Abstract
Major brassinosteroid (BR) effects such as BR-induced growth are mediated through genomic pathways because RNA synthesis inhibitors and protein synthesis inhibitors interfere with these processes. A limited number of BR-regulated genes have been identified hitherto. The majority of genes (such as BRU1, CycD3, Lin6, OPR3, and TRIP-1) were identified by comparisons of BR-treated versus control-treated plants. However, altered transcript levels after BR application may not reflect normal physiological events. A complementary approach is the comparison of BR-deficient plants versus wild-type plants. No artificial treatments interfere with endogenous signaling pathways, but a subset of phenotypic alterations of phytohormone-deficient plants most probably is secondary. To identify genes that are subject to direct BR regulation, we analyzed CPD antisense and dwf1-6 (cbb1) mutant plants. Both show a mild phenotype in comparison with BR-deficient mutants such as cpd/cbb3, det2, and dwf4. Plants were grown under two different environments to filter out BR deficiency effects that occur only at certain environmental conditions. Finally, we established expression patterns after BR treatment of wild-type and dwf1-6 (cbb1) plants. Ideally, a BR-regulated gene displays a dose-response relationship in such a way that a gene with decreased transcript levels in BR-deficient plants is BR inducible and vice versa. Expression profile analysis of above ground part of plants was performed by means of Affymetrix Arabidopsis Genome Arrays.
Brassinosteroids (BRs) are integrated in a complex signaling network and numerous BR effects appear to be mediated via a modulation of levels and sensitivities of other phytohormones. BR activity was demonstrated in almost all auxin assays (e.g. Yopp et al., 1981; Takeno and Pharis, 1982; Katsumi, 1985) and in selected GA bioassays (e.g. Yopp et al., 1979; Mandava et al., 1981). BRs influence ethylene levels (e.g. Schlagnhaufer et al., 1984; Arteca et al., 1985; Woeste et al., 1999), potentially via the regulation of genes involved in ethylene synthesis (Yi et al., 1999), and BRs potentially affect oxylipin metabolism (Müssig et al., 2000). Correspondingly, BR-deficient and -insensitive mutants display strong pleiotropic phenotypic alterations. The phenotypic characteristics of Arabidopsis mutants include dwarfism, small dark-green leaves, a compact rosette structure, delayed flowering and senescence, and reduced fertility. In comparison with mutants affected in reactions specific to BR biosynthesis such as dwf4 (Azpiroz et al., 1998; Choe et al., 1998) and cpd/cbb3 (Kauschmann et al., 1996; Szekeres et al., 1996), mutants of the steroid metabolism providing precursors to the specific BR pathway such as dwf5 (Choe et al., 2000), dwf7 (Choe et al., 1999), and dwf1/dim/cbb1 (Feldmann et al., 1989; Takahashi et al., 1995; Kauschmann et al., 1996; Klahre et al., 1998) have a rather mild phenotype for hitherto unknown reasons.
The growth promoting effect of BRs results primarily from the stimulation of cell elongation. Several genes encoding cell wall-modifying enzymes such as xyloglucan endotransglycosylases (e.g. meri5 and TCH4 [Kauschmann et al., 1996], LeBR1 [Koka et al., 2000], and BRU1 [Oh et al., 1998]) and EXPANSINS (A. Kauschmann, D.J. Cosgrove, and T. Altmann, unpublished data) are up-regulated after application of synthetic BRs. BR effects on cell division are less clear. The induction of CycD3 transcription by epibrassinolide may represent a mechanism by which BRs can drive cell division (Hu et al., 2000). Physiological responses to BR application include effects on primary carbon metabolism (e.g. Braun and Wild, 1984; Goetz et al., 2000), increased yield (Ikekawa and Zhao, 1981), enhanced stress tolerance, particularly with respect to cold stress (Wilen et al., 1995; Dhaubhadel et al., 1999), and stimulation of xylem formation (Clouse and Zurek, 1991; Iwasaki and Shibaoka, 1991; Yamamoto et al., 2001). The molecular basis of these effects is barely understood. The de-etiolated phenotype of seedlings of BR-deficient mutants such as det2 (Chory et al., 1991) and cpd/cbb3 suggested a role of BRs in light-regulated processes. The de-etiolation is accompanied by derepression of light-induced genes in the dark in a subset of mutants (e.g. Szekeres et al., 1996). Furthermore, BRs are connected to light signaling via the BAS1 gene. BAS1 regulates levels of active BRs via C26-hydroxylation and light responsiveness in Arabidopsis (Neff et al., 1999). A dark-induced small G protein (pea [Pisum sativum] Pra2) may mediate the cross talk between light and BRs in the etiolation process (Kang et al., 2001). However, the precise function of BRs in the control of photomorphogenesis and light-regulated gene expression is unclear.
The present study was conducted to shed more light on BR action and to lay the foundation for further in-depth analysis by the identification of genes that exhibit transcriptional regulation by BRs (i.e. the transcript levels of which show BR-related changes). To identify such genes, we studied BR-dependent gene expression by hybridization of Affymetrix (Santa Clara, CA) GeneChip oligonucleotide arrays representing approximately 8.200 genes. To this end, we set a couple of criteria with increasing stringency to define BR-regulated genes. In designing the experiments, we considered the following potential pitfalls: (a) BR-regulated genes may be identified by comparison of wild-type and BR-deficient mutants. Such analysis, however, might be compromised by secondary effects that arise in the mutants due to the long-term BR deficiency, resulting in morphological or physiological aberrations that in turn cause changes in expression of genes rather unrelated to the primary action of BRs. Such secondary effects may be most prevalent in mutants that exhibit very severe phenotypic alterations, such as extreme dwarfism. (b) BR-regulated genes are expected to show increased or decreased expression in wild-type plants upon exogenous BR application, but in some cases the responses may be rather limited due to the presence of appropriate endogenous BR levels and the genes therefore may escape identification. (c) Such a limitation may be overcome by the use of BR-deficient mutants, the expression profiles of which are compared between treated and untreated plants. In this situation, however, the release from a long-term “BR starvation” may trigger unusual responses. Such responses may be elicited by the sudden release from the block in cell expansion growth and concomitant changes in expression of genes required from rapid cell growth. (d) Major interest resides in the identification of “immediate response genes,” which are primary targets of the signal transduction cascade triggered by the regulatory substance under investigation. Such “immediate response genes” are expected to show changes in expression in the absence of any protein synthesis and thus are frequently looked for by analysis of gene expression patterns that occur in the presence of the protein synthesis inhibitor cycloheximide. Such analysis, however, may be compromised by the presence of short-lived repressors regulating the expression of the genes in search. Application of cycloheximide would derepress these genes regardless of the presence of the proper/specific inducer.
