Abstract
Throughout the body there are smooth muscle cells controlling a myriad of tubes and reservoirs. The cells show enormous diversity and complexity compounded by a plasticity that is critical in physiology and disease. Over the past quarter of a century we have seen that smooth muscle cells contain – as part of a gamut of ion-handling mechanisms – a family of cationic channels with significant permeability to calcium, potassium and sodium. Several of these channels are sensors of calcium store depletion, G-protein-coupled receptor activation, membrane stretch, intracellular Ca2+, pH, phospholipid signals and other factors. Progress in understanding the channels has, however, been hampered by a paucity of specific pharmacological agents and difficulty in identifying the underlying genes. In this review we summarize current knowledge of these smooth muscle cationic channels and evaluate the hypothesis that the underlying genes are homologues of Drosophila TRP (transient receptor potential). Direct evidence exists for roles of TRPC1, TRPC4/5, TRPC6, TRPV2, TRPP1 and TRPP2, and more are likely to be added soon. Some of these TRP proteins respond to a multiplicity of activation signals – promiscuity of gating that could enable a variety of context-dependent functions. We would seem to be witnessing the first phase of the molecular delineation of these cationic channels, something that should prove a leap forward for strategies aimed at developing new selective pharmacological agents and understanding the activation mechanisms and functions of these channels in physiological systems.
In this review we link two periods of discovery. The first began over 20 years ago when studies of mammalian smooth muscle tissues revealed the phenomena of store- and receptor-operated Ca2+-entry pathways (Van Breemen et al. 1978; Bolton, 1979; Casteels & Droogmans, 1981). Over the subsequent years a large number of reports have shown these or related phenomena in a wide range of smooth muscle types. Physiological roles are suggested to include the refilling of Ca2+ stores, neurotransmitter and hormone-evoked Ca2+ entry and depolarization, and tonic cationic entry – signals that may serve to modulate myogenic tone, stimulate contraction and modulate basic cellular systems such as those controlling proliferation, death, protein trafficking and gene transcription. There are implications for disease processes including those relating to blood pressure control, smooth muscle growth, blood vessel wall thinning, subarachnoid haemorrhage, asthma, premature labour, unstable bladder, lymphatic flow, and gastrointestinal motility.
The second period of discovery began roughly 15 years later with the emergence of at least 20 mammalian homologues of the Drosophila transient receptor potential (TRP) gene (Fig. 1) (Zhu et al. 1995; Wes et al. 1995; Minke & Cook, 2002; Montell et al. 2002; Vennekens et al. 2002; Zitt et al. 2002; Birnbaumer et al. 2003; Clapham, 2003; Alexander et al. 2004). Several of the smooth muscle non-selective cationic channels are clearly not encoded by TRP genes – the P2X1 ligand-gated ion channel (Vial & Evans, 2002), the channel carrying the hyperpolarization-activated cationic current (If or Ih) which is presumably encoded by a HCNx gene (Greenwood & Prestwich, 2002), and the inositol trisphosphate (IP3) and ryanodine receptors (Sanders, 2001; Fill & Copello, 2002). However, for many other non-selective cationic channels in smooth muscle there is reason to consider a TRP connection. First, heterologous expression of TRP genes induces cationic channel activity with strong permeability to K+ and Na+, and in most cases Ca2+ too. These TRP proteins are likely to be the ion pore components of the activity because they have structural homology to voltage-gated ion channels such as the Shaker K+ channel, which definitively carries ions (Fig. 2). Second, expression of at least six of the TRP genes has been commonly reported in smooth muscle tissues, and expression of others is indicated (Fig. 3). TRPM7 expression is said to be ubiquitous and necessary for cell survival (Nadler et al. 2001; Schmitz et al. 2003). Third, there are some general similarities in the properties of over-expressed TRP genes and the smooth muscle channels in question. These include non-selective cationic permeability and Ca2+ permeability, similarity in unitary conductance, and pharmacology including sensitivity to SKF96365 and 2-APB, and (in most cases) resistance to conventional Ca2+ antagonists (Fig. 4). There is also modest or weak voltage-gated activation, and a common – but by no means exclusive – ‘double-rectifying’ characteristic in the current–voltage-relationships (Clapham, 2003).
Figure 1. Members of the TRP family.
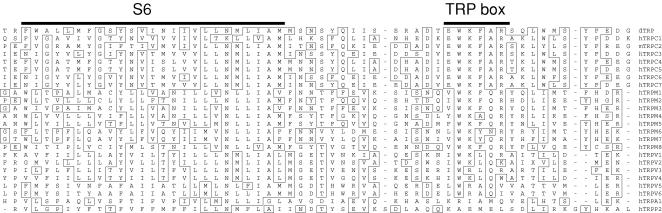
Single letter-code amino acid sequence alignment (Clustal W) for one of the most highly conserved region of TRPs – the distal element of the putative sixth membrane-spanning segment (S6) and the immediate C-terminus, which contains the so-called ‘TRP box’. Drosophila TRP (dTRP) is shown at the top. Thereafter, all sequences are human except for TRPC2, which is shown as mouse sequence because human TRPC2 would seem to be a pseudogene (i.e. not expressed as protein). TRPA (ANKTM1) and TRPP (polycystin) proteins show weak sequence similarity in this region and not all examples of these proteins are shown.
