Abstract
Critical illness myopathy is a disorder in which skeletal muscle becomes electrically inexcitable. We previously demonstrated that a shift in the voltage dependence of fast inactivation of sodium currents contributes to inexcitability of affected fibres in an animal model of critical illness myopathy in which denervated rat skeletal muscle is treated with corticosteroids (steroid-denervated; SD). In the current study we examined whether expression of Nav1.5 contributes to the altered voltage dependence of sodium channel inactivation in SD muscle. We used TTX and μ-conotoxin GIIIB to selectively block Nav1.4 in SD muscle and found that the level of Nav1.5 did not correlate closely with the shift in fast inactivation. Surprisingly, we found that the voltage dependence of inactivation of Nav1.4 was similar to that of Nav1.5 in skeletal muscle in vivo. In severely affected fibres, inactivation of both Nav1.4 and Nav1.5 was shifted towards hyperpolarized potentials. We examined the role of denervation and steroid treatment in the shift of the voltage dependence of inactivation and found that both denervation and steroid treatment contribute to the shift in inactivation. Our results suggest that modulation of the voltage dependence of inactivation of both Nav1.4 and Nav1.5 in vivo contributes to loss of electrical excitability in SD muscle.
Critical illness myopathy is a disorder of skeletal muscle in which acute weakness in patients is caused by failure of muscle fibres to generate action potentials (Rich et al. 1996, 1997; Bird & Rich, 2002). We have examined the cause of loss of muscle excitability in an animal model of critical illness myopathy in which denervated rat muscle is treated with corticosteroids in vivo (steroid-denervated; SD; Rich et al. 1998). We found that a hyperpolarized shift in the voltage dependence of sodium channel fast inactivation plays a central role in loss of muscle fibre excitability (Rich & Pinter, 2001, 2003). The cause of altered voltage dependence of sodium channel inactivation in SD muscle is unknown.
Innervated adult skeletal muscle expresses only the Nav1.4 sodium channel isoform (Yang et al. 1991; Rich et al. 1999). In SD muscle, however, RNA for a second sodium channel isoform (Nav1.5) is expressed at high levels, suggesting Nav1.5 protein may be abundant (Rich et al. 1999). In vitro, heterologously expressed Nav1.5 channels gate at more negative potentials than Nav1.4 channels so the presence of Nav1.5 could shift the voltage dependence of sodium current in SD muscle (Wang et al. 1996; Zhang et al. 1999). Thus, one explanation for the shift in the voltage dependence of sodium channel inactivation in SD muscle is the presence of high levels of Nav1.5. However, a study of sodium channel gating in denervated muscle found that although Nav1.5 contributed less than 30% of total sodium conductance, there was a 10 mV shift of sodium channel activation and inactivation towards more hyperpolarized potentials (Pappone, 1980). This raises the possibility that the voltage dependence of Nav1.4 gating is altered following denervation. Since the animal model of critical illness myopathy that we use involves denervation of skeletal muscle, we also considered a second possibility: that altered Nav1.4 gating contributes to the hyperpolarized shift in sodium channel inactivation in SD muscle.
To determine whether expression of Nav1.5 causes the shift in gating of sodium current in SD muscle we selectively blocked Nav1.4 with tetrodotoxin (TTX) and μ-conotoxin GIIIB. We found that hyperpolarized shifts in the voltage dependence of sodium current gating did not correlate closely with the amount of Nav1.5 present. Instead, in more severely affected fibres, there was a hyperpolarized shift in the voltage dependence of fast inactivation of both Nav1.4 and Nav1.5. Our data suggest that modulation of the voltage dependence of inactivation of the Nav1.4 and Nav1.5 sodium channel isoforms plays an important role in loss of muscle fibre excitability in the animal model of critical illness myopathy.
Methods
Tissue preparation and perfusion
Denervation, treatment of rats and viewing of muscle fibres were done as previously described (Rich et al. 1998; Rich & Pinter, 2001). Briefly, rat muscle was denervated by removing a 10-mm segment of the left sciatic nerve in anaesthetized (ketamine, 5 mg kg−1, and zylaxine, 15 mg kg−1, administered intraperitoneally) adult female Wistar rats (250–350 g body weight). Dexamethasone (5 mg kg−1) was injected daily intraperitoneally beginning on the day of denervation and continuing for 7–11 days. Rats were killed by carbon dioxide inhalation, the extensor digitorum longus (EDL) was dissected tendon to tendon and muscle fibres were labelled with 10 μm 4-(4-diethylaminostyrl)-N-methylpyridinium iodide (4-Di-2-ASP) and visualized using an upright epifluorescence microscope. For all experiments the recording chamber was continuously perfused with solution containing (mm): NaCl, 118; KCl, 3.5; CaCl2,1.5; MgSO4, 0.7; NaHCO3, 26.2; NaH2PO4, 1.7; glucose, 10.8 (pH 7.3–7.4, 20–22°C) equilibrated with 95% O2 and 5% CO2. All animal protocols were performed in accordance with Emory University IACUC guidelines.
