 may be identified and eliminated, to obliterate fluctuations in cyanide potential as a result of fertilizer application. In any case, a lower activity of CYP79A1 would lead to a lower cyanogenic potential of sorghum because this enzyme catalyzes the rate-limiting step in the synthesis of dhurrin. In addition, there would be no accumulation of the toxic intermediates because CYP79A1 is the first enzyme in the committed pathway for dhurrin synthesis.
MATERIALS AND METHODS
Plant Material
Seeds of sorghum (Sorghum bicolor L. Moench cv Sordan 1000) were imbibed in water (3 h, room temperature) and sown in vermiculite or soil. Seedlings and plants were grown in the light.
One-day-old seedlings to be used to study the effect of application of nitrogen fertilizer were grown in vermiculite supplied with 25 mm KNO3 for the time indicated. Seedlings for control experiments were grown in vermiculite supplied with either 25 mm KC1 or water. To study the effect of application of nitrogen fertilizer on 5-week-old plants, seeds were sown in pots containing 600 mL of soil (Weibulls enhetsjord K, Hammenhög, Sweden). Water was applied as required for optimal plant growth. After 5 weeks, the plants were watered with 200 mL of 25 mm KNO3 every 2nd d. Control plants were watered with 200 mL of 25 mm KC1 or water. Harvested plant material was weighed, homogenized in liquid N2, and stored at −80°C.
Determination of Dhurrin and Cyanide Content
To extract dhurrin, the plant material was boiled (5–10 min) in 90% (v/v) MeOH (approximately 10 mL g−1 plant material). The extraction was carried out twice, and an aliquot (10 μL) of the combined supernatants was used for determination of dhurrin by a method modified from Halkier and Møller (1989). In brief, 200 μg of almond β-glucosidase (Type II, Sigma, St. Louis, MO) in 200 μL of 50 mm MES (pH 6.5) was added to the extract in an Eppendorf tube (1.5 mL). The tube was immediately closed and incubated (2 h, 30°C), and the sample was frozen (liquid N2). NaOH (40 μL, 6 m) was added to each frozen sample. After an additional incubation period (20 min, room temperature), the cyanide released was determined spectrophotometrically as described by Halkier and Møller (1989), except that the absorption was measured at 585 and 750 nm.
Microsomal Preparations
Microsomes were prepared from the harvested frozen plant material (0.1–10 g) as described by Halkier and Møller (1989) with the following modifications: After homogenization and addition of isolation buffer, the homogenates were cleared by centrifugation (10,000g, 10 min, 4°C). The microsomal pellet was recovered from the supernatant by centrifugation (100,000g, 1 h, 4°C), resuspended in 50 mm Tricine (pH 7.9)/2 mm dithiothreitol, microdialyzed (1 h, 4°C, N2 atmosphere; Busk and Pagès, 1997) against 50 mm Tricine (pH 7.9)/2 mm dithiothreitol, and frozen (liquid N2). Protein concentration was determined according to Bradford (1976).
Enzyme Assays and Western Blots
Reaction mixtures (total volume, 200 μL) contained 10 to 50 μg of microsomal protein (10 μL of the microsomal preparation), 1 mm substrate, 1.5 mm NADPH, and 50 mm Tricine (pH 7.9). The assay mixtures were incubated (30 min, 30°C) in a closed Eppendorf tube. Control samples were without addition of substrate. The samples were frozen in liquid N2 after incubation, and the cyanide content was measured (Halkier and Møller, 1989). Radioassays (total volume, 12.5 μL) contained 12 μg of microsomal protein, 18 μm [UL-14C]labeled Tyr (specific activity of 443 Ci mmol−1), 1.2 mm NADPH, and 50 mm Tricine (pH 7.9). After incubation (1 h, 30°C), the composition of the reaction mixture was analyzed by TLC (Bak et al., 1998a).
Western blotting of microsomal proteins was carried out using polyclonal chicken antibodies raised against purified and electroeluted recombinant CYP79A1 (Sibbesen et al., 1994) and CYP71E1 (Bak et al., 1998a).
