Abstract
Peripheral nerves in the limbs stretch to accommodate changes in length during normal movement. The aim of this study was to determine how stretch is distributed along the nerve relative to local variations in mechanical properties. Deformation (strain) in joint and non-joint regions of rat median and sciatic nerves was measured in situ during limb movement using optical image analysis. In each nerve the strain was significantly greater in the joint rather than the non-joint regions (2-fold in the median nerve, 5- to 10-fold in the sciatic). In addition, this difference in strain was conserved in the median nerve ex vivo, demonstrating an in-built longitudinal heterogeneity of mechanical properties. Tensile testing of isolated samples of joint and non-joint regions of both nerves showed that joint regions were less stiff (more compliant) than their non-joint counterparts with joint: non-joint stiffness ratios of 0.5 ± 0.07 in the median nerve, and 0.8 ± 0.02 in the sciatic. However, no structural differences identified at the light microscope level in fascicular/non-fascicular tissue architecture between these two nerve regions could explain the observed tensile heterogeneity. This identification of localized functional heterogeneity in tensile properties is particularly important in understanding normal dynamic nerve physiology, provides clues to why peripheral nerve repair outcomes are variable, and suggests potential novel therapeutic targets.
Peripheral nerves are remarkable tissues that not only conduct electrical impulses, but also must bend and stretch to accommodate the movement of limbs. In order to achieve this they have a complex structure consisting of bundles of neurones packed into fascicles and surrounded by connective tissue layers, the perineurium and epineurium. Both neural and connective tissue elements are tethered proximally at the spinal cord and have numerous branch points allowing neurones from a single nerve trunk to synapse with various target organs. Despite some physiological connections to surrounding tissue, nerve trunks are largely free to glide along their length within their tissue bed. However, the nerve routes through the limbs tend to lie outside the plane of movement of the joints making some degree of length change inevitable during normal function. When this ability of nerves to stretch and glide freely is compromised by adhesion to surrounding tissues, for example after surgical repair, limb movement can result in localized increases in tension leading to loss of function, pain or fibrosis (Millesi et al. 1990; Hunter, 1991).
Numerous studies have been undertaken to define the limits to which nerves can be stretched before their function is compromised. It is known that straining nerves beyond the physiological tension range can alter their conduction properties (Wall et al. 1992; Ochs et al. 2000) and intraneural blood flow (Lundborg & Rydevik, 1973) and can result in permanent loss of function believed to be related to a breakdown in integrity of the perineurium (Rydevik et al. 1990; Kwan et al. 1992).
Identifying the maximum tension which nerves can withstand and understanding the origin of their mechanical resilience is of great importance to improving the outcome of surgical nerve repairs. Their behaviour under loading is viscoelastic and is likely to be dependent upon a number of factors such as the internal fluid pressure maintained by the impermeable perineurium (Low et al. 1977), the outer–inner layer integrity (Walbeehm et al. 2004), the number and arrangement of fascicles (Sunderland & Bradley, 1961), and the molecular structural elements of the extracellular matrix such as collagen and elastin (Ushiki & Ide, 1990; Tassler et al. 1994). However, the concept that nerves are mechanically homogeneous may be inappropriate and misleading since the mechanical environmental loading varies at points along the limb (relating to branch point, joint, muscle, tendon and fascia location).
Since understanding the upper limit to which nerves can be stretched is such an important clinical goal, the investigation of stretch properties within normal physiological conditions has been somewhat neglected. In particular, there is scant information available on whether increased tension generated by limb movement is focused around the articulation, or dissipated along the full length of the nerve. The transverse heterogeneity of the nerve trunk is often discussed in terms of the contribution of different concentric anatomical layers to overall mechanical behaviour, but little is known about the presence of any longitudinal variation in these structures. Variations in the number of fascicles have been observed along the length of human nerves (Sunderland & Bradley, 1949) and increased fasciculation has been suggested to be a protective feature of nerves crossing joints (Sunderland, 1991). Such variation could cause localized sections of tissue to exhibit distinct tensile properties, yet little regard has been paid to this possibility where studies of mechanical properties are concerned.
The aim of this study was to test the hypothesis that mechanical properties of the peripheral nerve are adapted to increased local strains resulting from joint articulation.
