Abstract
The study was designed to determine to what extent moderate elevation of renal perfusion pressure (RPP) via the mechanism of ‘pressure natriuresis’ contributes to the natriuresis induced by acute i.v. saline loading. Nine Beagle dogs maintained on ample sodium intake (5.5 mmol (kg body mass)−1 day−1) were chronically equipped with an aortic occluder to servocontrol RPP, a bladder catheter to measure renal function, and catheters for measurement of RPP and mean arterial blood pressure (MABP). A swivel system allowed free movement in the kennel during experiments. Isotonic saline loading (500 ml in 100 min) was studied as follows: with and without servocontrol of RPP, and these two protocols repeated in the presence of angiotensin-converting enzyme inhibition (ACEI, Enalapril, 2 mg (kg body mass)−1). Saline loading increased MABP by about 12 mmHg and sodium excretion from about 28 μmol min−1 up to about 350 μmol min−1. Without ACEI, servocontrol of RPP at 10% below control 24 h MABP slightly delayed the onset of the saline-induced natriuresis, but did not reduce peak sodium excretion or cumulative sodium excretion. The slight delay most probably resulted from pressure-controlled renin release because, with ACEI, servocontrol of RPP did not delay or reduce the saline-induced natriuresis. In conclusion, pressure natriuresis does not contribute to the natriuresis following acute saline loading.
The amount of body fluids and electrolytes are controlled within tight boundaries. The control of total body sodium (TBS) is of particular interest, since TBS is a major determinant of mean arterial blood pressure (MABP) (Guyton, 1991; Cowley, 1992; Hall et al. 1996; Reinhardt & Seeliger, 2000). In the face of considerable variations in Na+ intake and extrarenal Na+ losses, maintenance of TBS depends critically on control of renal Na+ excretion. Various hormonal, neuronal and physical factors impinge on renal Na+ excretion. Among these factors, however, arterial pressure has been considered to play an outstanding role (Guyton, 1990a, b, 1991; Cowley, 1992). It is well known that an increase of MABP may augment Na+ excretion via intrarenal effects of elevated renal perfusion pressure (RPP). This concept is termed ‘pressure natriuresis’. Conversely, decreased RPP may reduce Na+ excretion. The intrarenal mechanism underlying pressure natriuresis and putative low-pressure antinatriuresis is not fully understood (Cowley, 1992). However, pressure natriuresis has been assumed to be a dominating controller of Na+ excretion in long-term TBS control as well as in acute control (Guyton, 1990a, 1991; Cowley, 1992; Hall et al. 1996). It has been supposed that this mechanism operates at any pressure, and that even small changes in RPP would change Na+ excretion markedly (Guyton, 1990a, b, 1991).
The specific contribution of pressure natriuresis to control of TBS is still under debate, possibly because it is confounded by the interference with the renin–angiotensin–aldosterone system (RAAS). Renin release is also controlled by RPP (pressure-controlled renin release) (Hackenthal et al. 1990). Thus, alterations in RPP inevitably change the activity of the RAAS. Furthermore, renin release is also controlled by changes in TBS independent of arterial pressure (Seeliger et al. 1999). On the effector side, the RAAS controls TBS via renal action of angiotensin II (Ang II) and aldosterone (Aldo).
Studies in freely moving dogs, reviewed by Reinhardt & Seeliger (2000), helped to clarify the specific contributions to long-term TBS control of both pressure natriuresis and the RAAS. TBS can be controlled independently of pressure natriuresis and changes of the RAAS are very effective in controlling TBS. A significant contribution of pressure natriuresis could only be demonstrated under certain pathophysiological conditions, namely, during sustained elevation of TBS, e.g. induced by continued administration of antinatriuretic stimuli. The resulting long-term elevation of RPP by 25 mmHg or more facilitates Na+ excretion via pressure natriuresis mechanism. Long-term reduction of RPP by about 25 mmHg results in Na+ retention, but only via pressure-controlled renin release, not by the putative mechanism of low-pressure antinatriuresis.
In the light of these results, it appeared worthwhile to re-evaluate the contribution of pressure natriuresis to short-term TBS control in an experimental setting that includes moderate changes in RPP. We chose the well-known paradigm of natriuresis induced by acute isotonic i.v. NaCl-loading (sodium-loading natriuresis, SLN). It is known that pressure natriuresis is not a prerequisite for SLN: when small amounts of saline are slowly infused at rates insufficient to cause any blood pressure increase, Na+ excretion still increases markedly (Sandgaard et al. 2000). Therefore, we infused isotonic NaCl at a rate of 5 ml min−1, which has been shown in previous studies to increase pressure by about 10 mmHg (Andersen et al. 2000; Bie & Sandgaard, 2000).
SLN has been used before to assess the contribution of pressure natriuresis to TBS control; however, the results are conflicting, probably due to the specific experimental procedures. In one study, Na+ loading was performed while arterial pressure was prevented from rising by means of a servocontrolled infusion of sodium nitroprusside. SLN was reduced by about 30% (Andersen et al. 2000). However, prevention of blood pressure elevation by systemic administration of drugs, e.g. a NO donor, may influence Na+ excretion in several ways, e.g. by altering renal haemodynamics, tubular ion transports, and Aldo secretion (Persson et al. 1993; Manning et al. 1994; Qiu et al. 1998; Seeliger et al. 2001). In two other studies, suprarenal aortic constriction was used. The results of one study (Cowley & Skelton, 1991) appear to indicate that pressure natriuresis would not substantially contribute to SLN. However, in this study, Na+ loading was performed during administration of a cocktail of Ang II, Aldo, arginine vasopressin (AVP), and atrial natriuretic peptide (ANP) in dogs whose kidneys were bilaterally denervated. Thus, this study was not designed to assess the relative contribution of pressure natriuresis under normal conditions. In the other study (Kaczmarczyk et al. 1992), a 15% reduction of RPP was reported to reduce SLN by some 30%. However, this attenuation of natriuresis may stem from small increase of Ang II, as induced by the reduction of RPP, rather than from prevention of pressure natriuresis (Boemke et al. 1995).
In the present study in freely moving dogs, we used fast-acting servocontrolled aortic constriction to reduce RPP. We studied dogs with and without angiotensin-converting enzyme (ACE) inhibition in order to separate the effects of changes in RAAS activity resulting from pressure-controlled renin release from those of pressure natriuresis.
Methods
Nine chronically instrumented female Beagle dogs, ∼2 years of age, weighing 12–16 kg, were studied by standardized methods described in detail in previous papers (Reinhardt et al. 1990, 1994; Boemke et al. 1995). On completion of the experimental period, implants were removed and the dogs were given to suitable private individuals. The study was approved by the Berlin Government according to the German Animal Protection Law.
Surgery and maintenance
Each dog was equipped with a urinary bladder catheter, an inflatable cuff placed around the aorta above the renal arteries, one femoral vein catheter, and two femoral artery catheters. The right femoral artery catheter was advanced into the abdominal aorta directly below the renal arteries; the tip of the left femoral artery catheter was placed well above the renal arteries. The lines were exteriorized in the nape region. All operations were performed under aseptic conditions in an operating room. General anaesthesia was induced with methohexital (8 mg (kg body mass)−1 intravenously). After endotracheal intubation, anaesthesia was maintained under controlled ventilation (about 1.5 l min−1) with halothane (0.8–1.5%) and nitrous oxide–oxygen (2: 1). The depth of anaesthesia was clinically assessed, i.e. anaesthesia was increased by increasing inspiratory halothane concentration when the dog displayed sudden increases in heart rate or blood pressure during surgical procedures, started to breath spontaneously, or moved. The dogs were allowed at least 3 weeks for recovery. Catheter-related infections were prevented with a catheter-restricted antibiotic-lock technique (Palm et al. 1991). Daily assessments of general status, and daily measurements of body temperature, weight, and erythrocyte sedimentation rate ensured that only healthy dogs were studied. The dogs were housed individually in large kennels (9 m2) in a sound-protected, air-conditioned animal room. For reasons of social well-being, another dog in an adjacent kennel accompanied the dog under investigation (Boemke et al. 1995). Each dog was studied on four occasions (experimental days), which were separated by at least 1 day without an experiment.
