Abstract
Ghrelin, a gastric peptide hormone, has been reported to regulate growth hormone secretion and energy homeostasis. Here we show that ghrelin promotes neural proliferation in vivo and in vitro in the rat dorsal motor nucleus of the vagus (DMNV). Ghrelin receptor mRNA and immunoreactivity were detected in tissues from DMNV. Systemic administration of ghrelin (130 nmol kg−1) significantly increased 5-bromo-2′-deoxyuridine (BrdU) incorporation in the DMNV in adult rats with cervical vagotomy (BrdU positive cells; from 27 ± 4 to 69 ± 14 n = 5, P < 0.05). In vitro, exposure of cultured DMNV neurones to ghrelin significantly increased the percentage of BrdU incorporation into cells in both dose-dependent (10−9–10−6m), and time-dependent (6 h to 48 h) manners. Ghrelin significantly increased voltage-activated calcium currents in isolated single DMNV neurones from a mean maximal change of 141 ± 26 pA to 227 ± 37 pA. Upon removal of ghrelin, calcium currents slowly returned to baseline. Blocking L-type calcium channels by diltiazem (10 μm) significantly attenuated ghrelin-mediated increments in BrdU incorporation (n = 5, P < 0.05). Ghrelin acts directly on DMNV neurones to stimulate neurogenesis.
Ghrelin, a novel 28 amino acid peptide, is secreted by gastric oxyntic glands and circulates in blood (Kojima et al. 1999). It is an endogenous ligand for the growth hormone secretagogue receptor and has been reported to stimulate growth hormone release in human and rat, following either peripheral or central administration (Kojima et al. 1999; Wren et al. 2000; Takaya et al. 2000). Whereas ghrelin was originally reported to stimulate growth hormone release, the wide distribution of its receptor, growth hormone secretagogue receptor (GHS-R), has suggested a potentially broader array of actions. Expression of ghrelin and its related receptor in the kidney indicates that ghrelin may play an important role in the regulation of renal function (Mori et al. 2000). In rats, ghrelin has been found to stimulate food intake (Wren et al. 2000; Nakazato et al. 2001), to induce adiposity (Tschop et al. 2000), and to increase body weight (Wren et al. 2000; Tschop et al. 2000; Nakazato et al. 2001), suggesting a possible role in regulation of feeding behaviour and energy metabolism. The up-regulation of food intake by ghrelin is proposed to be mediated by a central mechanism involving the regulation of the neuropeptide Y (NPY) and proopiomelanocortin (POMC) neurones at the hypothalamic level (Nakazato et al. 2001; Cowley et al. 2003).
In the gastrointestinal system, ghrelin has been recently reported to regulate secretion of gastric acid (Masuda et al. 2000; Date et al. 2001), gastric motility (Masuda et al. 2000; Trudel et al. 2002), and pancreatic protein output (Zhang et al. 2001). These results suggest that ghrelin coordinates multiple functions in the gastrointestinal system, although the mechanisms involved remain largely unknown. The regulation of the gastrointestinal functions by ghrelin has been demonstrated to be mediated by a vagus nerve-dependent mechanism (Masuda et al. 2000; Date et al. 2001). A recent report by Faulconbridge et al. (2003) showed that direct application of ghrelin into the dorsal vagal complex (DVC) stimulates food intake. These studies suggest that ghrelin may act directly on the DVC to regulate the feeding behaviour.
Neurogenesis, a process through which precursor cells differentiate into a mature neuronal phenotype, persists in the discrete regions of the adult brain. While the neurogenesis in the subventricular zone (SVZ) (Alvarez-Buylla & Garcia-Verdugo, 2002) and the subgranular zone (SGZ) (Seri et al. 2001) of the hippocampal dentate gyrus has been described, it is unknown whether central parasympathetic nuclei in the adult brain contain precursor cells. The dorsal motor nucleus of the vagus (DMNV) contains neurones that provide the parasympathetic efferent outflow to the gastrointestinal system. Despite the DMNV having been demonstrated to undergo degeneration, regeneration and changes in gene expression in response to mechanical and chemical injury (Laiwand et al. 1987; Jacques et al. 1999; Chang et al. 2001; Yuan & Yang, 2002), the cellular and molecular mechanisms underlying the process of neural regeneration in the DMNV remain unknown. While a variety of extracellular growth factors have been reported to stimulate neurogenesis after brain injury (Seri et al. 2001; Alvarez-Buylla & Garcia-Verdugo, 2002; Jin et al. 2002a, b), the growth factors enhancing neural regeneration in the DMNV remain to be characterized. Previous studies (Gross et al. 1990; Hermann et al. 2001) have demonstrated that the DMNV directly responds to systemic endotoxin, suggesting that the DMNV may possess the characteristics of a circumventricular organ, which is essentially devoid of a blood–brain barrier. Since ghrelin is circulating in the blood, it is possible that the DMNV is exposed to, and directly responses to, ghrelin. Because ghrelin acts as a potent growth factor to stimulate cell proliferation (Pettersson et al. 2002; Zhang et al. 2004), we hypothesize that ghrelin stimulates neural proliferation in the DMNV.