Here we have shown that rat median and sciatic nerves undergo increased local strain in the region of articulations. This corresponds to an area of reduced stiffness relative to a region of the same nerve away from the joint. These variations in tensile properties do not correspond to variations in the distribution of fascicles or connective tissue as determined by histology, and so are likely to be due to adapted connective tissue architecture.
Methods
Two different rat nerves were selected for the experiments by virtue of the fact that they could be exposed with minimal disruption of their relationship to surrounding tissues, and contained distinct regions which ran around joints. The first was the median nerve in the rat forelimb, which runs around the inside of the elbow (joint region) then straight along the distal part of the limb (non-joint region). The second was the sciatic nerve, which runs around the outside of the hip joint (joint region) with straight (non-joint) regions proximal and distal.
Optical analysis of median nerve movement
In situ
Male Wistar rats (Harlan, UK), 200–250 g, were killed by asphyxiation and cervical dislocation according to UK Home Office guidelines and dissected within 30 min post mortem. To expose the median nerve, an incision was made midway between the biceps and the cubital fossa and extended superficially to the wrist, opening the skin and fascia down to the level of the muscles. Non-nerve-related connective tissue and fat were cleared from the area using microscopic dissection until the median nerve could be observed between the proximal side of the cubital fossa and the wrist. The exposed tissue was moistened continuously with phosphate buffered saline (PBS) at room temperature. The rat was pinned to a board such that the shoulder joint was immobilized in its extended position (defined as supine, shoulder abducted 90 deg, wrist 0 deg flexion/extension, elbow at 180 deg). Four optical location markers were spaced evenly along the exposed nerve, shown as A, B, C and D in Fig. 1A. The markers were 0.25 mm in diameter and were created by lightly applying Bonny's Blue tissue dye with the tip of a fine blunt glass needle. The first mark was placed midway between the distal limit of the biceps and the centre of the cubital fossa, then the other marks were made distally at 2 mm intervals with the limb in its extended position.
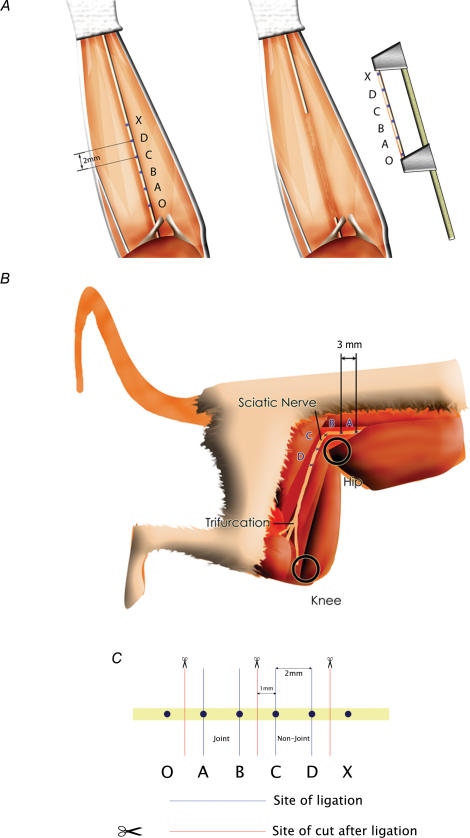
Figure 1.
Location of optical markings on median (A) (inset; jig for removal under native tension) and sciatic (B) nerves. C, the location of ligatures and transection sites on the median nerve for tensile testing.
Following marking of the nerve the forelimb was held with the elbow extended and photographed using a microscopic digital video camera (Scopeman® 504, Moritex, Cambridge, UK). Images were captured to a PowerMacintosh using Openlab software (Improvision, Coventry, UK) and calibrated against a millimetre scale positioned next to the nerve. The elbow was then flexed to an angle of 90 deg and another image was obtained. Distances between the centre points of the optical markers were measured using the digital images to give readings accurate to 50 μm. The anatomy of the joint region was such that upon flexion marker point A remained in the same plane as the other markers, allowing an accurate comparison to be made between the markers in the two positions. In this way the local change in length (strain) was measured across the ‘joint’ region of the median nerve (AB) and the ‘non-joint’ region 2 mm distal (CD) in both forelimbs of 5 rats.