Dietary regimen
Beginning at least 5 days before the studies, food intake was controlled with regard to daily feeding time, completeness of intake, and food composition. The dogs were offered the food once daily at 13.00 h (on experimental days, it was offered after the experiment). If a dog did not finish its meal within 20 min, the remainder was tube-fed to guarantee complete food and water intake. Thereafter the dog did not have access to food or water until the feeding the next day. The food provided 5.5 mmol of Na+, 3.5 mmol of K+, and 91 ml of water per day per kg of body mass (i.e. 82.5 mmol of Na+, 52.5 mmol of K+, and 1365 ml of water per day to a 15 kg dog). On experimental days, food composition was the same, except that only 0.5 mmol Na+ (kg body mass)−1 was given in order to compensate for the 76 mmol Na+ already administered on that day as part of the study (see below).
Experimental configuration
During the experiment, the chronic lines coming from the dog were connected to a swivel system which allowed free movement within the limits of the 9 m2 kennel (for details see Reinhardt et al. 1990; Boemke et al. 1995). The dogs were well accustomed to this equipment. From the swivel, the lines were led to the adjoining laboratory via a protective tube. Thus, all actions necessary to conduct the experiments were performed from the laboratory without drawing the attention of the dogs in the sound-protected animal room. Systemic mean arterial blood pressure (MABP, catheter above the aortic occluder) and RPP (catheter below the aortic occluder) were measured with pressure transducers integrated into the swivel (Reinhardt et al. 1990). Urine was collected by means of a computerized collection system (Reinhardt et al. 1990).
Experimental time schedule, protocols and interventions
Four protocols were performed in each dog in random order. All experiments were carried out in the morning (08.00–13.00 h). After connecting the dog to the swivel system, urine sampling (20 min sampling periods) and registration of haemodynamic variables were started and continued until the end of the experiment. Each protocol consisted of a 60 min ‘running-in’ period, followed by a 60 min control phase (three 20 min sampling periods C1, C2, C3), a 100 min phase during which Na+ loading was performed by infusion (five 20 min sampling periods I1 to I5), and a 40 min post-infusion phase (two 20 min sampling periods P1, P2).
NaCl loading.
NaCl loading was achieved by continuous i.v. infusion of a sterile isotonic solution containing 152 ± 1 mmol NaCl l−1, at a rate of 5 ml min−1, corresponding to 0.76 mmol Na+ min−1. Thus, the total amount infused within 100 min was 500 ml containing 76 mmol Na+.
Protocols.
Protocols differed (1) by servocontrolling RPP (scRPP) or leaving it free to change with MABP (fRPP), and (2) by inhibiting ACE with Enalapril (E) or leaving ACE unimpeded. Thus, the four protocols were termed fRPP, scRPP, fRPP + E, scRPP + E.
Servo control of RPP.
Servo control of RPP was achieved by regulating the degree of inflation of the aortic occluder using a fast-acting servocontrol device as described in detail by Nafz et al. (1992). Briefly, the electrical analog signal of the pressure transducer (Micro Switch 13PC066G1, Freeport, Illinois, USA) that reflects instantaneous RPP was fed into the electronic servocontrol apparatus. Here, the systolic maxima and diastolic minima of this signal were constantly compared with the higher and the lower limit of a pre-set pressure amplitude that was adjusted to the intended pre-set value of mean pressure. The digital outputs resulting from these comparisons drove two solenoid valves. One valve steered the inflow of compressed air into the pneumatic system, the other one steered the outflow from this system. The pneumatic system consisted of a windkessel, the Silastic tubing connecting the servocontrol apparatus with the aortic cuff, and the aortic cuff (produced in our laboratory with Dacron reinforced Silastic material). These elements have both resistance and dampening characteristics that decelerate the air flow into and from the cuff. The response velocity of the servocontrol system in vivo as determined by step changes in pre-set pressure is about 1–2 mmHg s−1 (Nafz et al. 1992). Servocontrol was initiated at the beginning of the running-in time and remained in constant operation until the end of the experiment. In protocol scRPP, the level of servocontrol was set 10% below the individual dog's 24 h mean value of MABP during a control study without infusion. This level of RPP was chosen because in freely moving dogs MABP is well known to vary considerably within the time frame of minutes through hours. Thus, RPP was set below the expected lowest level of MABP fluctuations. Accordingly, in protocol scRPP + E, the level of servocontrol was set 15% below this 24 h mean value, because ACE inhibition was expected to reduce MABP.
ACE inhibition.
ACE inhibition was achieved by i.v. bolus injection of Enalapril maleate (Sigma, St Louis, MO, USA; 2 mg (kg body mass)−1), administered at the beginning of the running-in time. The completeness of ACE inhibition was tested by bolus injections of Ang I (2 and 4 μg) immediately after completion of the postinfusion phase. Whereas this procedure resulted in marked increase of MABP (up to 35 mmHg) in dogs without ACE inhibition (tested in 4 experiments), it never increased MABP in dogs treated with Enalapril (10 experiments).
Measurements
MABP, RPP and heart rate.
MABP, RPP and heart rate (HR) were measured continuously; then mean values over 20 min (representing each urine sampling period) were calculated.
Urine collection and analysis.
Urine collections were performed continuously during the experiment. Urine volume was measured gravimetrically (Reinhardt et al. 1990). The urine collected during a 20 min period was analysed for Na+, K+, lithium and creatinine concentrations and osmolality.
Clearances.
Clearance of exogenous creatinine was used to assess the glomerular filtration rate (GFR), and lithium clearance used for calculation of proximal tubular reabsorption rate via fractional lithium excretion (FELi). As described by Corea et al. (1996), a priming dose of lithium (3 mmol Li2CO2; Quilonium retard oblong, SKD, Giessen, Germany) was fed 48 and 24 h prior of the experiment. An oral creatinine bolus of 50 ml creatinine 2% was given 60 min before starting the running-in time. During the running-in time and throughout the experiment, creatinine (26 μmol min−1) and lithium chloride (1.7 μmol min−1) were infused via the infrarenal aortic catheter.
Blood samples.
Blood samples were collected in periods C1, C3, I5, and P2 via the suprarenal aortic catheter. The blood withdrawn was always replaced by an equal amount of stored blood collected from the respective dog about 2 weeks before the experiments. Immediately after sampling, centrifugation for determination of haematocrit and separation of plasma took place. In each plasma sample, concentrations of sodium (PNa), potassium, lithium, creatinine, protein (PProt), aldosterone (PAC), atrial natriuretic peptide (ANP), antidiuretic hormone (AVP), as well as osmolality and plasma renin activity (PRA) were measured.