Loose patch voltage clamp
Loose patch voltage recording and analysis was performed as previously described (Rich & Pinter, 2001, 2003). Briefly, patch electrodes were made from soft glass (catalogue no. 22-358739, Fisher Scientific) using a horizontal pipette puller (Flaming/Brown type) and were heat polished. Patch pipettes were filled with the normal external solution (see above) containing 1 ng ml−1 sulforhodamine. The seal factor (Rs/(Rs+Rp), where Rs is the shunt resistance and Rp is the patch pipette resistance) was kept greater than 0.4. The leak current was compensated manually by adjusting the compensation current (Stuhmer et al. 1983). Shunt resistance was measured on-line immediately before the application of each voltage step and used to adjust the step amplitude.
Fibres were not impaled to measure the resting potential prior to seal formation. Instead the resting potential was assumed (based on an average of 5–10 fibres) during voltage protocols and then measured after patch recordings were complete. The difference between the assumed and measured resting potential was then used to correct step voltages used during data acquisition. By impaling the muscle fibre after patch recordings were complete, potential problems related to issues of muscle damage and depolarization due to impalement were avoided.
Tetrodotoxin and μ-conotoxin GIIIB application
Tetrodotoxin in citrate (TTX) was purchased from Alomone Laboratories (Jerusalem). Prior to application of TTX we measured the voltage dependence of fast inactivation in several fibres in each muscle. We then applied 300 nm TTX to the bath and used suction to fill the pipette with the same TTX solution. We then reformed seals on the same muscle fibres and measured sodium currents. Muscle fibres were re-identified using pictures we had taken during the initial recording of sodium currents (Fig. 2). To help identify fibres we stained endplates by applying 5 μg ml−1 rhodamine-conjugated bungarotoxin (Molecular Probes, Eugene OR, USA) for 30 s. Sodium currents were recorded near (within 100–200 μm), but never over muscle fibre endplates. By assuming a Kd of 1.9 μm for Nav1.5 (White et al. 1991) and 5 nm for Nav1.4 (Trimmer et al. 1989; for review see Goldin, 2001) one can estimate that 300 nm TTX blocked 97% of Nav1.4 and 23% of Nav1.5 (Lupa et al. 1995). We then solved for the percentage of Nav1.4 and Nav1.5 using the following equations:
|
|
where Natot is the amplitude of the sodium current before application of TTX and NaTTX is the sodium current after application of 300 nm TTX.
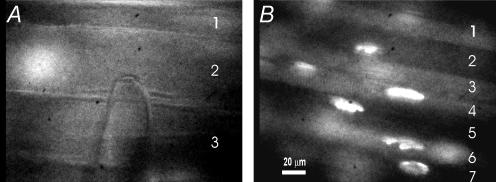
Figure 2. Images of control and SD fibres used for repeated formation of seals on the same fibres.
A, three fibres in a control muscle are shown running from left to right. An out of focus endplate stained with rhodamine-conjugated bungarotoxin is present on the left side of fibre 2. A patch electrode is shown contacting fibre 2. B, seven fibres are shown running diagonally in an SD muscle. Fibre diameter is much smaller than in control muscle. A number of endplates stained with rhodamine–bungarotoxin are seen in the field. The presence of endplates is useful in identifying individual fibres when reforming seals.
μ-Conotoxin GIIIB was purchased from Sigma (St Louis, MO, USA) and was applied to muscle fibres in the same way as TTX. In control fibres (n = 10) we found that the dose of μ-conotoxin GIIIB required to block close to 100% of the current carried by Nav1.4 was 600 nm.
Measurement of Inactivation of Nav1.4 and Nav1.5
Measures of fast inactivation were performed as previously described and all data was fitted to a Boltzmann function (Rich & Pinter, 2003). Briefly, measurement of fast inactivation was performed using a 50 ms pre-pulse to the test potential followed by a 10 ms pulse to −20 mV. For calculation of Nav1.4 inactivation, fibres were analysed in one of two ways. In SD fibres in which sodium current remaining after application of TTX or μ-conotoxin GIIIB was less than 10% of the amplitude prior to application of toxin, it was assumed that current was carried entirely by Nav1.4 since the error introduced by the presence of Nav1.5 was minimal. In fibres in which more than 10% of the sodium current remained after application of toxin, the voltage dependence of the Nav1.4 gating was calculated as follows: sodium current remaining after application of toxin (TTX currents were corrected for amplitude using the equations given above) was subtracted from the sodium current prior to toxin application at each voltage step. The current amplitudes after subtraction of current carried by Nav1.5 were then fitted with Boltzmann distributions and analysed.
