Abstract
Though all in vitro models of gamma frequency network oscillations are critically dependent on GABAA receptor-mediated synaptic transmission little is known about the specific role played by different subtypes of GABAA receptor. Strong expression of the α5 subunit of the GABAA receptor is restricted to few brain regions, amongst them the hippocampal dendritic layers. Receptors containing this subunit may be expressed on the extrasynaptic membrane of principal cells and can mediate a tonic GABAA conductance. Using hippocampal slices of wild-type (WT) and α5−/− mice we investigated the role of α5 subunits in the generation of kainate-induced gamma frequency oscillations (20–80 Hz). The change in power of the oscillations evoked in CA3 by increasing network drive (kainate, 50–400 nm) was significantly greater in α5−/− than in WT slices. However, the change in frequency of gamma oscillations with increasing network drive seen in WT slices was absent in α5−/− slices. Raising the concentration of extracellular GABA by bathing slices in the GABA transaminase inhibitor vigabatrin and blocking uptake with tiagabine reduced the power of gamma oscillations more in WT slices than α5−/− slices (43%versus 15%). The data suggest that loss of this GABAA receptor subunit alters the dynamic profile of gamma oscillations to changes in network drive, possibly via actions of GABA at extrasynaptic receptors.
Fast oscillations within the EEG gamma frequency band (20–80 Hz) arise in various cortical areas and are thought to play a role in certain forms of information processing (Singer & Gray, 1995). In the hippocampus they may be involved in aspects of learning and memory (Lisman & Idiart, 1995; Fell et al. 2001).
Gamma activity in the hippocampal formation requires inhibitory synaptic transmission mediated by GABAA receptors (Whittington et al. 1995; Fisahn et al. 1998; LeBeau et al. 2002). These receptors are composed of a number of gene family subunit proteins (Barnard et al. 1998; Bonnert et al. 1999), with the majority of native neuronal GABAA receptors containing a combination of at least one α, one β and one γ subunit (Pritchett et al. 1989; McKernan & Whiting, 1996). In recent years gene targeting techniques have clarified certain functions of individual subunits (McKernan et al. 2000). GABAA receptors containing the α5 subunit have an almost uniquely restricted expression pattern, being abundant extrasynaptically in the hippocampal CA1 and CA3 dendritic fields but only weakly expressed in other brain regions (Wisden et al. 1992; Fritschy & Mohler, 1995; Sur et al. 1999; Brunig et al. 2002; Ramos et al. 2004). The strong expression of this GABAA receptor subunit within the hippocampus is intriguing in view of the role of this structure in certain aspects of learning and memory processes (Eichenbaum, 2000) and mice devoid of α5-containing GABAA receptors exhibit superior performances in a water maze test of spatial learning (Collinson et al. 2002).
α5-containing GABAA receptors mediate a tonic inhibitory conductance similar to that previously described in hippocampal pyramidal neurones (Whittington et al. 1996; Bai et al. 2001) and dentate gyrus granule cells (Nusser & Mody, 2002). The small amount of spillover of GABA necessary to activate a tonic conductance via α5-containing receptors may occur during the firing of interneurones in rhythmic oscillatory activity (Scanziani, 2000). The presence of a tonic GABAergic conductance has been shown to reduce the power of gamma frequency oscillations in the hippocampus (Whittington et al. 1996).
In this study, we employ transgenic mice with a disrupted α5 gene (α5−/−) and compare the properties of gamma frequency network oscillations elicited in slices prepared from these mice and their wild-type littermates.
Methods
The generation of mice deficient for the α5 subunit of the GABAA receptor has been previously described (Collinson et al. 2002).