To cope with these potential limitations, it appeared inevitable to evaluate long-term effects of BR deficiency and short-term responses using plants of different genetic constitution. Therefore, we established expression profiles of the BR-deficient dwf1-6 mutant (cabbage1, Kauschmann et al., 1996), CPD-antisense (αCPD) plants (Schlüter et al., 2002), and the corresponding wild type. Both types of mutant plants display rather mild phenotypic alterations in comparison with mutants such as det2, cpd/cbb3, and dwf4 (Chory et al., 1991; Szekeres et al., 1996; Azpiroz et al., 1998). The extreme dwarfism and lack of organ formation in the latter may constitute secondary causes for altered gene expression patterns, e.g. the appearance of leaves in dark-grown dwf4 may be simply due to its short size and the culture conditions (Azpiroz et al., 1998). αCPD and dwf1-6 plants were grown either in agar-solidified synthetic medium or in soil, respectively. The combination of different growth conditions and BR-deficient genotypes provides a means to exclude changes in expression patterns, which are restricted to a specific environment or genotype. Furthermore, we established expression profiles of BR-treated and untreated wild-type and dwf1-6 plants to study short-term changes of gene expression patterns. Plants were harvested 1 h after BR application to avoid monitoring of secondary effects, which might be associated with longer periods. We expected that each of these sets of experiments in itself would reveal candidates for BR-regulated genes, but we reasoned that the genes most directly controlled by BRs would be identified as those showing consistent BR-dependent changes in transcript levels throughout all experiments. Thus, a core set of BR-regulated genes has been identified the transcript levels of which are decreased/increased in both BR-deficient backgrounds and growth conditions and are increased/decreased after BR application in wild-type and dwf1-6 plants. According to these strict criteria, BRs regulate the expression of genes encoding enzymes involved in (brassino) steroid synthesis, auxin response factors, nitrogen transport proteins, several transcription factors, and a few more proteins of different functions. This core set of genes is supplemented by genes that may reveal BR-related activities occurring only under certain environmental conditions or upon specific physiological states of the plants. These include genes involved in cell wall modification, phytohormone synthesis, phytohormone response, cold, and drought stress. Furthermore, BRs potentially regulate the expression of genes encoding chromatin components and several nuclear factors.
RESULTS
Technical Variability of Affymetrix Arabidopsis Genome Arrays
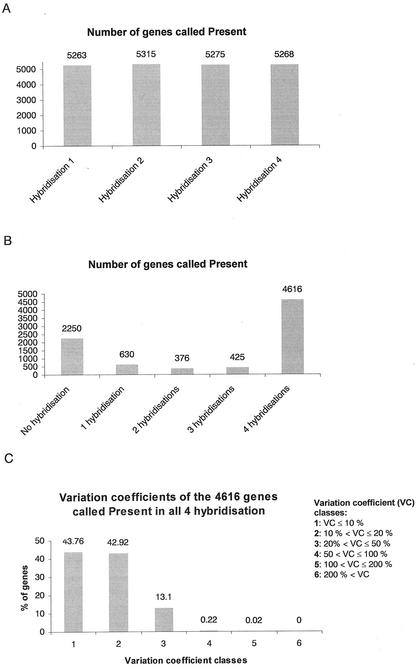
Technical and biological variability are deciding parameters that determine the meaningfulness of expression data. To suppress the effects of biological variability, large pools of plants raised independently were used for RNA isolation and probe synthesis. To evaluate the technical variability of the Affymetrix GeneChip technology, a series of test hybridizations was performed using one single wild-type RNA preparation for the synthesis of four independent probes (separate reactions for cDNA and cRNA synthesis with labeling). Four Arabidopsis Genome Arrays were hybridized and the obtained data were analyzed by means of the Microarray Suite (version 4.0, Affymetrix) software. To calculate absolute call metrics, the program includes three metrics (positive fraction, positive/negative ratio, and log average ratio) that serve to estimate cross hybridization and the signal specificity. In case certain critical values were exceeded, a gene is called present. Of approximately 8,200 genes, 4616 genes met this criterion in all four experiments, 2,250 genes were not detected in any of the hybridizations, and 1,431 genes were called present in only one, two, or three hybridizations (Fig. 1, A and B).
Figure 1.
Technical variability of gene expression analysis using Affymetrix Arabidopsis Genome Arrays. One single wild-type RNA isolation was used for the synthesis of four independently labeled samples that were hybridized to four Arabidopsis Genome Arrays. A, Number of genes called present in each of the four hybridizations according to the absolute call algorithm. B, Number of genes consistently called present in different hybridizations. C, Variation coefficients of expression values of the 4,616 genes termed present in all four hybridizations. Basis of the calculations are average difference values that serve as a relative indicator of the level of expression of a transcript. Variation coefficients (in %) were calculated by determining the ratios of the sds to the means for the 4,616 genes, multiplied by 100.
Remarkably, the 4,616 genes called present in all hybridizations showed very little variability with respect to the average difference measure, which serves as a relative indicator of the level of expression of a transcript. This metric can be used to determine the change in expression of a given gene between different experiments. The coefficient of variation was calculated for all genes called present in four experiments. The percentage of genes displaying a variation coefficient smaller than 20% was 86.7%, and 99.8% of the genes displayed a variation coefficient smaller than 50% (Fig. 1C). Thus, expression data of genes that meet the present criterion show low technical variability.
Influence of Growth Conditions on Gene Expression Patterns
Plants respond to environmental stimuli such as light and nutrients with morphological modifications and developmental switches. In this work, we applied two different growth conditions. On the one hand, plants were grown aseptically in agar-solidified one-half-concentrated Murashige and Skoog medium; on the other hand, plants were grown in soil in long days (see “Materials and Methods” for details). To obtain an impression of the impact of different growth conditions and developmental stages on gene expression profiles, large pools of Arabidopsis wild-type plants consisting of at least 50 individuals were harvested and used for probe preparation. Approximately 900 genes (present in both situations) complied with the “increase” (“I”) or “decrease” (“D”) outcome of the Affymetrix difference call algorithm. The difference call decision matrix is an algorithm that generates one of five outcomes (increase [“I”], marginal increase [“MI”], decrease [“D”], marginal decrease [“MD”], and no change [“NC”]), depending on four metrics that were entered into the calculation. The four metrics were derived from four algorithms that estimate changes of transcript levels in both experiments by means of different criteria.
In comparison with wild-type plants grown on agar, 407 genes show a decrease in soil-grown plants and 491 genes show an increase. Two hundred seventy-two and 299 of these genes display a fold change (“FC”) of ≤−2.0 and ≥2.0, respectively. Thus, the expression of one-fifth of all detected genes is affected by the environment and developmental stage. Because BR-deficient plants may respond to environmental stimuli in a way different than wild-type plants, the administration of two different growth conditions allowed us to discriminate against such effects.
Expression Profiles of BR-Deficient Plants
In addition to different growth conditions, two sets of BR-deficient plants were used. The dwf1-6 (cbb1) mutant (Kauschmann et al., 1996) is allelic to the dim and dwarf1 mutants (Feldmann et al., 1989; Takahashi et al., 1995). dim has been shown to accumulate 24-methylenecholesterol but is deficient in campesterol, an early precursor of brassinolide (Klahre et al., 1998). Nonetheless, dwf1-6, like the other dwf1 alleles, displays a rather mild phenotype (for hitherto unknown reasons), which can be rescued by BR feeding (Klahre et al., 1998), and produces viable seeds. The CPD gene product (CYP90A) mediates the second hydroxylation reaction in the BR side chain because teasterone and all further metabolites in the pathway normalize the growth defect (Szekeres et al., 1996). In contrast to the dwf1-6 mutant, the allelic cbb3 and cpd mutants (Kauschmann et al., 1996; Szekeres et al., 1996) display extreme dwarfism and sterility. cbb3 plants grown in soil frequently show stress symptoms that may be due to the reduced root system. The extreme dwarfism may likewise lead to secondary effects in aseptic culture (Azpiroz et al., 1998). We established CPD antisense plants that display phenotypic changes intermediate between cbb3 and wild-type plants (Schlüter et al., 2002). The transgenic plants exhibited short stems and petioles, small leaves, slight delays in flowering and senescence, and produced viable seeds. A representative line (termed line no. 2) was selected for the present study.