The object of this study was to determine whether ghrelin promotes neurogenesis in the DMNV. We report here for the first time that in the DMNV (1) ghrelin receptors are present; (2) systemic administration of ghrelin stimulates BrdU incorporation in neuronal precursors in adult rats; (3) in vitro, ghrelin increases BrdU incorporation in both a dose- and a time-dependent manner; (4) activation of ghrelin receptors increases voltage-sensitive calcium currents; and (5) blocking L-type calcium channels by diltiazem attenuates ghrelin-mediated increments in BrdU incorporation.
Methods
In vivo BrdU labelling and immunostainning
Male Sprague-Dawley rats (140–160 g, Harlan, Indianapolis, IN, USA) were kept in individual cages and were given chow food and water ad libitum. All experiments were performed in rats anaesthetized with intramuscular injection of a mixture of urethane and ketamine (13 and 87 mg (kg body weight)−1, respectively) and were approved by the University of Michigan Committee on Use and Care of Animals. Ghrelin was dissolved in 0.9% normal saline. Ghrelin (130 nmol kg−1) or 0.9% saline was injected intravenously into male rats twice daily 15 min before BrdU injection. BrdU (50 mg kg−1 in saline i.p.) or saline was given twice daily for 6 days. Unilateral cervical vagotomy was performed prior to drug administration. Through a midline cervical incision, right cervical vagal trunks were exposed and sectioned. In some animals, sham-operation was performed without sectioning vagus nerves.
After experiments, rats were anaesthetized by an intramuscular injection of sodium pentobarbital (75 mg (kg body weight)−1) and perfused transcardially, first for 10 min with 100 ml of 0.1 m phosphate buffer (pH 7.4) and then for 15 min with 150 ml of 4% paraformaldehyde in 0.1 m phosphate buffer. The brainstem was removed and postfixed for 2 days at 4°C, then kept in 0.1 m phosphate buffer containing 20% sucrose for 24 h. The medulla oblongata containing the DMNV was sectioned into 20 μm slices at the interaural level of −4.24 to −5.08 mm according to the atlas of Paxinos and Watson (1998), using a freezing microtome. All slices were incubated for 24 h at 4°C with a polyclonal rabbit anti-BrdU antibody (3.5 μg ml−1) (Roche Diagnostics Corporation, Indianapolis, IN, USA), then incubated at room temperature for 2 h with biotin-conjugated goat anti-rabbit IgG (1: 400) (Vector Laboratories Inc., Burlingame, CA, USA), and finally incubated for 30 min with Alexa Fluor 594 conjugated streptavidin (Vector Laboratories Inc.). BrdU positive cells were counted in three sections per animal at the level of area postrema and means of cells per section were calculated. Data were expressed as the mean ±s.e.m. Statistical differences between control and treatment were assessed by ANOVA with a post-test Tukey's multiple comparison test. Data were considered significant different when P < 0.05.
In vitro culture of DMNV
DMNV neurones were isolated from 3- to 5-day-old Sprague-Dawley rats (Harlan, Indianapolis, IN, USA). Rats were killed by an overdose of CO2, and the brainstem was rapidly removed and chilled at 0°C in a dissection solution containing (mm): NaCl 138, KCl 4, MgCl2 1, CaCl2 2, glucose 20, Hepes 10. Tissue blocks were prepared and sectioned transversely into 350 μm slice at the level around the obex using a Vibratome 3000 (Redding, CA, USA) and two slices were taken. The DMNV area, which lies immediately ventral to the nuclei of the solitary tract (NST) and dorsal to the XII nucleus was excised using a dissecting microscope. DMNV tissue was then digested in an enzyme solution containing protease type XIV (0.6 mg ml−1) and trypsin type I (0.4 mg ml−1) at 32°C for 40 min. The tissue was then dissociated by gentle trituration with fire-polished Pasteur pipettes. Cells were plated onto poly-d-lysine coated 35 mm culture dishes. Neurones were maintained and cultured at 37°C in an atmosphere of 5% CO2 in Neurobasal medium A (Gibco BRL, Grand Island, NY, USA) containing 2% B27 supplement (Gibco BRL), 2 mm glutamine, 1% penicillin and streptomycin, and 5 ng ml−1 fibroblast growth factor β (FGF-β). After 4 days, one-half of the medium was replaced and experiments were conducted at 6–7 days unless indicated otherwise.