Analyses, calculations, and statistics
Na+, K+, and Li+ concentrations in plasma and urine were measured by flame photometry, osmolality by freezing point depression, protein by biuret method, and creatinine by modified Jaffé reaction (Creatinine-Analyser II, Beckman Instruments, Galway, Ireland). PRA and PAC were measured with commercially available radioimmunoassays as described earlier (Reinhardt et al. 1994). For measurement of ANP a specific antibody (RAS 8798 Peninsula Laboratories) was used. The assay was carried out according to Schütten et al. (1987) with slight modifications (extraction elution with 80% ethanol, 4% acetic acid solution, separation of free and bound tracer with charcoal–ox plasma buffer solution). The detection limit was 2.0 pg ml−1 and the mean extraction recovery of unlabelled ANP added to plasma was 78%. To determine AVP, an antibody (AB3096; raised in rabbits in P. Bie's laboratory) was used at a final antibody dilution of 1: 800 000. The assay was performed according to Emmeluth et al. (1994) except for the said antibody and other minor changes (bovine albumin in assay buffer and 0.27% charcoal in buffer solution for separation of free and bound tracer). The detection limit was 0.15 pg ml−1 and the mean recovery of unlabelled AVP added to plasma was 69%.
Cumulative excretions were calculated by adding up excretion rates of the consecutive infusion and post-infusion periods (excretions during control time were not included). Clearances, fractional excretions, tubular loads, and amounts of tubular reabsorption were calculated by standard formulae.
Statistical comparisons were made using the NCSS statistical software (Hintze, Kaysville, UT, USA). Differences between the protocols fRPP versus scRPP, fRPP versus fRPP + E, scRPP versus scRPP + E, and fRPP + E versus scRPP + E, as well as among periods within one protocol were assessed by variance analysis (GLM ANOVA for repeated measurements) followed by Duncan's multiple comparison test with a significance level of P < 0.05. Data are given as means ±s.e.m.
Results
Haemodynamic variables
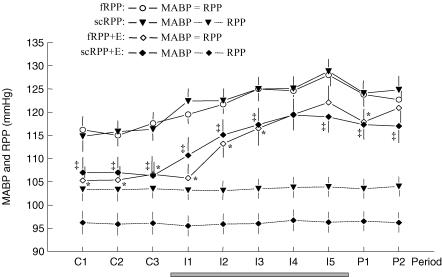
Without servocontrol of RPP (protocol fRPP), systemic mean arterial blood pressure (MABP; Fig. 1) averaged ∼116 mmHg during control periods. During saline infusion, MABP increased gradually by ∼12 mmHg. After the infusion, MABP returned towards baseline. With servocontrol of RPP (protocol scRPP) MABP did not differ measurably from that observed without servocontrol (fRPP). It averaged ∼116 mmHg during control periods, and increased by ∼13 mmHg during infusion. With Enalapril (protocol fRPP + E) control MABP was reduced by ∼11 mmHg and averaged ∼105 mmHg. It increased by ∼16 mmHg during infusion. With servocontrol during Enalapril (protocol scRPP + E) systemic MABP did not differ from that observed during the corresponding series without servocontrol (fRPP + E). It averaged ∼107 mmHg during control periods, and increased by ∼13 mmHg during infusion. Thus, irrespective of other interventions, saline infusion increased MABP by 12–16 mmHg while Enalapril pretreatment caused a reduction of 9–11 mmHg.
Figure 1. 20-min mean values of systemic mean arterial blood pressure (MAPB) and renal perfusion pressure (RPP).
Values are mean ±s.e.m. of 9 dogs studied in 4 protocols during 3 control periods (C1-C3), 5 periods with Na+ loading by i.v. infusion (I1–I5) and 2 postinfusion periods (P1, P2). Grey bar indicates period of infusion. RPP was free to change with MABP in protocols fRPP and fRPP + E (E = Enalapril), and was servocontrolled at pre-set reduced levels in protocols scRPP and scRPP + E. Individual pre-set levels of servocontrol of RPP were used to account for the interindividual variability of control MABP. For these protocols, RPP is plotted separately (error bars mirror individual pre-set levels). Significant differences obtained by GLM ANOVA for repeated measurements followed by Duncan's multiple comparison test: * significant versus fRPP, ‡ significant versus scRPP; within each protocol, MABP was higher during I2 through P2 as compared with control periods.
The servocontrol of renal perfusion pressure (RPP) was very effective (Fig. 1). Individual set points of RPP were applied to compensate for the interindividual variation of control MABP. Mean RPP was 103.5 mmHg without Enalapril (scRPP) and 96.0 mmHg during Enalapril (scRPP + E). The error bars of RPP in Fig. 1 mirror the scatter of the set points. Within every experiment, the servocontrol system kept RPP constant regardless of changes in MABP. In particular, RPP remained constant when MABP increased due to infusion. In contrast, without activation of the servocontrol (protocols fRPP and fRPP + E), RPP increased with MABP.
Heart rate (HR, data not shown) averaged ∼85 beats min−1 during control periods without Enalapril pretreatment (fRPP and scRPP). In both protocols HR increased by ∼10 beats min−1 with saline infusion, yet returned to control levels thereafter. HR was higher during the control periods after Enalapril (fRPP + E and scRPP + E, ∼100 beats min−1), and did not change significantly during saline infusion. However, it decreased by ∼15 beats min−1 during postinfusion periods.
Renal variables
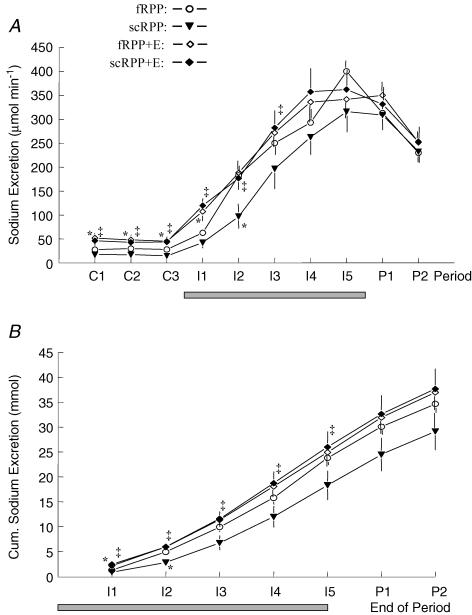
Urinary sodium excretion (UNaV) averaged ∼28 μmol min−1 during control periods without intervention (fRPP protocol, Fig. 2A). It increased during the first saline infusion period, and continued to increase throughout the infusion. Servocontrol of RPP (scRPP protocol) lowered control UNaV slightly but insignificantly (mean ∼17 μmol min−1). Subsequent saline infusion increased UNaV to levels similar to those observed without servocontrol (fRPP), except that the increase was slightly delayed (significantly lower UNaV in the second period of infusion). Enalapril per se (fRPP + E) increased UNaV markedly in control periods (∼48 μmol min−1). This Enalapril-induced difference vanished, however, during the saline infusion, i.e. the rates of UNaV were not different anymore beyond the first period of infusion (fRPP + E versus fRPP). Remarkably, compared with Enalapril alone, servocontrol of RPP during Enalapril did not affect UNaV with regard to control value (∼45 μmol min−1), or to the velocity of increase or the maximal value (fRPP + E versus scRPP + E). Hence, comparing the natriuretic responses in the two series with servocontrol of RPP (scRPP + E versus scRPP), it can be seen that Enalapril increased control UNaV markedly and augmented the natriuresis so that the difference in time course between the two series was present for 60 min.
Figure 2. Urinary sodium excretion (A) throughout the experiments, and cumulative urinary sodium excretion at the end of the respective infusion and postinfusion periods (B).
Grey bars indicate period of infusion. Values are mean ±s.e.m. of 9 dogs studied in 4 protocols. For protocols and periods see legend to Fig. 1. Significant differences: * significant versus fRPP, ‡ significant versus scRPP; within each protocol, sodium excretion was higher during I1 through P2 as compared with control periods.