RNA Purification, Northern Blotting, and Reverse Transcription PCR
Plant tissue was homogenized (liquid N2) with a mortar and pestle and RNA purified with the Trizol Reagent according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA). For northern blotting of total RNA (Sambrook et al., 1989), probes were isolated by agarose gel electrophoresis and labeled with the Random Primed DNA Labeling Kit (Roche Molecular Biochemicals, Summerville, NJ) and [α-32P]dCTP.
Reverse transcription was done with 2 to 5 μg of RNA (Wang and Mark, 1990). One-tenth of the volume of the reaction was used for PCR with the primers 5′-TGGACAACCCGTCGAACGCCGTG and 5′-GAGCTTCGGAATGTCCGACTCCTGCA for the CYP79A1 mRNA (Koch et al., 1995) and the primers 5′-ACGGCCTGGATGGCGACGTACATG and 5′-GCAGAAGGACGCCTACGTTGGTGAC for the sorghum ac1 actin gene (GenBank accession no. X79378). PCR reactions with [α-33P]dCTP (Cirera et al., 1998) were carried out using a Thermocycler Omnigene (Hybaid, Ashford, Middlesex, UK) with initial denaturation at 95°C for 2 min, followed by 25 cycles of 95°C for 5 s and 65°C for 5 s. Final elongation was 72°C for 5 min.
One-half of the volume of each PCR reaction was electrophoresed on a 5% (w/v) acrylamide gel (19:1 Acryl:bis) in 1× Tris-borate/EDTA. The gel was dried, and the radioactive bands were detected and quantitated on a Storm 840 phosphor imager (Molecular Dynamics, Sunnyvale, CA).
ACKNOWLEDGMENTS
We thank Dr. Peter Mackenzie (Flinders Medical Centre, Bedford Park, Australia) for assigning the pHMNGT as UGT85B1 according to the nomenclature rules. We thank Dr. Søren Bak (Plant Biochemistry Laboratory, Center for Molecular Plant Physiology, Royal Veterinary and Agricultural University, Frederiksberg, Denmark) for helpful discussions and technical help with the illustrations.
Footnotes
This work was supported by the Danish National Research Foundation, by the Danish Agricultural and Veterinary Research Council, and by Danish International Development Assistance.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.000687.
LITERATURE CITED
- Adewusi SRA. Turnover of dhurrin in green sorghum seedlings. Plant Physiol. 1990;94:1219–1224. doi: 10.1104/pp.94.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa T, Miljanich P, Conn EE. Studies on cyanogenic glycosides of Sorghum vulgare. Plant Physiol. 1960;35:535–538. doi: 10.1104/pp.35.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S, Feyereisen R. The involvement of two P450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol. 2001;127:108–118. doi: 10.1104/pp.127.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA. Cloning three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor L. Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of cyanogenic glucosides. Plant Mol Biol. 1998a;36:393–405. doi: 10.1023/a:1005915507497. [DOI] [PubMed] [Google Scholar]
- Bak S, Nielsen HL, Halkier BA. The presence of CYP79 homologues in glucosinolate-producing plants shows evolutionary conservation of the enzymes in the conversion of amino acid to aldoxime in the biosynthesis of cyanogenic glucosides and glucosinolates. Plant Mol Biol. 1998b;38:725–734. doi: 10.1023/a:1006064202774. [DOI] [PubMed] [Google Scholar]
- Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA. Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J. 1999;20:663–671. doi: 10.1046/j.1365-313x.1999.00642.x. [DOI] [PubMed] [Google Scholar]
- Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis thaliana. Plant Cell. 2001;13:101–111. doi: 10.1105/tpc.13.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough WA, Gander JE. Exogenous l-tyrosine metabolism and dhurrin turnover in sorghum seedlings. Phytochemistry. 1971;10:67–77. [Google Scholar]