RT-PCR
Total RNA was isolated from DMNV tissues prepared freshly from 3- to 5-day-old rats using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's directions. Single-strand cDNA synthesis was performed as follows: 30 μl of reverse transcription mixture contained 1 μg of DNase I pretreated total RNA, 0.75 μg of oligo d(T) primer, 6 μl of 5 × RT buffer, 10 mm dithiothreitol, 0.5 mm deoxynucleotides, 50 units of RNase inhibitor and 240 units of reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The RT reaction was carried out at 40°C for 70 min followed by heat inactivation at 95°C for 3 min. PCR primers used for ghrelin receptors mRNA detection were deduced from rat published sequences. β-Actin was used as an internal control. The nucleotide sequences of sense and antisense primers with the expected product size are follows: (1) ghrelin receptor: TCG GAT CTG CTC ATC TTC CT (sense, bp 259–278) and CAG CTC TCG CTG ACA AAC TG (antisense, bp 355–374), 116 bp product; (2) β-actin: GTC GTA CCA CTG GCA TTG TG (sense, bp 437–456) and CTC TCA GCT GTG GTG GTG AA (antisense, bp 598–617), 181 bp product. The cDNA was amplified by real time PCR, using the Smart Cycler system (Cepheid). PCR was performed in a total 25 μl volume, containing 2.5 μl cDNA, 5 mm MgCl2, 0.2 mm dNTPs, 0.25 μm each primer, 1.25 U Ampli Taq Polymerase (Roche), and 1 μl of a 800 × diluted SYBR Green I stock (Roche). The PCR program was as follows: hold 95°C for 2 min; three-temperature cycle repeat for 45 times, 94°C for 15 s, 70°C for 15 s, 72°C for 20 s. Melt curve analysis was from 60°C to 95°C at 0.2°C s−1 with Optics Ch1 On. PCR product was also visualized by 1.5% agarose gel electrophoresis. For negative controls, PCR reactions were performed for the primer pairs in the absence of transcript as others have reported. Total RNA from rat hypothalamus was used as positive control.
Whole cell patch-clamp recording
Experiments were performed at room temperature (22°C). Conventional whole cell patch-clamp recordings were made under voltage clamp on DMNV neurones cultured for 1–2 days using an Axopatch 200A amplifier (Axon Instruments, Union City, CA, USA). Patch pipettes (resistance 2–5 MΩ) were filled with an intracellular recording solution containing (mm): CsOH 112, CH3O4S 112, CsCl 13, MgCl2 1, EGTA 10, Hepes 30, ATP 2, GTP 0.2, pH adjusted to 7.3. The extracellular solution to isolate calcium current utilizing Ba2+ as a charge carrier contained (mm): tetraethylammonium chloride 120, BaCl2 10, MgCl2 1, Hepes 10, and glucose 10, pH adjusted to 7.3 with Tris. Data were filtered at 1 kHz, digitized via an Instrutech ITC-16 interface, leak subtracted, acquired and stored on a computer using Pulse Control software (Drs Jack Herrington and Richard Bookman, University of Miami Medical School, Miami, FL, USA).
Immunofluorescent staining
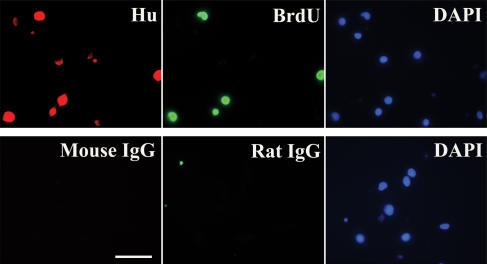
Cultured DMNV neurones were fixed with 4% paraformaldehyde for 10 min and processed for immunofluorescent staining. Neurones were incubated at 4°C for 24 h with one of the following primary antibodies: rabbit anti-microtubule-associated protein 2 (MAP-2) 1: 1000, Chemicon International, Temecula, CA, mouse anti-Hu (1: 100, Molecular Probes); rabbit anti-glial fibrillary acidic protein (GFAP; 1: 200, Santa Cruz Biotechnology Inc., Santa Cruz, CA). Neurones were then incubated at 22°C for 1 h with secondary antibodies: goat anti-rabbit FITC-conjugated IgG (1: 100) or goat anti-mouse FITC-conjugated IgG (1: 50) (Santa Cruz Biotechnology Inc.). Primary antibodies used for double staining were: mouse anti-Hu (1: 100, Molecular Probes) and rat anti-BrdU (Serotec Inc., Raleigh, NC, USA). Controls included omitting primary antibodies or substituting primary antibodies with mouse or rat IgGs.
Cell proliferation assay
BrdU incorporation was performed according to the manufacturer's protocol (Roche Diagnostics Corporation). In brief, BrdU (100 μm) was added to cultured DMNV neurones for 24 h and cultures were fixed with mixture of acetone and methanol (1: 1) at −20°C for 10 min. At room temperature, neurones were incubated with mouse monoclonal anti-BrdU (5 μg ml−1) for 2 h, followed by FITC-conjugated goat anti-mouse IgG (Vector Laboratories Inc., Burlingame, CA, USA). BrdU positive cells were counted in five 20 × microscope fields per well (at the 12-, 3-, 6-, and 9-o'clock positions and in the centre). Data were expressed as the percentage of total cells present.