Slice electrophysiology
Adult male mice (mixed background strain C57BL6–129SvEv; aged 9–39 weeks) deficient for the GABAA receptor α5 subunit (α5−/−) and their respective wild-type siblings were anaesthetized with inhaled isoflurane followed by injection of ketamine (> 100 mg kg−1) and xylazine (> 10 mg kg−1) i.p. Animals were perfused intracardially with ∼10 ml of modified artificial cerebrospinal fluid (ACSF) which was composed of (mm): 252 sucrose, 3 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2–4 MgSO4, 2 CaCl2 and 10 glucose. This procedure was in accordance with the UK Animals (Scientific Procedures) Act 1986. Horizontal slices 450 µm thick were prepared and were maintained at 34°C at the interface between warm/wetted 95% O2–5% CO2 and ACSF containing (mm): 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 24 NaHCO3, 10 glucose and 126 NaCl. Data were recorded with an Axoprobe-1A amplifier (Axon Instruments Inc., Union City, CA, USA). Signals were filtered at 1 kHz and digitized at 10 kHz using an Instrutech ITC-16 A/D board (Instrutech Corp., NY, USA). AxoGraph 4.6 software (Axon Instruments Inc.) was used to analyse the data.
Overall structure of the network model
The programme used to simulate persistent gamma oscillations, in a network of multicompartment neurones, was as in Traub et al. (2003). The network contained 3072 pyramidal neurones and 384 interneurones, with each model neurone containing a soma, branching dendrites, and a segment of axon. The ‘control’ simulation had a between-interneurone gap junction conductance of 1.84 nS. The effects of extrasynaptic GABAA receptors were simulated by using a uniform density of tonic GABAA conductance over the dendrites of the pyramidal cells (reversal potential −15 mV relative to resting potential). Values of the total tonic GABAA conductance were 0 and 10 nS. In addition, a model of interneurone network gamma rhythms was generated in the absence of pyramidal cell input to interneurones and the above tonic GABA conductance was applied to interneurones alone.
Results
Comparison of kainate-induced gamma oscillations in WT and α5−/− slices
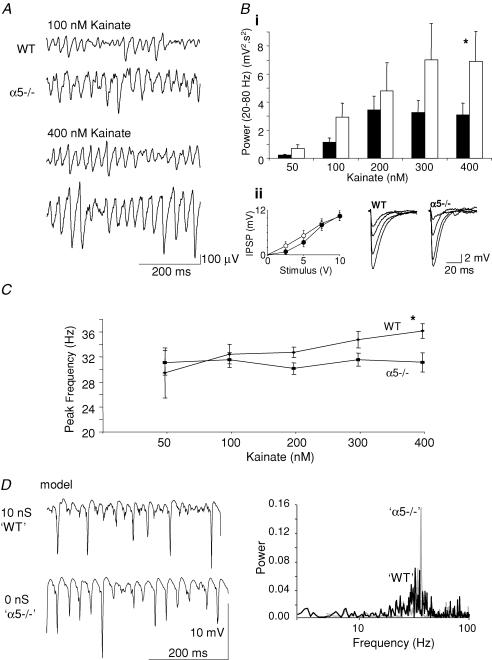
In order to assess the consequences of the loss of α5-containing GABAA receptors on neuronal network activity, gamma frequency network oscillations were elicited in slices prepared from WT and α5−/− mice. Nanomolar concentrations of the AMPA/kainate receptor agonist kainate (50–400 nm) were used to evoke a persistent gamma frequency population rhythm in a concentration-dependent manner (Fig. 1A and B). Incremental increases in agonist concentration yielded progressively larger amplitude network oscillations in WT slices up to 200 nm. Further increases in kainate concentration produced no further increase in power of the gamma oscillation. In α5−/− slices further increases in kainate concentration generated a greater power at gamma frequencies with the highest concentration tested (400 nm) generating oscillations with mean power double that in WT slices (Fig. 1Bi). This increase in the maximum power of gamma frequency oscillations was significant (P < 0.05, n = 6, two-way analysis of variance). Such changes in power could be due to changes in fast synaptic inhibition onto pyramidal cells. However, analysis of the strength of monosynaptic, electrically elicited IPSPs in CA3 pyramidal cells revealed no significant difference (P > 0.05, n = 7) between WT and α5−/− tissue at all stimulus intensities tested (0–10 V, Fig. 1Bii). Figure 1D summarizes data from a simulation in which the basic features of kainate-evoked gamma oscillations in WT and α5−/− slices were reproduced. By setting the tonic GABAA conductance on pyramidal cell dendritic membranes to 10 nS (as a model for tissue from WT) and 0 nS (as a model for α5−/−) the enhanced power of gamma activity in α5−/− slices could be accurately replicated.