- Boyd FT, Aamodt OS, Bohstedt G, Truog E. Sudan grass management for control of cyanide poisoning. J Am Soc Agron. 1938;30:569–582. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burbulis IE, Winkel-Shirley B. Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc Natl Acad Sci USA. 1999;96:12929–12934. doi: 10.1073/pnas.96.22.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busk PK, Pagès M. Microextraction of nuclear proteins from single maize embryos. Plant Mol Biol Rep. 1997;15:371–376. [Google Scholar]
- Casas AM, Kononowicz AK, Zehr UB, Tomes DT, Axtell JD, Butler LG, Bressan RA, Hasegawa PM. Transgenic sorghum plants via microprojectile bombardment. Proc Natl Acad Sci USA. 1993;90:11212–11216. doi: 10.1073/pnas.90.23.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicek M, Esen A. Structure and expression of a dhurrinase (β-glucosidase) from sorghum. Plant Physiol. 1998;116:1469–1478. doi: 10.1104/pp.116.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirera S, Julve J, Ferrer I, Mainou C, Bonet R, Martin-Campos JM, Gonzalez-Sastre F, Blanco-Vaca F. Molecular diagnosis of lecithin: cholesterol acyltransferase deficiency in a presymptomatic proband. Clin Chem Lab Med. 1998;36:443–448. doi: 10.1515/CCLM.1998.074. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Conn EE, Janzen DH. Developmental fate of the cyanogenic glucoside linamarin in Costa Rican wild lima bean seeds. Nature. 1979;278:343–344. [Google Scholar]
- Compton SG, Jones DA. An investigation of the responses of herbivores to cyanogenesis in Lotus corniculatus. Biol J Linn Soc. 1985;26:21–38. [Google Scholar]
- Conn EE. Biosynthesis of cyanogenic glycosides. Biochem Soc Symp. 1973;38:277–302. [PubMed] [Google Scholar]
- Cutler AJ, Hösel W, Sternberg M, Conn EE. The in vitro biosynthesis of taxiphyllin and the channeling of intermediates in Triglochin maritima. J Biol Chem. 1981;256:4253–4258. [PubMed] [Google Scholar]
- Erb N, Zinsmeister HD, Nahrstedt A. Die cyanogenen Glykoside von Triticum, Secale und Sorghum. Planta Med. 1981;41:84–89. doi: 10.1055/s-2007-971681. [DOI] [PubMed] [Google Scholar]
- Forslund K, Jonsson L. Cyanogenic glucosides and their metabolic enzymes in barley, in relation to nitrogen levels. Physiol Plant. 1997;101:367–372. [Google Scholar]
- Gillingham JT, Shirer MM, Starnes JJ, Page NR, McClain EF. Relative occurrence of toxic concentrations of cyanide and nitrate in varieties of sudangrass and sorghum-sudangrass hybrids. Agron J. 1969;61:727–730. [Google Scholar]
- Glassgen WE, Rose A, Madlung J, Koch W, Gleitz J, Seitz HU. Regulation of enzymes involved in anthocyanin biosynthesis in carrot cell cultures in response to treatment with ultraviolet light and fungal elicitors. Planta. 1998;204:490–498. doi: 10.1007/s004250050283. [DOI] [PubMed] [Google Scholar]
- Görlach J, Schmid J, Amrhein N. Abundance of transcripts specific for genes encoding enzymes of the prechorismate pathway in different organs of tomato (Lycopersicon exculentum L.) plants. Plant Physiol. 1994;193:216–223. doi: 10.1007/BF00192533. [DOI] [PubMed] [Google Scholar]
- Gorz HJ, Haskins FA, Morris R, Johnson BE. Identification of chromosomes that condition dhurrin content in sorghum seedlings. Crop Sci. 1987;27:201–203. [Google Scholar]
- Halkier BA, Du L. The biosynthesis of glucosinolates. Trends Plant Sci. 1997;2:425–431. doi: 10.1016/j.tplants.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Halkier BA, Møller BL. Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol. 1989;90:1552–1559. doi: 10.1104/pp.90.4.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, Du L, Naur P, Olsen CE, Axelsen KB, Hick AJ, Pickett JA, Halkier BA. CYP83B1 is the oxime-metabolizing enzyme in the glucosinolate pathway in Arabidopsis. J Biol Chem. 2001a;276:24790–24796. doi: 10.1074/jbc.M102637200. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Wittstock U, Olsen CE, Hick AJ, Pickett JA, Halkier BA. Cytochrome P450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J Biol Chem. 2001b;276:11078–11085. doi: 10.1074/jbc.M010123200. [DOI] [PubMed] [Google Scholar]
