Abstract
Intermittent hypoxia (IH) occurs in many pathophysiological conditions. The molecular mechanisms associated with IH, however, have received little attention. Previous studies have reported that the c-fos gene via formation of activator protein-1 (AP-1) transcription factor contributes to adaptive responses to continuous hypoxia. In the present study, using a cell culture model we examined whether IH activates c-fos and AP-1 and if so, by what mechanisms. Experiments were performed on rat phaeochromocytoma cells exposed to 21% O2 (normoxia) or 60 and 120 cycles of IH, each cycle consisting 15 s of hypoxia followed by 4 min of normoxia. IH resulted in a significant elevation of c-fos mRNA as well as transcriptional activation. IH was more potent and induced a longer lasting activation of c-fos than comparable cumulative duration of continuous hypoxia. IH increased AP-1 activity and tyrosine hydroxylase (TH) mRNA, an AP-1-regulated downstream gene, and these effects were prevented by antisense c-fos. Superoxide dismutase mimetic, a potent scavenger of superoxide anions, prevented IH-induced c-fos, AP-1 and TH activations. IH increased superoxide anion levels in mitochondria as evidenced by decreased aconitase enzyme activity and increased levels of hydrogen peroxide, a stable dismutated product of superoxide anions. Complex I of the mitochondrial electron transport chain was markedly inhibited in IH exposed cells. Pharmacological inhibitors of complex I mimicked the effects of IH during normoxia and occluded the effects of IH on c-fos activation, suggesting the involvement of the mitochondrial electron transport chain in the generation of superoxide anions during IH. These results suggest IH-induced c-fos-mediated transcriptional activation involves oxidative stress.
Hypoxia is a pervasive physiological stimulus that is encountered under many different circumstances. Continuous hypoxia, such as occurs with sojourns at high altitude, leads to phenotypic re-modelling and adaptive responses. The molecular mechanisms underlying the adaptive responses to continuous hypoxia have been extensively investigated, and activation of specific genes and resulting de novo protein synthesis are considered important for triggering adaptive responses (Bunn & Poyton, 1996; Semenza, 2000). Genes that are activated by continuous hypoxia, in general, fall into two classes: immediate early genes that are activated shortly after the onset of hypoxia, and late response genes activated following several hours of hypoxia. c-fos is one of the most extensively studied members of the immediate early gene family. Hypoxia induces c-fos expression both in intact animals (Erickson & Millhorn, 1994; Haxhiu et al. 1995) and in cell cultures (Prabhakar et al. 1995). Cell culture studies further showed that hypoxia-induced c-fos expression contributes to activator protein-1 (AP-1) transcription factor activity and stimulates AP-1 regulated downstream genes such as tyrosine hydroxylase (TH;Mishra et al. 1998). Consequently, it has been proposed that c-fos expression and the resulting AP-1 activation constitute one of the molecular mechanisms that trigger adaptations to continuous hypoxia (Cherniack et al. 1996).
People living at sea level, on the other hand, experience intermittent hypoxia (IH) in many situations including sleep disordered breathing manifested as recurrent apnoeas (obstructive sleep apnoeas or central apnoeas; Fletcher et al. 1985). Although both continuous hypoxia and IH lead to decreases in arterial blood oxygen, there are fundamental differences in the response of the physiological systems to both forms of hypoxia. While, physiological systems adapt to continuous hypoxia, people with chronic IH caused by recurrent apnoeas are prone to hypertension, myocardial infarctions and stroke as evidenced by epidemiological as well as cross-sectional studies (Nieto et al. 2000; Shahar et al. 2001). A previous study on experimental animals has shown that IH up-regulates c-fos expression in the central nervous system (Greenberg et al. 1999). However, neither the functional significance nor the mechanisms of c-fos activation by IH have been investigated.
The fact that, although both forms of hypoxia up-regulate c-fos (Erickson & Millhorn, 1994; Haxhiu et al. 1995; Greenberg et al. 1999), only IH leads to patho-physiological conditions, prompted us to hypothesize that the mechanisms of c-fos activation by IH differ from continuous hypoxia. To test this possibility, we developed a cell culture model, wherein cells are exposed to IH with duration of hypoxic episodes similar to that encountered during recurrent apnoeas. Our results demonstrated that IH activates c-fos, which is functionally coupled to AP-1 transcription factor and TH activation. Furthermore, there were striking differences in c-fos activation caused by IH and continuous hypoxia. IH-induced c-fos activation, as well as downstream gene activation, were associated with oxidative stress involving down-regulation of complex I activity of the mitochondria.
Methods
Cell cultures
Rat phaeochromocytoma cells (PC12 cells; original clone from Dr L. Green) and human umbilical vein endothelial (HUVEC) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% horse serum, 5% fetal bovine serum containing penicillin (100 U ml−1) and streptomycin (100 μg ml−1) under 21% O2 and 10% CO2 at 37°C. Once cells reached sub-confluence, they were placed in antibiotic-free medium for 48 h. All experiments were performed in serum-free medium. In the experiments involving treatment with drugs, cells were pre-incubated for 30 min with appropriate concentrations of either drug or vehicle.