Figure 1. Effects of α5 subunit deletion on hippocampal population gamma oscillations induced by kainate.
A, in wild-type (WT) and α5 knock-out (α5−/−) slices, superfusion with nanomolar concentrations of kainate (50–400 nm) resulted in the appearance of persistent gamma frequency network oscillations. The traces show extracellular field recordings from stratum radiatum of the CA3 area. Bi, agonist concentration versus spectral power (20–80 Hz) relationship of kainate-induced gamma frequency oscillations in wild-type (black bars) and α5−/− (white bars) slices showing enhanced power change with network drive. Data shown as mean ±s.e.m., n = 6 for WT, n = 7 for α5−/−. Bii, monosynaptic evoked IPSPs from CA3 pyramidal cells at stimulus intensities from 0 to 10 V n = 7, WT (•) and α5−/− (○). C, mean ±s.e.m. frequency changes with increased kainate concentration were absent in α5−/− slices. D, network model replicates basic experimental features of kainate-evoked oscillations in WT and α5−/− slices. The effects of pyramidal cell extrasynaptic GABAA receptors on network oscillations was simulated using tonic GABA conductances of 10 nS (‘WT’, black trace) and 0 nS (‘α5−/−’, grey trace) on dendritic compartments. Traces show simulated ‘field potential’ data. Power spectra reveal that decreased tonic GABAA conductance resulted in an increase in the power of network oscillations.
Despite the peak power of gamma frequency oscillations occurring at concentrations of kainate = 200 nm the frequency of the oscillations in slices from WT animals continued to increase throughout the concentration range used here. This effect was not seen in slices with no α5-containing GABA receptors. After superfusion of low concentrations of kainate, the peak frequency of gamma oscillations in WT and α5−/− slices was comparable.However, at higher concentrations, the peak frequency of gamma oscillations elicited by 400 nm kainate was unchanged in α5−/− slices but significantly increased in WT slices (WT median: 36.6 Hz; (interquartile range (IQR): 36.6, 34.2), n = 6; α5−/− median: 31.7 Hz; (IQR: 31.7, 31.7), n = 7; P < 0.05, Mann–Whitney rank sum test). Overall, the effect of kainate concentration on frequency change of gamma oscillations in WT and α5−/− slices was significantly different (P < 0.05, two-way analysis of variance, Fig. 1C).
Gamma oscillations generated in isolated interneurone networks in WT and α5−/− slices
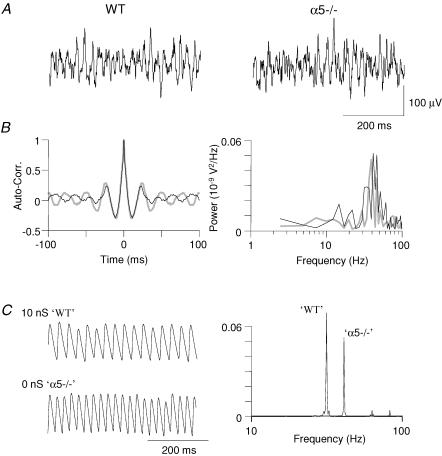
In order to establish whether absence of α5-containing GABA receptors was directly affecting interneurone networks we used a model of interneurone network gamma which did not rely on phasic interplay between principal cells and interneurones (Whittington et al. 1995). The inhibitory interneurone network was pharmacologically isolated from principal cell outputs by bath application of 50 mm D-AP5 (d-aminophosphonovalerate; NMDA receptor antagonist) and 20 mm NBQX (AMPA/kainate receptor antagonist). GABAB receptors were also blocked with 1 mm CGP55845. High molarity potassium solution (1.5 m KCH3SO4) was pressure-ejected onto stratum radiatum/lacunosum-moleculare of CA1 (Towers et al. 2002), and the oscillations were recorded in CA1 stratum radiatum (Fig. 2). Contrary to the increase in peak power of gamma oscillations seen in the persistent, kainate-induced, model