Figure 2. General structural themes of TRP proteins.
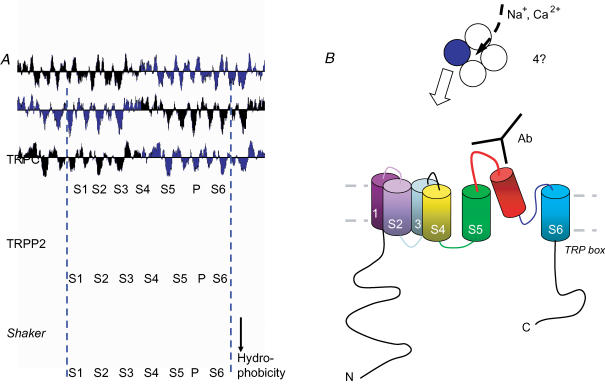
A, hydropathy plots for TRPC1 (accession P48995) and TRPP2 (accession XP-011124) compared with Shaker voltage-gated K+ channel (accession P08510). A theme of six membrane-spanning segments (S1-S6) and an ion selectivity filter (P-loop) is evident in each case. TRPC1 and other TRPCs have an extra hydrophobic segment towards the N-terminus. The general S1–S6 arrangement of TRPC1 has been supported by biochemical data (Dohke et al. 2004). All sequences have been clipped at the N (left) and C (right) termini. Hydropathy (Kyte-Doolittle) plots were created using Lasergene software (DNAStar Inc.). B, diagrams of the putative membrane-spanning arrangement of TRP proteins. The upper diagram is a plan view and suggests a tetrameric arrangement with ions flowing through a central pore – as for the Shaker K+ channel. The lower diagram is a classical depiction of a S1–S6 protein. Extracellular targeting of antibody (Ab; not to scale) is shown for inhibition of TRPC1 function (Xu & Beech, 2001a; Beech et al. 2003; Bergdahl et al. 2003).
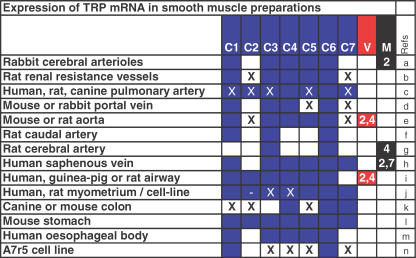
Figure 3. Expression of TRP mRNA species in smooth muscle cells, cell lines and whole tissue preparations.
Blue, red or black filled squares indicate mRNA was detected. Under the V and M columns, numbers indicate the subtype of TRPV or TRPM. A cross indicates mRNA could not be detected in a particular study. In some cases there is conflict between results from different research groups and so filled squares and crosses are superimposed. References: (a) Xu & Beech (2001a, b), Flemming et al. (2003); (b) Facemire et al. (2004); (c) Walker et al. (2001), McDaniel et al. (2001), Ng & Gurney (2001), Sweeney et al. (2002), Yu et al. (2003), Wang et al. (2004); (d) Inoue et al. (2001); (e) Facemire et al. (2003), Muraki et al. (2003); (f) Bergdahl et al. (2003); (g) Welsh et al. (2002), Earley et al. (2004), Waldron et al. (2004); (h) Jackson, et al. (2004), P. K. Jackson, B. Kumar, C. Munsch, C. D. Benham & D. J. Beech, unpublished observation; (i) Sweeney et al. (2002), Ong et al. (2003), Cloutier et al. (2003), Corteling et al. (2004), Jia et al. (2004); (j) Yang et al. (2002), Dalrymple et al. (2002), Babich et al. (2004); (k) Walker et al. (2001); (l) Lee et al. (2003); (m) Wang et al. (2003); (n) Jung et al. (2002).
Figure 4. Properties of some relevant non-selective cationic channel signals in smooth muscle.