Exposure to intermittent hypoxia
Cell cultures were exposed to alternating cycles of hypoxia (1.5% O2; 15 s) and normoxia (21% O2; 4 min) in a humidified Lucite chamber (dimensions in inches (cm); l = 12 (30); w = 12 (30); h = 7 (17.8)) at 37°C as previously described (Kumar et al. 2003). Briefly, the chamber was equilibrated with gases at a flow rate of 2.4 l min−1. The duration of hypoxia and normoxia was adjusted by using timed solenoid valves. O2 levels in the culture medium, ∼1 mm above the cell layer, were monitored with an O2 electrode (Lazar Research Laboratories Inc., CA, USA) and continuously recorded on a strip chart recorder.
Cell viability
Lactate dehydrogenase (LDH) activity in the medium was determined as an index of cell viability as previously described (Kumar et al. 1998). Briefly, cells were separated from the medium and pyruvate-dependent oxidation of NADH was monitored and expressed as micromoles of NADH oxidized per minute per milligram protein.
Northern blot and reporter gene assays
Total cellular RNA was isolated using the Rneasy mini kit method (Qiagen Inc., CA, USA) following the manufacturer's protocol. c-fos, TH and β-actin mRNAs were analysed by Northern blot analysis as previously described (Prabhakar et al. 1995; Mishra et al. 1998). Changes in mRNAs were quantified by optical density measurements. c-fos promoter and AP-1 activities were determined by reporter gene assay as previously described (Mishra et al. 1998; Premkumar et al. 2000a; Premkumar et al. 2000b). Changes in luciferase activity were normalized to β-galactosidase activity and the reporter gene assays were in the linear range.
The following plasmids were used: pfos-Luc, wild-type and c-fos promoter with point mutations at Calcium–calcium responsive element (Ca/CRE), Serum responsive element (SRE), and FAP cis-elements with luciferase reporter gene (Wang & Simonson, 1996). The 2 × AP-1 construct contains two AP-1 elements inserted in tandem upstream of the rat prolactin gene minimal promoter (Mishra et al. 1998). c-fos sense (pEMSV foss) and antisense (pEMSV fosAS) expression vectors were constructed by subcloning full length rat c-fos cDNA into the EcoR1 site of the pEMSV expression vector (Davis et al. 1987) The orientation of plasmids was confirmed by DNA sequencing. These constructs express sense or antisense RNA transcripts under the control of the Murine Sarcoma Virus. Plasmid pGL3 LUC was from Promega and β-galactosidase pRSV Lac Z was from the American Type Culture Collection (MacGregor et al. 1987).
Aconitase activity and H2O2 measurements in mitochondria
Mitochondria were isolated using procedures essentially the same as that reported (Lai & Clark, 1979) except that EDTA was substituted with 1 mm EGTA and the extraction volume was adjusted for smaller sample sizes. Aconitase enzyme activity was measured spectrophotometrically as previously described (Gardner et al. 1994) and was expressed in nanomoles of isocitrate formed per minute per milligram protein.
H2O2 was measured by monitoring H2O2-induced fluorescence of p-hydroxyphenylacetic acid in the presence of horseradish peroxidase (Type VIa, Sigma, St Louis, MO, USA) as previously described (Boveris et al. 2002). Basal production of H2O2 was measured by monitoring the fluorescence signal from the reaction medium for 6 min at 317 nm (excitation) and 414 nm (emission) wavelengths. The fluorescence signal was absent in the presence of catalase (100 units). The relative contribution of mitochondrial complexes to the generation of H2O2 was assessed by addition of rotenone (5 μm; complex I inhibitor) and antimycin A (10 μg ml−1; complex III inhibitor; Krahenbuhl et al. 1994), and changes in fluorescence intensity were recorded for additional 12 min. A standard curve relating the fluorescence signal to the amount of H2O2 generated was constructed. The rate of H2O2 production was expressed in picomoles per minute per milligram protein. The concentration of the stock H2O2 solution was determined spectrophotometrically using a molar extinction coefficient value of 43.6 m−1 cm−1 at 240 nm (Gardner et al. 1994; Boveris et al. 2002).
Mitochondrial complex activities
Complex I activity (NADH-ubiquinone reductase) was measured as previously described (Krahenbuhl et al. 1994). The complex I activity was measured as the rotenone (5 μm)-sensitive rate of NADH oxidation and expressed in nanomoles of NADH oxidized per minute per milligram protein. The complex III activity (ubiquinol–ferricytochrome c oxido-reductase) of the mitochondria was measured by a modification of the method as described (Krahenbuhl et al. 1994). The activity was measured in the presence and absence of antimycin A. Complex III activity was measured as the antimycin A (10 μg ml−1)-sensitive reaction and expressed as nanomoles of cytochrome c reduced per minute per milligram protein. Protein was determined as previously described (Redinbaugh & Turley, 1986).
Chemicals
All chemicals and reagents were of analytical grade and obtained from Sigma chemicals unless otherwise stated. Manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP) was obtained from Alexis Biochemicals (CA, USA).
Data analysis
The data are expressed as means ± s.e.m. from 4 to 6 individual experiments. Two-way analysis of variance (ANOVA) with repeated measures followed by Tukey's test was used to evaluate the statistical significance and P values less than 0.05 were considered significant.