of gamma oscillations, a decrease in the mean power of gamma oscillations was seen (WT: 5.09 ± 1.78 mV2 s−2, n = 15; α5−/−: 2.83 ± 0.59 mV2 s−2, n = 14). However, this was not a significant effect (P > 0.05). There was no significant effect either on mean peak frequency of interneurone network gamma oscillations (WT: 48.7 ± 3.2 Hz, n = 15; α5−/−: 45.1 ± 2.8 Hz, n = 14, P > 0.1) or rhythmicity of oscillations, measured as auto-correlation side peak amplitude (WT: 0.22 ± 0.02, n = 14; α5−/−: 0.20 ± 0.02, n = 14, P > 0.1). In contrast the computational model predicted that extrasynaptic GABAA receptor-mediated conductance specifically on interneurones would serve to increase field power and decrease frequency from 52 Hz (conductance = 0.0 nS) to 32 Hz (conductance = 10 nS).
Figure 2. Interneurone network gamma oscillations elicited in the CA1 area of WT and α5−/− slices.
A, example of interneurone network gamma oscillations (ING) elicited in CA1 of WT and α5−/− slices by pressure ejection of 1.5 m KCH3SO4 after superfusion of glutamate receptor blockers (50 μm D-AP5, 20 μm NBQX) and a GABAB receptor antagonist (1 μm CGP55845). B, corresponding pooled power spectra and auto-correlation data demonstrated that power, frequency and rhythmicity of the oscillations in both slices were not changed. C, model of interneurone network (synaptic connectivity between pyramidal cells and interneurones omitted from model used in Fig. 1). In contrast to the experimental data the model predicted large frequency changes in ING in the presence or absence of tonic GABA conductance. Data shown are ‘field potential’ recordings and corresponding power spectra.
Consequences of raising extracellular [GABA] on oscillations in WT and α5−/− slices
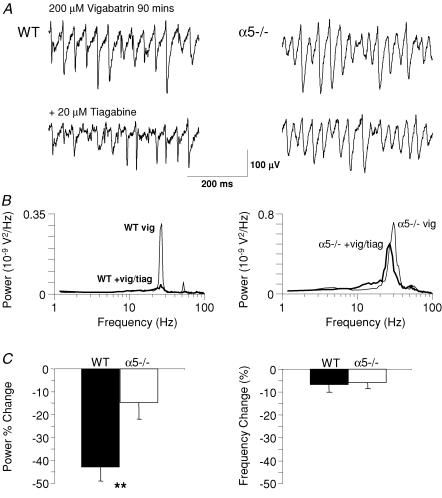
To investigate whether the effects of α5-containing GABA receptors could be mediated by GABA overspill from synapses we increased extracellular GABA concentration. Gamma oscillations were generated as in Fig. 1 with kainate (200 nm) but with GABAB receptors blocked with CGP55845 (1 μm) to prevent any effects of GABA overspill on this receptor subtype. Application of the GAT-1 GABA uptake inhibitor tiagabine (20 µm) (Borden et al. 1994) generated a similar modest reduction in gamma power in both WT (to 88.6 ± 15.6% of control, n = 9) and α5−/− (to 74.2 ± 20.4% of control, n = 5) slices (data not shown). There was no significant difference between the two groups (P > 0.05). Further elevation of extracellular GABA concentration by preincubation with the GABA transaminase inhibitor vigabatrin (200 μm), before tiagabine application, produced a significantly greater decrease in gamma power in slices from WT compared with α5−/− animals (Fig. 3). Following vigabatrin preincubation (90 min), a significantly greater reduction in the power of oscillations in WT slices than in α5−/− slices was observed 30 min after subsequent tiagabine application (WT: 42.9 ± 6.1%, n = 11; α5−/−: 14.7 ± 7.3%, n = 8; P < 0.01, t test; Fig. 3). As with tiagabine alone, there was no significant difference in the effect of vigabatrin alone on gamma oscillations (P > 0.05). This effect was accompanied, in both sets of slices, by a small, comparable reduction in oscillation peak frequency (WT: 6.7 ± 3.4%, n = 14; α5−/−: 5.9 ± 2.6%, P > 0.1, n = 11).