Ex vivo
In order to investigate whether region-specific stretch was due to intrinsic structure or the influence of surrounding tissue, median nerves were excised under controlled conditions (below) and tested in isolation. Rats were prepared as described above and, after marking, the median nerve was carefully mobilized by microscopic dissection. Two additional marks were placed 2 mm outside the other four and denoted O and X (Fig. 1A). The distance between O and X was measured both with the limb extended and 90 deg flexed using a vernier calliper. With the limb held in the extended position, the nerve was clamped at points O and X in a purpose built jig which maintained the nerve at the measured in situ elongated length, then the nerve was excised by transection each side of the clamp (Fig. 1A, inset). Digital images of the excised nerve were recorded as before, and the clamp gauge-length was then adjusted to the previously measured 90 deg flexed length, simulating the shortened nerve during flexion. Digital images were captured at the shorter length and the distances AB and CD were measured for both positions to allow calculation of strain.
Optical analysis of sciatic nerve movement
Similar experiments were carried out using the rat sciatic nerve (i) at the ‘joint region’ that curves around the hip and (ii) at the ‘non-joint’ regions each side of this. Eight Wistar rats (female, 250–400 g) were anaesthetized by inhalation of isoflurane and a mixture of O2–N2O and subsequently killed by cervical dislocation. The sciatic nerve was exposed between 10 mm proximal to the hip joint and the trifurcation at mid-femoral level. During dissection the nerve was not mobilized from the tissue bed. To indicate different regions on the nerve, epineurial marking sutures (10/0) were placed 3 mm apart, with the hip and knee joints positioned in 90 deg of flexion. Four regions were marked: proximal to the hip joint (A), at the level of the hip joint (B and C), and distal to the hip joint (D) (Fig. 1B). The distal edge of the tendinous insertion of the external obturator muscle was used as an anatomical landmark to determine the position of the initial marking suture between regions B and C.
Images of the marked nerve were captured, with the hind leg in two different positions, using a digital video camera (Sony, model DCR-TRV240E). In position 1 the hip, knee and ankle joints were manually flexed to 90 deg. In position 2 the hip and knee joints were manually extended to 180 deg, whilst the ankle was maintained at 90 deg flexion. To calibrate the recorded distances, a reference measuring scale was placed alongside the nerve. Images were captured to PC using Studio DV® software (Pinnacle Systems Inc., Mountain View, CA, USA) and length measurements were determined using Image J (NIH). The ratio of change in length after extension against original length (strain) was calculated for each of the four regions.
Region-specific tensile testing
In addition to the optical characterization of nerve stretch, direct tensile tests were carried out to compare the material stiffness at joint and non-joint regions of median and sciatic nerves. Three male Wistar rats were killed by CO2 inhalation followed by cervical dislocation, then immediately dissected to harvest the nerves. In order to preserve the internal fluid composition of the sections of nerve, the nerves were ligated prior to transection (Walbeehm et al. 2004).
Figure 1C shows the position of the ligatures and the sites where the nerves were cut for the median nerve. The joint and non-joint sections of the nerves were carefully dissected away from the surrounding tissue and ligated using 6/0 prolene sutures (Ethicon, Somerville, NJ, USA) placed 2 mm apart (Fig. 1C), then excised and stored in PBS prior to tensile testing. For the sciatic nerve, the joint region was defined as a 2 mm region centred on the position defined previously for the first marking suture used in the optical analysis. The non-joint region was a 2 mm section located distally with 4 mm between the two regions. For testing, the nerve sections were clamped firmly in a jig by the regions outside the ligatures and stretched from slack to breaking point at 10 mm min−1 using a tensile testing machine (Testometric 220 m, Testometric Co Ltd, Rochdale, UK). Force was measured using a 10 N load cell (TEDEA-Huntleigh Ltd, Cardiff, UK) and extension via a linear voltage differential transformer (LVDT). Force–extension signals from the transducers were digitized using an ADC-100 analog to digital converter (Pico Technology Ltd, St Neots, UK) and data were recorded on a personal computer. The data files were exported to Microsoft Excel for the production of force–extension curves. The gradient of the linear part of each force–extension curve was recorded as a measure of the stiffness of each region, and the stiffness ratio (joint stiffness/non-joint stiffness) was calculated for each nerve.
