Abstract
Nicotine affects the regulation of skin blood flow (SkBF), but the mechanisms involved are not well understood. We tested the hypothesis that acute exposure to nicotine inhibits both the initial neurally mediated component and the later sustained component of SkBF responses to local heating of non-glabrous skin in humans. SkBF (measured by laser-Doppler) responses to local heating of forearm skin from 32 to 42°C were measured in 11 chronic smokers. Heating occurred at one site over 15 min (RAMP) and over 90 s (STEP) at another site, and was maintained for an additional 30 min. STEP heating was also applied to a site pretreated with bretylium via iontophoresis to inhibit noradrenergic neurotransmission. Responses were measured before and after acute administration of nicotine via cigarettes or nasal spray in two experimental sessions. Nicotine decreased resting skin blood flow (P < 0.05); this response was inhibited by bretylium. During RAMP, nicotine increased the initial SkBF at 42°C (by ∼12%, P < 0.05). For STEP, nicotine increased the initial peak response (by ∼25%, P < 0.05), and decreased the sustained plateau value (by ∼10%, P < 0.05). In skin pretreated with bretylium, the increase caused by nicotine in the initial peak value persisted, but the plateau value was not different from pre-nicotine. These data suggest that in abstinent cigarette smokers, nicotine augments initial responses to both gradual and rapid non-painful heating of non-glabrous skin by sensitizing the sensory nerves that mediate the axon reflex associated with rapid vasodilatation. In contrast, nicotine decreases SkBF responses to prolonged heating by activating noradrenergic nerves.
Local heating of human non-glabrous skin increases skin blood flow (SkBF) via contributions from both local axon reflexes and the local release of mediators (Magerl & Treede, 1996; Kellogg et al. 1999; Minson et al. 2001; Stephens et al. 2001). Recent evidence suggests that it is possible to distinguish between these components of human SkBF responses using rapid, non-painful increases in local skin temperature (Kellogg et al. 1999; Minson et al. 2001). The neural component of this response, measured as an initial peak dilator response, is caused by the release of vasoactive peptides such as calcitonin gene-related peptide (CGRP) from a subpopulation of C-fibre nociceptive afferents via an axon reflex (Magerl & Treede, 1996; Schmelz et al. 1997; Klede et al. 2003). The neural element transducing this temperature-regulated mediator release is likely in the transient receptor potential (TRPV) channel subfamily (Caterina et al. 1997; Zygmunt et al. 1999; Schwarz et al. 2000; Xu et al. 2002), which includes the vanilloid receptor stimulated by capsaicin. The later component of sustained vasodilatation is caused, at least in part, by the local release of nitric oxide (NO) by mechanisms that are unknown (Kellogg et al. 1999; Minson et al. 2001).
Smoking a cigarette decreases resting SkBF (Roth et al. 1944; Eckstein et al. 1957; Richardson, 1987; Fushimi et al. 1992; Monfrecola et al. 1998), an effect mediated, at least in part, by an increase in central sympathetic outflow caused by nicotine (Narkiewicz et al. 1998). Nicotine could potentially have other effects on both components of the SkBF response to local heating. Although there is evidence that nicotinic acetylcholine receptors (nAChRs) are present on human nociceptors (Douglas & Ritchie, 1960; Parkhouse & Le Quesne, 1988; Dessirier et al. 1998), their physiological role is unknown. Some animal experiments suggest that stimulation of the nicotine receptors facilitates nociceptor axon reflexes (Grunfeld et al. 1991, 1993), which could increase SkBF. On the other hand, prejunctional nAChRs facilitate noradrenaline (norepinephrine) release from sympathetic postganglionic nerves, a factor that could inhibit SkBF responses to local warming (Starke, 1977; Kristufek et al. 1999). Nicotine may also affect the production of and responses to NO (Hashimoto, 1994; Sarabi & Lind, 2000; Gaenzer et al. 2001). The effects of smoking on SkBF responses to local heating are unknown. Such information is of clinical relevance because smoking is known to impair postoperative wound healing (Moller et al. 2002; Sorensen et al. 2003), which is affected by local SkBF and resulting tissue oxygenation.
With this information as a background, we tested the hypothesis that in habitual smokers deprived of nicotine for at least 12 h, acute exposure to nicotine delivered via cigarette smoke would inhibit both the initial neural component and the later sustained component of the SkBF response to local heating of human non-glabrous skin.
Methods
Subjects
This study was conducted in accordance with the standards in the Declaration of Helsinki, and was approved by the Mayo Foundation Institutional Review Board. Written informed consent was obtained from each subject.
The 11 subjects studied (9 female, 2 male) were all current smokers, defined as having smoked for at least 5 years, with an average daily cigarette consumption of > 10 over the last 6 months. Subjects were excluded if obese (body mass index > 30 kg m−2), hypertensive, or had a history of heart disease, diabetes, or autonomic disorders. Those taking serotonin-reuptake inhibitors were also excluded. Subjects abstained from the use of non-steroidal anti-inflammatory agents for 24 h before each study, and aspirin for 1 week before the study. All subjects abstained from smoking for at least 12 h before each study, as confirmed by exhaled carbon monoxide sampling in the morning of the study. All female subjects were studied in the early follicular phase of the menstrual cycle to control for variability due to reproductive hormone status (Charkoudian et al. 1999). All studies began in the morning, and subjects were seated throughout the session. Each subject was scheduled to participate in two experimental sessions separated by at least 24 h.
Instrumentation
Arterial blood pressure was measured by an oscillometric automated cuff (Agilent M1204R) providing mean arterial pressure. An intravenous catheter was inserted into an antecubital vein for blood sampling to measure plasma nicotine concentrations.