Soil-grown wild-type and dwf1-6 plants were compared by means of Affymetrix Genome Array hybridizations. Eighty-one genes (present in both profiles) displayed decrease (“D,” stronger expression in BR-deficient plants; 28 genes with a fold change [“FC”] of ≤−2.0) and 136 genes (present in both profiles) displayed increase (“I,” stronger expression in wild-type plants; 57 genes with FC of ≥2.0). In a comparison of wild-type and CPD antisense plants grown on agar, 138 genes displayed “I” (present in both profiles, stronger expression in wild-type plants, 50 genes with FC ≥ 2.0), and 77 genes displayed “D” (present in both profiles, stronger expression in αCPD plants, 29 genes with “FC” ≤ −2.0). Tables I and II give a summary of genes that displayed corresponding tendencies in wild-type versus dwf1-6 plants grown in soil. Numerous genes displayed no uniform changes across the two situations, e.g. are only up or down-regulated in soil-grown dwf1-6 plants or in CPD antisense plants grown on agar. This finding may reflect altered responses to the environment or secondary events restricted to one particular genotype.
Table I.
Genes affected by BR deficiency
| Affymetrix Identification/Accession No. | Gene | (Putative) Function | WT versus dwf1-6 | WT versus αCPD | WT + BR versus WT − BR | dwf1-6+ BR versus dwf1-6 − BR |
|---|---|---|---|---|---|---|
| Genes affected by BR deficiency: stronger expression in wild-type plants | ||||||
| 16522_at/X77500 | AAP4 | Amino acid transporter | NC 1.3 | I 2.1 | NC −1.3 | NC −1.8 |
| 19531_at/AL021960 | AAT1 | Amino acid transport protein | *I 3.5 | NC 1.8 | NC 1.2 | NC 1.4 |
| 17595_s_at/AF166352 | AGT3 | Ala:glyoxylate aminotransferase 2 homolog | NC 1.3 | I 2.6 | NC −1.5 | NC −1.6 |
| 15166_s_at/AF042195 | ARF7 | Auxin response factor | NC 1.3 | I 2.1 | NC 1.3 | NC 1.5 |
| 16411_s_at/AJ002280 | ASKβ | Shaggy-like protein kinase | NC 1.4 | I 3.2 | NC −1.1 | NC −1.2 |
| 17467_at/X75431 | ASK-γ | Homolog of the shaggy and GSK-3 protein kinases | I 1.9 | NC 1.6 | NC 1.5 | NC 1.1 |
| 13209_s_at/AF040632 | AXR3 | Auxin response | I 2.4 | NC 1.2 | NC 1.7 | NC 1.3 |
| 16669_at/U20810 | CIP1 | Cytoskeleton-associated protein | *NC ∼1.8 | I 2.1 | NC 1.0 | NC −2.2 |
| 15997_s_at/AB004872 | COR47 | Dehydration and cold-regulated gene | I 2.1 | I 1.7 | NC 1.2 | NC 1.3 |
| 16172_at/D78603 | CYP71B4 | Cytochrome P450 monooxygenase (CYP71B4) | I 1.5 | I 2.4 | NC 1.1 | NC 1.5 |
| 17023_s_at/U53860 | DET2 | Steroid reductase | NC 1.2 | I 3.3 | NC −1.1 | NC 1.1 |
| 18729_at/Y14072 | HMGβ1 | High-mobility group 1-like protein | NC 1.4 | I 1.9 | NC 1.1 | NC −1.2 |
| 13293_s_at/U18415 | IAA13 | Auxin response | NC 1.2 | I 1.7 | NC 1.1 | NC 1.0 |
| 13297_at/AF027157 | IAA2 | Auxin response | NC 1.4 | I 2.2 | NC −1.4 | NC −1.1 |
| 13300_at/U53672 | IAA22 | Auxin response | *I 2.4 | NC 1.8 | NC 1.0 | NC 1.2 |
| 13665_f_at/AF079587 | ICK1 | Cyclin-dependent kinase inhibitor | NC 1.4 | *I 1.8 | NC 1.0 | NC 1.2 |
| 18023_s_at/AJ011044 | Oas6 | Cys synthase | I 2.1 | NC 1.2 | NC −1.7 | NC −1.6 |
| 16038_s_at/L04173 | RAB18 | Gly-rich protein | *I 3.4 | I 4.9 | NC 1.4 | *NC ∼−2.1 |
| 16769_at/Z83312 | SAL2 | 5′-Bisphosphate nucleotidase | I 2.0 | I 3.4 | NC 1.0 | NC −1.3 |
| 17880_s_at/Z50752 | Sugar transporter | I 2.0 | I 1.6 | NC 1.2 | NC 1.2 | |
| 15761_at/AL021687 | Neoxanthin cleavage enzyme-like protein | I 1.7 | I 1.8 | NC 1.4 | NC −1.1 | |
| 18503_at/AL021889 | bHLH protein | *NC ∼3.1 | I 2.4 | NC 1.1 | NC 1.8 | |
| Genes affected by BR deficiency: stronger expression in BR-deficient plants | ||||||
| 17832_at/U94998 | AHB1 | Class 1 non-symbiotic hemoglobin | D −3.1 | *D ∼−2.5 | NC −1.2 | NC 1.5 |
| 16610_s_at/AB008490 | ARR7 | Response regulator 7 | NC −2.0 | *D ∼−5.3 | NC −1.6 | NC 1.1 |
| 12406_s_at/U31370 | ATCYP4 | Cyclophilin | NC −1.3 | D −2.1 | NC −1.4 | NC 1.3 |
| 16914_s_at/X89008 | Atosm34 | Osmotin | *D −6.6 | D −3.6 | D −1.6 | NC 1.2 |
| 15146_s_at/L38520 | DIM | Steroid synthesis | D −1.8 | NC 1.0 | NC −1.3 | NC 1.0 |
| 15194_s_at/U15683 | GASA4 | GA-regulated gene | D −3.5 | NC −1.7 | NC −1.3 | NC 1.1 |
| 15160_s_at/U75193 | GLP3b | Germin-like protein | D −2.2 | NC −1.2 | NC −1.2 | NC −1.0 |
| 15115_f_at/AF104330 | GRP3S | Gly-rich protein 3 | D −2.2 | NC −1.4 | NC 1.2 | NC −1.4 |
| 20347_at/Y12575 | H2A | Histone | D −2.7 | D −1.9 | NC 1.1 | NC 1.1 |
| 17409_at/M17131 | H3 | Histone | D −2.0 | NC −1.3 | NC 1.3 | NC 1.3 |
| 13680_at/L04637 | LOX1 | Lipoxygenase | NC −2.8 | D −1.8 | NC −1.0 | NC −1.5 |
| 15992_s_at/X16432 | NAEEFTU | Elongation factor 1-α | D −2.0 | NC −1.4 | NC −1.0 | NC −1.4 |
| 12926_s_at/L39954 | PRL | Cell division | D −2.5 | NC −1.4 | NC −1.1 | NC 1.6 |
| 14643_s_at/Z46823 | RAR047 | Sulfotransferase | *NC −1.5 | D −2.1 | NC 1.2 | MD −1.9 |
| 16981_s_at/U35829 | TRX5 | Thioredoxin h | NC −1.3 | D −2.1 | NC −1.1 | D −2.1 |
| 15658_at/D84417 | Monodehydroascorbate reductase | *D ∼−11.8 | D −2.1 | NC −1.2 | NC 1.1 | |
| 20547_at/AB021934 | Nicotianamine synthase | NC −1.4 | D −2.6 | NC −1.6 | NC 1.1 | |
I, Increase (according to the Affymetrix Difference Call algorithm); MI, marginal increase; D, decrease; MD, marginal decrease; NC, no change. Nos. give the fold change. All transcripts meet the presence criterion in both situations (exceptions indicated with an asterisk). ∼ Indicates background problems and the fold change is an approximation.