Results
Oxygen profiles and cell viability during IH
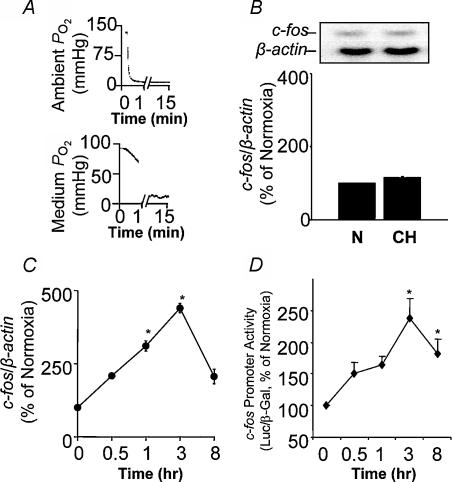
An example depicting changes in PO2 in the culture medium during IH is shown in Fig. 1. In presence of 3 × 106 cells per plate, medium PO2 during normoxia was 74 ± 3 mmHg (n = 6). The low PO2 in the medium was due to O2 consumption of the cells, because in their absence the medium PO2 was 146 ± 5 mmHg. During each hypoxic episode, medium PO2 decreased to 55 ± 5 mmHg (▵ decrease of 20 mmHg). Once the PO2 reached this level, it was maintained for 15 s. Re-introduction of normoxic gas restored the medium PO2 to pre-hypoxic value. O2 profiles remained constant during the course of a given experiment. pH and osmolarity of the medium were monitored before and after IH (120 cycles, maximum number of IH cycles used in this study). IH had no significant effect on these variables (P > 0.05). To determine the effect of IH on cell viability, LDH activity in the cell culture medium was determined. LDH activity remained unaltered following IH (control vs. IH, 0.53 ± 0.15 vs. 0.65 ± 0.12 μmol NADH oxidized min−1 (mg protein)−1; P > 0.05; n = 4). These observations suggest that IH decreases the medium PO2, albeit modestly, with each episode of hypoxia, and had no adverse effect on cell viability, or the pH or osmolarity of the medium.
Figure 1. Changes inPO2in the tissue culture chamber and the medium during intermittent hypoxia (IH).
A, representative tracings of PO2 during IH in the tissue culture chamber (top panel) and medium near the cells (bottom panel). B, expanded tracing of the PO2 changes during a single episode of IH. Note the decrease in medium PO2 with each episode of hypoxia.
Activation of c-fos by IH
c-fos mRNA increased in a stimulus-dependent manner as the duration of IH increased from 10 to 30–60 cycles (Fig. 2A). There was a 3.5-fold increase in c-fos mRNA with 60 cycles of IH (P < 0.01). Exposing cells to alternating cycles of normoxia instead of hypoxia (negative control) had no effect on c-fos mRNA (Fig. 2B). IH (60 cycles) also increased c-fos mRNA in HUVEC cells (3-fold increase; P < 0.01; n = 5), suggesting that the effects of IH on c-fos expression were not confined to PC12 cells only. All subsequent experiments were performed on PC12 cells exposed to either 60 or 120 cycles of IH.
Figure 2. Intermittent hypoxia up-regulates c-fos mRNA in PC12 cells.
A–D, top panels represent Northern blot analysis of c-fos and β-actin mRNAs and bottom panels average, normalized (c-fos/β-actin) data. A, effect of increasing the numbers of IH cycles on c-fos mRNA. B, effect of intermittent normoxia (IN, 60 cycles; control). C and D, effect of increasing the durations of individual hypoxic and normoxic episodes. Data presented are mean ± s.e.m. from n = 5 experiments in each group. *P < 0.01 (versus control). n.s. = not significant (P > 0.05).
The influence of varying the duration of each hypoxic and re-oxygenation episode on c-fos expression was examined. Increasing the duration of each hypoxic episode from 15 s to 1 min had no significant effect on the magnitude of c-fos mRNA expression (with a fixed normoxic exposure of 4 min; Fig. 2C). However, extending the duration of each re-oxygenation episode from 1 to 10 min, while maintaining the duration of hypoxic episodes at 15 s, resulted in a progressive increase in c-fos mRNA. There was a tendency for c-fos expression to decrease with 10 min of re-oxygenation, but this was not significant when compared to 4 min of re-oxygenation (P > 0.05; Fig. 2D).
Activation of c-fos transcription by IH
Increased c-fos mRNA could result from either increased transcription or mRNA stability or both. To demonstrate an increase in c-fos transcriptional activation, cells were transfected with a reporter gene in which the expression of firefly luciferase was driven by a c-fos promoter (−356 to +109 fragment) that contains the cis-elements necessary for eliciting c-fos activation by continuous hypoxia (Premkumar et al. 2000b). c-fos promoter activity increased following IH, although, relative to the induction of c-fos mRNA, the response was not as robust with 60 cycles, but required 120 cycles of IH to reach the same level. c-fos promoter activity was stimulated 3-fold in response to IH (P < 0.01; Fig. 3), whereas luciferase activity with the expression vector in the absence of c-fos promoter was unaffected (P > 0.05; n = 5).
Figure 3. Intermittent hypoxia stimulates c-fos promoter activity and requires Ca/CRE and SRE cis-elements.
Schematic representation of c-fos promoter constructs (left) and average, normalized (% of normoxia) data (mean ± s.e.m.) from 5 experiments run in duplicate (right). White bars, normoxic control; black bars, intermittent hypoxia (IH) for 60 cycles; grey bars, 20% fetal bovine serum (FBS) in normoxia. *P < 0.01 (versus control).