Colour coding: green, permeability; red, block; grey, no effect; blue, stimulation. ‘X’ indicates a weak effect, a mixed effect depending on the specific agent tested, or a conflict in data from different laboratories (see specific references for details). Data sets are grouped according to SOC (store-operated channel), ROC (receptor-operated channel), SAC (stretch-activated channel), CAC (Ca2+-activated channel), LAC (lipid-activated channel), BC (background channel). Asterisk indicates the possibility of a molecularly distinct subset of this channel type within one type of smooth muscle. References: (a) Loutzenhiser & Loutzenhiser (2000), Potocnik & Hill (2001), Curtis & Scholfield (2001), Guibert et al. (2002), Fellner & Arendshorst (2002), Flemming et al. (2002, 2003), Curtis et al. (2003); (b) Karaki et al. (1979), Tosun et al. (1998), Samain et al. (1999), Walter et al. (2000), Tanaka et al. (2000), Trepakova et al. (2000, 2001); (c) Hughes & Schachter (1994), Broad et al. (1999), Iwamura et al. (1999), Patterson et al. (1999, 2002), Moneer & Taylor (2002); (d) Casteels & Droogmans (1981), Golovina (1999), Doi et al. (2000), Golovina et al. (2001), Ng & Gurney (2001), Wilson et al. (2002), Kang et al. (2003); (e) Weirich et al. (2001); (f) Arnon et al. (2000); (g) Dreja et al. (2001); (h) Albert & Large (2002 a, b); (i) Lee et al. (2002); (j) Ohta et al. (2000); (k) Wang et al. (2003); (l) Ito et al. (2002), Sweeney et al. (2002); (m) Shlykov et al. (2003); (n) Patterson et al. (1999); (o) Broad et al. (1996); (p) Curtis & Scholfield (2001); (q) Stepien & Marche (2000); (r) Matsuoka et al. (1997); (s) Wayman et al. (1996a, b, 1998, 1999), Wallace et al. (1999), Gibson et al. (2001), McFadzean & Gibson (2002); (t) Guibert et al. (2004); (u) Snetkov et al. (2003); (v) Byrne & Large (1988), Amédee et al. 1990, Wang & Large (1991), Kitamura et al. (1992), Inoue & Kuriyama (1993), Oike et al. (1993), Helliwell & Large (1997, 1998), Aromolaran & Large (1999), Albert et al. (2001), Inoue et al. (2001), Large (2002), Albert & Large (2003a); (w) Van Renterghem & Lazdunski (1994), Nakajima et al. (1996), Iwasawa et al. (1997), Minowa et al. (1997), Iwamuro et al. (1998, 1999), Kawanabe et al. (2001, 2002), Jung et al. (2002), Moneer & Taylor (2002), Moneer et al. (2003); (x) Murray & Kotlikoff (1991), Wang & Kotlikoff (2000), Oonuma et al. (2000); (y) Amédee et al. (1990), Wang et al. (1993), Large (2002); (z) Welsh & Brayden (2001); (aa) Kim et al. (1995, 1997, 1998), Kang et al. (2001), Lee et al. (2003), So et al. (2003); (ab) Vogalis & Sanders (1990; (ac) Bayguinov et al. (2001); (ad) Benham et al. (1985), Inoue et al. (1987, 1994, 1995, 1998), Inoue & Isenberg (1990a, b), Inoue (1991), Chen et al. (1993), Zholos & Bolton (1994, 1995), Bakhramov (1995), Shi et al. (2003), Yan et al. (2003). (ae) Guibert et al. (2004). (af) Minowa et al. (1997), Iwamuro et al. (1998, 1999), Kawanabe et al. (2001, 2002); (ag) Muraki et al. (2003; (ah) Welsh et al. (2000), Slish et al. (2002; (ai) Wu & Davis (2001), Park et al. (2003), Wu et al. (2003; (aj) Wellner & Isenberg (1993a, b, 1994), Kushida et al. (2001); (ak) Loirand et al. (1991); (al) Jabr et al. (2000); (am) Terasawa et al. (2002); (an) Albert et al. (2003b); (ao) Bae et al. (1999); (ap) Zakharov et al. (1999, 2003); (aq) Hughes & Schachter (1994); (ar) Miyoshi et al. (2004); (as) Thorneloe & Nelson (2004).
Categorization as a family of TRP proteins may be taken as indicative of a high degree of similarity but there is considerable diversity in sequence and function. There is commonality in hydrophobicity profiles (e.g. Fig. 2) and in many, but not all, cases there is close amino acid sequence similarity in and immediately distal to the putative sixth membrane segment (Fig. 1). The distal element may contain a clear TRP box, a signature sequence highly conserved in Drosophila TRP and its canonical gene products the TRPC subfamily of vertebrate TRPs. The putative fourth membrane segment (S4) has only weak similarity to the voltage sensor of Shaker, and the K+ selectivity filter is absent.
Studies of native cationic channels in smooth muscle
Activation by store-depletion protocols – ‘SOCs’
It is now almost a quarter of a century since studies of smooth muscle and secretory gland cells led to the suggestion of specific plasma membrane Ca2+ channels that open in response to a stimulus from depleted intracellular stores formed by smooth endoplasmic reticulum (Casteels & Droogmans, 1981; Putney, 1986; Parekh & Penner, 1997). These are the so-called capacitative Ca2+ entry (CCE) or store-operated channels (SOCs). What is the evidence for such a channel in smooth muscle? Studies of smooth muscle are no exception in showing that depletion of stores in Ca2+-free medium substantially enhances the Ca2+ re-entry signal observed on re-admission of Ca2+ to the extracellular medium. Induction of such ‘Ca2+ entry’ has been demonstrated by numerous methods including the use of intracellular fluorescent dye to detect Ca2+, Ba2+, Sr2+, Mn2+ or Na+ entry, 45Ca2+ flux measurements, smooth muscle contraction and electrical current recording (data are referenced in Fig. 4). The use of such a range of methods means technical factors will explain some differences and inconsistencies between studies. For example, the reader should take due account of the fact that muscle contraction and Ca2+ indicator dye experiments are not direct measures of ion channel activity. Most of the ion channels under consideration have significant Na+ as well as Ca2+ permeability and so indirect effects, for example via Na+–Ca2+ exchange and Na+–K+-ATPase, are possible. Such studies should not, however, be dismissed without careful consideration of the underlying data sets because they have often correctly predicted the existence of ion channels and revealed important properties.
