Abstract
About 30 genes are predicted to encode degenerin/epithelial sodium channels (DEG/ENaCs) in Caenorhabditis elegans but the gating mode of these channels has not been determined. Using the whole-cell configuration of the patch-clamp technique in acutely dissected C. elegans, we investigated the effects of H+ as a potential activating factor of DEG/ENaCs on electrical properties of body wall muscle cells. Under current-clamp conditions, decreasing external pH from 7.2 to 6.1 led to a reversible depolarization of muscle cells associated with a decrease in input resistance which was partially inhibited by amiloride. Under voltage-clamp conditions, extracellular acidification activated an inward desensitizing current at −60 mV. In the absence of external Ca2+, H+-gated channels were found to be slightly more permeable to Na+ than to K+ and were blocked by amiloride with a K0.5 of 31 μm at −60 mV. An inward current could be also activated by protons in a GABA receptor null mutant in the presence of d-tubocurare and in an unc-105 null mutant. These results demonstrate that ion channels sharing common properties with mammalian acid-sensing ion channels (ASICs) are functional in C. elegans muscle which should prove useful for understanding proton sensing in animals.
The DEG/ENaC family represents a class of ion channels which exhibit an unusual functional diversity but share common properties such as non-voltage-gating, Na+ selectivity and inhibition by amiloride. Depending on their function in the cell, these channels are either constitutively active as epithelial Na+ channels, activated by protons as ASICs, neuropeptides like FaNaC, or possibly activated by mechanical stimuli as postulated for degenerins (Waldmann & Lazdunski, 1998; Bianchi & Driscoll, 2002; Kellenberger & Schild, 2002; Goodman & Schwarz, 2003; Krishtal, 2003). The first members identified in this superfamily were the genes involved in mechanosensitivity (mec) and degeneration (deg) in the nematode Caenorhabditis elegans. Six C. elegans genes, called degenerins, are thought to encode subunits of DEG/ENaCs, but up to now, only two degenerins, UNC-105 and MEC-4/MEC-10, have been successfully expressed in heterologous systems and demonstrated to function as amiloride-sensitive Na+ channels in their mutant form (Garcia-Anoveros et al. 1998; Goodman et al. 2002). Attempts to gate these channels were unsuccessful, most likely because the functional native environment of the channels could not be reconstituted in expression systems. Using an in situ patch-clamp technique on C. elegans body wall muscle cells, we recently demonstrated that UNC-105 was electrically silent and insensitive to mechanical stimuli (Jospin et al. 2004) despite its genetic interaction with the collagen LET-2 (Liu et al. 1996). These results emphasize the need to explore the behaviour of the products of genes predicted to encode ion channels in their native environment to properly assess their function. To date, apart from degenerins, 22 other genes in the C. elegans genome have been predicted to encode DEG/ENaCs (Goodman & Schwarz, 2003). The function of these proteins as well as the parameters that modulate their activity remain to be elucidated.
Using an in situ patch-clamp technique on body wall muscle cells, we give here the first experimental evidence for the presence in C. elegans of a new subfamily of DEG/ENaCs which display functional properties comparable to mammalian ASICs.
Methods
Experiments were performed on ventromedial body wall muscle cells from the N2 wild-type reference strain of C. elegans and the MT1685 strain carrying the unc-105(n490n786) mutation. DNA from unc-105(n490n786) animals was amplified by PCR using standard protocols and sequenced on a Megabace analyser (Amersham).
The dissection technique and patch-clamp recordings were performed as previously described (Jospin et al. 2002a). Cell capacitance, used to calculate the density of currents (A F−1), was determined by integration of a control current trace obtained with a −10 mV depolarizing pulse from −70 mV.
Data values are presented as means ±s.e.m. Data were statistically analysed using the Mann-Whitney test and were considered significant when P < 0.05.
Pipettes were filled with (mm): 120 KCl, 20 KOH, 4 MgCl2, 5 Tes, 4 Na2ATP, 36 sucrose, 5 EGTA (pH 7.2). The bath solution contained (mm): 140 NaCl, 5 KCl, 6 CaCl2 (or 0 Ca + 0.5 EGTA), 5 MgCl2, 11 glucose and 5 Hepes, buffered to pH 7.2 with NaOH. In the low pH solution, Hepes was replaced by Mes and the pH buffered to 6.1. TEACl, 4-aminopyridine (4-AP), d-tubocurare and amiloride (Sigma) were diluted to the required concentration in the bath solution. Voltages were not corrected for liquid junction potentials calculated to be lower than 5 mV with the different solutions used. Except under current-clamp conditions (Fig. 1), bath solutions were pressure applied to limit the delay for exchanging solutions. Exchange of control solution for pressure-ejected control solution was found to have no effect on membrane potential and current (see also Jospin et al. 2004).