To determine the cis-elements involved in IH-induced c-fos activation, experiments were performed with c-fos–luciferase constructs with point mutations at SRE (point mutation in the CArG sequence; SRE binding site), Ca/CRE (point mutation c-to-G conversion), and FAP cis-elements. Point mutations in SRE completely prevented IH-induced c-fos promoter activation, as well as c-fos activation by fetal bovine serum (12 h exposure to 20% FBS; positive control; Fig. 3). Mutations in Ca/CRE partially blocked (∼45%) c-fos promoter activation by IH, but not by FBS (negative control; Fig. 3). In contrast, mutations in the FAP cis-element had no effect on c-fos promoter activation by IH or FBS (P > 0.05; n = 5).
c-fos activation by continuous hypoxia
Cells were exposed to 15 min of continuous hypoxia, which was equivalent to the hypoxic duration accumulated during 60 episodes of IH (15 s episode −1× 60 episodes). Within 5 min of the onset of continuous hypoxia, the medium PO2 decreased from 80 ± 6 to 10 ± 2 mmHg (▵ decrease of 70 mmHg; Fig. 4A) and remained at this level during the ensuing 15 min of continuous hypoxic exposure. Despite the marked reduction in PO2, 15 min of continuous hypoxia had no effect on either c-fos mRNA (Fig. 4B) or c-fos transcriptional activity as determined by reporter gene assay (data not shown). Prolonging the duration of continuous hypoxia to more than 1 hour, however, increased c-fos mRNA as well as c-fos promoter activity (Fig. 4C and D). Maximal increases in c-fos activation were seen with 3 h of continuous hypoxia. Comparison of the time course of c-fos activation showed that IH required only minutes (60 cycles of IH = 15 min of hypoxia; Fig. 2A), whereas continuous hypoxia required hours to stimulate c-fos (Fig. 4C and D).
Figure 4. c-fos activation by continuous hypoxia (CH).
A, representative tracings of PO2 changes in the tissue culture chamber (top panel) and in the medium near the cells (bottom panel) during 15 min of continuous hypoxia (CH). B, 15 min of CH does not affect c-fos mRNA expression. Representative Northern blot (top panel) and average data (bottom panel) of c-fos mRNA expression. C and D, time course of c-fos mRNA and c-fos promoter activity to increasing durations of CH. Data are mean ± s.e.m. from 4–5 experiments. *P < 0.01.
Time courses of c-fos expression after terminating IH (60 cycles) and continuous hypoxia (3 h) were examined. Following termination of IH, c-fos mRNA remained elevated for at least 3 h (Fig. 5A). In contrast, c-fos mRNA returned to close to control levels within 1 h of terminating continuous hypoxia (Fig. 5B).
Figure 5. Long lasting activation of c-fos mRNA by intermittent hypoxia (IH).
A and B, time course of c-fos mRNA expression after termination of 60 cycles of intermittent hypoxia (IH) and 3 h of continuous hypoxia (CH). Top panels represent Northern blot assay of c-fos and β-actin mRNAs. Bottom panels represent average data presented as mean ± s.e.m. from 4 experiments in each group. *P < 0.01 (versus normoxic control).
Activation of AP-1 transcription factor and tyrosine hydroxylase (TH) by IH; involvement of c-fos
The AP-1 transcription factor complex is composed of either a heterodimer of c-Fos and Jun or a Jun homodimer (Angel & Karin, 1991). We examined whether IH activates AP-1, and if so, if this involves c-fos. We used a reporter gene assay using a luciferase expression vector driven by two copies of AP-1 cis-element. Cells transfected with AP-1 luciferase construct were exposed to increasing episodes of IH. IH increased AP-1 activity and the magnitude of activation increased progressively with increasing number of IH episodes (Fig. 6A). Significant increases in AP-1 activity were seen with 60 and 120 cycles of IH. Parallel experiments with cells transfected with a pGL3 luciferase construct lacking AP-1 sequence showed no increase in activity with IH (control; 120 cycles of IH; P > 0.05).
Figure 6. Intermittent hypoxia-induced c-fos contributes to activator protein-1 (AP-1) transcriptional activity.
A, schematic representation of AP-1 construct (top panel), and average, normalized AP-1 cis-element activity with increasing number of intermittent hypoxia (IH) cycles (bottom panel). B, antisense c-fos (c-fosAS) prevents IH-induced (120 cycles) AP-1 cis element activity in a concentration-dependent manner. Data presented are mean ± s.e.m from 6 experiments, and *P < 0.05; **P < 0.01.