Statistics and generation of modelled Boltzmann curves
All data other than linear fits were compared using Student's t test to determine statistical significance. Bonforroni correction was applied in all instances where multiple comparisons were made between treatment groups. All means are given ± the s.e.m. Fitting of the data analysing correlations between parameters was performed using linear regression analysis. All fits (linear and Boltzmann) were performed using Origin software (OriginLab Corp., Northampton, MA, USA). Boltzmann curves for simulation of Nav1.4 and Nav1.5 gating were generated, scaled and added using Origin software. For example, to generate a curve made up of 75% Nav1.4, the values from the Nav1.4 curve were multiplied by 0.75 and added to the 0.25 times the values from the Nav1.5 curve. Curves generated by mixing various percentages of Nav1.4 and Nav1.5 were then fitted with Boltzmann curves to estimate the midpoint and slope of the sodium current carried by the mix of sodium channel isoforms.
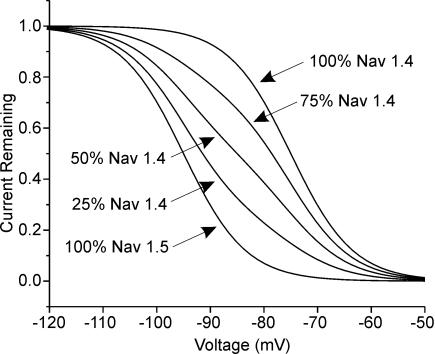
Results
Both Nav1.4 and Nav1.5 sodium channel isoforms are present in steroid-treated, denervated rat muscle (SD muscle) so the voltage dependence of sodium current inactivation is the result of inactivation of both sodium channel isoforms (Rich et al. 1999). Studies in vitro have found that fast inactivation of Nav1.5 occurs at potentials 20 mV more hyperpolarized than for Nav1.4 (Wang et al. 1996; Zhang et al. 1999). The shift in the voltage dependence of fast inactivation in affected SD muscle that we found previously was slightly more than 10 mV (Rich & Pinter, 2001, 2003). To better understand the effect of mixing two populations of sodium channels with a 20 mV difference in the voltage dependence of inactivation, we modelled the effect of increasing the percentage of Nav1.5 on gating of the mix of sodium channels. As shown in Fig. 1, increasing the percentage of Nav1.5 gradually shifts the voltage dependence towards hyperpolarized potentials. In order to shift the voltage dependence of fast inactivation by 10 mV, 50% of sodium current had to be carried by Nav1.5. However, increasing the percentage of Nav1.5 also reduced the slope of the voltage dependence of fast inactivation. In the simulation we used a slope of 5.8 for both Nav1.4 and Nav1.5 (Wang et al. 1996; Zhang et al. 1999). When 50% of the current was carried by Nav1.5 the slope decreased to 8.8. Even when only 25% of current was carried by Nav1.5 such that the shift in the midpoint of inactivation was only 4.4 mV, the slope of the Boltzmann fit decreased to 7.9. In our previous studies we found no evidence of a decrease in the slope of fast inactivation in SD fibres with hyperpolarized voltage dependence of fast inactivation (Rich & Pinter, 2001, 2003).
Figure 1. Simulation of the voltage dependence of sodium current inactivation in SD fibres with increasing percentages of Nav1.5.
Values for the voltage dependence of inactivation were taken from Wang et al. (1996) and Zhang et al. (1999). The midpoint of inactivation used for Nav1.4 (μ1, SkM1) was −75 mV with a slope of 5.8. For Nav1.5 (Hh1) the values were −95 mV and 5.8, respectively. When 25% of sodium current was carried by Nav1.5 the midpoint of inactivation was shifted to −79.5 mV with a slope of 7.9; when 50% of current was carried by Nav1.5 the midpoint was −85 mV with a slope of 8.8 and when 75% of current was carried by Nav1.5 the midpoint was −90.5 mV with a slope of 7.9.
To examine the role of Nav1.5 expression in the hyperpolarized shift of sodium current inactivation we measured fast inactivation in 23 SD fibres in the absence and presence of 300 nm TTX. We confirmed that 300 nm TTX blocked nearly all sodium current carried by Nav1.4 (Lupa et al. 1995) in six control fibres (data not shown). In each SD muscle we recorded from several fibres in the absence of TTX and then recorded from the same series of fibres in the presence of TTX (see methods). Fibres were visually re-identified for recording (Fig. 2) so that TTX was only applied once during each experiment.