Table II.
BR-regulated genes (consistently affected by BR treatment and BR deficiency)
| Affymetrix Identification/Accession No. | Gene | (Putative) Function | WT versus dwf1-6 | WT versus αCPD | WT + BR versus WT − BR | dwf1-6+ BR versus dwf1-6 − BR |
|---|---|---|---|---|---|---|
| Genes showing consistent responses to BR deficiency and BR treatment: BR-up-regulated genes | ||||||
| 17572_s_at/AF083036 | AMT1;2 | Ammonium transporter | I 3.6 | NC 1.5 | I 2.8 | NC −1.2 |
| 19653_g_at/AJ003215 | GTL1 | DNA-binding protein | I 1.5 | I 1.6 | NC 1.4 | NC 1.8 |
| 18950_at/U09335 | HAT2 | Homeobox protein | I 2.1 | NC 1.5 | NC 1.4 | I 1.8 |
| 16465_at/Y08892 | Hsc70-G8 | Heat shock protein | I 2.0 | NC 1.7 | I 2.2 | I 2.2 |
| 13301_at/U18406 | IAA3 | Auxin-inducible RNA | I 2.1 | NC 1.2 | I 3.2 | I 6.1 |
| 17961_at/AF053345 | KCS1 | Fatty acid elongase 3-ketoacyl-CoA synthase 1 | *I 2.5 | *NC 2.0 | I 3.8 | I 6.8 |
| 15124_s_at/U59508 | PRO1 | Osmotic stress-induced Pro DH | NC 1.0 | I 2.0 | I 2.2 | I 3.3 |
| 18038_i_at/AJ011637 | SPL10 | Squamosa promoter binding protein-like 10 | NC 1.3 | I 2.5 | I 2.5 | NC 1.1 |
| 17072_s_at/L39650 | ZFP7 | Zinc finger protein | I 1.8 | NC 1.3 | I 2.4 | NC 1.1 |
| 16555_at/AF079503 | H-protein promoter binding factor-2a | NC 1.2 | I 2.1 | NC 2.9 | *NC 2.3 | |
| Genes showing consistent responses to BR deficiency and BR treatment: BR-down-regulated genes | ||||||
| 13212_s_at/M90509 | BG2/PR2 | β-1,3-glucanase | NC −1.2 | D −2.1 | D −2.3 | D −1.9 |
| 16042_s_at/X87367 | CPD | Steroid 23-hydroxylase | NC −1.6 | I 2.0 | D −4.2 | D −4.2 |
| 13870_at/AF044216 | DWF4 | Steroid 22-hydroxylase | *MD −3.0 | NC −1.7 | D −2.7 | D −6.7 |
| 12736_f_at/Z97048 | MYB13 | R2R3-MYB transcription factor | NC −1.4 | NC −1.4 | D −3.1 | D −2.8 |
| 18738_f_at/Z95741 | MYB14 | R2R3-MYB transcription factor | NC −1.3 | *NC −1.7 | D −2.6 | D ∼−4.9 |
| 14240_s_at/X13434 | NIA1 | Nitrate reductase NR1 | NC −1.2 | D −2.9 | *D −7.3 | D −2.9 |
| 16535_s_at/AB008097 | ROT3 | Steroid synthesis | *D −3.7 | D −1.9 | *D −5.1 | D −1.9 |
| 14654_s_at/AF105034 | STE1/DWF7 | Δ7 Sterol C-5 desaturase | NC −1.1 | D −2.3 | D −4.1 | NC −1.7 |
I, Increase (according to the Affymetrix Difference Call algorithm); MI, marginal increase; D, decrease; MD, marginal decrease; NC, no change. Nos. give the fold change. All transcripts meet the presence criterion in both situations (exceptions indicated with asterisk). ∼ Indicates background problems and the fold change is an approximation.
Genes with altered basal transcript levels in both genotypes and environmental conditions potentially are involved in BR responses. BRs appear to be required for proper expression of stress-related genes (e.g. RAB18 and COR47), genes involved in nitrogen transport (e.g. AMT1;2, and AAT1), and several nuclear factors (e.g. HAT2, GTL1, and PRL). Altered histone transcript levels may indicate an altered chromatin composition. Several genes previously identified as auxin-, GA-, and cytokinin-regulated genes display altered transcript levels (e.g. IAA22, GASA4, ARR7, and ARR5). Decreased ferritin expression and increased nicotianamine synthase expression in BR-deficient plants may point to altered iron levels in BR-deficient plants.
CPD antisense plants show clearly decreased CPD transcript levels. In contrast, dwf1-6 displays higher CPD expression due to the negative feedback regulation of the CPD promoter (Mathur et al., 1998). DWF4 transcript levels are increased in both genotypes. This finding indicates a regulation comparable with the CPD gene. DET2 transcript levels are clearly decreased in αCPD plants. Potentially, αCPD plants accumulate metabolites such as cathasterone and campestanol, which may inhibit DET2 expression. DIM transcript levels are unaffected in αCPD plants but clearly increased in the dwf1-6 mutant. The mutant DIM gene of the dwf1-6 mutant carries a Ds insertion (Altmann et al., 1995). Detectable missense mRNA is produced and the accumulation of metabolic precursors such as 24-methylene-cholesterol may trigger DIM expression. ROT3 transcript levels are increased in αCPD and dwf1-6 plants.
BR deficiency does not result in significantly altered transcript levels of other phytohormone biosynthetic genes (represented on the array), with the exception of a gene encoding a (predicted) neoxanthin cleavage enzyme, which is involved in abscisic acid biosynthesis.
Expression Profiles of BR-Treated Plants
A further indication for direct BR regulation is short-term changes of transcript levels after BR application. Because synthetic events are required, periods of minutes may be too short for detection of altered transcript levels. In contrast, periods of several hours may result in the observation of secondary effects (e.g. caused by BR-induced growth). Therefore, wild-type and dwf1-6 plants grown on agar were treated with 300 nm 24-epibrassinolide and a control solution, respectively, and plants were harvested 1 h after treatment.