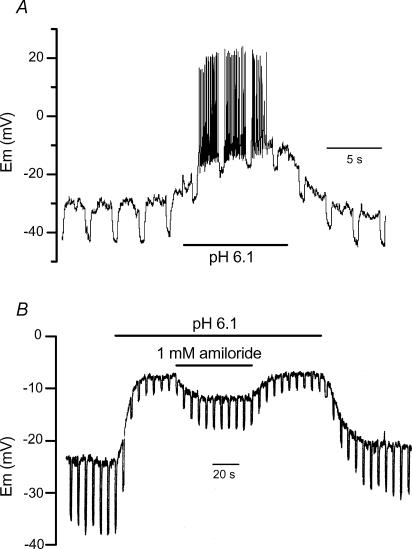
Figure 1. Effect of H+ and amiloride on membrane potential and input resistance.
Recordings of two different muscle cells stimulated in current-clamp mode at 0.2 Hz by 1 s duration negative current steps of −10 pA (A) and −20 pA (B). The pH of the control solution was 7.2.
Results
Under current-clamp conditions, decreasing external pH from 7.2 to 6.1 led to a reversible depolarization of muscle cell, initiating a burst of spontaneous overshooting spikes, associated with a decrease in the input resistance (Fig. 1A). Figure 1B shows in another cell that the depolarization and the decrease in the input resistance induced by a drop in external pH were reversibly inhibited by 1 mm amiloride. Since, in a preceding study, we demonstrated that amiloride had no effect on the electrical properties of body wall muscle cells (Jospin et al. 2004), these data probably indicate that protons activate an amiloride-sensitive inward current in C. elegans muscle cells. Figure 1B also shows that amiloride did not totally reverse the H+-induced depolarization. However acidic pH brought the membrane potential (Em) to values slightly above the threshold of activation of voltage-dependent Ca2+ and K+ channels (−30 mV; see Jospin et al. 2002a, b), so that the resulting current through these channels might have influenced the membrane potential recorded at acidic pH. To circumvent this phenomenon, the effects of a drop in external pH and of amiloride were examined on membrane currents under voltage-clamp conditions.
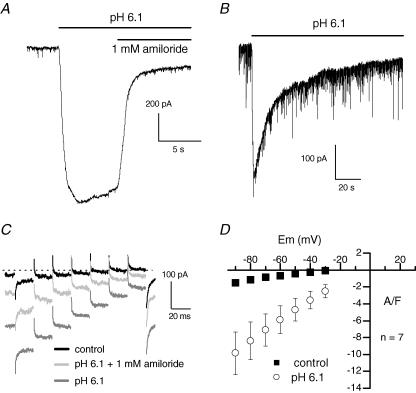
At a holding potential of −60 mV, extracellular acidification to pH 6.1 activated a large inward current which was inhibited by 85% by 1 mm amiloride (Fig. 2A). A lower pH value (5.1) was able to further increase the current (not shown). The magnitude of the H+-induced current was highly variable from one cell to another (mean current of 5.4 ± 1 A F−1, n = 23) with minimal and maximal amplitudes of 0.5 and 17.4 A F−1, respectively (Fig. 4). On average, the H+-induced current was inhibited by 66 ± 5% (n = 9) and 43 ± 8% (n = 8) by 1 and 0.5 mm amiloride, respectively, and was not affected by 10 and 100 μm (Fig. 3C). Figure 2B shows on a longer time scale that, in the continuous presence of an acidic pH, after a peak, the H+-induced current slowly declined. Fitting the falling phase of current with a single exponential indicated a time constant of 45 s. On average, the time constant of inactivation of the H+-induced current was 44.4 ± 3 s (n = 7).
Figure 2. Effect of H+ and amiloride on membrane currents.