The available evidence supports the view that store-depletion protocols activate a particular ion channel type that is otherwise inactive. The evoked Ca2+ entry pathway is pharmacologically distinguishable from background pathways (Flemming et al. 2002, 2003) and ion channels are involved because discrete unitary current events are observed in membrane patches (Fig. 4). Unitary conductance (depending on ionic conditions) is 1.5–7 pS in three independent studies and this relatively small conductance is an emerging feature distinguishing SOCs from receptor-operated channels (Fig. 4). However, there are also similarities with receptor-operated channels (see below), and one report describes a SOC unitary conductance of 30 pS – strikingly close to that of the receptor-operated channels (Fig. 4).
The name ‘SOC’ implies the channels are some how controlled by signals from the stores. Proof of such a link is non-trivial and depends on an ability to make a measurement at the plasma membrane while at the same time making a highly specific manipulation of the stores. Recordings from plasma membrane can be made unequivocally but our ability to specifically manipulate the stores is open to scrutiny. Importantly, the two chemically unrelated agents thapsigargin and cyclopiazonic acid are potent inhibitors of endoplasmic reticulum Ca2+-ATPases (SERCAs) and have in common the capacity to deplete stores and activate SOCs in smooth muscle (Wayman et al. 1996a, 1998, 1999; Wallace et al. 1999; Fig. 4). Thapsigargin does not inhibit plasma membrane Ca2+-ATPases (Thastrup et al. 1990; Kennedy & Mangini, 1996) and mutagenesis of a SERCA-like protein in yeast stimulates Ca2+ entry (Locke et al. 2000). Thapsigargin and cyclopiazonic acid have no effect on smooth muscle SOCs in excised patches of plasma membrane where intracellular stores are likely to be absent, and so they are not direct SOC activators (Albert & Large, 2002a). Use of another chemical agent to deplete stores (BAPTA-AM) activates the same smooth muscle SOCs, which is considered an important result because it argues against Ca i2+ elevation as the activation signal (Trepakova et al. 2001; Albert & Large, 2002b). An elegant bioassay has also shown smooth muscle SOCs in inside-out membrane patches activated by plasma membrane-permeabilized thapsigargin-treated platelets (Trepakova et al. 2000). These results favour the hypothesis that there is communication between depleted stores and the SOCs of the smooth muscle cell plasma membrane. Whatever the truth of this, it is indisputable that a discrete Ca2+ entry signal is evoked by thapsigargin or cyclopiazonic acid in, probably, every type of smooth muscle as well as many other cell types. We need to understand the molecular basis of this phenomenon if we are to know its physiological significance.
SOC signals have now been studied in a range of smooth muscle cells and different properties are apparent (Fig. 4). Although the comparisons are not within a single study, some differences are strong, giving reason to suspect multiple types of SOC (Fig. 4). This diversity may not, however, extend to the CRAC type of SOC. The Ca2+ release-activated channel (CRAC) of lymphocytes is one of the most studied SOCs and a highly Ca2+-selective small-conductance inwardly rectifying channel (Parekh & Penner, 1997; Lewis, 1999). So far, it has not been apparent in studies of smooth muscle. Instead, a non-selective cationic SOC is most common – a channel that not only has Ca2+ permeability but also high Na+ permeability and a relatively linear current–voltage relationship (Golovina et al. 2001; Trepakova et al. 2001; Albert & Large, 2002b).