Skin blood flow was measured by laser-Doppler flowmetry (LDF) (Perimed Periflux System 5000, Stockholm, Sweden) at sites on the anterior aspect of the left forearm. The laser-Doppler probes were placed in the centre of a specialized probe holder that both measured and controlled local temperature over 7 cm2 of the skin surface (Peritemp 4005, Stockholm, Sweden). After placement, probes were not moved until the end of the experimental session.
Based on the results of the first three subjects, in the last eight subjects bretylium was administered by iontophoresis to inhibit cutaneous adrenergic nerve transmission (Kellogg et al. 1989). A plastic iontophoresis chamber (Mayo Clinic Section of Engineering) similar to that described by Kellogg and colleagues (Kellogg et al. 1989) was attached to the forearm and filled with a 10 mm solution of bretylium tosylate dissolved in propylene glycol. A current of 250 μA was applied for 10 min, providing a current density of 400 μA cm−2. This regimen has been shown to provide complete blockade of adrenergic neurotransmission for up to 6 h (Pergola et al. 1996). At least 1 h elapsed between iontophoresis and LDF measurements to allow for the bretylium effect and the resolution of current-induced hyperaemia.
Local heating regimens
LDF was measured in at least two sites in all subjects. At one site, local heating was performed by increasing the set heating element temperature from a baseline of 32°C to 42°C in 0.5°C increments every 45 s (i.e. over 15 min), referred to hereafter as the RAMP protocol. At the other site, local heating was performed by increasing the set heating element temperature from a baseline of 32°C to 42°C in 0.5°C increments every 4.5 s (i.e. over 90 s), referred to hereafter as the STEP protocol (Minson et al. 2001). As shown in prior work, this rate of temperature change is not perceived as painful (Kellogg et al. 1999; Minson et al. 2001). For both heating protocols, heating element temperature was maintained at 42°C for 30 min. In previous work using similar experimental equipment, it was shown that LDF measurements at the conclusion of the STEP protocol are approximately 90% of the maximal values produced by intradermal microdialysis of 50 mm sodium nitroprusside (Minson et al. 2001), and that local skin temperature, as measured directly by thermocouples, is approximately 40°C under these conditions. The temperatures reported hereafter refer to those of the heating elements, which were consistent across conditions.
In the last eight subjects, LDF was measured at a third site pretreated with bretylium to investigate the possible involvement of noradrenergic neurotransmission in the responses. The STEP heating protocol was applied to this site.
Protocol
Following instrumentation, a 10 min baseline recording was obtained with the heating element temperature maintained at 32°C. Heating protocols were then applied to sites as described above. Following this initial measurement of responses (requiring a total of approximately 50 min), heating element temperature was returned to 32°C, and 1 h allowed for recovery. At the end of this recovery period, SkBF measurements had consistently returned to their pre-heating values.
After this recovery period, the subjects received nicotine. On the first experimental day, they smoked their usual brand of cigarette, taking one puff every minute (requiring approximately 8 min). Thirty minutes after beginning the first cigarette, they smoked a second cigarette in the same fashion. This regimen was designed using published pharmacokinetic data to provide a relatively sustained plasma nicotine concentration over the following hour (Gourlay & Benowitz, 1997). On the second experimental day, they received 2 mg of intranasal nicotine spray (Nicotrol NS, Parmacia Consumer Heathcare), followed 30 min later by a second dose of 2 mg. This method of administration was chosen to achieve approximately the same plasma nicotine concentration as produced by cigarette smoking (Gourlay & Benowitz, 1997). The nasal spray produced transient mucosal irritation that resolved within 10 min. On both days, the response of SkBF to the administration of nicotine via cigarette smoke or nasal spray was noted with heating element temperature maintained at 32°C.
Approximately 20 min after the initiation of the second cigarette or the second intranasal nicotine administration, the heating protocols were repeated. Thus, approximately 110 min elapsed from the completion of the first heating of a site to the commencement of the second heating. At the conclusion of the second heating protocol, venous blood samples were obtained to measure plasma nicotine concentrations by isotope dilution gas chromatography–mass spectrometry (Baskin et al. 1998). The lower level of detection for this assay is 2 ng ml−1.
We have previously shown that neither the initial peak vasodilatation nor the maximal response to sustained local heating change when the STEP heating protocol is repeated at least 1 h apart in the absence of interventions in normal subjects (Charkoudian et al. 2002). To evaluate whether this is also true in smokers and with the RAMP heating protocol, in three subjects nicotine was not administered between the two heating protocols on the first experimental day. Thus, in these three subjects the first day provided control data for the effect of repeated heating. On the second experimental day, they received nicotine nasal spray as described above.
Data analysis
LDF was divided by mean arterial pressure to derive an index of cutaneous vascular conductance (CVC), averaged at 1 s intervals. The reference CVC for each site was defined for this study as the mean CVC over the last 5 min of the initial (before nicotine) heating protocol (either STEP or RAMP). CVC values were expressed as a percentage of the reference value for that site.
Responses to RAMP heating were quantified by averaging CVC and heating element temperature for each individual run over 1 min intervals. A sigmoidal curve was then fitted to the relationship between temperature and CVC for each subject (Sigma Plot). In each case the correlation coefficient exceeded 0.992. Parameters from this sigmoidal relationship were then used to determine CVC at intervals of 1°C between 32 and 42°C for each subject, and to determine the temperature (T50) at which CVC reached half of the difference between the values at 32 and 42°C. The difference between CVC at 32 and 42°C was also noted.
The initial phase of the response to STEP heating was quantified as the initial peak of the CVC response, which occurred within 4–6 min after the onset of local warming.
Data are presented as means ±s.d. Paired comparisons were performed using the signed rank test, as the assumptions of the paired t test were not satisfied for all data. P < 0.05 was taken to be statistically significant.