Results
Expression of ghrelin receptor
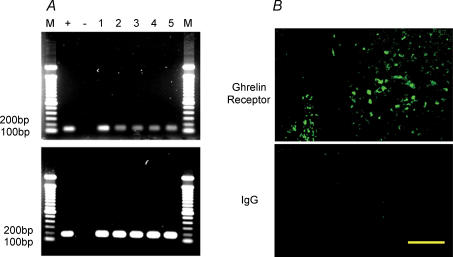
Ghrelin receptor, also known as growth hormone secretagogue receptor, contains seven transmembrane (TM) domains and belongs to a family of G-protein-coupled receptors (Howard et al. 1996; McKee et al. 1997). Two types of ghrelin receptor cDNA have been identified: type 1a, encoding a functional protein containing 7 TM domains, and type 1b, encoding a protein containing TM-1 through 5 with no measurable functional activity. Ghrelin receptors are widely distributed among both central and peripheral tissues including pituitary gland (Howard et al. 1996), hypothalamus (Howard et al. 1996), pancreas (Date et al. 2002), stomach and intestine (Date et al. 2000). cDNAs for human, rat, mouse and swine ghrelin receptor have been sequenced (Howard et al. 1996; McKee et al. 1997). To determine if ghrelin receptor is present in DMNV neurones, RT-PCR analysis was performed using primers generated from published coding rat ghrelin receptor sequences. As shown in Fig. 1A, mRNA corresponding to ghrelin receptor was detected in DMNV tissues isolated from 3- to 5-day-old rats (n = 5). Sequence analysis indicates that these mRNAs matched known rat nucleotide sequences. As a control for expression level, the house-keeping gene β-actin mRNA was detected in all samples. Expression of ghrelin receptor was further verified by localization of the ghrelin receptor immunoreactivity in DMNV neurones using a specific antibody against the C-terminal sequence 330–366 of the human ghrelin receptor 1a (Fig. 1B).
Figure 1. Expression of ghrelin receptor in DMNV.
A, RT-PCR products of RNA extracted from 3- to 5-day-old rat DMNV tissues corresponding to ghrelin receptor coding sequences (116 bp) are shown in lanes numbered 1 to 5 (each lane represents result from one individual rat, the numbers correspond to each rat). Lanes M show molecular size markers. Other lanes represent the positive control (+) from hypothalamus and the negative control (–) (RT-PCR performed without RNA reverse transcription). The upper panel represents ghrelin receptor mRNA, whereas the lower panel shows β-actin mRNA (181 bp). B, ghrelin receptor immunoreactivity was localized in DMNV neurones by a specific antibody against the 330–366 amino acid sequence of the human ghrelin receptor 1a (upper panel). Control tissue incubated with rabbit IgG showed no significant staining (lower panel). Scale bar represents 100 μm.
Stimulation of neuronal proliferation in vivo
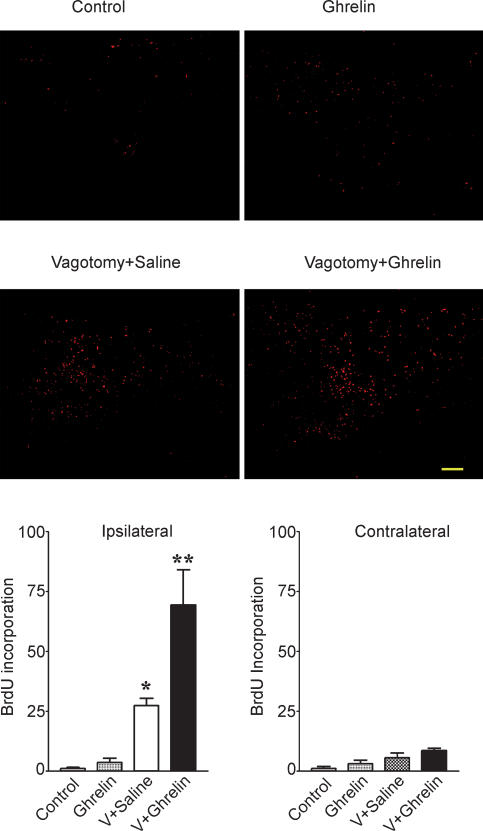
In order to study neurogenesis in the DMNV, we assayed the BrdU incorporation in adult rats. As shown in Fig. 2, BrdU labelling (1 ± 0.5, n = 4) was rare in the DMNV. Systemic administration of ghrelin (130 nmol kg−1) demonstrated no significant increase in the BrdU incorporation (3.5 ± 2, n = 4, P > 0.05 as compared to control). With cervical vagotomy, there was a significant increase in BrdU labelling in the ipsilateral DMNV. When ghrelin (130 nmol kg−1) was systemically administrated in adult rats undergoing vagotomy, BrdU incorporation was significantly increased relative to rats with cervical vagotomy that were administrated 0.9% NaCl (69 ± 14 versus 27 ± 4, P < 0.05).