One hundred eighty-four and 199 genes (present in both profiles) were BR induced according to the difference call algorithm in wild-type and dwf1-6 plants, respectively (indicated by “I”). Ninety-eight and 94 genes, respectively, displayed an FC of ≥2.0. Conversely, 260 and 193 genes were repressed after BR treatment (indicated by ‘D’), 127 and 118 genes displayed a FC of ≤−2.0 in wild-type and dwf1-6 plants, respectively. However, only a limited number of genes were common to both sets and showed consistent induction or repression. This finding indicates genotype-dependent responses to BRs most probably related to the different endogenous BR levels in the two sets of plants. Tables II and III give a summary about differentially expressed genes in both genotypes. Table IV gives a summary of genes that are not consistently affected by BR treatment and BR deficiency. Only genes with an assigned function are shown.
Table III.
Genes affected by BR treatment
| Affymetrix Identification/Accession No. | Gene | (Putative) Function | WT versus dwf1-6 | WT versus αCPD | WT + BR versus WT − BR | dwf1-6+ BR versus dwf1-6 − BR |
|---|---|---|---|---|---|---|
| Genes affected by BR treatment: stronger expression in BR-treated plants | ||||||
| 16617_s_at/AF029980 | A37 | Ortholog of SNZ growth arrest response genes | NC −1.1 | NC −1.3 | I 1.8 | I 1.7 |
| 14732_at/AF016100 | ACO2 | 1-Aminocyclopropane-1-carboxylic acid oxidase | *NC 1.6 | NC −1.1 | I 3.2 | NC 1.3 |
| 15107_s_at/AF060874 | AGP4 | Arabinogalactan protein | NC 1.2 | NC 1.6 | I 2.5 | I 1.9 |
| 15154_at/L29083 | ASN1 | Asn synthetase | NC −1.5 | I 2.4 | I 1.6 | I 3.7 |
| 17525_at/AF007778 | AtTPPA | Trehalose-6-phosphate phosphatase | NC −1.0 | NC −1.5 | I 2.4 | I 2.7 |
| 15122_at/U39485 | dTIP | Tonoplast integral protein | NC −1.4 | NC −1.0 | I 2.6 | I 1.8 |
| 17494_s_at/U30478 | EXP5 | Expansin | NC 1.5 | NC 1.0 | I 2.0 | I 2.8 |
| 17378_at/U89512 | Glossy8 | β-keto acyl reductase | NC 1.0 | NC −1.1 | I 2.2 | I 1.6 |
| 15960_at/Y17053 | Hsc70-3 | Heat shock protein | NC 1.2 | NC −1.3 | I 1.8 | I 1.8 |
| 16466_s_at/Y08903 | Hsc70-G7 | Heat shock protein | NC 1.2 | NC −1.2 | I 1.8 | I 1.6 |
| 13277_i_at/Y14070 | HSF3 | Heat shock protein | NC −1.3 | NC 1.6 | I 1.6 | I 3.7 |
| 13284_at/AJ002551 | HSP70 | Heat shock protein | NC 1.3 | NC 1.3 | I 3.2 | I 4.3 |
| 13285_at/M62984 | HSP83 | Heat shock protein | NC 1.2 | NC 1.3 | I 2.5 | I 4.7 |
| 13296_at/U49075 | IAA19 | Auxin response | NC 1.7 | NC −1.2 | I 2.1 | I 2.6 |
| 18723_at/X99809 | MIXTA | Myb transcription factor | NC −1.1 | NC 1.6 | NC 1.5 | I 2.1 |
| 16099_at/U26936 | MYB6 | Myb transcription factor | NC 1.1 | NC 1.2 | I 1.6 | I 2.1 |
| 19203_s_at/AJ010475 | RH28 | DEAD box RNA helicase | ∼NC −1.2 | NC −1.1 | I 2.6 | NC 1.7 |
| 17094_s_at/U49453 | ROF1 | FK506 binding protein FKBP62 | NC 1.2 | NC −1.1 | I 1.8 | I 1.9 |
| 13027_at/Y14423 | SEB1 | Cell wall protein | NC −1.2 | NC 1.3 | NC 1.5 | I 1.8 |
| 16620_s_at/AF051338 | TCH4 | Xyloglucan endotransglycosylase | NC −1.1 | NC 1.2 | I 3.2 | I 10.3 |
| 16583_s_at/L39651 | ZFP8 | Zinc finger protein | NC −1.0 | NC −1.0 | I 2.1 | I 1.8 |
| 16082_s_at/AF144382 | Glutathione S-transferase | NC 1.5 | NC −1.1 | I 2.0 | I 1.9 | |
| 16598_s_at/U63373 | Polygalacturonase isoenzyme 1 β-subunit homolog | NC −1.1 | NC −1.0 | NC 1.8 | I 2.0 | |
| Genes affected by BR treatment: weaker expression in BR-treated plants | ||||||
| 18909_s_at/AF055848 | AIR3 | Subtilisin-like protease | NC −1.1 | NC −1.1 | D −1.7 | D −1.8 |
| 14763_at/X86958 | AK13 | Protein kinase | *NC 1.5 | *NC ∼1.2 | D −3.2 | D −3.1 |
| 16163_s_at/U40154 | AKT2 | Potassium channel | NC 1.1 | NC 1.1 | *D −2.1 | *D −2.9 |
| 16606_at/U89771 | ARF1-BP | ARF1 binding protein | NC 1.2 | NC 1.1 | D −1.5 | D −2.0 |
| 20389_at/X67033 | Athb-5 | Homeodomain protein | NC 1.0 | NC 1.4 | D −4.7 | D −2.3 |
| 16613_s_at/AF012657 | AtKT2 | Potassium transporter | NC 1.1 | NC −1.1 | NC −1.6 | D −2.9 |
| 16119_s_at/AF029876 | AtKUP1 | Potassium transporter | NC −1.4 | NC −1.2 | D −2.6 | D −7.8 |
| 20210_g_at/AF017056 | BRI1 | BR receptor | NC 1.2 | NC 1.4 | D −2.3 | D −1.8 |
| 17514_s_at/AF076277 | ERF1 | Ethylene-responsive element binding factor 1 | *NC −1.2 | NC ∼2.3 | D −3.4 | D −6.0 |
| 16609_at/AB008104 | ERF2 | Ethylene-responsive element binding factor 2 | NC 1.4 | NC 1.4 | D −1.7 | D −2.3 |
| 17089_s_at/U38416 | FAH1 | Ferulate-5-hydroxylase | NC 1.2 | NC −1.2 | NC −1.2 | D −2.1 |
| 12500_s_at/AF081067 | IAR3 | Auxin conjugate hydrolase | NC 1.2 | NC −1.1 | D −1.6 | D −2.5 |
| 12712_f_at/Z95774 | MYB51 | R2R3-MYB transcription factor | *NC 1.4 | *NC −1.2 | D −4.5 | NC −1.9 |
| 16078_at/AF088281 | PAP1 | Phytochrome-associated protein 1 | NC −1.2 | NC 1.1 | D −2.8 | NC −1.7 |
| 16559_s_at/AF088280 | PAP3 | Phytochrome-associated protein 3 | NC −1.5 | NC 1.1 | NC −1.2 | D −2.2 |
| 14630_s_at/AF100166 | PIF3 | Phytochrome-interacting factor 3 | NC −1.7 | NC 1.3 | D −1.6 | D −1.9 |
| 20462_at/U82399 | PK1 | Protein kinase | NC −1.4 | NC −1.2 | D −3.6 | D −4.0 |
| 16927_s_at/AF035384 | SEN4 | Endo-xyloglucan transferase | NC −1.1 | NC 1.4 | D −2.4 | D −2.3 |
| 18217_g_at/X95573 | STZ | Salt tolerance zinc finger protein | NC 1.3 | NC 1.3 | D −2.0 | D −2.8 |
| 15178_s_at/U43489 | XTR7 | Xyloglucan endotransglycosylase | D −1.9 | I 3.4 | D −22.6 | *D −12.3 |
| 13211_s_at/M38240 | Basic chitinase | *NC 1.9 | NC 1.8 | D −2.2 | D −2.1 | |
| 15142_at/AB016819 | UDP-Glc glucosyltransferase | NC 1.2 | NC 1.2 | MD −3.0 | D −2.1 | |
I, Increase (according to the Affymetrix Difference Call algorithm; MI, marginal increase; D, decrease; MD, marginal decrease; NC, no change. Nos. give the fold change. All transcripts meet the presence criterion in both situations (exceptions indicated with asterisk). ∼ Indicates background problems and the fold change is an approximation.