Results
All 11 subjects completed the first experimental day. On this first day, 8 subjects received nicotine delivered via cigarette smoking, and 3 received repeated heating without nicotine administration. Of the 8 subjects who smoked on the first experimental day, one subject elected not to complete the second experimental day (nicotine delivered via nasal spray), and data from the second day of another subject were lost due to equipment failure. Thus, data concerning nicotine effects are reported from 8 subjects who received nicotine via cigarette smoke on the first experimental day, and 9 subjects who received nicotine via nasal spray on the second experimental day (of these, 6 subjects received nicotine via both routes on these two separate days).
The plasma nicotine concentration produced by smoking two cigarettes (9.1 ± 2.1 ng ml−1) was not significantly different from that produced by the two administrations of nicotine nasal spray (8.2 ± 2.2 ng ml−1).
Administration of nicotine via a single cigarette or set of nasal sprays significantly increased mean arterial pressure (from 82 ± 8 to 88 ± 8 mmHg and from 84 ± 8 to 91 ± 6 mmHg for cigarettes and nasal sprays, respectively, P < 0.01 for each). There was no significant difference in the increases produced by the two modes of nicotine administration. The second administration of nicotine had no further effect on MAP (data not shown).
Resting SkBF
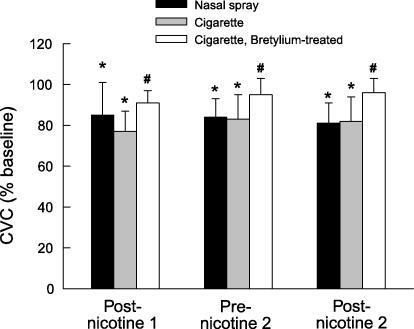
Administration of nicotine via a single cigarette or set of nasal sprays decreased resting SkBF measured at a heating element temperature of 32°C (P < 0.008 for both, Fig. 1). The second administration of nicotine had no further effect on SkBF. There was no significant difference in the decreases produced by the two modes of nicotine administration. Pretreatment of the skin with bretylium significantly inhibited this decrease (P < 0.04 for each, Fig. 1).
Figure 1.
Changes in cutaneous vascular conductance (CVC) during two administrations of nicotine either via cigarettes (n = 8 experiments) or nasal sprays (n = 9). In some subjects, one site was treated with bretylium before cigarette smoking (n = 5). Values are means ±s.d. and are expressed as a percentage of baseline measured before the first administration of nicotine. * Significant difference from baseline, signed-rank test, P < 0.05. # Significant difference from site not treated with bretylium, signed-rank test, P < 0.05.
RAMP heating protocol
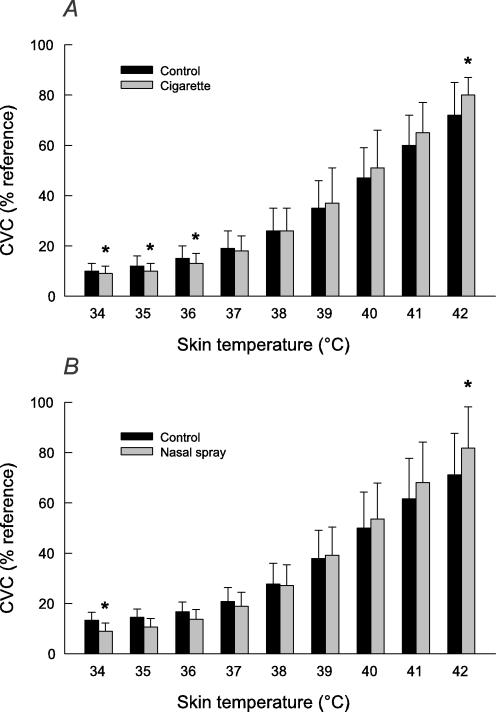
Increasing heating element temperature from 32 to 42°C over 15 min increased SkBF (Fig. 2). Nicotine, whether administered via cigarettes or nasal spray, significantly affected this response. At lower temperatures, SkBF was significantly decreased after nicotine, consistent with the effect observed on baseline SkBF. At the midrange of temperatures, SkBF was similar before and after nicotine administration. At the highest temperature (42°C), SkBF (measured over the first minute after achieving this temperature) was greater after nicotine administration (P = 0.008 for cigarettes and P = 0.039 for nasal spray).
Figure 2.
Cutaneous vascular conductance (CVC) as a function of local skin temperature during gradual RAMP heating (over 15 min), before (Control) and after nicotine given via cigarettes (A, n = 8) or nasal spray (B, n = 9). Values are means ±s.d. and are expressed as a percentage of the reference CVC for that site. * Significant difference from control, signed-rank test, P < 0.05.
For both cigarette smoking and nasal spray, the difference between SkBF measured at 42 and 32°C was greater after nicotine (62 ± 13 versus 72 ± 9% of maximal CVC before and after cigarettes, respectively, P = 0.008, and 58 ± 14 versus 73 ± 16% of reference CVC before and after nasal spray, respectively, P = 0.004). The temperature (T50) at which CVC reached half of the difference between the values at 32 and 42°C was not significantly affected by nicotine (39.4 ± 0.7 versus 39.5 ± 0.9°C before and after cigarettes, respectively, and 39.4 ± 0.6 versus 39.4 ± 0.7°C before and after nasal spray, respectively). The plateau CVC measured 30 min after achieving 42°C was significantly decreased after nicotine administration (to 89 ± 10% of reference CVC after cigarettes, P = 0.008, and 82 ± 11% of reference CVC after nasal spray, P = 0.004).
The temperature at which warmth was first perceived during RAMP heating tended to be less after nicotine administration, a difference that was significant after nasal spray (36.4 ± 2.4 versus 35.4 ± 2.0°C before and after cigarettes, respectively, P = 0.078, and 36.9 ± 2.2 versus 35.5 ± 1.1°C before and after nasal spray, respectively, P = 0.039).