- Hegnauer R. Chemotaxonomie der Pflanzen. Vol. 7. Basel: Birkhäuser Verlag; 1986. Cyanogene Verbindungen; pp. 345–374. [Google Scholar]
- Herrmann KM. The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell. 1995;7:907–919. doi: 10.1105/tpc.7.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska AJ. Cyanogenic glucosides as defense compounds: a review of the evidence. J Chem Ecol. 1988;14:2213–2217. doi: 10.1007/BF01014026. [DOI] [PubMed] [Google Scholar]
- Jones DA. Chemical defense: primary or secondary functions? Am Nat. 1979;113:445–451. [Google Scholar]
- Jones DA. Why are so many food plants cyanogenic? Phytochemistry. 1998;47:155–162. doi: 10.1016/s0031-9422(97)00425-1. [DOI] [PubMed] [Google Scholar]
- Jones PR, Andersen MD, Nielsen JS, Høj PB, Møller BL. The biosynthesis, degradation, transport and possible function of cyanogenic glucosides. In: Romeo JT, Ibrahim R, Varin L, DeLuca V, editors. Evolution of Metabolic Pathways. Recent Advances in Phytochemistry. Vol. 34. Amsterdam: Pergamon; 2000. pp. 191–247. [Google Scholar]
- Jones PR, Møller BL, Høj PB. The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor: isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem. 1999;274:35483–35491. doi: 10.1074/jbc.274.50.35483. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Bak S, Svendsen I, Halkier BA, Møller BL. Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol. 1997;115:1661–1670. doi: 10.1104/pp.115.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Fahrendorf T, Halkier BA, Møller BL. Substrate specificity of the cytochrome P450 enzymes CYP79A1 and CYP71E1 involved in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys. 1999;363:9–18. doi: 10.1006/abbi.1998.1068. [DOI] [PubMed] [Google Scholar]
- Koch BM, Sibbesen O, Halkier BA, Svendsen I, Møller BL. The primary sequence of cytochrome P450tyr, the multifunctional N-hydroxylase catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys. 1995;323:177–186. doi: 10.1006/abbi.1995.0024. [DOI] [PubMed] [Google Scholar]
- Lechtenberg M, Nahrstedt A. Cyanogenic glucosides. In: Ikan R, editor. Naturally Occurring Glucosides. Chichester, UK: John Wiley; 1999. pp. 147–191. [Google Scholar]
- Lieberei R, Biehl B, Giesemann A, Junqueira NTV. Cyanogenesis inhibits active defense reactions in plants. Plant Physiol. 1989;90:33–36. doi: 10.1104/pp.90.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell W. Sorghum poisoning. Qld Agric J. 1903;13:93–98. [Google Scholar]
- Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indoleglucosinolates and indole-3-acetic acid. J Biol Chem. 2000;275:33712–33717. doi: 10.1074/jbc.M001667200. [DOI] [PubMed] [Google Scholar]
- Møller BL, Conn EE. The biosynthesis of cyanogenic glucosides in higher plants: N-hydroxytyrosine as an intermediate in the biosynthesis of dhurrin by Sorghum bicolor (Linn) Moench. J Biol Chem. 1979;254:8575–8583. [PubMed] [Google Scholar]
- Møller BL, Conn EE. The biosynthesis of cyanogenic glucosides in higher plants: channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (Linn) Moench. J Biol Chem. 1980;255:3049–3056. [PubMed] [Google Scholar]
- Møller BL, Seigler DS. Biosynthesis of cyanogenic glycosides, cyanolipids, and related compounds. In: Singh BK, editor. Plant Amino Acids. Biochemistry and Biotechnology. New York: Marcel Dekker; 1999. pp. 563–609. [Google Scholar]
- Nahrstedt A. Relationships between the defense systems of plants and insects: the cyanogenic system of the moth Zygaena trifolii. In: Romeo JT, editor. Recent Advances in Phytochemistry. Vol. 33. New York: Plenum Press; 1996. pp. 217–230. [Google Scholar]
- Nelson CE. Hydrocyanic acid content of certain sorghums under irrigation as affected by nitrogen fertilizer and soil moisture stress. Agron J. 1953;45:615–617. [Google Scholar]