Table IV.
Genes not consistently affected by BR treatment and BR deficiency
| Affymetrix Identification/Accession No. | Gene | (Putative) Function | WT versus dwf1-6 | WT versus αCPD | WT + BR versus WT − BR | dwf1-6+ BR versus dwf1-6 − BR |
|---|---|---|---|---|---|---|
| Genes showing inconsistent responses to BR deficiency and BR treatment: stronger expression in wild-type plants, weaker expression in BR-treated plants | ||||||
| 17567_at/AF055372 | AT4 | Pi starvation inducible Mt4 homolog | NC 1.1 | I 1.6 | D −1.2 | D −1.7 |
| 16031_at/X94248 | AtFer1 | Ferritin | I 2.0 | I 3.1 | NC 1.0 | D −2.2 |
| 15695_s_at/U73781 | His1-3 | Histone | I 1.8 | I 2.0 | NC −1.2 | D −4.0 |
| 18701_s_at/X55053 | COR6.6 | Cold-regulated gene | I 2.0 | NC −1.3 | NC −1.5 | D −2.0 |
| 15611_s_at/L22567 | COR78 | Dehydration and cold-regulated gene | I 2.2 | NC 1.4 | *D ∼−9.9 | MD −3.2 |
| 16048_at/X78586 | Dr4 | Drought stress-regulated gene | I 3.2 | NC 1.3 | NC −1.2 | D −2.6 |
| 17922_at/U71122 | Pdc2 | Pyruvate decarboxylase-2 | I 2.1 | NC 1.5 | NC −1.1 | MD −1.5 |
| 16482_s_at/D61395 | γ-VPE | Vacuolar-processing enzyme | I 1.8 | I 1.7 | NC −1.4 | D −1.7 |
| 18968_at/AF163823 | XTR3 | Endoxyloglucan transferase | *MI ∼6.1 | *I 2.1 | NC ∼−2.1 | *D ∼−2.0 |
| 18953_at/AF077955 | Branched-chain alpha keto-acid dehydrogenase E1 α-subunit | NC −1.2 | I 2.3 | D −2.4 | D −3.3 | |
| Genes showing inconsistent responses to BR deficiency and BR treatment: stronger expression in BR-deficient plants, stronger expression in BR-treated plants | ||||||
| 15184_s_at/AB008488 | ARR5 | Response regulator 5 | NC −1.6 | *D −4.0 | NC 1.2 | I 1.9 |
| 18683_s_at/L27158 | FAD8 | ω-3 Fatty acid desaturase | NC 1.0 | D −1.6 | I 2.2 | I 2.0 |
I, Increase (according to the Affymetrix Difference Call algorithm); MI, marginal increase; D, decrease; MD, marginal decrease; NC, no change. Nos. give the fold change. All transcripts meet the presence criterion in both situations (exceptions with asterisk). ∼ Indicates background problems and the fold change is an approximation.
DISCUSSION
Complementary Approaches to Identify BR-Regulated Genes
To identify BR-regulated genes, previous approaches compared BR-treated plants with control plants (e.g. Zurek et al., 1994; Hu et al., 2000; Müssig et al., 2000). These studies provided valuable hints to the potential mode of action of BRs; however, their physiological relevance remained uncertain because the rate of uptake and the degree of distribution of the exogenously applied BRs are unknown and thus is the actual dose of BRs and the tissues reached. Furthermore, only single developmental stages were tested. A complementary approach is the comparison of BR-deficient plants and wild-type plants. So far, this approach was applied to analyze the expression of only a limited number of genes such as rbcS, cab, and psbA (Chory et al., 1991) or stress-related genes (Szekeres et al., 1996). We established expression profiles using both approaches. To reduce the incidence of detecting secondary effects due to extreme dwarfism in several BR mutants, we analyzed BR-deficient plants with mild phenotypic alterations (the dwf1-6 mutant and CPD antisense plants). In addition, we applied two different growth conditions (dwf1-6 in soil and αCPD in synthetic medium) to take into account that BR deficiency may result in specific changes only at a particular environmental situation.
For expression profiling experiments, we used Affymetrix Arabidopsis Genome Arrays. The expression data variability of genes that meet the criterion present, which is imposed by technical error, turned out to be very low. The average difference metric of only 0.24% of genes displayed a variation coefficient of more than 50%, and 86.7% of genes had a variation coefficient of 20% or less. Thus, the average difference metric, which serves as a relative indicator of the expression level of a transcript, is highly reproducible. The average difference is the basis for the fold change metric, which estimates the difference of expression levels between different samples. Therefore, even small fold change values—of genes called present in both (all) samples analyzed—point to reliable differences in transcript levels. The expression of a subset of genes that displayed minor fold change values in one situation (such as CPD, GASA4, and GLP3b) was checked by northern-blot or reverse northern-blot analysis. In all cases, the Affymetrix data were confirmed qualitatively (data not shown). The most stringent criterion of the Affymetrix Microarray Suite program is the difference call, which is based on several high stringency quality control measures and corresponding statistics which produce four comparison metrics: (a) the number of probe pairs that have changed in a certain direction, (b) the ratio of increased probe pairs over decreased probe pairs, (c) the log average ratio change, and (d) the Dpos-Dneg ratio (if a transcript is present in both the baseline and experimental samples, the metrics c and d may be close to zero and cause the outcome no change despite an increase or decrease in the level of the transcript). Although changes of transcript levels detected as increase or decrease are highly trustworthy (little or no false positives), many genes with true differential expression are dismissed as no change (many false negatives) if only this measure is used with a concomitant loss of valuable information.