STEP heating protocol
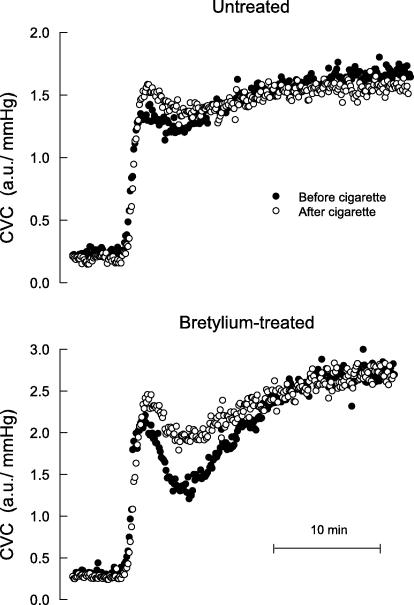
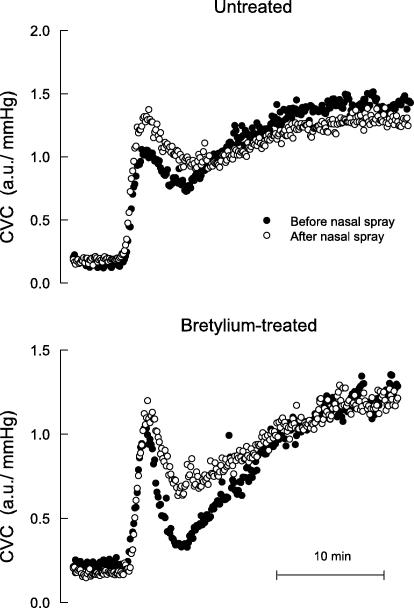
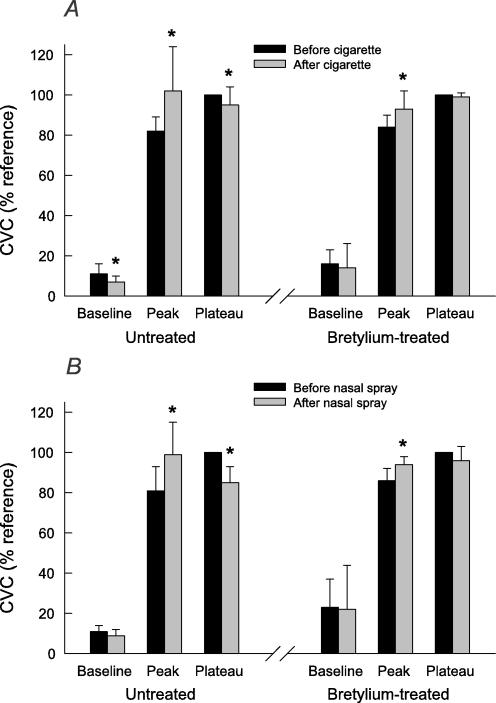
Increasing the heating element temperature from 32 to 42°C over 90 s produced a biphasic increase in SkBF, characterized by a rapid increase to a peak value, then a moderate decrease, followed by a slower phase vasodilatation that attained a plateau. Figures 3 and 4 show a representative example of SkBF responses before and after cigarettes and nasal spray, respectively, and Fig. 5 shows mean baseline, peak and plateau SkBF values for all subjects. Nicotine, whether administered via cigarettes or nasal spray, significantly increased the initial peak value, and significantly decreased the plateau value. In skin pretreated with bretylium, nicotine still increased the initial peak value, but had no effect on the plateau value (Figs 3–5).
Figure 3.
Cutaneous vascular conductance (CVC) over time during rapid STEP heating in one subject before (•) and after (^) cigarette smoking, measured at separate sites not treated and treated with bretylium delivered via iontophoresis.
Figure 4.
Cutaneous vascular conductance (CVC) over time during rapid STEP heating in one subject (the same subject whose data are depicted in Fig. 3) before (•) and after (^) nicotine nasal spray, measured at separate sites not treated and treated with bretylium delivered via iontophoresis.
Figure 5.
Cutaneous vascular conductance (CVC) measured at three times during rapid STEP heating: at baseline before heating (averaged over the 5 min prior to heating), at the peak of the initial response, and at a plateau of the sustained response after 30 min maintained at 42°C (averaged over the last 5 min of this period). The effect of cigarette smoking on these values is shown in A, and that of nicotine nasal spray in B, both in separate sites not treated (n = 8 for cigarettes and n = 9 for nasal spray) and treated with bretylium via iontophoresis (n = 5 for cigarettes and n = 6 for nasal spray). Values are means ±s.d. and are expressed as a percentage of the reference CVC for that site. * Significant difference from before nicotine administration, signed-rank test, P < 0.05.
Effect of repeated heating in the absence of nicotine
Repeated local STEP or RAMP heating protocols, separated by 110 min with no other intervention, produced similar vasodilator responses in three subjects (Table 1). Thus, the repetition of heating in the absence of nicotine did not affect SkBF responsiveness.
Table 1.
Responses to repeated heating in the absence of nicotine
| Response to first heating (%ref CVC) | Response to second heating (%ref CVC) | |
|---|---|---|
| RAMP heating (°C) | ||
| 34 | 9 ± 2 | 6 ± 1 |
| 35 | 11 ± 3 | 8 ± 1 |
| 36 | 16 ± 4 | 13 ± 2 |
| 37 | 23 ± 7 | 20 ± 4 |
| 38 | 34 ± 10 | 33 ± 8 |
| 39 | 48 ± 14 | 49 ± 13 |
| 40 | 62 ± 17 | 64 ± 16 |
| 41 | 75 ± 17 | 77 ± 17 |
| 42 | 83 ± 16 | 85 ± 15 |
| STEP heating | ||
| Untreated | ||
| Peak | 91 ± 17 | 89 ± 19 |
| Plateau | 100 | 95 ± 17 |
| Bretylium-treated | ||
| Peak | 84 ± 6 | 84 ± 4 |
| Plateau | 100 | 93 ± 8 |
Responses (% reference CVC) were determined at three sites: one exposed to RAMP heating (see text for description), one exposed to STEP heating, and a third exposed to STEP heating after bretylium iontophoresis. CVC: cutaneous vascular conductance. Values are means ±s.d. from three subjects.