Figure 2. BrdU incorporation into the dorsal vagal complex .
Sections through the dorsal vagal complex were stained for BrdU and visualized with Alexa fluor 594. BrdU labelling was increased in the ipsilateral DVC 6 days after cervical vagotomy. In rats with cervical vagotomy (n = 5), administration of ghrelin significantly increased BrdU labelling in the DVC relative to controls receiving saline. Results are representative of five experiments. The bar graphs show the average BrdU labelling in the DMNV as means ±s.e.m.*P < 0.05 versus control. **P < 0.01 versus vagotomy + saline.
Stimulation of neuronal proliferation in vitro
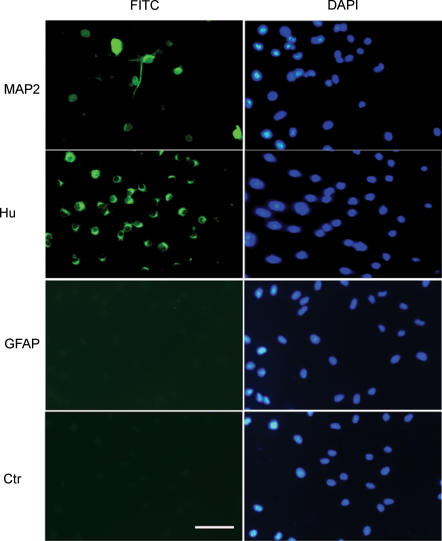
To study the mechanism of neurogenesis stimulated by ghrelin, we established an in vitro model of culture of DMNV neurones and assayed the effect of ghrelin on cultured DMNV neural proliferation. DMNV neurones cultured for 6–7 days contained mature neurones as demonstrated by expression of neuronal marker, MAP-2 (13 ± 4%, n = 3), as well as precursor cells as demonstrated by expression of the immature neuronal marker, Hu in 78 ± 9% of cells (Fig. 3). Glia fibrillary acidic protein (GFAP) immunopositive cells in these cultures were rare (∼1%), indicating that cultures were free of contaminating astrocytes. When cultured neurones were induced to differentiate by growing in medium without βFGF, the number of fully differentiated neurones increased significantly as demonstrated by the increment of MAP-2 positive cells (49 ± 8%, n = 4). Exposure of cultured cells to ghrelin (10−7m) did not demonstrate any effect on neuronal differentiation (48 ± 9% of cells stained positive for MAP-2, n = 4) relative to control.
Figure 3. Neural precursor cells in cultured DMNV neurones .
DMV neurones cultured for 6–7 days contained neural precursor cells as demonstrated by expression of the immature neuronal marker Hu: 78 ± 9% of cells expressed Hu immunoreactivity; 13 ± 4% of cells stained positive for MAP-2, identifying them as post-mitotic cells; GFAP-immunopositive cells, indicative of cells of glial lineage, were rare (∼1%). Cell nuclei were counterstained with DAPI. Scale bar represents 100 μm.
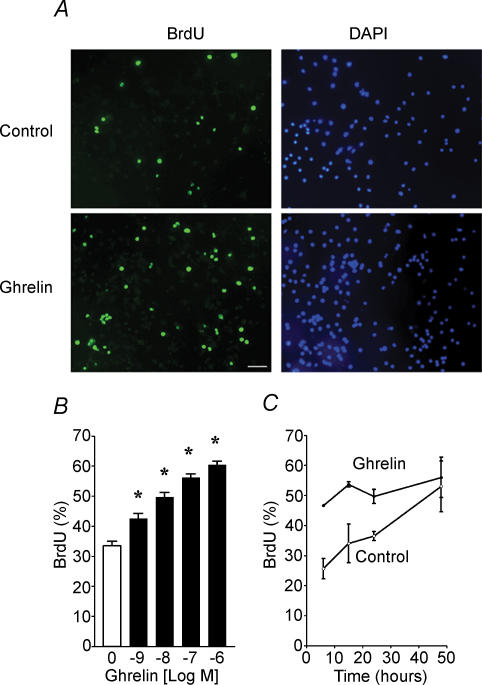
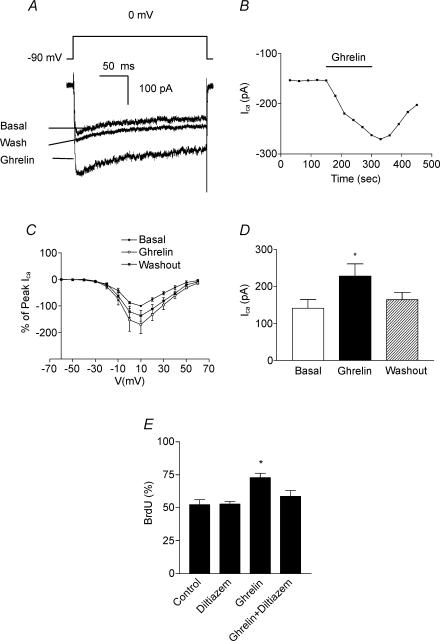
DMNV neurones were cultured under serum-free conditions and cell proliferation was assayed. When cultures were treated with ghrelin for 24 h in vitro, the number of neurones increased 2.1 ± 0.1-fold, which was significantly different from control (1.4 ± 0.2, n = 5, P < 0.05). To further characterize the mitotic effect of ghrelin, we used BrdU labelling to identify cells undergoing proliferation. As shown in Fig. 4A, increased incorporation of BrdU into cells was detected. The ghrelin-mediated increase in BrdU incorporation was dose dependent within the range of 10−9–10−6m (Fig. 4B), and time dependent from 6 h to 48 h (Fig. 4C).