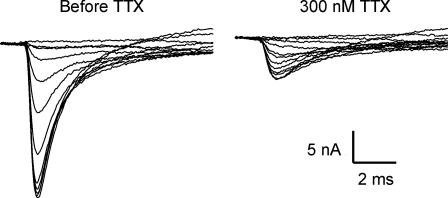
Based on published data, greater than 50% of sodium current would need to be carried by Nav1.5 in order to cause a shift of fast inactivation of greater than 10 mV (Fig. 1) (Wang et al. 1996; Zhang et al. 1999). It is estimated that a dose of 300 nm TTX blocks 97% of Nav1.4 current and 23% of Nav1.5 current (Lupa et al. 1995). We found that the majority of sodium current in most fibres was blocked by a dose of 300 nm TTX. An example of a fibre in which close to 30% of the current remained after TTX application is shown in Fig. 3. The voltage dependence of fast inactivation in the fibre prior to application of TTX was −72.7 mV with a slope of 4.6. These values are similar to those obtained from control muscle which has no Nav1.5, and suggest that, in the fibre shown, having more than a third of the sodium current carried by Nav1.5 was not sufficient to induce a hyperpolarized shift in fast inactivation of the total current.
Figure 3. Fast inactivation in an SD fibre before and after application of 300 nm TTX.
After application of 300 nm TTX, close to 70% of the sodium current is blocked. Despite having a relatively high level of Nav1.5, the midpoint of inactivation prior to TTX application was −72.4 mV; a value similar to that of control muscle. Furthermore, the voltage dependence of inactivation before and after TTX application was similar (midpoint of inactivation after TTX =−70.5 mV). This suggests that the voltage dependence of inactivation of Nav1.4 and Nav1.5 are similar in this fibre.
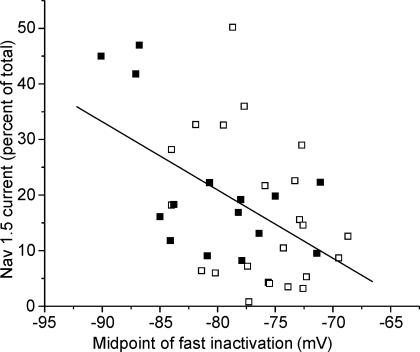
To examine the effect of differing amounts of Nav1.5 on the voltage dependence of inactivation, we plotted the percentage of current carried by Nav1.5 versus the midpoint of fast inactivation in SD fibres and found a correlation (Fig. 4, R =−0.49, P < 0.01). However, in a number of fibres there were hyperpolarized shifts in the voltage dependence of inactivation in the absence of significant expression of Nav1.5. This suggests that a shift in the voltage dependence of Nav1.4 may also contribute to the shift in voltage dependence of inactivation. We thus determined the voltage dependence of both Nav1.4 and Nav1.5 in SD muscle. In 7/23 fibres too little current remained after application of TTX to determine the voltage dependence of Nav1.5. However, in 16 fibres we were able to measure the voltage dependence of sodium current inactivation after application of 300 nm TTX. In these fibres, the current carried by Nav1.5 was subtracted from the total current to leave only current carried by Nav1.4 (see Methods). This allowed for comparison of inactivation of Nav1.4 relative to that of Nav1.5 in individual fibres. There was no significant difference between the calculated midpoint of fast inactivation of Nav1.4 and Nav1.5. This finding differed dramatically from previous studies that have compared gating of Nav1.4 and Nav1.5 in vitro (Wang et al. 1996; Zhang et al. 1999). We thus further examined inactivation of Nav1.4 relative to Nav1.5 in skeletal muscle in vivo.
Figure 4. An increase in the percentage of sodium current carried by Nav1.5 does not fully account for the shift in the voltage dependence of fast inactivation in SD fibres.
The percentage of Nav1.5 present in individual fibres is plotted against the midpoint of fast inactivation for the fibre. Superimposed on the graph is the best linear fit of the data (R =−0.49, P < 0.01). Although there was a correlation between the percentage of current carried by Nav1.5 and the midpoint of inactivation, there were many fibres with little Nav1.5 in which there were large shifts in the voltage dependence of inactivation. ▪, data from fibres treated with μ-conotoxin GIIIB; □, data from fibres treated with TTX.