Expression Profiles Point to BR Effects
A core set of BR-regulated genes has been identified the transcript levels of which are decreased/increased in both BR-deficient backgrounds and growth conditions and are increased/decreased after BR application in wild-type and dwf1-6 plants. Remarkably, numerous BR-inducible genes such as TCH4 and EXP5 do not display significantly altered transcript levels in BR-deficient plants. Similarly, numerous genes with altered basal transcript levels in BR-deficient plants such as RAB18, SAL2, or AHB1 displayed no clear induction or repression after BR application. On that account and according to the criteria set in this study, these genes cannot be regarded as primary targets of BR action (although a later induction or repression might occur and may well represent specific BR effects). The subset of genes that meets all criteria is shown in Table II. BR regulation is most significant for the (brassino) steroid synthesis pathway. The expression of the ROT3, DWF4, and CPD genes is clearly down-regulated, which is in agreement with the negative feedback regulation model of BR biosynthesis (Mathur et al., 1998). A BR regulation of several auxin response genes exists. Reduced ARF7, AXR3, IAA3, IAA2, IAA13, and IAA22 transcript levels in BR-deficient plants and BR-induced expression of IAA3 and IAA19 may be the consequence of altered auxin levels. Previous studies demonstrated higher levels of indole-3-acetic acid (IAA) in BR-treated hypocotyls (Eun et al., 1989) and the BR-deficient lkb mutants have a reduction in IAA levels (Nomura et al., 1997). However, the clear repression of IAR3 expression and the clear induction of IAA3 and IAA19 expression observed within 1 h may point to a mechanism different from alterations of auxin levels. The IAR3 gene encodes an auxin conjugate hydrolase (Davies et al., 1999) and potentially is involved in the release of storage or inactivation forms of auxin. Its repression points to a reduced release of free IAA. The short-term regulation of auxin-inducible genes by BRs points to a direct regulatory effect that does not require altered auxin-levels. Thus, BRs and auxin have partly identical regulatory functions. The reduced ARF1-BP (ARF1-binding protein; Ulmasov et al., 1997) expression in BR-treated plants provides further evidence for this finding. ARF1 is a transcription factor that binds to auxin response elements. The precise function of ARF1-BP is unclear, but the protein appears to regulate ARF1 activity.
Furthermore, BRs appear to be directly involved in the regulation of genes encoding N-transport proteins (such as AMT1;2) and several nuclear factors (GTL1, HAT2, MYB13, and MYB14). BR-regulated transcription factors may provide important insights into BR actions. The MYB13 gene promoter is active in the shoot meristem region, in axillary buds, and at the basis of flowers. The expression of MYB13 is regulated by drought, abscisic acid, light and wounding (Kirik et al., 1998) and ectopic expression results in altered inflorescence architecture. Kirik et al. suggested a function of the MYB13 gene product in linking shoot morphogenic activity with environmental and intrinsic signals. Because MYB13 expression is clearly down-regulated by exogenous BRs, and slightly increased in BR-deficient plants, BRs may influence these morphogenic events. The GTL1, HAT2, and MYB14 genes are barely characterized. Furthermore, BR-regulated genes such as KCS1 and BG2 point to different interesting potential BR functions related to wax biosynthesis and pathogen defense.
This core set of genes is supplemented by genes that may reveal BR-related activities occurring only under certain environmental conditions or upon specific physiological states of the plants and that have been identified either by expression monitoring of BR-deficient or -treated plants. These include genes involved in cell wall modification (e.g. SEN4, TCH4, XTR7, and EXP5), phytohormone synthesis (e.g. FAD8, ACO2, and neoxanthin cleavage enzyme), phytohormone response (e.g. GASA4, ARR5, ARR7, ERF1, and ERF2), and cold and drought stress responses (e.g. Atosm34, COR47, and COR78). Furthermore, BRs potentially regulate the expression of chromatin components (e.g. different histones and HMGβ1), further transcription factors (e.g. ZFP8, STZ, Athb5, and MYB51), and light-signaling genes such as PIF3 and CIP1. Decreased AtFer1 and increased nicotianamine synthase expression indicate lower iron levels or altered iron signaling in BR-deficient plants.
CONCLUSIONS
The expression monitoring experiments described in this study have identified several new BR-regulated genes. These genes display increased/decreased transcript levels in BR-deficient plants and decreased/increased transcript levels in BR-treated plants. The expression of hundreds of genes is significantly altered after BR application; however, wild-type and dwf1-6 plants display clearly different responses to exogenous BRs. The expression profiles of soil-grown dwf1-6 plants and CPD antisense plants grown on agar clearly differ from expression profiles of wild-type plants grown in parallel. Only a subset of genes displays corresponding changes in both situations. Thus, specific changes occur in dependency of growth conditions and genotypes. A complete set of data from this study is downloadable at our Web site (http://www.mpimp-golm.mpg.de/BR_reg_gene_expression/). To get a clearer picture of interactions with other growth regulators and to identify further BR-regulated genes, a more detailed analysis (e.g. of specific tissues) is required, because whole plants expression profiles hide changes which may occur in specific organs or cell types. The identification of numerous BR-regulated genes provides the basis for the identification of cis-acting elements in promoters that mediate BR effects.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Two growth conditions were applied. First, Arabidopsis cv C24 (wild type), the BR-deficient mutant dwf1-6 (cbb1, Kauschmann et al., 1996), and transgenic plants carrying a construct for antisense inhibition of CPD expression (Schlüter et al., 2002) were grown in one-half-concentrated Murashige and Skoog medium supplemented with 1% (w/v) Suc and solidified with 0.7% (w/v) agar under a 16-h day (140 μmol m−2 s−1, 22°C)/8-h night (22°C) regime. Plants were harvested 20 ± 1 d after sowing. Roots were discarded. Second, Arabidopsis cv C24 and the BR-deficient mutant dwf1-6 (Kauschmann et al., 1996) plants were grown in soil under long-day conditions (16 h of fluorescent light, 180 μmol m−2 s−1, 20°C, 60% relative humidity/8 h of dark, 16°C, 75% relative humidity). Above ground organs were harvested 50 ± 1 d after sowing.
The BR 24-epibrassinolide (CID-tech Research Inc., Cambridge, ON) was applied as a 300 nm solution to 20-d-old wild-type and dwf1-6 plants grown on agar. Plants were harvested 1 h after treatment and roots were discarded. Five hundred milliliters of aqueous epibrassinolide-solution contained approximately 25 μL of Sapogenat T-110 (Hoechst, Frankfurt). The control solution had the same composition but lacked epibrassinolide.
Hybridization of Affymetrix Genome Arrays
Total RNA was isolated as described previously (Müssig et al., 2000). The quality and quantity was checked using the Bioanalyzer 2100 (Agilent Technologies, Böblingen, Germany) and MOPS-formaldehyde agarose gels. Twenty micrograms of total RNA was used for double-stranded cDNA synthesis (SuperScript Choice system, Gibco BRL, Karlsruhe, Germany). Biotin-labeled cRNAs were synthesized using the BioArray High Yield RNA Transcript Labeling Kit (Enzo, New York). All cRNA samples were checked for degradation by gel analysis according to the Affymetrix technical manual. In addition, most of the targets were checked by hybridizations of Test 3 arrays (part no. 900341). Only bona fide probes were used for Arabidopsis Genome Array (part no. 900292) hybridizations. Hybridization, washing, staining, and scanning procedures were performed as described in the Affymetrix technical manual. Expression analysis via the Affymetrix Microarray Suite software (version 4.0) was performed with standard parameters. The output of every experiment was multiplied by a scaling factor to adjust its average intensity to a target intensity of 1,000. Thus, scaling allows comparisons between any two experiments. Basic principles of Affymetrix oligonucleotide arrays were reviewed by Lipshutz et al. (1999) and Lockhart et al. (1996).
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011003.