For direct comparison of magnitude and velocity of UNaV responses to infusion, cumulative sodium excretion during infusion and postinfusion periods is depicted in Fig. 2B. Without intervention (fRPP) a total amount of ∼35 mmol of Na+ was excreted before termination of the experiment, equivalent to ∼46% of the amount infused. Neither servocontrol of RPP (scRPP), Enalapril (fRPP + E), nor the combination thereof (scRPP + E) changed this amount significantly. Thus, the differences observed among the protocols in earlier periods (e.g. I1, I2) mirror different velocities of the UNaV responses only. For instance, less Na+ is excreted within the first 20 min of infusion (I1) in protocol fRPP than fRPP + E. However, within the following 20 min (I2), UNaV increased more in the former than in the latter; thus, the total amount excreted within these two periods (I1 + I2) did not differ.
Urine flow (UF, Table 1) in general changed parallel to Na+ excretion within and between protocols. Urinary potassium excretion (data not shown) increased 2- to 3-fold during saline infusion but did not differ among the protocols.
Table 1.
Urine flow (UF) and urine osmolality (UOsmol)
| Protocols | |||||
|---|---|---|---|---|---|
| Period | fRPP | scRPP | fRPP + E | scRPP + E | |
| UF (ml min−1) | C1 | 0.42 ± 0.06§ | 0.30 ± 0.07 | 0.98 ± 0.22* | 0.64 ± 0.13 †‡ |
| C2 | 0.50 ± 0.10§ | 0.30 ± 0.05 | 0.81 ± 0.12* | 0.59 ± 0.10‡ | |
| C3 | 0.41 ± 0.08 | 0.30 ± 0.08 | 0.59 ± 0.12 | 0.50 ± 0.08 | |
| I1 | 0.89 ± 0.12§ | 0.73 ± 0.14§ | 1.53 ± 0.23§* | 1.83 ± 0.31§ | |
| I2 | 2.21 ± 0.10§ | 1.49 ± 0.25§* | 2.47 ± 0.32§ | 2.27 ± 0.36§‡ | |
| I3 | 2.96 ± 0.25§ | 2.14 ± 0.27§* | 3.00 ± 0.26§ | 2.71 ± 0.22§ | |
| I4 | 3.15 ± 0.17§ | 2.75 ± 0.18§ | 2.85 ± 0.28§ | 3.05 ± 0.36§ | |
| I5 | 3.56 ± 0.21§ | 2.69 ± 0.32§* | 2.81 ± 0.31§ | 3.11 ± 0.28§ | |
| P1 | 2.44 ± 0.18§ | 2.40 ± 0.31§ | 2.52 ± 0.15§ | 2.53 ± 0.23§ | |
| P2 | 1.56 ± 0.27§ | 1.67 ± 0.29§ | 1.74 ± 0.18§ | 1.79 ± 0.31§ | |
| UOsmol (mosmol kg−1) | C1 | 508 ± 56 | 620 ± 73 | 357 ± 79* | 459 ± 52‡ |
| C2 | 486 ± 54 | 646 ± 59* | 396 ± 78 | 492 ± 46 | |
| C3 | 504 ± 60 | 645 ± 74* | 460 ± 40 | 477 ± 39 | |
| I1 | 428 ± 44 | 605 ± 77* | 334 ± 26 | 356 ± 26§ | |
| I2 | 297 ± 23§ | 376 ± 62§ | 262 ± 38§ | 302 ± 16§ | |
| I3 | 250 ± 13§ | 313 ± 17§ | 267 ± 28§ | 293 ± 27§ | |
| I4 | 256 ± 20§ | 281 ± 21§ | 302 ± 40§ | 347 ± 29§ | |
| I5 | 294 ± 16§ | 324 ± 13§ | 306 ± 17§ | 304 ± 25§ | |
| P1 | 336 ± 18§ | 360 ± 18§ | 358 ± 21 | 332 ± 17§ | |
| P2 | 443 ± 40 | 420 ± 23§ | 401 ± 31 | 405 ± 26 | |
Values are mean ±s.e.m. of 9 dogs studied in 4 protocols during 3 control periods (C1–C3), 5 periods with Na+ loading by i.v. infusion (I1–I5) and 2 postinfusion periods (P1, P2). For protocols see Methods. Significant differences obtained by GLM ANOVA for repeated measurements + Duncan's multiple comparison test:
significant versus fRPP
significant versus fRPP + E
significant versus scRPP;
significant change versus control periods within the respective protocol.
Urine osmolality (UOsmol, Table 1) decreased with saline infusion in each protocol and was a mirror image of UF. Compared with non-pretreated conditions (fRPP) control samples showed slightly higher osmolality during servocontrol (scRPP), but slightly reduced values after Enalapril (fRPP + E).
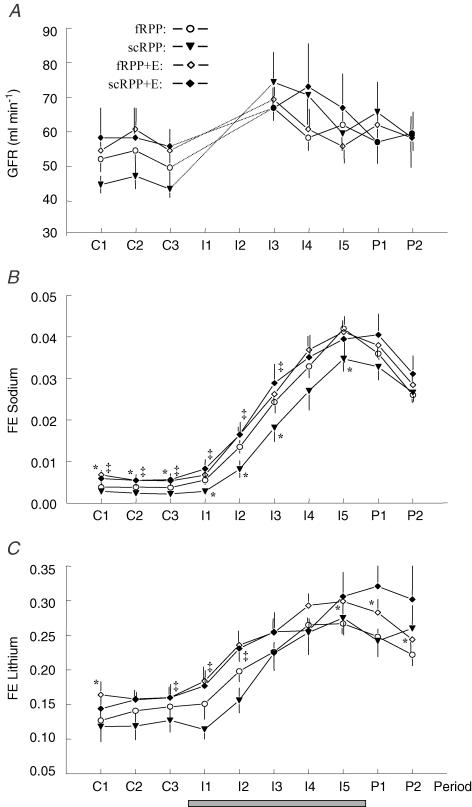
GFR (Fig. 3A) did not differ among the protocols during control conditions. With the onset of infusion (I1 and I2), a sharp increase in creatinine clearance was measured in each protocol. Since this increase most probably does not reflect a true increase of GFR, but at least in part represents an artifact due to the increase of urine flow, i.e. wash out of the dead space (renal tubules, renal pelvis, ureters), the values for these two periods are not included in Fig. 3. However, clearance values obtained during subsequent periods (beyond I2), are assumed to be reliable measures of GFR. Irrespective of protocol, GFR in these periods was 10–20% higher than during control periods and thus independent of changes in RPP or ACE blockade.
Figure 3. GFR (exogenous creatinine clearance, A), fractional sodium excretion (B) and fractional lithium excretion (C).
Grey bars indicate period of infusion. Values are mean ±s.e.m. of 9 dogs studied in four protocols. For protocols and periods see legend to Fig. 1. Significant differences: * significant versus fRPP, ‡ significant versus scRPP; within each protocol, GFR was higher during I3 through P2, and FeNa and FeLi were higher during I2 through P2 as compared with control periods.
The patterns of fractional sodium excretion over time (FENa, Fig. 3B), in general, resembled UNaV. In each protocol, FENa increased gradually with infusion, reaching peak values 8- to 12-fold above control values. Control FENa did not differ between fRPP and scRPP. However, servocontrol of RPP (scRPP protocol) delayed the increase in FENa during saline infusion, and, in addition, reduced peak FENa (period I5). Enalapril increased control FENa as compared with the respective protocol without Enalapril.