Although TTX is an excellent tool for distinguishing between Nav1.4 and Nav1.5, at doses needed to block Nav1.4 there is block of a portion of Nav1.5 channels. Since Nav1.5 currents are generally small to begin with, this partial block makes measurement of Nav1.5 inactivation difficult. To further evaluate inactivation of Nav1.5 we performed a set of experiments in which μ-conotoxin GIIIB was used to selectively block Nav1.4. μ-Conotoxin GIIIB is selective for Nav1.4 (Cruz et al. 1985; Li et al. 2003), and thus allows for measurement of larger Nav1.5 currents. We confirmed that we could block greater than 99% of sodium current carried by Nav1.4 using 600 nmμ-conotoxin in 10 control muscle fibres (data not shown). When we blocked Nav1.4 using 600 nmμ-conotoxin GIIIB in SD muscle, slightly more current remained than after block with TTX (21.4%versus 14.9%). The estimate of the amount of sodium current carried by Nav1.5 was similar in the two groups (21.4%versus 16.2%, P = 0.25). Thus, data following application of 300 nm TTX was pooled with data following application of 600 nmμ-conotoxin GIIIB.
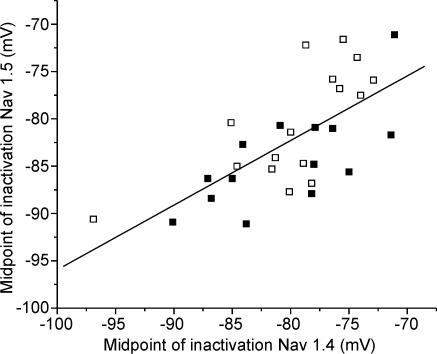
Not all SD fibres have a hyperpolarized shift in the voltage dependence of inactivation (Rich & Pinter, 2001, 2003). The reason for the variation between fibres is unknown, but it provided a way to compare the voltage dependence of inactivation of Nav1.4 to that of Nav1.5 in both mildly and severely affected fibres. We found that the voltage dependence of inactivation of Nav1.4 and Nav1.5 were shifted in parallel in more severely affected SD fibres (Fig. 5, R = 0.68, P < 0.01). This suggests that the process underlying the shift in inactivation affects both Nav1.4 and Nav1.5.
Figure 5. The voltage dependence of inactivation of both Nav1.4 and Nav1.5 is shifted towards hyperpolarized potentials in affected SD fibres.
The plot of midpoint of inactivation of Nav1.5 versus the midpoint of inactivation of Nav1.4 in individual SD fibres reveals a significant correlation between the midpoints of inactivation of the two sodium channel isoforms (R = 0.68, P < 0.01). ▪, data from fibres treated with μ-conotoxin GIIIB; □, data from fibres treated with TTX.
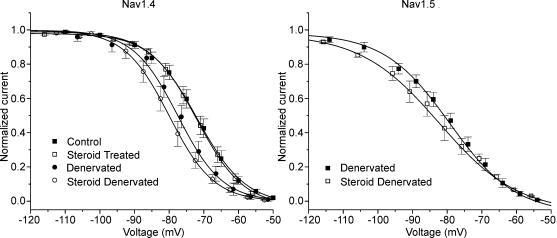
To determine whether the similarity in the voltage dependence of inactivation of Nav1.4 and Nav1.5 was caused by SD treatment we measured inactivation of Nav1.4 and Nav1.5 in both SD and denervated muscle (Table 1, Fig. 6). We have previously found that both denervation and SD treatment of muscle shift inactivation in a subset of fibres (Rich & Pinter, 2003). The primary difference is that SD treatment causes the shift in a greater percentage of fibres. Steroid treatment had no effect on the voltage dependence of inactivation of Nav1.4 in innervated muscle. In control and steroid-treated innervated muscle, there was very little current carried by Nav1.5, so the effect of steroid treatment on inactivation of Nav1.5 in innervated muscle could not be studied. Following SD treatment, there was a shift in the voltage dependence of inactivation of Nav1.4 (P < 0.01). Denervation alone did not cause a statistically significant shift in the gating of Nav1.4 (P = 0.10), suggesting that both denervation and treatment with steroids contribute to the shift. This agrees with our previous finding that steroid treatment amplifies the effect of denervation on the midpoint of inactivation (Rich & Pinter, 2003).
Table 1.
Voltage dependence of inactivation of Nav1.4 and Nav1.5
| Control | Steroid treated | Denervated | SD | |
|---|---|---|---|---|
| −72.6 ± 1.0 | −72.4 ± 1.2 | −76.4 ± 1.2 | −80.0 ± 1.1 | |
| Nav1.4 | 6.2 ± 0.4 | 5.7 ± 1.0 | 6.5 ± 0.4 | 5.0 ± 0.9 |
| n = 19 | n = 11 | n = 15 | n = 30 | |
| −79.3 ± 1.6 | −82.3 ± 1.1 | |||
| Nav1.5 | No current | No current | 9.5 ± 0.7 | 11.8 ± 0.7 |
| n = 15 | n = 30 |
Values shown for the voltage dependence of inactivation in control, steroid-treated innervated, denervated, and steroid-treated denervated muscle. The top value in each block represents the midpoint of inactivation (mV), the second value is the slope of inactivation (mV), and the third value is the number of fibres studied. Values shown represent the means ±s.e.m.