Histological comparison of site-specific cross-sectional morphology
Samples of the joint and non-joint regions were harvested from fresh rat median and sciatic nerve specimens. The nerves were gently dissected out and layers of fascia connecting the nerve to surrounding tissue were trimmed away as close as possible to the outer layer of the nerve. After fixation in 4% paraformaldehyde, the sciatic nerve samples were wax embedded for routine histological sectioning and 6 μm transverse sections were stained with haematoxylin–eosin. The median nerve specimens were embedded in OCT tissue-embedding medium (Tissue-Tek®, Sakura Finetek Europe BV, Zoeterwoude, the Netherlands) and frozen in liquid nitrogen. Cryostat sections of 8 μm were stained with haematoxylin–eosin. Equivalent digital micrograph images were obtained from a typical joint and non-joint section of each nerve. For each section total nerve area, fascicular, and non-fascicular area were determined using Adobe Photoshop 4.0 (Adobe Systems Inc., San Jose, CA, USA) and the number of fascicles recorded. The ratio of non-fascicular: total nerve area was calculated and compared between the joint and non-joint region using Student's paired t test.
Results
Optical analysis of differential elongation
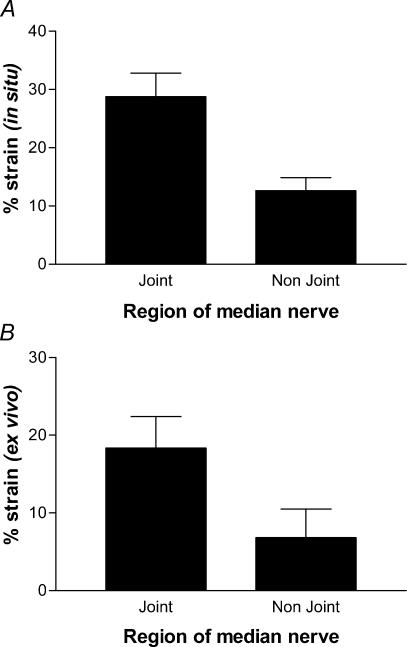
Analysis of the movement of marker points on the nerves gave an indication of how different regions responded to changes in overall length after repositioning the limbs. The change in displacement between the markers showed the strain at each nerve region. This strain is shown for the median nerve in Fig. 2. When the forelimb was moved in situ the nerve segment at the joint underwent more than twice the strain as the non-joint region (Fig. 2A) (P < 0.05). In order to establish whether this difference reflected a difference in material properties of the nerve tissue itself, or was a function of the internal limb environment, the procedure was repeated with the nerves excised (Fig. 2B). Once again the local mean deformation (strain) in the joint region was significantly greater (by a similar 2-fold factor to that in situ).
Figure 2. The joint region of the median nerve experiences more strain than the non-joint region.
Median nerves were extended in situ by limb movement (A) or stretched after excision (B). Local strain at two regions showed the same distinctive difference both in situ and ex vivo. Data are means ± s.e.m., n = 5 or 6 nerves for A and B, respectively, P = 0.02 and 0.006, respectively (paired t test).
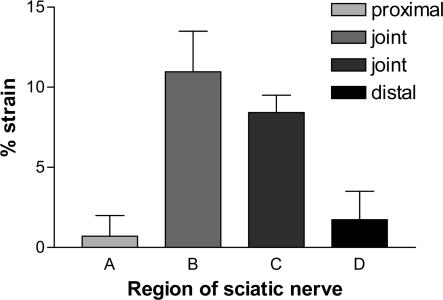
To confirm that this site-specific difference in nerve deformation was not a special feature of the median nerve, measurements were repeated in situ on the sciatic nerve. In this case local anatomy made it possible to compare regions both proximal and distal to the joint region (in the median nerve the region proximal to the joint is obscured by muscle). In the sciatic nerve the joint region spanned two of the segments under test. Figure 3 shows that the mean strain measured over the joint was between 5- and 10-fold greater than the flanking non-joint segments. Interestingly these differences in strain between nerve segments were seen at much lower levels of total applied strain (approx 1/3 that seen in median nerve). The difference between the joint region (B) and the proximal and distal non-joint regions was significant (P < 0.01).
Figure 3. In situ strain in the sciatic nerve during flexion and extension.