LITERATURE CITED
- Altmann T, Felix G, Jessop A, Kauschmann A, Uwer U, Peña-Cortés H, Willmitzer L. Ac/Ds transposon mutagenesis in Arabidopsis thaliana: mutant spectrum and frequency of Ds insertion mutants. Mol Gen Genet. 1995;247:646–652. doi: 10.1007/BF00290357. [DOI] [PubMed] [Google Scholar]
- Arteca RN, Bachman JM, Yopp JH, Mandava NB. Relationship of steroidal structure to ethylene production by etiolated mung bean segments. Physiol Plant. 1985;64:13–16. [Google Scholar]
- Azpiroz R, Yewen W, LoCascio JC, Feldmann KA. An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P, Wild A. The influence of brassinosteroid on growth and parameters of photosynthesis of wheat and mustard plants. J Plant Physiol. 1984;116:189–196. doi: 10.1016/S0176-1617(84)80088-7. [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE et al. The Arabidopsis dwf7/ste1 mutant is defective in the Δ7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell. 1999;11:207–221. [PMC free article] [PubMed] [Google Scholar]
- Choe S, Tanaka A, Noguchi T, Fujioka S, Takatsuto S, Ross AS, Tax FE, Yoshida S, Feldmann KA. Lesions in the sterol Δ7 reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 2000;21:431–443. doi: 10.1046/j.1365-313x.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Zurek D. Molecular analysis of brassinolide action in plant growth and development. In: Cutler HG, Yokota T, Adam G, editors. Brassinosteroids; Chemistry, Bioactivity and Applications, ACS Symposium Series. Washington, DC: American Chemical Society; 1991. pp. 122–140. [Google Scholar]
- Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B. IAR3 encodes an auxin conjugate hydrolase from Arabidopsis. Plant Cell. 1999;11:365–376. doi: 10.1105/tpc.11.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P. Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol. 1999;40:333–342. doi: 10.1023/a:1006283015582. [DOI] [PubMed] [Google Scholar]
- Eun J-S, Kuraishi S, Sakurai N. Changes in levels of auxin and abscisic acid and the evolution of ethylene in squash hypocotyls after treatment with brassinolide. Plant Cell Physiol. 1989;30:807–810. [Google Scholar]
- Feldmann KA, Marks MD, Christianson ML, Quatrano RS. A dwarf mutant of Arabidopsis generated by T-DNA insertion mutagenesis. Science. 1989;243:1351–1354. doi: 10.1126/science.243.4896.1351. [DOI] [PubMed] [Google Scholar]
- Goetz M, Godt DE, Roitsch T. Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J. 2000;22:515–522. doi: 10.1046/j.1365-313x.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Bao F, Li Y. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000;24:693–701. doi: 10.1046/j.1365-313x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Ikekawa N, Zhao Y-J. Application of 24-epibrassinolide in agriculture. In: Cutler HG, Yokota T, Adam G, editors. Brassinosteroids: Chemistry, Bioactivity and Applications, ACS Symposium Series. Washington DC: American Chemical Society; 1981. pp. 280–291. [Google Scholar]
- Iwasaki T, Shibaoka H. Brassinosteroids act as regulators of tracheary-element differentiation in isolated Zinnia mesophyll cells. Plant Cell Physiol. 1991;32:1007–1014. [Google Scholar]
- Kang J-G, Yun J, Kim D-H, Chung K-S, Fujioka S, Kim J-I, Dae H-W, Yoshida S, Takatsuto S, Song P-S et al. Light and brassinosteroid signals are integrated via a dark-induced small G protein in etiolated seedling growth. Cell. 2001;105:625–636. doi: 10.1016/s0092-8674(01)00370-1. [DOI] [PubMed] [Google Scholar]
- Katsumi M. Interaction of a brassinosteroid with IAA and GA3 in the elongation of cucumber hypocotyl sections. Plant Cell Physiol. 1985;26:615–625. [Google Scholar]
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:701–713. [Google Scholar]
- Kirik V, Kölle K, Wohlfarth T, Miséra S, Bäumlein H. Ectopic expression of a novel MYB gene modifies the architecture of the Arabidopsis inflorescence. Plant J. 1998;13:729–742. doi: 10.1046/j.1365-313x.1998.00072.x. [DOI] [PubMed] [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua N-H. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka N, Takatsuto S, Yoshida S, Clouse SD. A putative role for the tomato gene DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol. 2000;122:85–98. doi: 10.1104/pp.122.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz RJ, Fodor SPA, Gingeras TR, Lockhart DJ. High density synthetic oligonucleotide arrays. Nat Genet. 1999;21:20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton M et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Mandava NB, Sasse JM, Yopp JH. Brassinolide, a growth-promoting steroidal lactone II. Activity in selected gibberellin and cytokinin bioassays. Physiol Plant. 1981;53:453–461. [Google Scholar]
- Mathur J, Molnár G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C et al. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313x.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- Müssig C, Biesgen C, Lisso J, Uwer U, Weiler EW, Altmann T. A novel stress-inducible 12-oxophytodienoate reductase from Arabidopsis thaliana provides a potential link between brassinosteroid-action and jasmonic-acid synthesis. J Plant Physiol. 2000;157:143–152. [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S et al. BAS1: A gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M-H, Romanow WG, Smith RC, Zamski E, Sasse J, Clouse SD. Soybean BRU1 encodes a functional xyloglucan endotransglycosylase that is highly expressed in inner epicotyl tissues during brassinosteroid-promoted elongation. Plant Cell Physiol. 1998;39:124–130. [Google Scholar]
- Schlagnhaufer C, Arteca RN, Yopp JH. A brassinosteroid-cytokinin interaction on ethylene production by etiolated mung bean segments. Physiol Plant. 1984;60:347–350. [Google Scholar]
- Schlüter U, Köpke D, Altmann T, Müssig C. Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid deficient cbb1 mutant. Plant Cell Environ. 2002;25:783–791. [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua N-H. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Takeno K, Pharis RP. Brassinosteroid-induced bending of the leaf lamina of dwarf rice seedlings: an auxin-mediated phenomenon. Plant Cell Physiol. 1982;23:1275–1281. [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. ARF1, a transcription factor that binds to auxin response elements. Science. 1997;276:1865–1868. doi: 10.1126/science.276.5320.1865. [DOI] [PubMed] [Google Scholar]
- Wilen RW, Sacco M, Gusta LV, Krishna P. Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. Physiol Plant. 1995;95:195–202. [Google Scholar]
- Woeste KE, Vogel JP, Kieber JJ. Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol Plant. 1999;105:478–484. [Google Scholar]
- Yamamoto R, Fujioka S, Demura T, Takatsuto S, Yoshida S, Fukuda H. Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol. 2001;125:556–563. doi: 10.1104/pp.125.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT. Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.) Plant Mol Biol. 1999;41:443–454. doi: 10.1023/a:1006372612574. [DOI] [PubMed] [Google Scholar]
- Yopp JH, Colclasure GC, Mandava N. Effects of brassin-complex on auxin and gibberellin mediated events in the morphogenesis of the etiolated bean hypocotyl. Physiol Plant. 1979;46:247–254. [Google Scholar]
- Yopp JH, Mandava NB, Sasse JM. Brassinolide, a growth-promoting steroidal lactone: I. Activity in selected auxin bioassays. Physiol Plant. 1981;53:445–452. [Google Scholar]
- Zurek DM, Rayle DL, McMorris TC, Clouse SD. Investigation of gene expression, growth kinetics, and wall extensibility during brassinosteroid-regulated stem elongation. Plant Physiol. 1994;104:505–513. doi: 10.1104/pp.104.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]