Figure 6. The voltage dependence of fast inactivation of Nav1.4 and Nav1.5 following denervation and steroid treatment.
On the left, the normalized current carried by Nav1.4 is plotted versus the pre-pulse potential for control, denervated, steroid-treated and SD muscle fibres. The inactivation curves of Nav1.4 from control and steroid-treated fibres are nearly superimposed. The inactivation curve for denervated fibres is moderately shifted towards more hyperpolarized potentials; the curve for SD fibres is the most shifted. On the right, the normalized current carried by Nav1.5 is plotted versus the pre-pulse potential for denervated and SD muscle fibres. Too little Nav1.5 current was present to study in control and steroid treated muscle. Error bars represent the s.e.m. Control (n = 19), steroid treated (n = 11), denervated (n = 15), steroid denervated (n = 30).
In neither denervated nor SD fibres was there a statistically significant difference between the voltage dependence of inactivation of Nav1.4 and Nav1.5 (Table 1). There was also no significant difference in the voltage dependence of inactivation of Nav1.5 between denervated and SD fibres. Thus it is unclear whether steroid treatment contributes to the shift of inactivation of Nav1.5 that occurs in more severely affected fibres (Fig. 5). It does appear, however, that the voltage dependence of inactivation of Nav1.4 and Nav1.5 is similar in both denervated and SD fibres.
Discussion
We report that a hyperpolarized shift in the voltage dependence of fast inactivation of both Nav1.4 and Nav1.5 is present in affected fibres in an animal model of critical illness myopathy. Surprisingly, we found that the voltage dependence of inactivation of Nav1.4 is similar to that of Nav1.5 in skeletal muscle in vivo. Our data suggest that modulation of the voltage dependence of inactivation of both Nav1.4 and Nav1.5 contributes to muscle fibre electrical inexcitability in the animal model of critical illness myopathy.
The role of Nav1.5 expression on excitability of SD muscle
We have previously demonstrated that loss of electrical excitability in patients with critical illness myopathy can be replicated by combining denervation and corticosteroid treatment in rat muscle in vivo (steroid-denervated; SD) (Rich et al. 1998; Rich & Pinter, 2001). In this model there are a number of factors that contribute to reduced excitability of muscle. Depolarization of the resting potential following denervation is one of the most important factors since it increases inactivation of sodium channels (Rich & Pinter, 2003). However, changes in the specific membrane resistance as well as a reduction in sodium current density are also contributing factors (Rich et al. 1998). Finally, there is a 10–15 mV hyperpolarized shift in the voltage dependence of fast inactivation that appears to play a central role in loss of excitability (Rich & Pinter, 2001, 2003). The current study was aimed at determining the cause of this shift.
In normal muscle fibres, only Nav1.4 is present (Yang et al. 1991). In SD muscle, however, mRNA for both the Nav1.4 and Nav1.5 is present at high levels (Rich et al. 1999). The voltage dependence in vitro of fast inactivation of the Nav1.5 has been found to be close to 20 mV more negative than for Nav1.4 (Wang et al. 1996; Zhang et al. 1999). Thus, it seemed reasonable to expect that higher levels of Nav1.5 might underlie the hyperpolarized shift in the voltage dependence of fast inactivation in affected SD fibres. However, we found that higher levels of Nav1.5 correlated only loosely with a more hyperpolarized voltage dependence of inactivation.
Comparison of the voltage dependence of inactivation of Nav1.4 to that of Nav1.5 in vivo revealed a similar voltage dependence of inactivation. These data suggest that the voltage dependence of inactivation of Nav1.5 may be different in vivo from what has been reported in vitro. One explanation for the difference is that most studies of the voltage dependence of Nav1.5 have used traditional gigaseal patch recording whereas we are using loose patch. It has been found that gigaseal patch recording shifts the voltage dependence of Nav1.5 in isolated cardiac myocytes by close to −20 mV whereas loose patch does not (Eickhorn et al. 1994). If Nav1.5 activates and inactivates at similar potentials to Nav1.4, its re-expression in denervated and SD muscle might be expected to have little effect on excitability. However, Nav1.5 has been found to be less susceptible to slow inactivation (Richmond et al. 1998; Vilin et al. 2001). Since both denervated and SD muscle fibres have relatively depolarized resting potentials, the resistance of Nav1.5 to slow inactivation may serve to increase excitability.