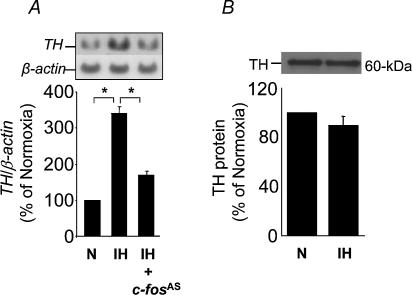
An antisense c-fos strategy was used to assess the contribution of c-fos in IH-induced AP-1 activation. The antisense vector directs transcription of a full-length antisense RNA transcript that hybridizes with c-fos mRNA by either a ribonuclease based mechanism or by translational arrest (Russell & Liebhaber, 1993). Cells were transfected with the antisense c-fos along with AP-1–luciferase construct and β-galactosidase. IH-induced AP-1 activity was significantly reduced in cells cotransfected with antisense c-fos (Fig. 6B). On the other hand, antisense c-fos had no effect on basal AP-1 activity (P > 0.05; control). We then examined whether IH activates TH, an AP-1-regulated downstream gene (Norris & Millhorn, 1995; Mishra et al. 1998), and if so, if this involves c-fos. IH resulted in a 3.5-fold increase in TH mRNA and cotransfecting cells with antisense c-fos attenuated TH activation by IH (Fig. 7A). Control experiments with sense c-fos (1 μg) showed increases in basal AP-1 activity as well as TH mRNA (∼3- to 3.5-fold). In the presence of sense c-fos IH did not further stimulate either AP-1 activity or TH mRNA. Although 120 cycles of IH up-regulated TH mRNA, there was no concomitant increase in TH protein (Fig. 7B). Taken together, these findings show that IH-induced c-fos activation is coupled to activation of AP-1 and TH, an AP-1 regulated downstream gene.
Figure 7. Intermittent hypoxia (IH) activates tyrosine hydroxylase (TH) mRNA.
A, intermittent hypoxia (IH, 120 cycles) stimulated TH, and antisense c-fos (c-fosAS; 1 μg) prevented this effect. B, IH (120 cycles) had no effect on TH protein expression. Top panels in A and B represent Northern and Western blot analysis of TH, respectively. Bottom panels represent average normalized data presented as mean ±s.e.m from 6 experiments. *P < 0.01.
Involvement of superoxide (O2·−) anion in IH-induced gene activation
Recent studies on intact animals suggest that increased generation of superoxide (O2·−) anion contributes to IH-induced augmentation of long-term facilitation (LTF) of respiratory motor activity (Peng & Prabhakar, 2003) as well as in the induction of sensory LTF in the carotid body (Peng et al. 2003). To test whether O2·− also plays a role in IH-induced gene activation, cells pretreated with MnTMPyP, a stable superoxide dismutase (SOD) mimetic that scavenges O2·−, were exposed to IH. SOD mimetic (25 μm) attenuated or abolished IH-induced c-fos mRNA, c-fos promoter activation, AP-1 activation, and TH (Fig. 8A–D).
Figure 8. Reactive oxygen species contribute to gene activation by intermittent hypoxia (IH).
Superoxide dismutase mimetic (MnTMPyP; 25 μm), scavenger of O2·− prevents activation of c-fos mRNA (A), c-fos promoter activity (B), AP-1 cis-element activity (C) and TH mRNA by IH (D). Top panels in A and D illustrate representative Northern blots of c-fos, TH, and β-actin mRNAs, respectively. Bottom panels represent average data (mean ± s.e.m) from n = 5 individual experiments. *P < 0.01.
Mitochondria as a source of O2·− generation induced by IH
The results described above suggest that IH increases cellular generation of O2·−. Mitochondria as well as several cytosolic and membrane bound oxidases are known to contribute to O2·− generation (Halliwell & Gutteridge, 1990; Ambrosio et al. 1993). As an initial step towards identifying the cellular sources of O2·− generation during IH, we focused on mitochondria. O2·− generation in the mitochondria was determined by monitoring aconitase enzyme activity as an index of O2·− generation (Gardner et al. 1994). Aconitase activity was ∼78% less in mitochondria harvested from IH (60 cycles) exposed cells compared to mitochondria from normoxic cells (P < 0.01; n = 5), suggesting increased generation of O2·−. Exposure to continuous hypoxia (15 min), however, had no significant effect on mitochondrial aconitase activity (P > 0.05; n = 5). In another series of experiments, the rate of O2·− generation in the mitochondria was quantified indirectly by measuring the rate of formation of H2O2, a stable dismutated product of O2·−. Basal H2O2 levels were significantly higher in IH mitochondria compared to controls (control vs. IH, 358 ± 2 vs. 410 ± 8 pmol min−1 (mg protein)−1; P < 0.01; n = 5).