Figure 4. Stimulation of BrdU incorporation into cultured DMNV neurones by ghrelin .
A, immunodetection of BrdU labelling in control (upper) and in the presence of ghrelin (lower). Nuclei were counterstained with DAPI. The figure shown is representative of three different experiments. Scale bar represents 100 μm. B, effect of increasing concentrations of ghrelin on BrdU incorporation in cultured DMNV neurones. C, time dependent increases in percentage of BrdU positive cells responding to ghrelin (10−7m). Values are means ±s.e.m.*P < 0.05.
In order to identify the nature of proliferating cells, double immunofluorescent staining was performed. As shown in Fig. 5, mitotic cells, identified by BrdU labelling, also stained positive for Hu, a marker for immature and mature neurones.
Figure 5. Stimulation of BrdU incorporation by ghrelin in neurones .
Cultured DMNV cells were treated with ghrelin (10−7m) for 24 h. Colocalization of BrdU labelling (Green) with immunoreactivity for the neuronal marker Hu (Red) was demonstrated by double immunofluorescent staining. Nuclei were counterstained with DAPI (Blue). Scale bar represents 50 μm.
Activation of voltage-dependent calcium channels
Postsynaptic L-type voltage-dependent calcium currents have been reported to activate the MAPK pathway which then stimulates expression of genes that are essential for neural survival and plasticity, including nuclear transcription factor cAMP response element binding protein (CREB) (Dolmetsch et al. 2001). It is possible that ghrelin stimulates neurogenesis via the mediation of L-type calcium channels. To test this possibility, we investigated whether ghrelin affects calcium channels on DMNV neurones using a whole-cell patch-clamp technique. Experimental conditions were set to isolate calcium currents from other voltage-dependent currents. Calcium current was evoked by stepping the membrane potential from a holding potential of −90 mV to potentials between −90 and +60 mV. Calcium current was first evident with voltage steps to −30 mV, and reached an average maximal current amplitude of 308 ± 78 pA (n = 13) with membrane depolarization at 10 mV (Fig. 6A). The current was sustained during the time of recording. As shown in Fig. 6A, application of ghrelin (10−8m) significantly increased both the peak and the plateau phases of the voltage-dependent calcium currents in 35 ± 9% of DMNV neurones tested (n = 16). The effect of ghrelin was time dependent with the peak response at 2.5 min (Fig. 6B). Current–voltage relationship analysis demonstrated no shift of voltage activation with ghrelin, suggesting that it does not alter the voltage sensitivity of activation (Fig. 6C). As shown in Fig. 6D, ghrelin increased the mean maximal change of calcium currents from 141 ± 26 pA to 227 ± 37 pA, when membrane potential was depolarized to 0 mV to induce submaximal calcium current.
Figure 6. Calcium currents contribute to ghrelin-induced increment of BrdU incorporation in cultured DMNV neurones .
A, representative calcium current activated by 50 ms voltage step from a holding potential of −90 mV to 0 mV prior to, during and following washout of ghrelin (10−8m). n = 16. B, representative time course of change in current following to application of ghrelin. C, current–voltage relationship for calcium current before, during and following washout of ghrelin (n = 3). D, mean change of calcium current in the absence of ghrelin (open bar), in the presence of ghrelin (filled bar), and following 2 min washout of ghrelin (stippled bar). n = 6. *P < 0.05. E, treatment of cultured DMNV neurones with 10 μm diltiazem, a specific L-type voltage-dependent calcium channel blocker, significantly attenuated the increment of BrdU incorporation induced by ghrelin (10−7m). Values are means ±s.e.m.n = 5. *P < 0.05 versus ghrelin.