Discussion
The major new finding of this study is that in habitual smokers abstinent from cigarettes for at least 12 h, nicotine augmented initial cutaneous vasodilator responses (measured at a heating element temperature of 42°C) to both gradual and rapid warming of non-glabrous skin by mechanisms not dependent on noradrenergic nerves. In contrast, nicotine inhibited the sustained vasodilatation produced by local heating (i.e. the response after 30 min at a heating element temperature of 42°C) by a mechanism dependent on local noradrenergic neurotransmission.
The observed reduction in resting SkBF (measured at 32°C) caused by cigarette smoking is consistent with many prior studies (Roth et al. 1944; Eckstein et al. 1957; Richardson, 1987; Fushimi et al. 1992; Monfrecola et al. 1998). Most suggest that vasoconstriction is caused primarily by nicotine rather than other components of smoke (Maddock & Coller, 1932; Weatherby, 1942; Roth et al. 1944; Fushimi et al. 1992), which is consistent with our results, although some studies show that smoking cigarettes with very low nicotine can also reduce blood flow (Evans & Stewart, 1943; Richardson, 1987). Circulating catecholamines do not appear to contribute significantly to this vasoconstriction (Coffman, 1967; Cryer et al. 1976). Rather, smoking increases sympathetic outflow to the skin (Narkiewicz et al. 1998). In addition, prejunctional nAChRs may enhance noradrenaline release from postganglionic sympathetic neurones (Miao et al. 1996; Kristufek et al. 1999; Si & Lee, 2002). The ability of bretylium to attenuate nicotine-induced decreases in resting SkBF is consistent with nicotine-induced enhancement of local noradrenaline release via either or both of these mechanisms (Cryer et al. 1976; Grassi et al. 1994).
Nicotine also attenuated the sustained vasodilatation produced by local heating (measured 30 min after achieving a probe temperature of 42°C). This sustained vasodilator response is mediated at least in part by nitric oxide, as it is inhibited by preventing NO formation in the skin (Kellogg et al. 1999; Minson et al. 2001). The finding that bretylium blocked nicotine-induced attenuation of the sustained response suggests that this attenuation was mediated by enhanced noradrenergic neurotransmission rather than by the inhibition of endothelial NO release, a postulated mechanism in other vascular beds (Hashimoto, 1994; Habler et al. 1999; Sarabi & Lind, 2000). The ability of sympathetic activity to modulate vascular axon reflex responses in skin is established (Hornyak et al. 1990; Habler et al. 1997). However, Pergola et al. (1993) found that increased sympathetic activation produced by whole-body cooling did not affect SkBF responses of human forearm skin to a local temperature of 42°C. It is possible that the ability of nicotine to reduce SkBF under similar conditions reflects the ability of prejunctional nAChRs to increase noradrenaline release, as discussed above.
In contrast to these reductions in resting SkBF and the sustained SkBF response to local heating, nicotine augmented the initial phase of SkBF responses to local non-painful heating from 32 to 42°C, whether this increase in temperature occurred over 90 s (STEP heating) or 15 min (RAMP heating). The mechanism producing this effect did not involve noradrenergic neurotransmission, as it was still present after bretylium pretreatment. For at least the more rapid heating protocol, the initial increase in SkBF is mediated by an axon reflex (Minson et al. 2001). The observed effect of nicotine thus implies that it modulated this reflex, which is mediated by primary nociceptive afferents (Holzer, 1992). These afferents can respond to noxious mechanical stimulation (causing the perception of pain), chemical, cold, and heat stimuli. The neural elements transducing temperature-regulated mediator release are members of the transient receptor potential (TRPV) channel subfamily that include the vanilloid receptor (VR1) activated by capsaicin, as well as other capsaicin-insensitive receptors that may mediate responses to higher temperatures (Caterina et al. 1997; Zygmunt et al. 1999; Schwarz et al. 2000; Xu et al. 2002). Receptor stimulation causes vasodilatation (Parkhouse & Le Quesne, 1988; Wardell et al. 1993; Magerl & Treede, 1996; Stephens et al. 2001) via the release of neuropeptides such as CGRP and pituitary adenylate cyclase-activating polypeptide (PACAP), which coexists with CGRP in capsaicin-sensitive afferents (Mulder et al. 1994; Zygmunt et al. 1999). Both compounds produce cutaneous vasodilatation in humans (Dorner et al. 1998) and may regulate other key elements of the response to local tissue trauma (Schaffer et al. 1998; Smith & Liu, 2002).
Application of acetylcholine or nicotine to tissues produces pain and inflammation (Dessirier et al. 1998). Exploration of this observation has revealed the presence of nAChRs on primary nociceptors in several tissues, including skin (Douglas & Ritchie, 1960; Steen & Reeh, 1993; Dessirier et al. 1998; Jinks & Carstens, 1999). Activation of these receptors, which are found on both capsaicin-sensitive and -insensitive afferents (Roberts et al. 1995), can produce the release of neuropeptides (Jinno et al. 1994; Puttfarcken et al. 1997), vasodilatation (Grunfeld et al. 1991), and plasma extravasation (Miao et al. 2001). The latter effect can occur at subnanomolar local nicotine concentrations in animal experiments. Although their physiological function is obscure, many tegmental cells (including skin keratinocytes) synthesize and secrete acetylcholine (Conti-Fine et al. 2000), which may act in a paracrine fashion to mediate responses to tissue injury.