Fractional lithium excretion (FELi, Fig. 3C) increased gradually with infusion in each protocol, reaching peak values 2-fold above control values. Servocontrol of RPP did not alter this pattern significantly (fRPP versus scRPP). The effects of Enalapril on control FELi were not consistent. However, with Enalapril FELi was increased in the last infusion period and in both postinfusion periods (fRPP + E versus fRPP). Li+ clearance was used to calculate proximal and postproximal tubular Na+ reabsorption rates based on the generally accepted assumptions that (i) Li+ in the proximal tubules is reabsorbed (roughly) in parallel with Na+, and (ii) in segments distal to the proximal tubules Li+ is not reabsorbed in appreciable amounts. Irrespective of the protocol, saline infusion was associated with the following changes in segmental Na+ handling (data not shown). Due to the increase in GFR (see Fig. 3A), filtered Na+ load was persistently increased by 10–20%. Absolute proximal Na+ reabsorption was only transiently increased, but returned to values indistinguishable from baseline even within the time of infusion (periods I4 and I5). Thus, saline infusion decreased fractional proximal reabsorption progressively (see FELi in Fig. 3). In consequence, postproximal Na+ delivery increased by up to 140%. Absolute postproximal reabsorption also increased. However, because this increase was smaller (90%) than the increase in delivery, fractional postproximal reabsorption decreased markedly (from 0.97 during control periods to 0.86 during infusion period 5).
Plasma variables
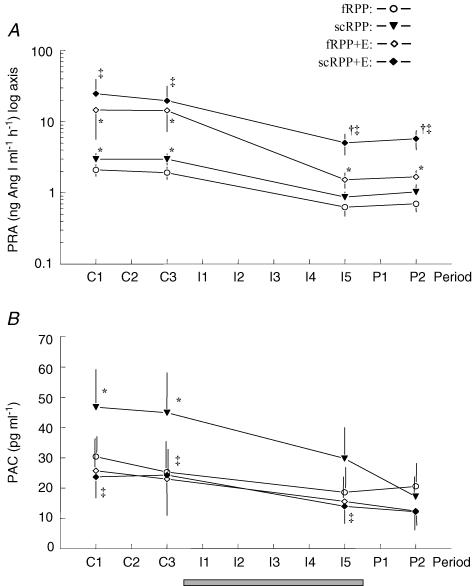
Control PRA (Fig. 4A) averaged ∼2 ng Ang I ml−1 h−1 without intervention (control period of fRPP), and was increased to ∼3 ng Ang I ml−1 h−1 by servocontrol of RPP (control period of scRPP). Enalapril increased PRA about 7-fold, i.e. to ∼14 and ∼22 ng Ang I ml−1 h−1 with and without servocontrol (fRPP + E and scRPP + E, respectively). During saline infusion, PRA decreased in every protocol. Without Enalapril (fRPP and scRPP), PRA reached 30 and 22% of the respective control levels. With Enalapril (fRPP + E) the relative fall tended to be more pronounced (to 10% of control values). However, due to high initial levels, PRA remained above the corresponding results obtained without Enalapril (fRPP) and close to the control values obtained in these experiments. Likewise, with servocontrol and Enalapril, PRA remained well above the levels measured in the corresponding experiments without servocontrol (scRPP + E versus fRPP + E), despite a decrease to 22% of control.
Figure 4. Plasma renin activity (PRA, log axis, A), and plasma aldosterone concentration (PAC, B).
Grey bars indicate period of infusion. Values are mean ±s.e.m. of 9 dogs studied in four protocols. For protocols and periods see legend to Fig. 1. Significant differences: * significant versus fRPP, † significant versus fRPP + E, ‡ significant versus scRPP; within each protocol, PRA was lower in I5 and P2 as compared with control periods; in scRPP, PAC was lower in I5 and P2 as compared with control periods.
Control concentrations of aldosterone (PAC, Fig. 4C) averaged ∼28 pg ml−1 without pretreatment (fRPP), and was increased to ∼45 pg ml−1 by servocontrol (scRPP). Enalapril did not change PAC significantly (fRPP + E versus fRPP). However, Enalapril completely prevented the increase of PAC induced by servocontrol of RPP (scRPP + E versus scRPP). During saline infusion, PAC decreased significantly when RPP was servocontrolled (scRPP), while the decrease was insignificant with the other protocols.
Plasma vasopressin (AVP, Table 2) did not change significantly during saline infusion. Plasma concentrations of ANP (Table 2) increased during saline infusion in each protocol. Sodium concentrations (PNa, Table 2) increased slightly but significantly during infusion in every protocol. Similar small increases of plasma osmolality (data not shown) did reach significance only in one series of experiments (fRPP + E). Plasma potassium concentrations did not change (data not shown). Plasma protein (PProt, Table 2) and haematocrit (data not shown) decreased significantly during infusion in every protocol.
Table 2.
Plasma concentrations of AVP, ANP, sodium (PNa), and protein (PProt)
| Protocols | |||||
|---|---|---|---|---|---|
| Period | fRPP | scRPP | fRPP + E | scRPP + E | |
| AVP (pg ml−1) | C1 | 1.01 ± 0.20 | 0.97 ± 0.22 | 1.37 ± 0.64 | 1.12 ± 0.15 |
| C3 | 0.99 ± 0.21 | 1.02 ± 0.24 | 1.83 ± 0.93 | 1.06 ± 0.20 | |
| I5 | 1.03 ± 0.20 | 1.17 ± 0.15 | 1.72 ± 0.69 | 1.18 ± 0.17 | |
| P2 | 1.24 ± 0.27 | 1.14 ± 0.22 | 2.12 ± 0.86 | 1.29 ± 0.19 | |
| ANP (pg ml−1) | C1 | 65.0 ± 7.7 | 54.1 ± 5.5 | 48.3 ± 4.8* | 51.8 ± 5.1 |
| C3 | 63.9 ± 5.9 | 54.7 ± 5.7 | 48.8 ± 5.9 | 62.1 ± 8.4 | |
| I5 | 78.1 ± 7.3§ | 87.2 ± 7.5§ | 64.8 ± 11.2§ | 73.8 ± 8.1§ | |
| P2 | 74.9 ± 6.9§ | 80.1 ± 6.5§ | 66.5 ± 10.5§ | 68.7 ± 6.9§ | |
| PNa (mmol l−1) | C1 | 150.2 ± 0.6 | 150.6 ± 0.6 | 151.6 ± 0.7* | 150.2 ± 0.6† |
| C3 | 150.6 ± 0.6 | 151.2 ± 0.6 | 151.2 ± 0.7 | 150.3 ± 0.7 | |
| I5 | 153.5 ± 0.7§ | 152.6 ± 0.7§ | 153.8 ± 0.7§ | 151.8 ± 0.9§† | |
| P2 | 152.4 ± 0.8§ | 152.0 ± 0.5 | 153.8 ± 1.0§ | 151.1 ± 0.8† | |
| PProt (g%) | C1 | 5.90 ± 0.12 | 5.81 ± 0.14 | 6.07 ± 0.13 | 5.99 ± 0.08 |
| C3 | 5.92 ± 0.20 | 5.86 ± 0.16 | 6.03 ± 0.11 | 5.90 ± 0.11 | |
| I5 | 5.32 ± 0.15§ | 5.18 ± 0.14§ | 5.39 ± 0.09§ | 5.24 ± 0.09§ | |
| P2 | 5.52 ± 0.19§ | 5.39 ± 0.16§ | 5.62 ± 0.11§ | 5.46 ± 0.12§ | |
Values are mean ±s.e.m. of 9 dogs studied in 4 protocols. For protocols and periods see legend to Table 1. Significant differences:
significant versus fRPP
significant versus fRPP + E;
significant change versus control periods within the respective protocol.