The general mechanisms linking stores to SOC activation have been a subject of intrigue (Venkatachalam et al. 2002). Several features of the mechanism have emerged from studies of smooth muscle SOCs. There is an essential role for phospholipase C-γ in smooth muscle cell lines (Patterson et al. 2002), and in freshly isolated portal vein smooth muscle cells protein kinase C is necessary (Albert & Large, 2002a). Curtis et al. (2003) also suggest a role for protein kinase C, but an inhibitory one. The involvement of specialized subplasma membrane signalling microdomains is inferred from discrete non-contractile Ca2+ entry events of SOCs (Flemming et al. 2002) and the impact of cholesterol depletion on SOC function, indicating association with the signalling domains of caveolae (Bergdahl et al. 2003). Inhibition by jasplakinolide (Patterson et al. 1999), an agent that stabilizes actin polymerization, indicates involvement of the cytoskeleton – suggesting a structural rearrangement near the channels either as part of an acute activation mechanism or for shuttling of key proteins in the vicinity of the SOC channels. Smooth muscle studies have also seen a major focus on the hypothesis that SOC activation depends on diffusion of a soluble factor of unknown chemical identity (called calcium influx factor, or CIF) from the emptied stores to SOCs (Trepakova et al. 2000; Smani et al. 2003). Smani et al. (2004) suggest CIF does not act directly on the SOCs but displaces calmodulin from iPLA2 (a calcium-independent phospholipase A2), releasing the iPLA2 from constitutive inhibition by calmodulin. A requirement for iPLA2 is indicated because antisense DNA targeted to the iPLA2 mRNA and a chemical inhibitor of iPLA2 (bromoenol lactone) inhibit SOC activation in smooth muscle cells derived from mouse aorta (Smani et al. 2003, 2004). iPLA2 is also activated when smooth muscle stores are depleted (Wolf et al. 1997). iPLA2 generates arachidonic acid and lysophospholipids, and liposomal application of lysophosphoinositol was shown to enhance SOC-like channels in inside-out membrane patches (Smani et al. 2004). The inference that arachidonic acid must also be generated is, however, intriguing because arachidonic acid is suggested to be an inhibitor of SOCs and a signal mediating activation of receptor-operated channels in smooth muscle (Fig. 4, and text below). Although Smani et al. (2004) found no effect of arachidonic acid, we observe activation of cationic channels by arachidonic acid in mouse aorta smooth muscle cells (Jackson et al. 2004). Therefore, there is exciting progress in the field of SOC activation but still much to be worked out. For example, how do phospholipase C and protein kinase C fit into the iPLA2 pathway? How can arachidonic acid produced by iPLA2 fail to inhibit SOCs or activate receptor-operated channels? What is CIF, how is it produced, and do smooth muscle cells produce it?
Activation by agonists at G-protein-coupled receptors – ‘ROCs’
Many G-protein-coupled receptors are linked to phospholipase Cβ and so agonists at these receptors evoke IP3 production and then Ca2+ release. Receptor activation may therefore lead to SOC activity. However, there are several reasons to conclude there are also separate receptor-operated channels (ROCs) – channels that require agonist binding to a G-protein-coupled receptor but that are not dependent on the Ca2+ release event. The first reason to conclude there are ROCs – that are not SOCs – is that store-depletion does not prevent subsequent activation of cationic channels by a receptor agonist (Wang & Large, 1991; Guibert & Beech, 1999; Zholos et al. 2004). Second, heparin, which blocks IP3 receptors does not prevent ROC activation (Albert & Large, 2003a). Third, ROCs have a unitary conductance about 5 times larger than that of the primary SOC (Fig. 4). Fourth, SOCs and ROCs have been pharmacologically distinguished in the same cell type (Iwamuro et al. 1999).
ROCs have been studied extensively in several types of smooth muscle (Fig. 4). Examples receiving considerable attention include those coupled to α1-adrenergic receptors in rabbit portal vein, M2 and M3 muscarinic receptors in guinea-pig ileum, endothelin ET-A receptors in arterial smooth muscle cells, and vasopressin V1a receptors in A7r5 cells. As with SOCs, differences are apparent and so there may be multiple types of ROC (Fig. 4). For example: lanthanum activates the ileal ROC but blocks the ROC in portal vein; and LOE908 blocks ROCs in aorta-derived A7r5 cells but not in freshly isolated intrapulmonary arterioles. However, where determined, the unitary conductances are similar and it may be premature to decide on whether ‘different’ ROCs are molecularly distinct entities or whether the signalling networks of different receptors confer different properties on the same channels. Nevertheless, it is striking that Iwamuro et al. (1998) pharmacologically distinguished two types of ROC activated by the same agonist in the same cell type.
Numerous intriguing observations have been made on the activation pathway for ROCs. Involvement of G-proteins is suggested by the common observation that agonist effects are mimicked by intracellular dialysis with the hydrolysis-resistant guanosine triphosphate (GTP) analogue GTP-γ-S (Inoue & Isenberg, 1990; Zholos & Bolton, 1994). Furthermore, pertussis toxin or intracellular dialysis with antibodies that inhibit G-protein function suppress agonist activation of ROCs (see Yan et al. 2003 and references therein). These studies further narrow down the type of G-protein involved to the Gi, Go or Gq/11α-subunits of heterotrimeric G-proteins (Kim et al. 1998; Wang & Kotlikoff, 2000; Lee et al. 2003; Yan et al. 2003). The chemical and widely used phospholipase C inhibitor U73122 suppresses ROC activation (Fig. 4). This may mean that the next step after G-protein activation is a classical stimulation of phospholipase C and generation of a phosphatidylinositol bisphosphate (PIP2) product that activates the ROCs. Consistent with this hypothesis, the portal vein ROC is activated by exogenous diacyglycerol analogues – an effect that is facilitated by inositol phosphates (Helliwell & Large, 1997; Albert & Large, 2003a). Diacyglycerol activation of protein kinase C is required in some but not other blood vessels (Kitamura et al. 1992; Helliwell & Large, 1997; Slish et al. 2002). In contrast, the ileal ROC is not affected by diacylglycerol or inositol phosphates, even though U73122 inhibits its activation (Zholos et al. 2004). Given the widespread effects of PIP2 on ion channels (Hilgemann et al. 2001; Runnels et al. 2002), such an apparent anomaly might be explained if a fall in PIP2 levels is a significant regulatory factor, or if a non-enzymatic function of phospholipase C is involved. Additional possibilities have emerged from studies of ROC activation by vasopressin in A7r5 cells where arachidonic acid produced by diacylglycerol lipase is suggested to stimulate nitric oxide synthase (NOS), producing nitric oxide, which then activates ROCs (Moneer & Taylor, 2002; Moneer et al. 2003). Although there is support for part of this hypothesis in a recent study of 5HT-activated ROCs in intrapulmonary arterioles, the expression of NOS in physiological contractile smooth muscle cells is controversial (Guibert et al. 2002, 2004). Not reviewed in detail here are important regulatory effects of Ca2+, Mg2+, H+ and tyrosine phosphorylation (Zholos & Bolton, 1995,1997; Helliwell & Large, 1998; Albert et al. 2001; Large, 2002).