To determine if activation of calcium channels directly contributes to cell proliferation, cultured DMNV neurones were treated with diltiazem, a specific L-type voltage-dependent calcium channel blocker. As shown in Fig. 6E, treatment of cultured DMNV neurones with 10 μm diltiazem significantly attenuated ghrelin-induced increases in BrdU incorporation (n = 5, P < 0.05).
Discussion
The major finding of the present study is that ghrelin stimulates neuronal proliferation in DMNV both in vivo and in vitro. This observation constitutes the first report of neurogenesis stimulated by ghrelin. This conclusion is supported by two distinct observations: (1) systemic administration of ghrelin increases vagotomy-induced BrdU incorporation in the DMNV in adult rats; and (2) cultured DMNV neurones respond to ghrelin with increased BrdU incorporation in both dose- and time-dependent manners.
Neurogenesis has been demonstrated in the adult nervous system both under physiological conditions and subsequent to ischaemic brain injury (Seri et al. 2001; Alvarez-Buylla & Garcia-Verdugo, 2002; Jin et al. 2002a, b). Several studies have shown that global or focal cerebral ischaemia stimulates neurogenesis, typically defined by increased incorporation of BrdU into cells that express neuronal marker proteins (Jin et al. 2002a, b). Although the molecular mechanisms remain unknown, neurogenesis has been reported to be generated through the proliferation of radial cells located in the floor of cerebral ventricles (Seri et al. 2001; Alvarez-Buylla & Garcia-Verdugo, 2002). The present study examines neurogenesis in adult brain nuclei surrounding the 4th cerebral ventricle such as DMNV, the NST and the area postrema (AP). Although lacking active proliferation under basal conditions, the dorsal vagal complex (DVC) responds to perturbation induced by vagotomy with an increase in cell proliferation. It has been previously reported that mechanical or chemical injury such as vagal axotomy, hypoxia and hypoglycemia results in multiple neuronal responses in the DVC including activation of c-fos expression (Yuan & Yang, 2002) and neuronal degeneration (Chang et al. 2001), suggesting neuronal plasticity in the DVC.
Neurogenesis in embryos and in adult neural stem cells is regulated by a group of growth factors including stem cell factor (Jin et al. 2002a), vascular endothelial growth factor (VEGF) (Jin et al. 2002b), fibroblast growth factor (FGF) (Munoz-Sanjuan & Brivanlou, 2002), insulin-like growth factor (IGF) (Munoz-Sanjuan & Brivanlou, 2002) and bone morphogenetic protein (BMP) (Munoz-Sanjuan & Brivanlou, 2002). Based on both in vivo and in vitro data, ghrelin produced a significant increase in BrdU incorporation, suggesting its potential to promote neuronal development and regeneration. Both the organization and phenotype of DMNV neurones projecting to the gastrointestinal tract undergo extensive specification and reorganization in the perinatal period (Powley et al. 2001). The molecular mechanisms that control alteration in vagal innervation are not clear. In rats, ghrelin-immunoreactive cells were found to be first expressed at a relatively low level in the fetal stomach from pregnancy day 18 (Hayashida et al. 2002). The number of ghrelin-immunoreactive cells and plasma ghrelin levels increase significantly in the early postnatal period (Hayashida et al. 2002; Sakata et al. 2002), suggesting that ghrelin may play a role in neonatal development. It is possible that ghrelin-mediated mitotic effects on DMNV neurones play a role in the development of the vagal innervation. Since ghrelin immunoreactivity is not detected in the DVC (data not shown) and DVC is devoid of brain–blood barrier, the source of ghrelin responsible for stimulation of neurogenesis in DMNV may come from the circulating blood.
The detection of both ghrelin receptor mRNA and immunoreactivity in DMNV suggests that ghrelin may act as an extracellular signal molecule to activate its receptor. The ghrelin receptor has been reported to be expressed in a variety of tissues ranging from the hypothalamus, pituitary gland and cerebral cortex in the central nervous system (Yokote et al. 1998) to the gastrointestinal system (Date et al. 2000), pancreas (Date et al. 2002), kidney (Mori et al. 2000) and the cardiovascular system (Papotti et al. 2000) in the peripheral tissues. Activation of the ghrelin receptors accounts for the diversity of functions that ghrelin exerts in these tissues.