Discussion
Whereas TBS has long been recognized as a major determinant of arterial blood pressure, the contribution of arterial pressure to the control of TBS is still under debate (Reinhardt & Seeliger, 2000). While ‘pressure natriuresis’ has been assumed to be a major controller of Na+ excretion in long-term as well as acute control (Guyton, 1990a, 1991; Cowley, 1992; Hall et al. 1996), it is also well known that renal Na+ excretion may change many-fold without arterial pressure changes. For instance, the diurnal changes in Na+ excretion due to intermittent Na+ intake occur unrelated to changes in arterial pressure, and spontaneous RPP fluctuations do not change Na+ excretion (Palm et al. 1992; Corea et al. 1996; Reinhardt et al. 1996). As outlined in the introduction, previous studies that attempted to assess the role of pressure natriuresis in relation to short-term control of TBS with acute i.v. saline loading (Cowley & Skelton, 1991; Kaczmarczyk et al. 1992; Andersen et al. 2000) provided conflicting results.
The present experiments reveal that in freely moving dogs a moderate acute increase in RPP induced by i.v. isotonic NaCl loading does not contribute substantially to the associated natriuretic response (SLN). A 500 ml saline loading at a rate of 5 ml per min was used to increase MABP by about 12 mmHg (Fig. 1), while servocontrol of RPP by a fast-acting aortic occluder allowed us to quantify the role of pressure natriuresis. In its present form this control not only prevented RPP from rising with increased MABP, but RPP was set at 10% below individual 24 h mean arterial pressure for the duration of the experiment (protocol scRPP) to ensure that it was lower than the nadirs of the somewhat fluctuating arterial pressure of the freely moving dog. Nevertheless, the magnitude of SLN hardly differed between scRPP and fRPP (Fig. 2); at most there was a slight delay at the onset of the natriuresis. Thus, the contribution of pressure natriuresis, if any, seems very small under these conditions.
Moreover, the slight delay in natriuresis is easily explained by pre-stimulation of RAAS. As RPP was servocontrolled below control values, pressure-controlled renin release resulted in increases in pre-infusion plasma renin activity and plasma Aldo concentration (Fig. 4), and, thus presumably likewise increased plasma Ang II (not measured). To evaluate the effects of this pre-stimulation, ACE inhibition was used in the second set of experiments. With ACE inhibition, the SLN response was not affected by servocontrol (scRPP + E versus fRPP + E), with regard neither to velocity of increase nor to peak excretion rate. Thus, the delay in natriuresis observed with servocontrol of RPP without ACE inhibition (scRPP) is most likely related to the pre-stimulation of the RAAS. This is in line with long-term studies in dogs demonstrating that 20% reduction in RPP does not induce Na+ retention by the mechanism of low-pressure antinatriuresis, but rather via pressure-controlled renin stimulation (Boemke et al. 1995). In conclusion, the mechanism of pressure natriuresis does not appear to contribute to SLN.
From previous studies with similar Na+ loading in conscious dogs (Bie & Sandgaard, 2000) as well as in humans (Singer et al. 1991; 1994) suppression of the RAAS was interpreted to be the prime cause of SLN, because (i) RAAS activity decreased substantially during salt loading, and (ii) low dose infusion of Ang II (e.g. in amounts required to sustain control blood pressure after ACE inhibition; normotensive Ang II clamp) eliminated more than 90% of SLN. One earlier study in dogs (Cowley & Skelton, 1991) that appears to contradict this notion will be discussed below. In both our protocols without ACE inhibition, Na+ loading decreased PRA (Fig. 4), and a similar decrease of Ang II is assumed to have occurred.
Obviously, the decrease of PRA is induced by the increase of TBS, which, until the end of infusion, increased by about 55 mmol (about 10% of calculated exchangeable TBS in a 15 kg dog). Interestingly, this TBS-driven inhibition of renin release was operative in the face of ongoing pressure-driven stimulation of renin release induced by moderate RPP reduction (scRPP, Fig. 4), i.e. TBS-driven inhibition overcomes pressure-driven stimulation of renin release. Furthermore, the TBS-driven renin inhibition occurred irrespective of changes in RPP, as the decrease in PRA was similar both with an increase in RPP up to 10% above control (fRPP) and with a RPP fixed at 10% below control (scRPP). This confirms previous results demonstrating that TBS-controlled renin release is not mediated by changes in RPP (Seeliger et al. 1999). It is well known that changes of distal tubular NaCl load influence renin release (Hackenthal et al. 1990). In the present experiments, GFR increased by 10–20%. This increased distal Na+ load considerably as indicated by the concomitant increase in fractional excretion rate for lithium and would thus be expected to inhibit renin secretion. However, the time schedule of the present experiments does not allow us to determine whether the GFR increase preceded the decrease in PRA and could thus have contributed to the observed renin inhibition. With ACE inhibition, the control values of PRA were markedly elevated as a consequence of the absence of negative feed-back of Ang II on renin release (Hackenthal et al. 1990). However, the increase of TBS by the saline infusion decreased PRA markedly also in the absence of this feed-back.
During ACE inhibition, suppression of PRA is assumed to have no effects, i.e. mechanisms different from Ang II and Aldo must be responsible for the SLN. Moreover, it is completely possible that such mechanisms are active even without ACE inhibition. For instance, in the present study, both direct pressure effects (pressure natriuresis) and indirect effects exerted by pressure-controlled renin release were excluded by servocontrol and ACE inhibition. However, increased systemic pressure may still have lowered tubular Na+ reabsorption, e.g. via carotid baroreceptor stimulation resulting in decreased renal sympathetic nerve activity (Lohmeier et al. 1999). In this context it is notable that the absolute rate of proximal reabsorption returned to control levels during the saline infusion after an initial increase. ‘Atrial natriuresis’, i.e. volume receptor-mediated natriuresis independent from ANP (Johansen et al. 1997; Reinhardt & Seeliger, 2000), may operate through the same efferent pathway beside its primary action via renin inhibition. Furthermore, it is conceivable that even during complete ACE inhibition changes in intrarenal Ang II concentrations may still occur. In anaesthetized rats, renal interstitial concentrations of Ang II higher than concomitant plasma concentrations were found; these interstitial concentrations were not reduced by ACE inhibition or volume expansion, both of which lowered circulating levels of Ang II (Nishiyama et al. 2002). It appears therefore that intrarenal formation of Ang II, e.g. by non-ACE pathways, does not play a significant role in SLN in rats. However, in the face of considerable species differences of RAAS components (Hollenberg, 2000), the contribution of intrarenal Ang II to SLN in other species including dogs remains to be determined. Finally, NaCl loading reduced plasma protein concentration (Table 2), thus reducing colloid osmotic pressure (COP). This could be one reason behind the increase in GFR observed in all protocols. In addition, decreased COP might have contributed to the reduction of fractional tubular Na+ reabsorption (Fig. 3) by altering peritubular physical forces including interstitial hydrostatic pressure (Granger, 1986).