The finding that the voltage dependence of inactivation of Nav1.5 can be modified in a model of critical illness myopathy raises the question as to whether patients with critical illness myopathy may have cardiac problems caused by increased inactivation of Nav1.5. We previously found that severe sepsis can cause critical illness myopathy (Rich et al. 1997; Bird & Rich, 2002) as well as abnormalities on ECG that are consistent with a reduction in cardiac sodium current (Rich et al. 2002). Such a reduction in cardiac sodium current may contribute to the decrease in cardiac contractility that is found in sepsis (Parker et al. 1984; Ognibene et al. 1988). It is thus possible that an alteration in the voltage dependence of inactivation of Nav1.5 contributes to cardiac dysfunction in septic patients.
Possible factors modulating voltage dependence of inactivation of Nav1.4 and Nav1.5
There is evidence suggesting that gating of Nav1.4 is modulated in vivo from work studying the voltage dependence of sodium channel gating in different fibre types. It has been found that slow twitch fibres have a more positive voltage dependence of inactivation (Ruff & Whittlesey, 1993; Ruff, 1996). Since Nav1.4 is the only sodium channel present in adult muscle (Yang et al. 1991; Rich et al. 1999), this suggests that gating of Nav1.4 is different in fast and slow fibres. We used the extensor digitorum longus muscle in which the vast majority of fibres are fast twitch (Ruff et al. 1982). Thus, in our study the shift we see in fast inactivation is occurring in fibres that had a fast phenotype prior to denervation and steroid treatment.
A number of secondary modifications have been found to modulate sodium channel function in vitro. Phosphorylation by PKA and PKC has been shown to reduce peak sodium conductance (Cantrell & Catterall, 2001) through a process that is similar to slow inactivation (Carr et al. 2003). However, in general, phosphorylation does not cause major changes in the voltage dependence of fast inactivation (Cantrell & Catterall, 2001). Glycosylation shifts the voltage dependence of fast inactivation of both Nav1.4 and Nav1.5 toward hyperpolarized potentials through what appears to be a surface charge mechanism (Bennett et al. 1997; Zhang et al. 1999). However, the shift in the voltage dependence of fast inactivation caused by glycosylation appears modest. Nitric oxide has also been found to inhibit neuronal sodium currents through hyperpolarized shifts in both fast and slow inactivation (Li et al. 1998; Bielefeldt et al. 1999). It is not known whether nitric oxide can affect gating of Nav1.4 or Nav1.5. It has recently been found that calcium-dependent binding of calmodulin enhances slow inactivation of Nav1.5 sodium channels (Tan et al. 2002). Calmodulin binds Nav1.4 in a calcium-independent manner, but appears to have little effect on the voltage dependence of activation and fast inactivation (Herzog et al. 2003; see, however, Deschenes et al. 2002). Further studies in SD muscle will be necessary to determine whether any of these mechanisms are involved in the altered voltage dependence of Nav1.4 and Nav1.5 inactivation in this disorder.
Our work suggests that a hyperpolarized shift in the voltage dependence of inactivation of Nav1.4 and Nav1.5 contribute to muscle fibre inexcitability in the animal model of critical illness myopathy. Other situations in which abnormal regulation of excitability occurs in the setting of genetically normal sodium channels include neuropathic pain and neuronal response to demyelination (Waxman et al. 2000; Lai et al. 2003). However, in these situations it appears that regulation of sodium channel isoform expression may be the most important change. It thus appears that loss of muscle fibre excitability in critical illness myopathy represents a new kind of ion channel disease in which the defect is caused by neither a mutation in the channel nor a change in isoform expression.
Acknowledgments
This work was supported by the National Institutes of Health, NS-40826 (M.R.). We thank Dr Martin Pinter for helpful discussions.