Based on this information and our present results, we propose that the stimulation of nAChRs on nociceptive afferents following smoking or nasal spray enhances the release of vasodilating neuropeptides from these fibres in response to stimulation of TRPV-subfamily receptors by heat. Such an effect would be analogous to the function of presynaptic nAChRs that modulate the release of neurotransmitters in the central nervous system (Wonnacott, 1997). Support for this mechanism is provided by the observations of Bernardini et al. (2001a, b). Using an isolated saphenous nerve–skin preparation from the rat, they found that nicotine excited nociceptive afferents and caused the release of CGRP. Furthermore, nicotine also augmented heat-induced increases in afferent activity in a dose-dependent manner. Chronic exposure of rats to systemic nicotine causes enhanced SkBF responses to iontophoresed acetylcholine, providing support for the concept that systemic nicotine can also modulate axon reflexes in vivo (Grunfeld et al. 1991, 1993).
In human non-glabrous skin, iontophoresis of acetylcholine also produces cutaneous vasodilatation mediated at least in part by an axon reflex (Parkhouse & Le Quesne, 1988; Berghoff et al. 2002). However, this vasodilatation can be blocked by atropine (Kellogg et al. 1995), suggesting involvement of muscarinic rather than nicotinic mechanisms in responses to high local concentrations of acetylcholine. The activation of nicotinic receptors in skin appears to be facilitatory rather than a primary stimulus for vasodilatation, at least for the degree of stimulation produced by systemic nicotine. Indeed, in the present study nicotine produced vasoconstriction in the absence of heating, suggesting either that stimulation by systemic nicotine was insufficient to trigger axon reflexes in the absence of heating, or that increased noradrenaline release produced by nicotine overcame any reflex vasodilatation.
Sensitization of nociceptive afferents is also suggested by the finding that nicotine tended to decrease the temperature at which warmth was first perceived during gradual heating. This interpretation assumes that perception is related to afferent activity, such that stimulation of nicotine receptors reduced the heat for TRPV receptor activation to reach perception threshold. A similar phenomenon has been observed when capsaicin was used to stimulate nociceptors in the skin, producing a decrease in the temperature of initial warmth perception during heating (Stephens et al. 2001). However, unlike that previous study we did not observe a shift in the position of the curve describing the relationship between skin temperature and SkBF. This may be related to the effect nicotine has of reducing baseline SkBF, making the interpretation of T50 in these terms problematic.
Although our findings are consistent with our proposed mechanism, given the ubiquity of nicotinic receptors in other systems responsible for SkBF regulation (e.g. autonomic ganglia) and the complex regulation of SkBF during heating, other mechanisms cannot be excluded. Further studies will be necessary to confirm that direct nicotinic enhancement of axon reflexes is the salient mechanism for the observed augmentation of initial SkBF responses to local heating.
It is also important to note that these findings may be specific to chronic smokers. Exposure to nicotine produces both acute and chronic tolerance to its effects that is specific to the response examined (e.g. psychological effects versus cardiovascular effects) (Perkins et al. 1994). Acute tolerance may develop within minutes, and resolve within hours (Porchet et al. 1988). Chronic tolerance requires years to develop, and may not change even after years of abstinence (Perkins et al. 2001). In animal models, tolerance is generally associated with an increase in central nAChR number but a decrease in function (Marks et al. 1993). There is no information regarding how chronic nicotine exposure affects peripheral nAChRs. Thus, our results must be interpreted in the context of possible chronic changes in nAChR function. For example, if the function of nAChRs on nociceptive afferents is decreased in habitual smokers, the increased peak vasodilatation may simply represent a restoration of a more normal response. On the other hand, the increased response may be indicative of an upregulation of peripheral nAChR function (‘sensitization’) caused by chronic nicotine exposure. Thus, nicotine effects on SkBF may differ in non-smoking subjects. However, such studies may prove challenging, as in naive subjects nicotine produces symptoms of anxiety, nausea, and dysphoria (Heishman & Henningfield, 2000), additional central actions which may themselves affect autonomic function and skin blood flow responses.
In summary, we observed that acute exposure to nicotine alters locally mediated vasodilatation in the skin of chronic smokers. The results suggest that peripheral nAChRs on primary nociceptive afferents in humans chronically exposed to nicotine can modulate the release of vasodilator neuropeptides that mediate the initial SkBF response to local heating. Nicotine also affects SkBF responses by causing the release of noradrenaline from noradrenergic nerves. These effects of nicotine on skin blood flow may have important implications for processes such as wound healing, in which local changes in skin blood flow are essential for normal function.
Acknowledgments
This work was supported by National Institutes of Health grants HL-46493, NS-32352, HL-73884, and RR-00585 (to the Mayo Clinic), and a grant from the Minnesota Partnership for Action Against Tobacco (RC 0020-2000). The authors thank Anita Baumgartner and Lavonne Liedl for their excellent technical assistance.