In an early elaborate study in dogs, Cowley & Skelton (1991) used isotonic NaCl loading (400 ml in 30 min) to evaluate the contribution of various factors for SLN. The authors found SLN almost unimpeded (i) by infusion of AVP, (ii) by renal denervation (RDX) plus infusion of AVP, (iii) by RDX plus infusion of a cocktail of AVP, Ang II, Aldo and ANP, and (iv) by RDX plus the hormone cocktail plus servocontrol of RPP. They concluded that neither suppression of AVP, renal sympathetic traffic, or the RAAS, nor an increase in ANP or RPP, nor the combination of these conditions would substantially contribute to SLN. Because NaCl loading resulted in a decrease in COP, they infused blood instead of saline and found that, with unchanged COP, sodium and water excretion did not exceed pre-infusion levels. It was concluded that SLN is predominantly a result of reduction in COP. With regard to the roles of ANP, AVP, and renal nerves, these conclusions have been corroborated by various studies (e.g. Andersen et al. 2000; Sandgaard et al. 2000; Bie & Sandgaard, 2000). Other results and conclusions, however, could not be corroborated. As mentioned above, the results from dog and human studies (Singer et al. 1991, 1994; Bie & Sandgaard, 2000) with similar Na+ load and similar Ang II infusion rates favour a major role of RAAS suppression in SLN. Remarkably, in these studies Ang II infusion almost abolished SLN despite a decrease in COP similar to that observed by Cowley & Skelton (1991). Furthermore, saline infusion with iso-oncotic solutions was found to result in profuse natriuresis (Morita & Vatner, 1985), and Na+ excretion is not increased during water loading despite decreased COP (e.g. Bie et al. 1984). These results are incompatible with a dominating role of COP in SLN. One may speculate that the puzzling results of Cowley & Skelton are related to the specific experimental procedures. For instance, the blood infused could have contained antinatriuretic substances, e.g. Ang II. Furthermore, in all protocols, dogs were studied after an overnight infusion of about 1.5 l of saline generating basal rates of sodium excretion of 100–150 μmol min−1, i.e. more than double the rates observed even during ACE inhibition in our dogs. In this context it is remarkable, that their acute NaCl loading at a rather high rate failed to increase MABP or GFR (for discussion see also Sandgaard et al. 2000). In conclusion, the contribution of COP to SLN remains to be quantified.
In the present study, creatinine and lithium clearances were used to assess the role of different nephron segments in SLN (see Fig. 3). During control periods, neither ACE inhibition, nor reduction of RPP, nor the combination thereof affected GFR measurably. Thus, physiological Ang II levels were not essential in controlling GFR. In accordance with previous long-term studies (Boemke et al. 1995), moderate reduction of RPP did not compromise the control of GFR either. FENa, however, was increased by ACE inhibition during control periods independent of changes in RPP. The increase in FELi, reflecting proximal tubular Na+ outflow (Atherton et al. 1987), and the decrease in fractional distal reabsorption, were smaller and statistically inconsistent. Thus, the marked increase in FENa induced by ACE inhibition reflects less pronounced reductions of proximal and distal fractional Na+ reabsorption, an observation in accord with studies in humans (Vos et al. 1993).
Irrespective of the protocol, SLN was associated with changes of GFR, and proximal and distal Na+ reabsorption. GFR increased by 10–20% in all protocols, i.e. independent of physiological changes of Ang II and RPP. The increase in GFR was associated with an increase in tubular Na+ load and an increase in the absolute tubular reabsorption rate of sodium. Thus, increase in Na+ load may be an important factor in SLN. Fractional proximal Na+ reabsorption decreased markedly. Despite the resulting increase in distal tubular load, fractional distal reabsorption also decreased markedly in every protocol. Thus, a marked part of natriuresis was due to the decrease in distal fractional Na+ reabsorption. In summary, SLN was achieved by an increase in the absolute values of all tubular Na+ transport rates (filtered load, absolute proximal reabsorption, distal load, absolute distal reabsorption, urinary excretion), concomitant with substantial decreases in both (proximal and distal) fractional reabsorption rates.
Perspectives
The present study clearly indicates that, at moderately elevated RPP, ‘pressure natriuresis’ does not contribute to acute NaCl loading natriuresis in the freely moving dog. Previous studies comparing the diurnal time courses of Na+ excretion and pressure revealed that Na+ excretion changes many-fold without RPP changes, and that moderate spontaneous RPP changes do not affect Na+ excretion. (Palm et al. 1992; Corea et al. 1996; Reinhardt et al. 1996). Long-term balance studies reviewed by Reinhardt & Seeliger (2000) demonstrated that TBS can be controlled independently of pressure natriuresis. A significant contribution of pressure natriuresis to TBS control could only be demonstrated under certain pathophysiological conditions, namely during sustained elevation of TBS as induced, e.g. by continued administration of antinatriuretic stimuli. The resulting long-term elevation of RPP by 25 mmHg or more facilitated Na+ excretion via pressure natriuresis mechanism, but did not restore TBS to normal. Long-term reduction of RPP by about 25 mmHg resulted in Na+ retention, but only via pressure-controlled renin release, not by the putative mechanism of low-pressure antinatriuresis. It appears that, at least in the conscious dog, pressure natriuresis is not operative at lowered, normal, or moderately elevated pressures when neurohumoral control of Na+ excretion is operating normally, a notion that is also supported by the present results. Thus, pressure natriuresis might be viewed as a compensating mechanism, which becomes active only under conditions of sustained significant elevation of RPP. Clearly, further studies are needed to clarify if this notion is correct.
Acknowledgments
We thank K. Dannenberg and R. Mohnhaupt for maintenance of the laboratory equipment, D. Bayerl, S. Molling and C. Pfaff for technical assistance, and C. Kasprzak and J. Wagner for animal care. This study was in part supported by the Deutsche Forschungsgemeinschaft.