A and B, cells were held at −60 mV under voltage-clamp conditions. Note the high level of current noise in B, probably resulting from a high spontaneous excitatory synaptic activity in this cell (see Richmond & Jorgensen, 1999). C, membrane currents were recorded in response to 20 ms voltage steps from −90 to −30 mV in 10 mV increments from a holding potential of −70 mV under voltage-clamp conditions, in the presence of the indicated solutions. The dotted line indicates the zero current level. D, mean current–voltage relationship of the control current and of the H+-induced current after subtraction of the control. Error bars are smaller than symbols in control. The pH of the control solution was 7.2.
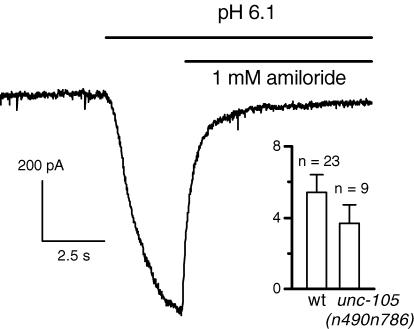
Figure 4. H+-induced currents in unc-105(n490n786) double mutant cells.
The cell was held at −60 mV in a Ca2+-free solution. The pH of the control solution was 7.2. Inset shows the average and the scatter of amplitudes of the H+-induced current at −60 mV for wild-type (wt) and mutant cells.
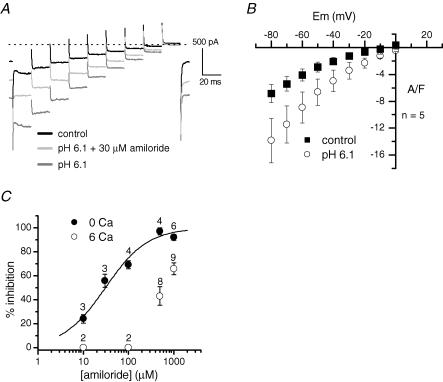
Figure 3. Voltage dependence and amiloride sensitivity of the H+-induced current in the absence of external Ca2+.
A, membrane currents were recorded in response to 20 ms voltage steps from −80 to 0 mV in 10 mV increments from a holding potential of −60 mV under voltage-clamp conditions, in the presence of the indicated solutions. B, mean current–voltage relationship of the control current and of the H+-induced current after subtraction of the control. C, dose–response histograms of amiloride block at −60 mV in the presence and absence of Ca2+ (numbers indicate the number of cells tested). The curve was fitted using a Hill equation, with a Hill coefficient of 0.93 and a K0.5 of 31 μm. The pH of the control solution was 7.2.
Body wall muscles are polyinnervated both by excitatory acetylcholine and inhibitory γ-aminobutyric acid (GABA) motor neurones (Richmond & Jorgensen, 1999). During the dissection of the worms, the ventral nerve cord that innervates body wall muscle cells was preserved, so that the response to acidification could be indirectly mediated by a H+-induced depolarization of the motor neurones which in turn would release acetylcholine or GABA (also excitatory in our high intracellular Cl− conditions) onto muscle cells. However, we observed that an inward current could still be elicited by protons in a Ca2+-free solution (see below), which is expected to strongly inhibit synaptic transmission. The mean amplitude of the H+-induced current at −60 mV (5.4 ± 0.8 A F−1, n = 11) was the same as the one recorded in standard saline, probably suggesting that protons directly depolarize muscle cells. Additionally, an inward current could be also evoked by protons in the unc-49(e407) GABA receptor null mutant, in the presence of the nicotinic receptor blocker d-tubocurare, confirming that the H+-induced current was of muscular origin (not shown).
The effects of external acidification were then tested on currents elicited every 0.5 s by a series of short voltage steps delivered from −90 mV to a maximum of −30 mV to prevent Ca2+ and K+ voltage-dependent conductances from being activated. At all potentials tested, external acidification produced inward currents which were partially reduced by 1 mm amiloride (Fig. 2C). The slope of the mean current–voltage relationship of the H+-induced current component was largely increased compared to control (Fig. 2D). However, the small range of voltages tested did not allow extrapolation of the value of the reversal potential, so that the ionic basis of the H+-evoked current could not be evaluated. In order to explore the current over more positive voltages, experiments were performed in a Ca2+-free external solution containing 20 mm TEA and 3 mm 4-AP. Under these conditions, the current could be recorded in response to depolarizations up to 0 mV without being contaminated by voltage-activated Ca2+ and K+ currents (Fig. 3A). Figure 3B shows that the H+-induced current rectified in the inward direction and was still inward at 0 mV. Given the composition of the extra- and intracellular solutions used, the Na+ and K+ equilibrium potentials were +72 and −84 mV, respectively. The fact that the H+-evoked current was negative at 0 mV indicated that the reversal potential was closer to the Na+ equilibrium potential and, as a result, that the H+-gated channels were slightly more permeable to Na+ than to K+. In addition, ion substitution experiments confirmed that both K+ and Cs+ ions could permeate the channels (not shown).