References
- Baskin LB, Anderson RW, Charlson JR, Hurt RD, Lawson GM. A solid phase extraction method for determination of nicotine in serum and urine by isotope dilution gas chromatography/mass spectrometry with selected ion monitoring. Ann Clin Biochem. 1998;35:522–527. doi: 10.1177/000456329803500406. [DOI] [PubMed] [Google Scholar]
- Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–788. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- Bernardini N, Reeh PW, Sauer SK. Muscarinic M2 receptors inhibit heat-induced CGRP release from isolated rat skin. Neuroreport. 2001a;12:2457–2460. doi: 10.1097/00001756-200108080-00034. [DOI] [PubMed] [Google Scholar]
- Bernardini N, Sauer SK, Haberberger R, Fischer MJ, Reeh PW. Excitatory nicotinic and desensitizing muscarinic (M2) effects on C-nociceptors in isolated rat skin. J Neurosci. 2001b;21:3295–3302. doi: 10.1523/JNEUROSCI.21-09-03295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol. 1999;87:1719–1723. doi: 10.1152/jappl.1999.87.5.1719. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Vella A, Reed AS, Minson CT, Shah P, Rizza RA, Joyner MJ. Cutaneous vascular function during acute hyperglycemia in healthy young adults. J Appl Physiol. 2002;93:1243–1250. doi: 10.1152/japplphysiol.00345.2002. [DOI] [PubMed] [Google Scholar]
- Coffman JD. The attenuation by reserpine or guanethidine of the cutaneous vasoconstriction caused by tobacco smoking. Am Heart J. 1967;74:229–234. doi: 10.1016/0002-8703(67)90282-7. [DOI] [PubMed] [Google Scholar]
- Conti-Fine BM, Navaneetham D, Lei S, Maus AD. Neuronal nicotinic receptors in non-neuronal cells: new mediators of tobacco toxicity? Eur J Pharmacol. 2000;393:279–294. doi: 10.1016/s0014-2999(00)00036-4. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Dessirier JM, O'Mahony M, Sieffermann JM, Carstens E. Mecamylamine inhibits nicotine but not capsaicin irritation on the tongue: psychophysical evidence that nicotine and capsaicin activate separate molecular receptors. Neurosci Lett. 1998;240:65–68. doi: 10.1016/s0304-3940(97)00930-0. [DOI] [PubMed] [Google Scholar]
- Dorner GT, Wolzt M, Eichler HG, Schmetterer L. Effect of pituitary adenylate cyclase activating polypeptide 1–27 on ocular, cerebral and skin blood flow in humans. Naunyn-Schmiedebergs Arch Pharmacol. 1998;358:657–662. doi: 10.1007/pl00005308. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ritchie JM. The excitatory action of acetylcholine on cutaneous non-myelinated fibres. J Physiol. 1960;150:501–514. doi: 10.1113/jphysiol.1960.sp006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein JW, Wood JE, Wilkins RW. Comparative vasoconstrictor effects of inhaling tobacco smoke in warm and cool environments and before and after abstinence from tobacco. Am Heart J. 1957;53:455–462. doi: 10.1016/0002-8703(57)90180-1. [DOI] [PubMed] [Google Scholar]
- Evans WF, Stewart HJ. Effect of smoking cigarets on the peripheral blood flow. Am Heart J. 1943;26:79–91. [Google Scholar]
- Fushimi H, Inoue T, Yamada Y, Matsuyama Y, Kameyama M. Profound vasoconstrictive effect of cigarette smoking in diabetics with autonomic neuropathy. Diabetes Res Clin Pract. 1992;16:191–195. doi: 10.1016/0168-8227(92)90116-9. [DOI] [PubMed] [Google Scholar]
- Gaenzer H, Neumayr G, Marschang P, Sturm W, Kirchmair R, Patsch JR. Flow-mediated vasodilation of the femoral and brachial artery induced by exercise in healthy nonsmoking and smoking men. J Am Coll Cardiol. 2001;38:1313–1319. doi: 10.1016/s0735-1097(01)01575-3. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. 1994;90:248–253. doi: 10.1161/01.cir.90.1.248. [DOI] [PubMed] [Google Scholar]
- Grunfeld JA, Tiedemann GJ, Westerman RA. Chronic nicotine exposure enhances cutaneous axon reflexes in the rat. Neuroreport. 1991;2:421–424. doi: 10.1097/00001756-199108000-00002. [DOI] [PubMed] [Google Scholar]
- Grunfeld JA, Tiedemann GJ, Westerman RA. Maternal nicotine exposure enhances cutaneous axon reflexes in the neonatal rat. Neuroreport. 1993;4:635–638. doi: 10.1097/00001756-199306000-00008. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Timmermann L, Stegmann JU, Janig W. Involvement of neurokinins in antidromic vasodilatation in hairy and hairless skin of the rat hindlimb. Neuroscience. 1999;89:1259–1268. doi: 10.1016/s0306-4522(98)00322-4. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Wasner G, Janig W. Interaction of sympathetic vasoconstriction and antidromic vasodilatation in the control of skin blood flow. Exp Brain Res. 1997;113:402–410. doi: 10.1007/pl00005594. [DOI] [PubMed] [Google Scholar]
- Hashimoto H. Impaired microvascular vasodilator reserve in chronic cigarette smokers – a study of postocclusive reactive hyperemia in the human finger. Jpn Circ J. 1994;58:29–33. doi: 10.1253/jcj.58.29. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology. 2000;152:321–333. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- Holzer P. Peptidergic sensory neurons in the control of vascular functions: mechanisms and significance in the cutaneous and splanchnic vascular beds. Rev Physiol Biochem Pharmacol. 1992;121:49–146. doi: 10.1007/BFb0033194. [DOI] [PubMed] [Google Scholar]
- Hornyak ME, Naver HK, Rydenhag B, Wallin BG. Sympathetic activity influences the vascular axon reflex in the skin. Acta Physiol Scand. 1990;139:77–84. doi: 10.1111/j.1748-1716.1990.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Activation of spinal wide dynamic range neurons by intracutaneous microinjection of nicotine. J Neurophysiol. 1999;82:3046–3055. doi: 10.1152/jn.1999.82.6.3046. [DOI] [PubMed] [Google Scholar]
- Jinno S, Hua XY, Yaksh TL. Nicotine and acetylcholine induce release of calcitonin gene-related peptide from rat trachea. J Appl Physiol. 1994;76:1651–1656. doi: 10.1152/jappl.1994.76.4.1651. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol. 1989;257:H1599–H1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-L-arginine-methyl ester on neuropeptide-induced vasodilation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- Kristufek D, Stocker E, Boehm S, Huck S. Somatic and prejunctional nicotinic receptors in cultured rat sympathetic neurones show different agonist profiles. J Physiol. 1999;516:739–756. doi: 10.1111/j.1469-7793.1999.0739u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock WG, Coller FA. Peripheral vasoconstriction by tobacco demonstrated by skin temperature changes. Proc Soc Exp Biol Medical. 1932;29:497–488. [Google Scholar]
- Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol. 1996;497:837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266:1268–1276. [PubMed] [Google Scholar]
- Miao FJ, Benowitz N, Levine JD. Neural and endocrine circuits mediating inhibition of bradykinin-induced plasma extravasation by subcutaneous and spinal-intrathecal nicotine. J Pharmacol Exp Ther. 1996;277:1510–1516. [PubMed] [Google Scholar]
- Miao FJ, Benowitz NL, Levine JD. Endogenous opioids suppress activation of nociceptors by sub-nanomolar nicotine. Br J Pharmacol. 2001;133:23–28. doi: 10.1038/sj.bjp.0704031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114–117. doi: 10.1016/S0140-6736(02)07369-5. 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- Monfrecola G, Riccio G, Savarese C, Posteraro G, Procaccini EM. The acute effect of smoking on cutaneous microcirculation blood flow in habitual smokers and nonsmokers. Dermatology. 1998;197:115–118. doi: 10.1159/000017980. [DOI] [PubMed] [Google Scholar]
- Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, Sundler F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63:307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Van De Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation. 1998;98:528–534. doi: 10.1161/01.cir.98.6.528. [DOI] [PubMed] [Google Scholar]
- Parkhouse N, Le Quesne PM. Quantitative objective assessment of peripheral nociceptive C fibre function. J Neurol Neurosurg Psychiatry. 1988;51:28–34. doi: 10.1136/jnnp.51.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola PE, Johnson JM, Kellogg DL, Jr, Kosiba WA. Control of skin blood flow by whole body and local skin cooling in exercising humans. Am J Physiol. 1996;270:H208–H215. doi: 10.1152/ajpheart.1996.270.1.H208. [DOI] [PubMed] [Google Scholar]
- Pergola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Sanders M, Grobe J, Fonte C, Cherry C, Wilson A, Jacob R. Quitting cigarette smoking produces minimal loss of chronic tolerance to nicotine. Psychopharmacology. 2001;158:7–17. doi: 10.1007/s002130100850. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Goettler J, Caggiula AR, Reynolds WA, Stiller RL, Scierka A, Jacob RG. Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans. J Pharmacol Exp Ther. 1994;270:628–638. [PubMed] [Google Scholar]
- Porchet HC, Benowitz NL, Sheiner LB. Pharmacodynamic model of tolerance: application to nicotine. J Pharmacol Exp Ther. 1988;244:231–236. [PubMed] [Google Scholar]
- Puttfarcken PS, Manelli AM, Arneric SP, Donnelly-Roberts DL. Evidence for nicotinic receptors potentially modulating nociceptive transmission at the level of the primary sensory neuron: studies with F11 cells. J Neurochem. 1997;69:930–938. doi: 10.1046/j.1471-4159.1997.69030930.x. [DOI] [PubMed] [Google Scholar]
- Richardson D. Effects of tobacco smoke inhalation on capillary blood flow in human skin. Arch Environ Health. 1987;42:19–25. doi: 10.1080/00039896.1987.9935790. [DOI] [PubMed] [Google Scholar]
- Roberts RG, Stevenson JE, Westerman RA, Pennefather J. Nicotinic acetylcholine receptors on capsaicin-sensitive nerves. Neuroreport. 1995;6:1578–1582. doi: 10.1097/00001756-199507310-00028. [DOI] [PubMed] [Google Scholar]
- Roth GM, McDonald JB, Sheard C. The effect of smoking cigarettes and of the intravenous administration of nicotine on the electrocardiogram, basal metabolic rate, cutaneous temperature, blood pressure and pulse rate of normal persons. JAMA. 1944;125:761–767. [Google Scholar]
- Sarabi M, Lind L. Short-term effects of smoking and nicotine chewing gum on endothelium-dependent vasodilation in young healthy habitual smokers. J Cardiovasc Pharmacol. 2000;35:451–456. doi: 10.1097/00005344-200003000-00016. [DOI] [PubMed] [Google Scholar]
- Schaffer M, Beiter T, Becker HD, Hunt TK. Neuropeptides: mediators of inflammation and tissue repair? Arch Surg. 1998;133:1107–1116. doi: 10.1001/archsurg.133.10.1107. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Luz O, Averbeck B, Bickel A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neurosci Lett. 1997;230:117–120. doi: 10.1016/s0304-3940(97)00494-1. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Greffrath W, Busselberg D, Treede RD. Inactivation and tachyphylaxis of heat-evoked inward currents in nociceptive primary sensory neurones of rats. J Physiol. 2000;528:539–549. doi: 10.1111/j.1469-7793.2000.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si ML, Lee TJ. Alpha7-nicotinic acetylcholine receptors on cerebral perivascular sympathetic nerves mediate choline-induced nitrergic neurogenic vasodilation. Circ Res. 2002;91:62–69. doi: 10.1161/01.res.0000024417.79275.23. [DOI] [PubMed] [Google Scholar]
- Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–291. doi: 10.1007/s00441-001-0477-8. [DOI] [PubMed] [Google Scholar]
- Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1–5. doi: 10.1097/01.SLA.0000074980.39700.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW. Actions of cholinergic agonists and antagonists on sensory nerve endings in rat skin, in vitro. J Neurophysiol. 1993;70:397–405. doi: 10.1152/jn.1993.70.1.397. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R894–R901. doi: 10.1152/ajpregu.2001.281.3.R894. [DOI] [PubMed] [Google Scholar]
- Wardell K, Naver HK, Nilsson GE, Wallin BG. The cutaneous vascular axon reflex in humans characterized by laser Doppler perfusion imaging. J Physiol. 1993;460:185–199. doi: 10.1113/jphysiol.1993.sp019466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherby JH. Skin temperature changes caused by smoking and other sympathomimetic stimuli. Am Heart J. 1942;24:17–30. [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]