- Nielsen JS, Møller BL. Biosynthesis of cyanogenic glucosides in Triglochin maritima and the involvement of cytochrome P450 enzymes. Arch Biochem Biophys. 1999;368:121–130. doi: 10.1006/abbi.1999.1258. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, Møller BL. Cloning and expression of cytochrome P450 enzymes catalyzing the conversion of tyrosine to p-hydroxyphenylacetaldoxime in the biosynthesis of cyanogenic glucosides in Triglochin maritima. Plant Physiol. 2000;122:1311–1321. doi: 10.1104/pp.122.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole OSA, Onabolu AO, Link H, Rosling H. Persistence of tropical ataxic neuropathy in a Nigerian community. J Neurol Neurosurg Psychiatry. 2000;69:96–101. doi: 10.1136/jnnp.69.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn AE. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckney RM. Effects of nitrate applications upon hydrocyanic acid content of sorghum. J Agric Res. 1924;27:717. [Google Scholar]
- Piotrowski M, Schönfelder S, Weiler EW. The Arabidopsis thaliana isogene NIT4 and its orthologs in tobacco encode β-cyano-l-alanine hydratase/nitrilase. J Biol Chem. 2001;276:2616–2621. doi: 10.1074/jbc.M007890200. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Seigler DS. Cyanide and cyanogenic glucosides. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. San Diego: Academic Press; 1991. pp. 35–77. [Google Scholar]
- Selmar D. Biosynthesis of cyanogenic glucosides, glucosinolates and nonprotein amino acids. In: Wink M, editor. Biochemistry of Plant Secondary Metabolism, Ann Plant Rev. Vol. 2. Sheffield Academic Press, CRC Press; 1999. pp. 79–150. [Google Scholar]
- Selmar D, Lieberei R, Biehl B. Mobilization and utilization of cyanogenic glucosides: the linustatin pathway. Plant Physiol. 1988;86:711–716. doi: 10.1104/pp.86.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbesen O, Koch B, Halkier BA, Møller BL. Isolation of the heme-thiolate enzyme cytochrome P-450TYR, which catalyses the committed step in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Proc Natl Acad Sci USA. 1994;91:9740–9744. doi: 10.1073/pnas.91.21.9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbesen O, Koch B, Halkier BA, Møller BL. Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem. 1995;270:3506–3511. doi: 10.1074/jbc.270.8.3506. [DOI] [PubMed] [Google Scholar]
- Siebert M, Sommer S, Li S-m, Wang Z-x, Severin K, Heide L. Genetic engineering of plant secondary metabolism. Accumulation of 4-hydroxybenzoate glucosides as a result of the expression of the bacterial ubiC gene in tobacco. Plant Physiol. 1996;112:811–819. doi: 10.1104/pp.112.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NA, Singh SP, Wang M, Stoutjesdijk PA, Green AG, Waterhouse PM. Total silencing by intron-spliced hairpin RNAs. Nature. 2000;407:319–320. doi: 10.1038/35030305. [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Høj PB, Møller BL. Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science. 2001;293:1826–1828. doi: 10.1126/science.1062249. [DOI] [PubMed] [Google Scholar]
- Wang AM, Mark DF. Quantitative PCR. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols. A Guide to Methods and Applications. San Diego: Academic Press; 1990. pp. 70–75. [Google Scholar]
- Wittstock U, Halkier BA. Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of l-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J Biol Chem. 2000;275:14659–14666. doi: 10.1074/jbc.275.19.14659. [DOI] [PubMed] [Google Scholar]
- Zhao Z-y, Cai T, Tagliani L, Miller M, Wang N, Pang H, Rudert M, Schroeder S, Hondred D, Seltzer J et al. Agrobacterium-mediated sorghum transformation. Plant Mol Biol. 2000;44:789–798. doi: 10.1023/a:1026507517182. [DOI] [PubMed] [Google Scholar]
- Zhu T, Peterson DJ, Tagliani L, St Clair G, Baszczynski CL, Bowen B. Targeted manipulation of maize genes in vivo using chimeric RNA/DNA oligonucleotides. Proc Natl Acad Sci USA. 1999;96:8768–8773. doi: 10.1073/pnas.96.15.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]