A striking observation was that, in the Ca2+-free solution, the effectiveness of the amiloride block was strongly increased. Figure 3A shows that the H+-induced current at −60 mV was inhibited by 53% with 30 μm amiloride in the Ca2+-free solution. Using a Hill equation, the best fit to the mean percentages of block by different concentrations of amiloride indicated a K0.5 of 31 μm at −60 mV (Hill coefficient of 0.93) (Fig. 3C). The block was found to be voltage dependent, since at −80 and −40 mV, the K0.5 values of amiloride were 21 and 48 μm, respectively (not shown).
Among the 28 genes predicted to encode DEG/ENaCs, unc-105 is the only degenerin gene which has been shown to be expressed in C. elegans body wall muscle (Liu et al. 1996). We then tested the possibility that UNC-105 could be the channel carrying the H+-induced current. Gain-of-function mutations in the unc-105 gene causes muscle hypercontraction and paralysis (Park & Horvitz, 1986). Park & Horvitz (1986) isolated a series of intragenic revertants of these gain-of-function mutations which were postulated to correspond to null mutations of unc-105. We sequenced the DNA from several of these mutants and found that the n786 mutation consisted of a stop codon at amino acid 427, in the second conserved cysteine-rich region in the extracellular loop linking the two transmembrane domains, suggesting that the encoded UNC-105 protein in n490n786 double mutants was not functional. The mean resting potential of these double mutant cells was −24.4 ± 1 mV (n = 15), a value not significantly different from that of wild-type cells (−24.5 ± 0.95 mV, n = 16, P = 0.94). This result confirmed that the gain-of-function mutation n490 was reversed by the associated n786 mutation, since otherwise the n490 mutation could have led to a persistent depolarization as observed with the less severe n506 mutation (Jospin et al. 2004). Figure 4 shows that acidification induced an amiloride-sensitive inward current in these double mutant muscle cells in a Ca2+-free solution. The mean amplitude of the H+-induced current at −60 mV was not significantly different from that recorded in wild-type cells (3.7 ± 1 A F−1, n = 9, P = 0.51) while scattering of amplitudes was the same (Fig. 4).
Discussion
Taken together, our results demonstrate that external protons activate ion channels in body wall muscle cells of C. elegans that share similar properties with the mammalian ASIC subfamily of DEG/ENaCs. Experiments performed in a Ca2+-free solution containing K+ channel blockers showed that C. elegans H+-gated channels were slightly more permeable to Na+ than to K+, although K+ and Cs+ were also found to permeate the channels. Comparable ion selectivities have been observed for ASIC1b (Chen et al. 1998) and for the heteromultimeric association of ASIC2a+2b and ASIC2b+3 which poorly discriminated between Na+ and K+ (Lingueglia et al. 1997). In the absence of external Ca2+, C. elegans H+-gated channels were inhibited by amiloride with a similar efficiency to that found with other ASICs (Kellenberger & Schild, 2002). The block was strongly reduced by external Ca2+, indicating that Ca2+ might interact with the amiloride binding site while it appeared not to affect the amplitude of the pH response. Finally, as observed for ASICs, C. elegans H+-gated channels were found to desensitize in the continued presence of acid but with a long time constant (about 45 s), again comparable to that reported for ASIC3 or heteromultimeric associated ASIC2a+2b (Lingueglia et al. 1997; Waldmann et al. 1997a).