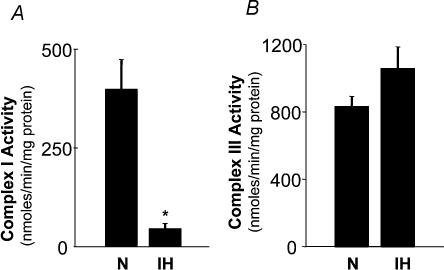
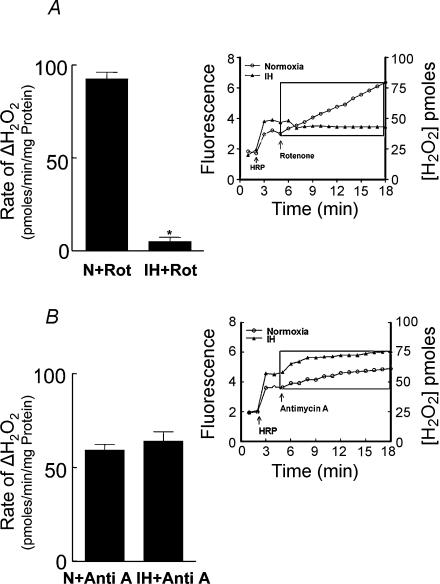
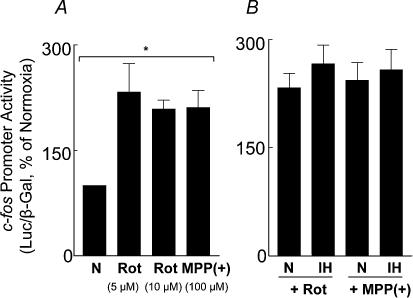
Inhibition of complexes I and/or III of the electron transport chain has been proposed to generate O2·− in the mitochondria (Ambrosio et al. 1993). We examined whether IH affects complex I and/or complex III and, if so, whether it contributes to increased generation of O2·−. Analysis of complex I and III activities showed selective down-regulation of complex I by IH; whereas complex III was unaffected (Fig. 9). Remarkably, a comparable cumulative duration of continuous hypoxia had no effect either on complex I or complex III (P > 0.05; n = 4). It is known that inhibition of complex I results in an increased generation of O2·− (Ambrosio et al. 1993). If IH has already down-regulated complex I, then inhibitors of complex I should have no impact on the rate of O2·− production in mitochondria from IH cells. Indeed, rotenone (5 μm), an inhibitor of complex I, had no effect on the rate of H2O2 production (an indirect measure of O2·−) in mitochondria from IH-exposed cells, whereas in control mitochondria, rotenone resulted in a progressive increase in H2O2 levels (Fig. 10A). On the other hand, antimycin A (10 μg ml−1), an inhibitor of complex III, increased H2O2 production in both IH and control mitochondria (Fig. 10B), further confirming the lack of effect of IH on complex III. To further assess whether inhibition of complex I contributed to c-fos activation by IH, the effects of complex I inhibitors on c-fos promoter activity were examined. Rotenone (5 μm), as well as 1-methyl-4-phenylpyridinium ion (MPP(+), 100 μm), potent inhibitors of complex I, stimulated c-fos promoter activity under normoxia and occluded IH-induced c-fos activation (Fig. 11).
Figure 9. Inhibition of mitochondrial electron transport at complex I by intermittent hypoxia (IH).
IH inhibits complex I (A) but not complex III (B) activity of the mitochondrial electron transport chain. Data are mean ± s.e.m. from four experiments. *P < 0.01.
Figure 10. Effect of mitochondrial complex I and III inhibitors on H2O2 generation in mitochondria from PC12 cells exposed to intermittent hypoxia (IH).
The effects of rotenone (5 μm, complex I inhibitor) and antimycin A (10 μg ml−1) on H2O2 production (indirect measure of O2·−; for details see Methods) were determined in mitochondria harvested from control (normoxia, N) and IH cells. Insets in A, and B represent analysis of H2O2 generation with mitochondrial complex inhibitors in control and IH cells. The rate of H2O2 was averaged for 12 min after the addition of rotenone or antimycin A (depicted as boxes in insets in A, and B). Average data are presented as rate of change of H2O2 (inhibitor – basal rate of H2O2 production; mean ± s.e.m. from n = 5 experiments). *P < 0.01.
Figure 11. Mitochondrial complex I inhibitors mimic the effects of intermittent hypoxia (IH).
A, the effect of increasing concentrations of rotenone and MPP(+) (100 μm) on c-fos during normoxia (N) is shown. B, rotenone and MPP(+) occlude c-fos activation by intermittent hypoxia (IH). Data presented are mean ± s.e.m from six experiments. *P < 0.01.
Discussion
The major findings of the present study are: (a) IH activates c-fos in cell cultures, and c-fos activation is functionally coupled to AP-1 transcription factor and TH, an AP-1-regulated downstream gene, (b) there were striking differences in c-fos activation by IH and continuous hypoxia, and (c) oxidative stress involving down-regulation of complex I activity of the mitochondria plays an essential role in IH-induced c-fos as well as downstream gene activation.
The following observations suggest that c-fos activation by IH in cell cultures is due to periodic decreases in PO2 rather than to secondary changes in other variables. First, the PO2 near the cells decreased (∼20 mmHg) during each episode of hypoxia. Second, intermittent normoxia under identical experimental conditions had no effect on c-fos expression, implying that the effects of IH were unlikely to be due to a high rate of gas flow. Third, IH had no effect on the pH, or osmolarity of the culture medium, or on cell viability, suggesting that c-fos activation was not due to changes in these variables. Greenberg et al. (1999) reported up-regulation of c-Fos protein in central neurones of rats exposed to a similar protocol of IH. However, neither the mechanisms nor the functional significance of c-fos activation were ascertained in this study, probably due to inherent limitations associated with whole animal studies. The cell culture model of IH used in this study permitted analysis of the mechanisms associated with IH-induced c-fos activation. Our data with reporter gene analysis suggest that c-fos activation by IH is in part due to transcriptional activation and requires SRE and Ca/CRE cis-elements. Furthermore, experiments using the antisense c-fos strategy revealed that IH-induced c-fos activation is functionally coupled to the AP-1 transcription factor as well as downstream gene activation, i.e. TH. Consistent with a previous report (Kumar et al. 2003), we found no change in TH protein with 120 cycles of IH, although TH mRNA was up-regulated. It is likely that a longer duration of IH exposure (more than 120 cycles) is required to elicit increases in TH protein. Alternatively, IH might have enhanced the rate of TH protein turnover, masking the increase in net protein levels. These possibilities, however, require further investigation. None-the-less, taken together the present results demonstrate that IH activates c-fos, which in turn stimulates AP-1-regulated downstream genes. Humans experiencing IH as a result of recurrent apnoeas exhibit elevated catecholamines (Ziegler et al. 1997). It is likely that up-regulation of TH via c-fos activation might contribute to elevated catecholamine levels reported in recurrent apnoea subjects experiencing chronic IH.