Four adjacent regions were monitored during flexion and extension of the rat sciatic nerve, proximal to the hip joint (A), level with the hip joint (B and C) and distal to the hip joint (D). Data shown are means + s.e.m. where n = 4 (A and C) or 6 (B and D). One way analysis of variance (ANOVA) showed a significant difference between the means (P < 0.01) and Tukey's multiple comparison test showed significant differences where groups A and B or B and D were compared.
Tensile testing
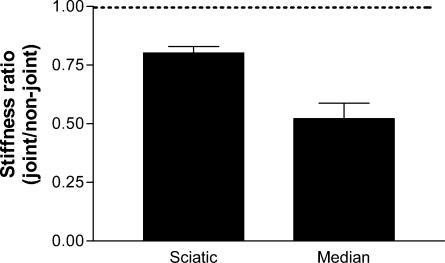
To compare directly the material properties of nerve tissue at joint and non-joint regions it was necessary to measure their stiffness. Force–extension curves were plotted for each region of each nerve tested, the gradient of which corresponded to the stiffness. From these data a ratio of joint: non-joint zone stiffness was obtained for each nerve (Fig. 4). In all cases the ratio was less than 1, indicating a clear trend of lower stiffness (greater compliance) in the joint segments. The mean ratio for median nerves was 0.5 ± 0.07, and 0.8 ± 0.02 for the sciatic indicating a significantly greater stiffness at sites away from joints (P < 0.01, t test comparing each set of experimental ratio data to null stiffness ratio of 1).
Figure 4. Stiffness ratios for joint and non-joint regions are less than 1 in both sciatic and median nerves.
Stiffness ratios were calculated from the slopes of the force–extension curves obtained from stretching joint and non-joint regions of sciatic and median nerves. Data are means + s.e.m., n = 5 (sciatic) or 6 (median). Dotted line (ratio of 1) indicates position of null stiffness ratio (i.e. no difference between regions).
Histology
Histological examination revealed more fascicles present in the non-joint than the joint regions of the sciatic nerve, but little difference between the two regions of the median nerve (Table 1). The proportion of the cross-sectional area of each nerve sample which comprised fascicular endoneurium, and that which comprised interfascicular epineurium (referred to as non-fascicular tissue) was calculated. Table 1 shows the proportion of the cross-section from each nerve specimen which was made up of non-fascicular tissue compared to the total cross section (non-fascicular + fascicular tissue area). In the median nerve there was no significant difference between the proportion of non-fascicular tissue measured in the joint (0.41 ± 0.04) compared to the non-joint (0.46 ± 0.03) samples. Interestingly there was almost twice as much non-fascicular tissue in the median than the sciatic nerve by proportion. The difference between the relative areas in the sciatic nerve was significant with a mean non-fascicular tissue proportion of 0.25 ± 0.006 in the joint region and 0.36 ± 0.02 in the non-joint region (P ≤0.005). There was no significant difference between the mean total cross-sectional area at the joint and the non-joint region in either of the nerves which is consistent with the lack of branching between the regions.
Table 1.
Histological comparison of non-fascicular (connective tissue) area relative to the total nerve area in transverse sections of the joint and non-joint region of median and sciatic nerves
| Ratio of non-fascicular area: total area | |||
|---|---|---|---|
| Nerve | Joint region | Non-joint region | No. of fascicles joint: non-joint |
| Median nerve | |||
| 1 | 0.45 | 0.40 | 3 : 3 |
| 2 | 0.52 | 0.43 | 3 : 3 |
| 3 | 0.34 | 0.42 | 2 : 3 |
| 4 | 0.31 | 0.55 | 2 : 3 |
| 5 | 0.43 | 0.48 | 1 : 3 |
| Mean (s.e.m.) | 0.41 (0.04) | 0.46 (0.03) | — |
| Sciatic nerve | |||
| 1 | 0.24 | 0.29 | 1 : 2 |
| 2 | 0.23 | 0.30 | 1 : 2 |
| 3 | 0.26 | 0.37 | 1 : 2 |
| 4 | 0.23 | 0.40 | 1 : 2 |
| 5 | 0.27 | 0.36 | 1 : 3 |
| 6 | 0.24 | 0.43 | 1 : 2 |
| Mean (s.e.m.) | 0.25 (0.006) | 0.36 (0.02) | — |
The difference in the mean ratio of joint versus non-joint was significant in the sciatic nerve (P < 0.005), but not in the median nerve using a paired t test. Number of fascicles is also shown as determined for the two regions of each nerve.