In mammalians, ASICs are predominantly found in sensory neurones and in brain (Waldmann et al. 1997b). To our knowledge, there is no evidence for their presence in muscles. Although we cannot totally exclude that a small potentiated excitatory neuronal input contributes to the global pH response, our results obtained in Ca2+-free solution and in GABA receptor null mutants in the presence of a nicotinic receptor blocker suggest that the H+-induced current in C. elegans flowed in muscle cells rather than in neurones.
unc-105 is the only C. elegans gene predicted to encode DEG/ENaCs whose expression pattern as well as in vivo recordings have revealed that the protein is produced in body wall muscles (Liu et al. 1996; Jospin et al. 2004). We found that the associated mutation n786 in the mutant unc-105(n490) consists of a stop codon between the two predicted transmembrane domains which probably made the UNC-105 channel protein non-functional. The H+-evoked current in these double mutant cells was found to display comparable mean amplitude and same variability with wild-type cells. It cannot be totally excluded that UNC-105 might be partially involved because a subtle reduction in the amplitude of the response in mutants may have not been resolved. Nevertheless, our data firmly demonstrate that another muscle DEG/ENaC is mainly responsible for the H+-activated current.
To date, H+-gated conductances have not been found in animals less complex than amphibians (Krishtal, 2003). To our knowledge, this work is the first demonstration of the presence of an ASIC in invertebrates. Interestingly, nematodes have been shown to avoid acidic environments and this avoidance behaviour was found to be reduced by amiloride (Sambongi et al. 2000). Although the acid avoidance behaviour seems to mainly involve chemosensory neurones, a depolarizing response of body wall muscle to acidic pH may reinforce the escape behaviour by promoting Ca2+ influx through voltage-gated Ca2+ channels and eventually muscle contraction (Jospin et al. 2002a).
C. elegans has been established as an animal model highly amenable to molecular and classic genetic techniques. Our experimental evidence for the existence of ASICs in this model system will undoubtedly contribute to a better understanding of the mechanism of proton sensing in animals.
Acknowledgments
We are indebted to M.-C. Mariol and L. Ségalat for providing us with wild-type and unc-105 worms and for DNA sequencing, and A. Bilbaut, R. Bonvallet, C. Chouabe, V. Jacquemond and E. Jorgensen for helpful comments. We also thank J.-L. Bessereau for the gift of unc-49. This work was supported by the Centre National de la Recherche Scientifique, the Ministère de la Recherche (Action Concertée Incitative 2003), the Université Claude Bernard Lyon 1, and the Association Française contre les Myopathies.
References
- Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron. 2002;34:337–340. doi: 10.1016/s0896-6273(02)00687-6. [DOI] [PubMed] [Google Scholar]
- Chen C-C, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Garcia JA, Liu JD, Corey DP. The nematode degenerin UNC-105 forms ion channels that are activated by degeneration- or hypercontraction-causing mutations. Neuron. 1998;20:1231–1241. doi: 10.1016/s0896-6273(00)80503-6. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Ann Rev Physiol. 2003;65:429–452. doi: 10.1146/annurev.physiol.65.092101.142659. [DOI] [PubMed] [Google Scholar]
- Jospin M, Jacquemond V, Mariol MC, Ségalat L, Allard B. The L-type voltage-dependent Ca2+ channel EGL-19 controls body wall muscle function in Caenorhabditis elegans. J Cell Biol. 2002a;159:337–347. doi: 10.1083/jcb.200203055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jospin M, Mariol MC, Ségalat L, Allard B. Characterization of K+ currents using an in situ patch clamp technique in body wall muscle cells from Caenorhabditis elegans. J Physiol. 2002b;544:373–384. doi: 10.1113/jphysiol.2002.022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jospin M, Mariol MC, Ségalat L, Allard B. Patch clamp study of the UNC-105 degenerin and its interaction with the LET-2 collagen in Caenorhabditis elegans muscle. J Physiol. 2004;557:379–388. doi: 10.1113/jphysiol.2003.057687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators. Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatry subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Liu J, Schrank B, Waterston RH. Interaction between a putative mechanosensory membrane channel and a collagen. Science. 1996;273:361–364. doi: 10.1126/science.273.5273.361. [DOI] [PubMed] [Google Scholar]
- Park EC, Horvitz HR. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics. 1986;113:821–852. doi: 10.1093/genetics/113.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambongi Y, Takeda K, Wakabayashi T, Ueda I, Wada Y, Futai M. Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport. 2000;11:2229–2232. doi: 10.1097/00001756-200007140-00033. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton gated channel involved in acid sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]