Activation of ghrelin receptors has been associated with an increase of intracellular calcium concentration ([Ca2+]i) in several types of cells, including pancreatic β cells, HEK-293 cells and CHO cells expressing ghrelin receptor (Kojima et al. 1999; Bednarek et al. 2000; Date et al. 2002). The increase in [Ca2+]i is mediated by release of IP3-sensitive stores. Whole-cell patch-clamp recording suggests that voltage-sensitive calcium channels are present and may account for the ghrelin-mediated increase in the [Ca2+]i in DMNV neurones. Previous studies (Chen et al. 1996) using somatotropes demonstrated that binding of GHS ligand to the ghrelin receptor leads to an inhibition of potassium channels, which results in sustained depolarization of the somatotropes. This depolarization results in the entry of Ca2+ through voltage-gated L- and T-type channels. It is still unclear whether ghrelin activates L-type voltage-dependent calcium channels in DMNV neurones by inhibition of potassium currents. The physiological significance of the [Ca2+]i signalling in DMNV neurones is not clearly understood. However, [Ca2+]i is an important intracellular second messenger that regulates many short-term and long-term neuronal functions ranging from secretion to cell survival. In the present study, we demonstrated that the mitotic effect of ghrelin on DMNV neurones is attenuated by blocking L-type calcium channels by diltiazem.
Acknowledgments
This study was supported by National Institutes of Health Grant 2RO1DK043225-14.
References
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- Chang H-M, Lue J-H, Wen C-Y, Shieh J-Y. Axotomy along with hypoxia enhances the neuronal NADPH-d/NOS expression in lower brain stem motor neurons of adult rats. Exp Neurol. 2001;171:116–126. doi: 10.1006/exnr.2001.7731. [DOI] [PubMed] [Google Scholar]
- Chen C, Wu D, Clarke IJ. Signal transduction systems employed by synthetic GH-releasing peptides in somatotrophs. J Endocrinol. 1996;148:381–386. doi: 10.1677/joe.0.1480381. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. Ghrelin is present in pancreatic a cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Kojima M, Kuroiwa T, Matsukura S, Kangawa K, Nakazato M. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol. 2002;173:239–245. doi: 10.1677/joe.0.1730239. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Emch GS, Tovar CA, Rogers RC. c-fos generation in the dorsal vagal complex after systemic endotoxin is not dependent on the vagus nerve. Am J Physiol. 2001;280:R289–R299. doi: 10.1152/ajpregu.2001.280.1.R289. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Charles I, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Skepper JN, Navaratnam V. Fibroblast growth factor-1 improves the survival and regeneration of rat vagal preganglionic neurons following axon injury. Neurosic Lett. 1999;276:197–200. doi: 10.1016/s0304-3940(99)00832-0. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Greebberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002a;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002b;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Laiwand R, Werman R, Yarom Y. Time course and distribution of motoneuronal loss in the dorsal motor vagal nucleus of guinea pig after cervical vagotomy. J Comp Neurol. 1987;256:527–537. doi: 10.1002/cne.902560405. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- McKee KK, Palyha OC, Feighner SD, Hreniuk DL, Tan CP, Phillips MS, et al. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol Endocrinol. 1997;11:415–423. doi: 10.1210/mend.11.4.9908. [DOI] [PubMed] [Google Scholar]
- Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, et al. Kidney produces a novel acylated peptide, ghrelin. FEBS Lett. 2000;486:213–216. doi: 10.1016/s0014-5793(00)02308-5. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nature Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matrukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pettersson I, Muccioli G, Granata R, Deghenghi R, Ghigo E, Ohlsson C, et al. Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J Endocrinol. 2002;175:201–209. doi: 10.1677/joe.0.1750201. [DOI] [PubMed] [Google Scholar]
- Powley TL, Martinson FA, Phillips RJ, Jones S, Baronowsky EA, Swithers SE. Gastrointestinal projection maps of the vagus nerve are specified permanently in the perinatal period. Brain Res Dev Brain Res. 2001;129:57–72. doi: 10.1016/s0165-3806(01)00183-3. [DOI] [PubMed] [Google Scholar]
- Sakata I, Tanaka T, Matsubara M, Yamazaki M, Tani S, Hayashi Y, et al. Postnatal changes in ghrelin mRNA expression and in ghrelin-producing cells in the rat stomach. J Endocrinol. 2002;174:463–471. doi: 10.1677/joe.0.1740463. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Yokote R, Sato M, Matsubara S, Ohye H, Niimi M, Murao K, Takahara J. Molecular cloning and gene expression of growth hormone-releasing peptide receptor in rat tissues. Peptides. 1998;19:15–20. doi: 10.1016/s0196-9781(97)00263-5. [DOI] [PubMed] [Google Scholar]
- Yuan P-Q, Yang H. neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexues by insulin hypoglycemia. Am J Physiol Endocrinol Metab. 2002;283:E436–E448. doi: 10.1152/ajpendo.00538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chen M, Chen X, Segura BJ, Mulholland MW. Inhibition of pancreatic protein secretion by ghrelin in rat. J Physiol. 2001;537:231–236. doi: 10.1111/j.1469-7793.2001.0231k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao L, Lin TR, Chai B, Fan Y, Gantz I, et al. Inhibition of adipogenesis by ghrelin. Mol Biol Cell. 2004;15:2484–2491. doi: 10.1091/mbc.E03-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]