Figure 3. Elevated concentrations of [GABA]o reduce the power of gamma oscillations less in α5−/− slices than in WT slices.
A, example of kainate-evoked oscillations after superfusion of 200 μm vigabatrin for 90 min, and after subsequent block of GABA uptake with tiagabine (20 μm). B, corresponding power spectra indicate a substantial reduction in power of the oscillations in the WT slices and a smaller reduction in the α5−/− slices. Thin lines = vigabatrin control; thick lines = vigabatrin + tiagabine. C, group data demonstrating the change in power (20–80 Hz) and peak frequency of oscillations in WT (black bars) and α5−/− slices (white bars) after tiagabine application (expressed as percentage change from control). Tiagabine application resulted in a significantly larger reduction in the power of oscillations in WT compared to α5−/− slices (**P < 0.01, t test).
Discussion
Alterations in dynamic range of oscillations in α5−/− slices
This study provides the first insight into the role of a specific GABAA receptor subunit protein in the generation of hippocampal network oscillations. The mean peak power of kainate-induced gamma oscillations in hippocampi devoid of α5 subunit-containing GABAA receptors was higher than in the WT. This finding was accompanied by no significant change in the strength of fast GABAA receptor-mediated monosynaptic inhibitory events in principal cells, implying that during rhythmic network oscillations, activation of these receptors by increased ambient GABA mediates a predominantly tonic inhibitory conductance that serves to limit the change in power of gamma activity in response to increasing network drive. In contrast, gamma oscillations in WT slices showed a change in frequency with increasing network drive that was absent in α5−/− slices. Thus, the presence of α5 subunit-containing GABAA receptors appeared to limit increases in gamma power while facilitating an increase in gamma frequency in response to increased network excitation in this model. In the absence of these receptors, increased network drive is represented as an increase in gamma power only. Power of these field oscillations is dependent upon the local potential changes generated by synchronous synaptic inputs to pyramidal cells. For a given synaptic conductance a smaller field response would be seen with a lower membrane resistance. Thus, in the case of phasic IPSPs underlying gamma oscillations, an accompanying tonic GABAergic conductance would reduce the influence of these IPSPs on principal cells and reduce the relative power of the consequent field. The frequency of gamma rhythms is dependent on the kinetics and amplitude of phasic inhibition. Collinson et al. (2002) showed that the bi-exponential decay in WT mice was seen to a lesser extent in α5−/− mice. Given this we suggest that the more labile frequency dynamics in WT mice may come from the relative proportions of the fast and slow decays with changing levels of GABA release as network drive increases. Some confusion exists as to the extent of involvement of the α5 subunit in phasic synaptic responses. Collinson et al. (2002) demonstrated a significant decrease in small, spontaneous IPSCs but not the 5- to 10-fold larger evoked responses. Caraiscos et al. (2004) also reported no change in larger IPSCs. This difference may reflect the high affinity of GABA for α5-containing receptors, with relatively small amounts of GABA release preferentially activating these receptors over those containing other α subunits. With larger amounts of GABA release the higher number of non-α5-containing subsynaptic receptors may make the majority contribution to postsynaptic currents.