Activation by membrane stretch – ‘SACs’
Responses to membrane-stretch are one of the key elements in muscle tone regulation. Such responses involve sustained membrane depolarization that is resistant to conventional Ca2+ antagonists and due to opening of non-selective cationic channels that we refer to as stretch-activated channels (SACs). In cell-attached and inside-out patch recording modes, membrane-stretch applied through the recording pipette activates cationic channels in toad stomach, pig and human coronary artery, rat and hamster mesenteric artery, rabbit pulmonary and coronary artery, and guinea-pig urinary bladder (Kirber et al. 1988; Davis et al. 1992; Wellner & Isenberg, 1993a,b; Ohya et al. 1998; Wu et al. 2003; Park et al. 2003). SACs are blocked by gadolinium, unitary conductance ranges from 27 to 40 pS (charge carried by monovalent ions), and several other general properties are similar to those of ROCs (Fig. 4). In whole-cell recordings, application of longitudinal cell-stretch or cell swelling by pressure on the patch pipette or hypotonic bath solution evokes Ca2+-permeable cationic channel activity in coronary artery and urinary bladder (Davis et al. 1992; Wellner & Isenberg, 1994). In smooth muscle the mechanisms coupling membrane stretch to channel opening seem likely to involve bioactive intermediates rather than direct mechanical activation because activation takes as long as one second – much slower than the submillisecond response time of mechano-gated channels in the fly (Walker et al. 2000; Gillespie & Walker, 2001). Consistent with such a hypothesis is the blocking effect of U73122, indicating a role for a signalling cascade involving phospholipase C (Fig. 4).
Linking native cationic channels to TRP homologues
The potential for a link between native channels and TRP homologues is addressed in three stages. First, we have asked if there is evidence for expression of the protein and whether it is at the plasma membrane. Second, we have only focused on the protein if there is ‘a direct link’– by which we mean the use of a TRP-specific tool to study the smooth muscle cationic channel. This might mean the use of gene disruption, RNA targeting with antisense DNA, or a specific blocking antibody. Some conventional pharmacological agents inhibit TRP function but none have the specificity that can provide a direct link. Lastly, we compare the properties of the native cationic channel with channel activity generated by heterologously over-expressed cDNA.
TRPC1 and TRPC4/5
The TRPC1 gene is widely expressed as mRNA and protein, including in a range of smooth muscle cell types (Fig. 3) (Xu & Beech, 2001a; Dalrymple et al. 2002; Ong et al. 2002; Sweeney et al. 2002). The protein is localized to the plasma membrane, although intracellular localization is also evident (Xu & Beech, 2001a; Dalrymple et al. 2002). Glycosylation of TRPC1 has been suggested (Ong et al. 2002) but the consensus N-linked glycosylation site is weak and between S5 and S6 (Fig. 1). We have not been able to detect glycosylation of TRPC1 (S. Z. Xu & D. J. Beech, unpublished obsevation). The apparent molecular mass is about 90 kDa (Beech et al. 2003). Splice variants have been reported but the functional significance is unknown (Beech et al. 2003).
Three studies provide evidence of a direct link between TRPC1 and Ca2+-permeable cationic channels of vascular smooth muscle. In each case, TRPC1 has only been linked to Ca2+ entry associated with store depletion. In two of the studies a functional anti-TRPC1 antibody was used to specifically inhibit TRPC1 (Xu & Beech, 2001a; Bergdahl et al. 2003). This antibody is targeted to the putative outer vestibule of the ion-pore (Fig. 2), acts extracellularly, and is specific for a 90 kDa protein in vascular smooth muscle samples. In one of the studies the antibody was shown to partially inhibit the Ca2+ re-entry signal in store-depleted smooth muscle cells of rabbit arterioles, without effect on background Ca2+ entry (Xu & Beech, 2001a). In the other, on rat caudal artery, the antibody inhibited contractions that had a pharmacological profile like that of store-operated Ca2+ entry (Bergdahl et al. 2003). Antisense DNA targeted to TRPC1-encoding mRNA has also been applied to human pulmonary artery smooth muscle cells cultured to passages 4–6 (Sweeney et al. 2002). After 24 h treatment there was a reduction in RNA, as well as protein detected with anti-TRPC1 antibody. The labelled protein was large, at 220 kDa, and suggested to be a dimer of TRPC1 – an aggregating effect that is common with membrane proteins. The functional effect of the antisense DNA was a 50–70% reduction in the amplitude of the non-selective cationic current evoked by store depletion. In support of a role for TRPC1 in the smooth muscle SOC phenomenon, over-expression of TRPC1 enhanced pulmonary artery contraction evoked by cyclopiazonic acid but not 40 mm K+ (Kunichika et al. 2004).