References
- Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J General Physiol. 1997;109:327–343. doi: 10.1085/jgp.109.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Whiteis CA, Chapleau MW, Abboud FM. Nitric oxide enhances slow inactivation of voltage-dependent sodium currents in rat nodose neurons. Neurosci Lett. 1999;271:159–162. doi: 10.1016/s0304-3940(99)00553-4. [DOI] [PubMed] [Google Scholar]
- Bird SJ, Rich MM. Critical illness myopathy and polyneuropathy. Curr Neurol Neurosci Rep. 2002;2:527–533. doi: 10.1007/s11910-002-0041-2. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- Deschenes I, Neyroud N, D, Marban E, Yue DT, Tomaselli GF. Isoform-specific modulation of voltage-gated Na+ channels by calmodulin. Circ Res. 2002;90:E49–E57. doi: 10.1161/01.res.0000012502.92751.e6. [DOI] [PubMed] [Google Scholar]
- Eickhorn R, Dragert C, Antoni H. Influence of cell isolation and recording technique on the voltage dependence of the fast cardiac sodium current of the rat. J Mol Cell Cardiol. 1994;26:1095–1108. doi: 10.1006/jmcc.1994.1129. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Liu C, Waxman SG, Cummins TR. Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties. J Neurosci. 2003;23:8261–8270. doi: 10.1523/JNEUROSCI.23-23-08261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Hunter JC, Porreca F. The role of voltage-gated sodium channels in neuropathic pain. Curr Opin Neurobiol. 2003;13:291–297. doi: 10.1016/s0959-4388(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee HC, Abboud FM. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron. 1998;20:1039–1049. doi: 10.1016/s0896-6273(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Li RA, Ennis IL, Xue T, Nguyen HM, Tomaselli GF, Goldin AL, Marban E. Molecular basis of isoform-specific micro-conotoxin block of cardiac, skeletal muscle, and brain Na+ channels. J Biol Chem. 2003;278:8717–8724. doi: 10.1074/jbc.M210882200. [DOI] [PubMed] [Google Scholar]
- Lupa MT, Krzemien DM, Schaller KL, Caldwell JH. Expression and distribution of sodium channels in short- and long-term denervated rodent skeletal muscles. J Physiol. 1995;483:109–118. doi: 10.1113/jphysiol.1995.sp020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene FP, Parker MM, Natanson C, Shelhamer JH, Parrillo JE. Depressed left ventricular performance. Response to volume infusion in patients with sepsis and septic shock. Chest. 1988;93:903–910. doi: 10.1378/chest.93.5.903. [DOI] [PubMed] [Google Scholar]
- Pappone PA. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve. 1997;20:665–673. doi: 10.1002/(sici)1097-4598(199706)20:6<665::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rich MM, Kraner SD, Barchi RL. Altered gene expression in steroid-treated denervated muscle. Neurobiol Dis. 1999;6:515–522. doi: 10.1006/nbdi.1999.0257. [DOI] [PubMed] [Google Scholar]
- Rich MM, McGarvey ML, Teener JW, Frame LH. ECG changes during septic shock. Cardiology. 2002;97:187–196. doi: 10.1159/000063120. [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol. 2001;50:26–33. doi: 10.1002/ana.1016. [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol. 2003;547:555–566. doi: 10.1113/jphysiol.2002.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol. 1998;43:171–179. doi: 10.1002/ana.410430207. [DOI] [PubMed] [Google Scholar]
- Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology. 1996;46:731–736. doi: 10.1212/wnl.46.3.731. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Featherstone DE, Hartmann HA, Ruben PC. Slow inactivation in human cardiac sodium channels. Biophys J. 1998;74:2945–2952. doi: 10.1016/S0006-3495(98)78001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand. 1996;156:159–168. doi: 10.1046/j.1365-201X.1996.189000.x. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Martyn D, Gordon AM. Glucocorticoid-induced atrophy is not due to impaired excitability of rat muscle. Am J Physiol. 1982;243:E512–E521. doi: 10.1152/ajpendo.1982.243.6.E512. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Whittlesey D. Na+ currents near and away from endplates on human fast and slow twitch muscle fibers. Muscle Nerve. 1993;16:922–929. doi: 10.1002/mus.880160906. [DOI] [PubMed] [Google Scholar]
- Stuhmer W, Roberts WM, Almers W. The loose-patch clamp. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1983. pp. 123–132. [Google Scholar]
- Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, Anderson ME, Balser JR. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Cooperman SS, Tomiko SA, Zhou JY, Crean SM, Boyle MB, Kallen RG, Sheng ZH, Barchi RL, Sigworth FJ, et al. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989;3:33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Vilin YY, Fujimoto E, Ruben PC. A single residue differentiates between human cardiac and skeletal muscle Na+ channel slow inactivation. Biophys J. 2001;80:2221–2230. doi: 10.1016/S0006-3495(01)76195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, George AL, Jr, Bennett PB. Comparison of heterologously expressed human cardiac and skeletal muscle sodium channels. Biophys J. 1996;70:238–245. doi: 10.1016/S0006-3495(96)79566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and their genes: dynamic expression in the normal nervous system, dysregulation in disease states (1) Brain Res. 2000;886:5–14. doi: 10.1016/s0006-8993(00)02774-8. [DOI] [PubMed] [Google Scholar]
- White MM, Chen LQ, Kleinfield R, Kallen RG, Barchi RL. SkM2, a Na+ channel cDNA clone from denervated skeletal muscle, encodes a tetrodotoxin-insensitive Na+ channel. Mol Pharmacol. 1991;39:604–608. [PubMed] [Google Scholar]
- Yang JS, Sladky JT, Kallen RG, Barchi RL. TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron. 1991;7:421–427. doi: 10.1016/0896-6273(91)90294-a. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hartmann HA, Satin J. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. J Membr Biol. 1999;171:195–207. doi: 10.1007/s002329900571. [DOI] [PubMed] [Google Scholar]