References
- Andersen JL, Andersen LJ, Sandgaard NC, Bie P. Volume expansion natriuresis during servo control of systemic blood pressure in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2000;278:R19–R27. doi: 10.1152/ajpregu.2000.278.1.R19. [DOI] [PubMed] [Google Scholar]
- Atherton JC, Green R, Hughes S, McFall V, Sharples JA, Solomon LR, Wilson L. Lithium clearance in man: effects of dietary salt intake, acute changes in extracellular fluid volume, amiloride and frusemide. Clin Sci. 1987;73:645–651. doi: 10.1042/cs0730645. [DOI] [PubMed] [Google Scholar]
- Bie P, Munksdorf M, Warberg J. Renal effects of overhydration during vasopressin infusion in conscious dogs. Am J Physiol. 1984;247:F103–F109. doi: 10.1152/ajprenal.1984.247.1.F103. [DOI] [PubMed] [Google Scholar]
- Bie P, Sandgaard NC. Determinants of the natriuresis after acute, slow sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1–R10. doi: 10.1152/ajpregu.2000.278.1.R1. [DOI] [PubMed] [Google Scholar]
- Boemke W, Seeliger E, Rothermund L, Corea M, Pettker R, Mollenhauer G, Reinhardt HW. ACE inhibition prevents Na and water retention and MABP increase during reduction of renal perfusion pressure. Am J Physiol. 1995;269:R481–R489. doi: 10.1152/ajpregu.1995.269.3.R481. [DOI] [PubMed] [Google Scholar]
- Corea M, Seeliger E, Boemke W, Reinhardt HW. Diurnal pattern of sodium excretion in dogs with and without chronically reduced renal perfusion pressure. Kidney Blood Press Res. 1996;19:16–23. doi: 10.1159/000174041. [DOI] [PubMed] [Google Scholar]
- Cowley AW. Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Skelton MM. Dominance of colloid osmotic pressure in renal excretion after isotonic volume expansion. Am J Physiol. 1991;261:H1214–H1225. doi: 10.1152/ajpheart.1991.261.4.H1214. [DOI] [PubMed] [Google Scholar]
- Emmeluth C, Drummer C, Gerzer R, Bie P. Natriuresis in conscious dogs caused by increased carotid [Na+] during angiotensin II and aldosterone blockade. Acta Physiol Scand. 1994;151:403–411. doi: 10.1111/j.1748-1716.1994.tb09760.x. [DOI] [PubMed] [Google Scholar]
- Granger JP. Regulation of sodium excretion by renal interstitial hydrostatic pressure. Fed Proc. 1986;45:2892–2896. [PubMed] [Google Scholar]
- Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990a;259:R865–R877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Renal function curves and control of body fluids and arterial pressure. Acta Physiol Scand Suppl. 1990b;591:107–113. [PubMed] [Google Scholar]
- Guyton AC. Blood pressure control – special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- Hall JE, Brands MW, Shek EW. Central role of the kidney and abnormal fluid volume control in hypertension. J Hum Hypertens. 1996;10:633–639. [PubMed] [Google Scholar]
- Hollenberg NK. Implications of species difference for clinical investigation: studies on the renin-angiotensin system. Hypertension. 2000;35:150–154. doi: 10.1161/01.hyp.35.1.150. [DOI] [PubMed] [Google Scholar]
- Johansen LB, Bie P, Warberg J, Christensen NJ, Hammerum M, Videbaek R, Norsk P. Hemodilution, central blood volume, and renal responses after an isotonic saline infusion in humans. Am J Physiol. 1997;272:R549–R556. doi: 10.1152/ajpregu.1997.272.2.R549. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk G, Schröder K, Lampe D, Mohnhaupt R. Role of renal arterial pressure in the regulation of extracellular volume in conscious dogs. Clin Sci. 1992;82:247–254. doi: 10.1042/cs0820247. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension. 1999;33:487–492. doi: 10.1161/01.hyp.33.1.487. [DOI] [PubMed] [Google Scholar]
- Manning RD, Hu L, Williamson TD. Mechanisms involved in the cardiovascular-renal actions of nitric oxide inhibition. Hypertension. 1994;23:951–956. doi: 10.1161/01.hyp.23.6.951. [DOI] [PubMed] [Google Scholar]
- Morita H, Vatner SF. Effects of volume expansion on renal nerve activity, renal blood flow, and sodium and water excretion in conscious dogs. Am J Physiol. 1985;249:F680–F687. doi: 10.1152/ajprenal.1985.249.5.F680. [DOI] [PubMed] [Google Scholar]
- Nafz B, Persson PB, Ehmke H, Kirchheim HR. A servo-control system for open- and closed-loop blood pressure regulation. Am J Physiol. 1992;262:F320–F325. doi: 10.1152/ajprenal.1992.262.2.F320. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- Palm U, Boemke W, Bayerl D, Schnoy N, Juhr NC, Reinhardt HW. Prevention of catheter-related infections by a new, catheter-restricted antibiotic filling technique. Laboratory Anim. 1991;25:142–152. doi: 10.1258/002367791781082469. [DOI] [PubMed] [Google Scholar]
- Palm U, Boemke W, Reinhardt HW. Rhythmicity of urinary sodium excretion, mean arterial blood pressure, and heart rate in conscious dogs. Am J Physiol. 1992;262:H149–H156. doi: 10.1152/ajpheart.1992.262.1.H149. [DOI] [PubMed] [Google Scholar]
- Persson PB, Baumann JE, Ehmke H, Hackenthal E, Kirchheim HR, Nafz B. Endothelium-derived NO stimulates pressure-dependent renin release in conscious dogs. Am J Physiol. 1993;264:F943–F947. doi: 10.1152/ajprenal.1993.264.6.F943. [DOI] [PubMed] [Google Scholar]
- Qiu C, Muchant D, Beierwaltes WH, Racusen L, Baylis C. Evolution of chronic nitric oxide inhibition hypertension: relationship to renal function. Hypertension. 1998;31:21–26. doi: 10.1161/01.hyp.31.1.21. [DOI] [PubMed] [Google Scholar]
- Reinhardt HW, Corea M, Boemke W, Pettker R, Rothermund L, Scholz A, Schwietzer G, Persson PB. Resetting of 24-h sodium and water balance during 4 days of servo-controlled reduction of renal perfusion pressure. Am J Physiol. 1994;266:H650–H657. doi: 10.1152/ajpheart.1994.266.2.H650. [DOI] [PubMed] [Google Scholar]
- Reinhardt HW, Palm U, Mohnhaupt R, Dannenberg K, Boemke W. Computer-assisted long-term measurements of urinary output and other biological data. Am J Physiol. 1990;258:R274–R280. doi: 10.1152/ajpregu.1990.258.1.R274. [DOI] [PubMed] [Google Scholar]
- Reinhardt HW, Seeliger E. Toward an integrative concept of control of total body sodium. News Physiol Sci. 2000;15:319–325. doi: 10.1152/physiologyonline.2000.15.6.319. [DOI] [PubMed] [Google Scholar]
- Reinhardt HW, Seeliger E, Lohmann K, Corea M, Boemke W. Changes of blood pressure, sodium excretion and sodium balance due to variations of the renin-angiotensin-aldosterone system. J Auton Nerv Syst. 1996;57:184–187. doi: 10.1016/0165-1838(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Sandgaard NC, Andersen JL, Bie P. Hormonal regulation of renal sodium and water excretion during normotensive sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2000;278:R11–R18. doi: 10.1152/ajpregu.2000.278.1.R11. [DOI] [PubMed] [Google Scholar]
- Schütten HJ, Johannessen AC, Torp-Pedersen C, Sander-Jensen K, Bie P, Warberg J. Central venous pressure – a physiological stimulus for secretion of atrial natriuretic peptide in humans. Acta Physiol Scand. 1987;131:265–272. doi: 10.1111/j.1748-1716.1987.tb08236.x. [DOI] [PubMed] [Google Scholar]
- Seeliger E, Lohmann K, Nafz B, Persson PB, Reinhardt HW. Pressure-dependent renin release: effects of sodium intake and changes of total body sodium. Am J Physiol. 1999;277:R548–R555. doi: 10.1152/ajpregu.1999.277.2.R548. [DOI] [PubMed] [Google Scholar]
- Seeliger E, Persson PB, Boemke W, Mollenhauer G, Nafz B, Reinhardt HW. Low-dose nitric oxide inhibition produces a negative sodium balance in conscious dogs. J Am Soc Nephrol. 2001;12:1128–1136. doi: 10.1681/ASN.V1261128. [DOI] [PubMed] [Google Scholar]
- Singer DR, Markandu ND, Morton JJ, Miller MA, Sagnella GA, MacGregor GA. Angiotensin II suppression is a major factor permitting excretion of an acute sodium load in humans. Am J Physiol. 1994;266:F89–F93. doi: 10.1152/ajprenal.1994.266.1.F89. [DOI] [PubMed] [Google Scholar]
- Singer DR, Shirley DG, Markandu ND, Miller MA, Buckley MG, Sugden AL, Sagnella GA, MacGregor GA. How important are suppression of aldosterone and stimulation of atrial natriuretic peptide secretion in the natriuretic response to an acute sodium load in man. Clin Sci. 1991;80:293–299. doi: 10.1042/cs0800293. [DOI] [PubMed] [Google Scholar]
- Vos PF, Boer P, Koomans HA. Effects of enalapril on renal sodium handling in healthy subjects on low, intermediate, and high sodium intake. J Cardiovasc Pharmacol. 1993;22:27–32. doi: 10.1097/00005344-199307000-00005. [DOI] [PubMed] [Google Scholar]