We cannot be sure of the properties of TRPC1 because it is difficult to obtain substantial functional signals over background in response to over-expression of TRPC1 alone (reviewed in Beech et al. 2003). It also may not be relevant to focus on such properties because TRPC1 probably does not act physiologically on its own (i.e. as a homomeric channel assembly). There is ample evidence that TRPC1 forms heteromeric arrangements, particularly with TRPC4 and 5, but also involving TRPC3 and TRPP2 (Tsiokas et al. 1999; Lintschinger et al. 2000; Strübing et al. 2001, 2003; Goel et al. 2002; Hofmann et al. 2002; Xu et al. 2002) (Fig. 3). For example, in HEK-293 cells, TRPC1 trafficked poorly to the plasma membrane unless coexpressed with TRPC4β (Hofmann et al. 2002). The over-expression studies yielding a functional TRPC1 signal may have been positive because there were sufficient native TRPs to support function. If we nevertheless assume these were pure TRPC1 signals, TRPC1 would seem to be a non-selective cationic channel subunit with permeability to Ca2+ and barium but not strontium or N-methyl-d-glucamine (NMDG+). It is blocked by gadolinium, lanthanum (IC50 1–10 μm), 80 μm 2-aminoethoxydiphenyl borate (2-APB), 20 μm xestospongin C or high concentrations of extracellular Ca2+, but not by 1 mm zinc (reviewed in Beech et al. 2003). Negative feedback results from Ca2+ binding to calmodulin and there is activation by the calmodulin inhibitor 1 μm calmidazolium (Delmas et al. 2002; Singh et al. 2002). The current–voltage relationship has only mild rectification over a wide voltage range. Stimulation of TRPC1 by diacylglycerols was apparent in the absence but not presence of extracellular Ca2+ (Lintschinger et al. 2000; Delmas et al. 2002), a weak effect when compared with those on TRPC3, TRPC6 and TRPC7 (Hofmann et al. 1999; Okada et al. 1999). Regulation of TRPC1 by a range of accessory proteins has been proposed (see Fig. 6 for references).
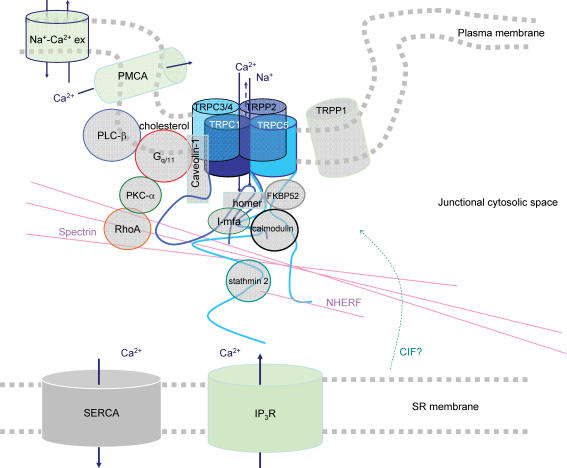
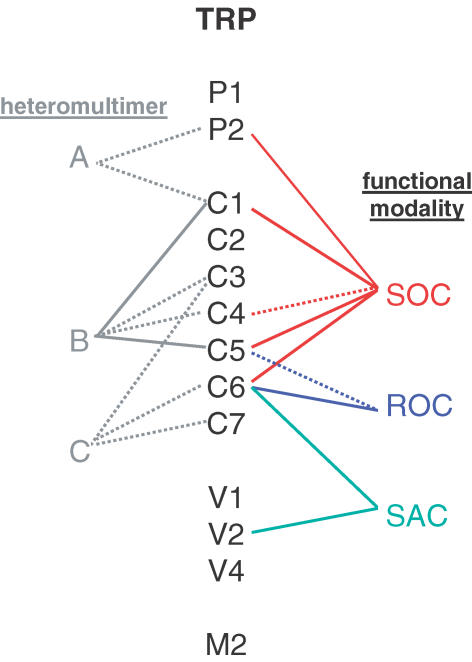
Figure 6. Putative local protein and signalling network associated with TRPC1.