Discussion
In situ experiments in both the sciatic and median nerves showed that during normal extension and flexion the joint regions underwent greater deformation than the corresponding non-joint regions. Whilst this may seem intuitive, and was suggested as likely by Sir Sydney Sunderland more than a decade ago (Sunderland, 1991), it has not previously been demonstrated in an experimental system. Here we have shown quantitatively, in two different nerves in the rat, that there are regions near joints which undergo greater elongation than other areas during limb movement. However, this is not a simple function of greater loading over joints but rather reflects distinct material properties of the two zones, joint regions being inherently more compliant.
When median nerve strain was optically analysed ex vivo, it was found that the joint region stretched more than the non-joint region. This demonstrated that the structure of the nerve varied along its length in terms of its capacity to respond to tensile loads comparable to those generated during limb movement. This is in contrast to a previous study in which no differences could be detected in the stretch properties of different segments of median nerve harvested from human cadavers (Millesi et al. 1990), although the experimental details in that study were unclear and fixation may have affected the tissue.
Here we have confirmed the presence of distinct regional heterogeneities in terms of functional tensile properties, by direct stiffness measurement in isolated tissues. This revealed that the joint regions of both nerves were more compliant than the comparator (non-joint) regions. There are a number of key differences between the sciatic and median nerve in the rat. In particular, the sciatic nerve is of greater diameter and runs around the outside of the hip joint whereas the median nerve follows the inside of the elbow joint. Also, the regions chosen for comparison in the sciatic nerve were adjacent, but more widely spaced in the median nerve. The results presented here do not seek to compare these two nerves to each other, but use each as an independent demonstration of localized heterogeneity. Since both the sciatic and the median nerve exhibit a number of branch points along their length which may influence the local mechanical environment, care was taken to ensure the regions chosen for stiffness comparison were free from branching, both by direct examination and consultation with an anatomical reference (Hebel & Stromberg, 1986).
Histological data showed that differences in stiffness cannot be explained simply by the number of fascicles present. Previous work by Sunderland & Bradley (1949), who used similar analyses to explore nerves in human cadavers, showed that more fascicles were present (and therefore more non-fascicular connective tissue) in regions where the nerves passed near joints, leading to the later suggestion that this was a protective feature by which vulnerable areas of nerves resisted mechanical injury (Sunderland, 1991). In contrast we show here that in two regions of the rat median nerve with differences in mechanical properties there were no differences in the proportion of connective (non-fascicular) tissue between the joint and non-joint regions. In the sciatic nerve there were more fascicles (and more non-fascicular connective tissue) in the non-joint region than at the joint. This observation is opposite to that which might be predicted from Sunderland's work on human nerves, and does not support the idea that increased fasciculation is a means by which nerves reduce strain around joints. There is no simple relationship here between morphology, connective tissue volume and tensile properties. However, this is not unusual in biomechanics and only suggests that functional tensile testing is a more appropriate measure of the mechanical behaviour of nerves than histology.
Mechanical tension is an important issue for reconstructive surgery and nerve repair and consequently a number of studies have investigated changes in tension in human cadaveric nerves during limb movement (Kleinrensink et al. 1995; Wright et al. 2001; Hicks & Toby, 2002; Byl et al. 2002). Previous investigators have sought to place values on the degree to which nerves can be stretched before they become compromised through changes in conduction, blood flow, or integrity of intraneural structures (Lundborg & Rydevik, 1973; Rydevik et al. 1990; Wall et al. 1992; Kwan et al. 1992; Millesi et al. 1995; Ochs et al. 2000). These studies have failed to agree on an absolute strain-limit value, chiefly due to (i) difficulties in determining original length and disagreement on what constitutes nerve resting tension and (ii) differences in post mortem treatments (fixation, ligation, incubation, etc.). This study identifies a further cause in that longitudinal heterogeneity will lead to variance in tensile testing experiments unless properly controlled.