An important finding of the present study is the striking difference in c-fos activation by IH and continuous hypoxia. Although IH resulted in only a modest decrease of PO2 (∼20 mmHg) near the cells, it produced a significant stimulation of c-fos. On the other hand, whilst a comparable cumulative duration of continuous hypoxia caused a more pronounced drop in PO2 (∼70 mmHg) near the cells, it failed to activate c-fos. The lack of c-fos activation by a comparable duration of continuous hypoxia was unexpected in view of the general belief that the more severe the hypoxia, the greater the magnitude of gene induction. The absence of c-fos stimulation by continuous hypoxia (15 min) might be due to severe hypoxia near the cells relative to that caused by IH. Such a possibility, however, seems unlikely because: (a) 15 min exposure to less severe continuous hypoxia (PO2−30 mmHg) was equally ineffective in eliciting c-fos activation, and (b) c-fos could be activated by extending the duration of severe continuous hypoxia for 3 h (Fig. 4C). It follows from these observations that IH is a more robust activator of c-fos than a comparable cumulative duration of continuous hypoxia.
A novel observation of the present study was that IH resulted in longer lasting activation of c-fos that persisted for several hours after terminating the stimulus (Fig. 5A). Such long-lasting activation was not evident with continuous hypoxia. Studies on intact animals have documented long-lasting activation of physiological systems by IH that persist several minutes to hours after termination of the stimulus. The long-lasting activation is termed as ‘long-term facilitation’ or LTF. For instance, IH results in LTF of respiratory motor output (Mitchell et al. 2001; Peng & Prabhakar, 2003) and sensory LTF of the carotid body (Peng et al. 2003). LTF is unique to IH, because it is not elicited by a comparable duration of continuous hypoxia (Mitchell et al. 2001). The longer lasting c-fos activation observed in the present study is reminiscent of IH-induced LTF of systemic responses reported in intact animals. Thus, the use of a cell culture model of IH has allowed the unravelling of a hitherto uncharacterized LTF-like phenomenon even at the gene level. The mechanisms associated with the persistent activation of c-fos are clearly beyond the scope of the present investigation, and may involve sustained increases in the stability of mRNA and/or transcription.
What makes IH more effective in activating c-fos than continuous hypoxia? The feature that distinguishes IH from continuous hypoxia is the intervening periods of normoxia. It has been postulated that reactive oxygen species (ROS) released during intervening normoxic episodes facilitates oxygen sensing during IH (Prabhakar, 2001). Recent studies on intact animals support the role of ROS, especially O2·− in eliciting systemic responses to IH. (Peng et al. 2003; Peng & Prabhakar, 2003). In the present study, increasing the duration of intervening re-oxygenation periods, but not the hypoxic episode caused progressive enhancement of c-fos expression, suggesting that the durations of normoxic episodes are more critical in determining the magnitude of c-fos activation by IH than the absolute duration of individual hypoxic episodes. A role for ROS in IH-induced c-fos activation is supported by the following observations. First, IH increased ROS levels, especially O2·− as evidenced by down-regulation of aconitase activity and elevated H2O2 production (an indirect measure of O2·−). Second, a SOD mimetic, a scavenger of O2·−, prevented c-fos, AP-1 and TH activation by IH. Where does ROS come from during IH? Our results suggest that inhibition of mitochondrial complex I is one of the sources of O2·− generation during IH. Several proteins contribute to the assembly of complex I of the mitochondrial electron transport chain. It is likely that one or more of them are targets of IH and disruption of these proteins leads to inhibition of complex I activity. These possibilities, however, require further study. Although we identified mitochondrial complex I as one of the sources of ROS generation by IH, it is known that several cytosolic and membrane bound oxidase also generate ROS (Halliwell & Gutteridge, 1990). Whether IH also affects ROS generating oxidase remain to be investigated.
Reactive oxygen species include superoxide anions (O2·−), hydrogen peroxide (H2O2) and hydroxyl radicals (OH−). Which of these species contribute to IH-induced c-fos activation? Previous studies have shown that xanthine- xanthine oxidase, which generates O2·− anions, stimulates c-fos within 15 min, whereas H2O2 was without any effect (Maki et al. 1992). The fact that IH-induced c-fos, as well as AP-1 and TH activation, could be prevented by a SOD mimetic, which is a known scavenger of O2·−, suggests that O2·− anions play an important role in gene activation by IH. However, from the present data we cannot exclude the possible contribution of H2O2 to gene activation by IH. Recently, Hohler and coworkers (Hohler et al. 1999) questioned the importance of ROS in gene activation by hypoxia. These investigators found that although hypoxia increased ROS, scavengers of ROS failed to prevent up-regulation of TH mRNA with 6 h of continuous hypoxia. It is possible that during such long exposures to hypoxia (6 or more hours) mechanisms other than ROS may contribute to gene activation.