As kainate-induced persistent gamma oscillations are generated by an interplay between principal cells and populations of interconnected interneurones, the rhythmicity of the network oscillation could theoretically have been impaired by any reduction in the coherence of interneurone output onto principal cells, caused by removal of a subset of interneurone-expressing α subunits. However, two observations suggest this was not the case. Firstly the power and frequency of oscillations generated in pharmacologically isolated interneurone networks (interneurone network gamma) was unchanged in α5−/− slices. Secondly, the model predicted that inclusion of an α5-like conductance in interneurones would markedly affect the frequency of interneurone network rhythms. The experimental interneurone network gamma more closely resembled the model data in frequency when a tonic GABA conductance was present on interneurones. A tonic GABAergic influence on interneurones has been demonstrated to control interneurone excitability (Semyanov et al. 2003). However, α5 expression has been found in specific interneurone subtypes in early postnatal hippocampus (Ramos et al. 2004) but not in adult tissue, and there was no difference in the nature of the interneurone network rhythm when comparing tissue from WT and α5−/− mice. Thus the repertoire of interneurone GABAA receptor α subunits appeared to be little affected in slices from adult α5−/− mice.
Differential change in the power of gamma oscillations in WT and α5−/− slices after increasing extracellular [GABA]
In addition to studies suggesting that α5 subunit-containing GABAA receptors may have a predominantly extrasynaptic location (Brunig et al. 2002; Crestani et al. 2002) it has recently been reported that these receptors can be activated by low concentrations of GABA and mediate a form of tonic inhibition in hippocampal pyramidal neurones (Caraiscos et al. 2004). These results suggest therefore that spillover of synaptically released GABA during the generation of gamma oscillations is sufficient to activate a tonic α5-mediated conductance on pyramidal neurones. After using vigabatrin as a tool to raise the concentration of GABA in the tissue, blockade of GABA uptake reduced the power of the oscillatory activity to a significantly greater degree in WT slices. This provides support for the idea that α5-containing GABAA receptors in the hippocampus are activated by the synchronous release of GABA during rhythmic oscillations thus mediating a persistent tonic conductance that in turn governs the amplitude of the emergent activity. This hypothesis was supported by model simulations in which reducing the tonic GABAA conductance on the dendritic membrane of principal cells from 10 to 0 nS accurately reproduced the enhanced peak gamma power in α5−/− slices.
This study indicates that α5 subunit-containing GABAA receptors affect the dynamic response of hippocampal gamma rhythms to changes in network drive. The enhanced power of gamma oscillations in α5−/− slices may be due to removal of a population of α5-containing GABAA receptors that mediate a tonic inhibition of principal cell dendrites. This change in power was accompanied by an increased stability of the oscillation in the frequency domain. Since it is the temporal characteristics of network rhythms that have been proposed to underlie their role in neuronal network function it is of note that recent studies have described the pro-cognitive effects of either removal or inverse agonism of this receptor (Collinson et al. 2002; Chambers et al. 2003).
Acknowledgments
This work was supported by The Medical Research Council, UK and The Wellcome Trust.
References
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. θ, a novel γ-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci U S A. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of γ-aminobutyric acidA receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JL, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, Hobbs SC, Marshall G, Maubach KA, Pillai GV, Reeve AJ, MacLeod AM. Identification of a novel, selective GABAAα5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- LeBeau FE, Towers SK, Traub RD, Whittington MA, Buhl EH. Fast network oscillations induced by potassium transients in the rat hippocampus in vitro. J Physiol. 2002;542:167–179. doi: 10.1113/jphysiol.2002.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Ramos B, Lopez-Tellez JF, Vela J, Baglietto-Vargas D, Del Rio JC, Ruano D, Gutierrez A, Vitorica J. Expression of alpha5 GABA(A) receptor subunit in developing rat hippocampus. Brain Res Dev Brain Res. 2004;151:87–98. doi: 10.1016/j.devbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- Towers SK, LeBeau FE, Gloveli T, Traub RD, Whittington MA, Buhl EH. Fast network oscillations in the rat dentate gyrus in vitro. J Neurophysiol. 2002;87:1165–1168. doi: 10.1152/jn.00495.2001. [DOI] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, LeBeau FE, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci U S A. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Jefferys JG, Traub RD. Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro. Br J Pharmacol. 1996;118:1977–1986. doi: 10.1111/j.1476-5381.1996.tb15633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]