The observation that nerves exhibit longitudinal heterogeneity of material properties poses a number of intriguing questions regarding the way in which nerves are adapted to accommodate limb movement. The viscoelastic properties of nerves have been measured extensively (Sunderland & Bradley, 1961; Wall et al. 1991; Kwan et al. 1992; Millesi et al. 1995; Driscoll et al. 2002) but the key structural elements which permit this elasticity remain elusive. For many years some observers have believed nerve fibres exhibit a zigzag morphology which disappears upon stretching as the fibres straighten out. This has been related to the presence of the spiral bands of Fontana that also fade with stretching (Clarke & Bearn, 1972). However, the zigzag arrangement of fibres has not always been observed in vivo (Williams & Hall, 1970), and application of sufficient tension to straighten out the fibres ex vivo did not result in disappearance of the bands of Fontana, which only occurred following a further tension increase (Pourmand et al. 1994). Earlier work by Glees (1942) identified the incisures of Schmidt-Lantermann as sites at which the myelin sheath could telescope to accommodate stretch, and proposed that this could confer stretching ability. Further investigations are required in order to investigate the properties of nerve fibres which allow them to accommodate the strain experienced by the nerve trunk. At a molecular level, elastin fibres are present throughout the tissue layers of peripheral nerves (Tassler et al. 1994) and collagen fibres are arranged in such as way as to allow some degree of longitudinal stretch (Glees, 1942; Ushiki & Ide, 1990). The specialized arrangement of layers of collagen fibres is likely to be the underlying structural component, in conjunction with fluid pressure, which provides the nerve with its viscoelasticity. There may also be differential movement possible between fascicle and non-fascicle elements. Further studies are planned to investigate differences in collagen architecture in different regions of a nerve which could account for the local differences in stiffness.
Another matter for consideration, which arises from these findings, is the means by which regions of differing stiffness develop. It would be interesting to examine whether reduced stiffness is an intrinsic property of nerve regions adjacent to joints or whether this property is conferred upon the nerve by movement. An understanding of this phenomenon may yield important information when undertaking surgical repair of peripheral nerves. For example would movement be sufficient to decrease the stiffness of a graft at a joint region, and if not, would inappropriate local stiffness compromise the clinical outcome?
In conclusion, the results presented here demonstrate that the strain on nerves during limb movement is not equally distributed along their length, but is increased at articulations. Furthermore, the stiffness of nerve tissue varies longitudinally, with regions near joints being more compliant to tensile loading than elsewhere. However, these properties seem to result from complex tissue architecture rather than simple proportion of connective tissue or fascicular number.
Acknowledgments
The authors are grateful to Andrew McCulloch for technical assistance with the tensile testing experiments, Ellen Riem for assistance with the histology, and Professor Susan Hall for helpful comments on the manuscript. Financial support was from the EU framework 5 programme in neural tissue engineering (QLK3-CT-1999–00625).
References
- Byl C, Puttlitz C, Byl N, Lotz J, Topp K. Strain in the median and ulnar nerves during upper-extremity positioning. J Hand Surg. 2002;27:1032–1040. doi: 10.1053/jhsu.2002.35886. [DOI] [PubMed] [Google Scholar]
- Clarke E, Bearn JG. The spiral Bands of Fontana. Brain. 1972;95:1–20. doi: 10.1093/brain/95.1.1. [DOI] [PubMed] [Google Scholar]
- Driscoll PJ, Glasby MA, Lawson GM. An in vivo study of peripheral nerves in continuity: biomechanical and physiological responses to elongation. J Orthop Res. 2002;20:370–375. doi: 10.1016/S0736-0266(01)00104-8. [DOI] [PubMed] [Google Scholar]