In summary, using a cell culture model we have demonstrated that oxidative stress leading to increased generation of O2·− plays a critical role in c-fos and downstream gene activation by IH. Future studies with cell culture models of IH might provide further insight into the cellular mechanisms associated with the morbidity associated with IH caused by recurrent apnoeas.
Acknowledgments
This work was supported by grants from National Institutes of Health, Heart, Lung and Blood Institute (HL-25830 and HL-66448).
References
- Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty JT. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Boveris A, Alvarez S, Bustamante J, Valdez L. Measurement of superoxide radical and hydrogen peroxide production in isolated cells and subcellular organelles. Meth Enzymol. 2002;349:280–287. doi: 10.1016/s0076-6879(02)49342-1. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Shenoy PC, Mishra R, Simonson M, Prabhakar NR. Induction of immediate early response genes by hypoxia. Possible molecular bases for systems adaptation to low pO2. Adv Exp Med Biol. 1996;410:127–134. [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103:190–195. doi: 10.7326/0003-4819-103-2-190. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999;816:638–645. doi: 10.1016/s0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Strohl KP, Cherniack NS. The N-methyl-D-aspartate receptor pathway is involved in hypoxia-induced c-Fos protein expression in the rat nucleus of the solitary tract. J Auton Nerv Syst. 1995;55:65–68. doi: 10.1016/0165-1838(95)00029-w. [DOI] [PubMed] [Google Scholar]
- Hohler B, Lange B, Holzapfel B, Goldenberg A, Hanze J, Sell A, Testan H, Moller W, Kummer W. Hypoxic upregulation of tyrosine hydroxylase gene expression is paralleled, but not induced, by increased generation of reactive oxygen species in PC12 cells. FEBS Lett. 1999;457:53–56. doi: 10.1016/s0014-5793(99)00999-0. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994;230:177–187. doi: 10.1016/0009-8981(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Kim DK, Lee MS, Ramachandran R, Prabhakar NR. Activation of tyrosine hydroxylase by intermittent hypoxia: Involvement of serine phosphorylation. J Appl Physiol. 2003;95:536–544. doi: 10.1152/japplphysiol.00186.2003. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Overholt JL, Bright GR, Hui KY, Lu H, Gratzl M, Prabhakar NR. Release of dopamine and norepinephrine by hypoxia from PC12 cells. Am J Physiol. 1998;274:C1592–C1600. doi: 10.1152/ajpcell.1998.274.6.C1592. [DOI] [PubMed] [Google Scholar]
- Lai JC, Clark JB. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Meth Enzymol. 1979;55:51–60. doi: 10.1016/0076-6879(79)55008-3. [DOI] [PubMed] [Google Scholar]
- MacGregor GR, Mogg AE, Burke JF, Caskey CT. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat Cell Mol Genet. 1987;13:253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- Maki A, Berezesky IK, Fargnoli J, Holbrook NJ, Trump BF. Role of [Ca2+]i in induction of c-fos, c-jun, and c-myc mRNA in rat PTE after oxidative stress. Faseb J. 1992;6:919–924. doi: 10.1096/fasebj.6.3.1740241. [DOI] [PubMed] [Google Scholar]
- Mishra RR, Adhikary G, Simonson MS, Cherniack NS, Prabhakar NR. Role of c-fos in hypoxia-induced AP-1 cis-element activity and tyrosine hydroxylase gene expression. Brain Res Mol Brain Res. 1998;59:74–83. doi: 10.1016/s0169-328x(98)00139-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Norris ML, Millhorn DE. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol. 2003;94:2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during inttttthypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Shenoy BC, Simonson MS, Cherniack NS. Cell selective induction and transcriptional activation of immediate early genes by hypoxia. Brain Res. 1995;697:266–270. doi: 10.1016/0006-8993(95)00994-2. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Adhikary G, Overholt JL, Simonson MS, Cherniack NS, Prabhakar NR. Intracellular pathways linking hypoxia to activation of c-fos and AP-1. Adv Exp Med Biol. 2000a;475:101–109. doi: 10.1007/0-306-46825-5_10. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Mishra RR, Overholt JL, Simonson MS, Cherniack NS, Prabhakar NR. L-type Ca2+ channel activation regulates induction of c-fos transcription by hypoxia. J Appl Physiol. 2000b;88:1898–1906. doi: 10.1152/jappl.2000.88.5.1898. [DOI] [PubMed] [Google Scholar]
- Redinbaugh MG, Turley RB. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986;153:267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Russell JE, Liebhaber SA. Double-stranded RNA triggers generalized translational arrest in Xenopus oocytes. Biochem Biophys Res Commun. 1993;194:892–900. doi: 10.1006/bbrc.1993.1905. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Simonson MS. Voltage-insensitive Ca2+ channels and Ca2+/calmodulin-dependent protein kinases propagate signals from endothelin-1 receptors to the c-fos promoter. Mol Cell Biol. 1996;16:5915–5923. doi: 10.1128/mcb.16.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler MG, Nelesen R, Mills P, Ancoli-Israel S, Kennedy B, Dimsdale JE. Sleep apnea, norepinephrine-release rate, and daytime hypertension. Sleep. 1997;20:224–231. doi: 10.1093/sleep/20.3.224. [DOI] [PubMed] [Google Scholar]