- Glees P. Observations on the connective tissue sheaths of peripheral nerves. J Anat. 1942;77:153–159. [PMC free article] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy and Embryology of the Laboratory Rat. Germany: Biomed Verlag, Wörthsee; 1986. [Google Scholar]
- Hicks D, Toby EB. Ulner nerve strains at the elbow: the effect of in situ decompression and medial epicondylectomy. J Hand Surg. 2002;27:1026–1031. doi: 10.1053/jhsu.2002.35870. [DOI] [PubMed] [Google Scholar]
- Hunter JM. Recurrent carpal tunnel syndrome, epineural fibrous fixation, and traction neuropathy. Hand Clin. 1991;7:491–504. [PubMed] [Google Scholar]
- Kleinrensink GJ, Stoeckart R, Vleeming A, Snijders CJ, Mulder PGH. Mechanical tension in the median nerve. The effects of joint positions. Clin Biomech. 1995;10:240–244. doi: 10.1016/0268-0033(95)99801-8. [DOI] [PubMed] [Google Scholar]
- Kwan MK, Wall EJ, Massie J, Garfin SR. Strain, stress and stretch of peripheral nerve. Rabbit experiments in vitro and in vivo. Acta Orthop Scand. 1992;63:267–272. doi: 10.3109/17453679209154780. [DOI] [PubMed] [Google Scholar]
- Low P, Marchand G, Knox F, Dyck PJ. Measurement of endoneurial fluid pressure with polyethylene matrix capsules. Brain Res. 1977;122:373–377. doi: 10.1016/0006-8993(77)90305-5. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg Br. 1973;55:390–401. [PubMed] [Google Scholar]
- Millesi H, Zöch G, Rath T. The gliding apparatus of peripheral nerve and its clinical significance. Ann Hand Surg. 1990;9:87–97. doi: 10.1016/s0753-9053(05)80485-5. [DOI] [PubMed] [Google Scholar]
- Millesi H, Zöch G, Reihsner R. Mechanical properties of peripheral nerves. Clin Orthop. 1995;314:76–83. [PubMed] [Google Scholar]
- Ochs S, Pourmand R, Si K, Friedman RN. Stretch of mammalian nerve in vitro: Effect on compound action potentials. J Peripher Nerv Syst. 2000;5:227–235. doi: 10.1046/j.1529-8027.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Pourmand R, Ochs S, Jersild RA., Jr The relation of the beading of myelinated nerve fibres to the bands of Fontana. Neuroscience. 1994;61:373–380. doi: 10.1016/0306-4522(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Rydevik BL, Kwan MK, Myers RR, Brown RA, Triggs KJ, Woo SL, Garfin SR. An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res. 1990;8:694–701. doi: 10.1002/jor.1100080511. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Nerve Injuries and Their Repair. A Critical Appraisal. Edinburgh, London, Melbourne and New York: Churchill Livingstone; 1991. [Google Scholar]
- Sunderland S, Bradley KC. The cross-sectional area of peripheral nerve trunks devoted to nerve fibres. Brain. 1949;72:428–449. doi: 10.1093/brain/72.3.428. [DOI] [PubMed] [Google Scholar]
- Sunderland S, Bradley KC. Stress-strain phenomena in human peripheral nerve trunks. Brain. 1961;84:102–119. [Google Scholar]
- Tassler PL, Dellon AL, Canoun C. Identification of elastic fibres in the peripheral nerve. J Hand Surg (Br Eur) 1994;19B:48–54. doi: 10.1016/0266-7681(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Ushiki T, Ide C. Three-dimensional organisation of the collagen fibrils in the rat sciatic nerve as revealed by transmission- and scanning electron microscopy. Cell Tissue Res. 1990;260:175–184. doi: 10.1007/BF00297503. [DOI] [PubMed] [Google Scholar]
- Walbeehm ET, Afoke A, de Wit T, Holman F, Hovius SER, Brown RA. Mechanical functioning of peripheral nerves: linkage with the ‘mushrooming’ effect. Cell Tissue Res. 2004;316:115–121. doi: 10.1007/s00441-004-0867-9. [DOI] [PubMed] [Google Scholar]
- Wall EJ, Kwan MK, Rydevik BL, Woo SL, Garfin SR. Stress relaxation of a peripheral nerve. J Hand Surg (Am) 1991;16:859–863. doi: 10.1016/s0363-5023(10)80149-2. [DOI] [PubMed] [Google Scholar]
- Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR. Experimental stretch neuropathy. J Bone Joint Surg. 1992;74:126–129. doi: 10.1302/0301-620X.74B1.1732240. [DOI] [PubMed] [Google Scholar]
- Williams PL, Hall SM. In vivo observations on mature unmyelinated nerve fibres of the mouse. Changes in nerve conduction under tension. J Anat. 1970;107:31–38. [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Glowczewskie F, Cowin D, Wheeler DL. Ulner nerve excursion and strain at the elbow and wrist associated with upper extremity motion. J Hand Surg. 2001;26A:655–662. doi: 10.1053/jhsu.2001.26140. [DOI] [PubMed] [Google Scholar]