One possible heteromultimer is indicated. Homer is suggested to dissociate from inositol trisphosphate receptor (IP3R) when stores are depleted (Yuan et al. 2003). There is evidence CIF acts on SOCs but there is no direct information for TRP (Trepakova et al. 2000; see text). References: Tsiokas et al. (1999), Lintschinger et al. (2000), Lockwich et al. (2000), Tang et al. (2000, 2001), Trepakova et al. (2000), Strübing et al. (2001), Hofmann et al. (2002), Kunzelmann-Marche et al. (2002), Rosado et al. (2002), Singh et al. (2002), Vaca & Sampieri (2002), Beech et al. (2003), Bergdahl et al. (2003), Brazer et al. (2003), Cioffi et al. (2003), Greka et al. (2003), Ma et al. (2003), Mehta et al. (2003), Boulter et al. (2001), Qian et al. (2003b), Yuan et al. (2003), Ahmmed et al. (2004), Sinkins et al. (2004).
Although there are studies both in favour and against a role for TRPC1 in SOC phenomena we favour the conclusion that TRPC1 is a pore-forming subunit of the in situ non-selective cationic SOC of vascular smooth muscle cells (Xu & Beech, 2001a; Beech et al. 2003; Bergdahl et al. 2003). The failure of many investigators to find function when over-expressing cDNA encoding TRPC1 may mean that it cannot function alone. In this regard TRPC5 is of interest because TRPC1–TRPC5 heteromultimers are functional and have a conductance of about 5 pS (Strübing et al. 2001) – close to the unitary conductance of smooth muscle SOC (Fig. 4). TRPC5 was initially indicated to be primarily expressed in brain and some studies of smooth muscle have not detected TRPC5 mRNA (Fig. 3). However, an increasing number of reports reveal TRPC5 mRNA in smooth muscle and we have developed several anti-TRPC5 antibodies that show TRPC5 protein is present (Fig. 3) (Xu et al. 2002). Furthermore, an anti-TRPC5 antibody inhibits Ca2+ entry evoked by store depletion in arterioles (Beech et al. 2004), suggesting TRPC1 and TRPC5 are involved in smooth muscle SOCs (Figs 5 and 6). TRPC5 over-expressed alone has properties similar to those of the muscarinic cationic ROC of gastric smooth muscle (Lee et al. 2003) and thus TRPC5 may have multiple functional roles (Zeng et al. 2004). TRPC4 is the most closely related protein to TRPC5, has similar properties in over-expression systems, and can form a complex with TRPC1 (Strübing et al. 2001; Plant & Schaefer, 2003). The mRNA encoding TRPC4 is detected in smooth muscle preparations (Fig. 3) and antisense DNA treatment of cultured mesangial cells inhibits Ca2+ entry associated with store depletion (Wang et al. 2004). Although the smooth muscle α-actin marker is not expressed in normal adult mesangial cells, it appears in glomerular disease and cell culture (Stephenson et al. 1998). Therefore, TRPC4 and TRPC5 are candidates as subunits assisting TRPC1 in its function as an element of the SOC phenomenon in smooth muscle (Fig. 6).
Figure 5. Schematic diagram to illustrate how diversity might arise in TRP-dependent non-selective cationic channels.
Three origins of diversity are proposed: (i) expression of multiple independent TRP genes, each with distinct properties; (ii) heteromultimerization of different TRP proteins within a tetrameric complex, with diversity arising due to differing expression levels of component TRP subunits; (iii) ‘functional modality’, by which we mean that each TRP may have the capability to be activated by several different signals (also referred to as versatility or promiscuity of gating, or multiplicity of activation). Continuous lines indicated that there is direct evidence in a smooth muscle preparation. Dotted lines indicated speculation based on work on other cell types or where evidence is not direct. See text for references and supporting information.
TRPP1 and TRPP2
TRPP1 and TRPP2 are also called polycystin 1 and polycystin 2, or PKD1 and PKD2, or PC1 and PC2. They have general structural similarity to Shaker K+ channel and TRPC1 (Fig. 2), although they lack a distinct TRP box and high sequence identity with TRPCs (Fig. 1). Recent classifications include them in the TRP superfamily (Birnbaumer et al. 2003; Clapham, 2003). The C-terminal portion of TRPP1 also has similarity but is a much larger protein with an additional N-terminal feature. The names of these proteins derive from their role in polycystic kidney disease. However, the phenotypes resulting from TRPP mutations in humans and knock-out mice are complex and vascular abnormality is striking – particularly aneurysmal disease. Furthermore, there is good agreement that TRPP1 and TRPP2 are expressed as protein in smooth muscle cells (Griffin et al. 1997; Kim et al. 2000; Gao et al. 2004). Expression is observed in major arteries such as the aorta, and in intracranial arteries, the afferent arteriole, and many more (Boulter et al. 2001; Torres et al. 2001). TRPP1 is about 400 kDa and several forms may be expressed, especially early in development. TRPP2 is about 110 kDa and is consistently expressed through development and in the adult animal (Qian et al. 2003b). Glycosylation studies suggest TRPP1 is expressed in intracellular and plasma membranes, where as TRPP2 seems mostly in intracellular membranes (Qian et al. 2003b). Gold-labelling electron microscopy studies localize TRPP1 to the plasma membrane and both proteins near to dense plaques, which are associated with linkage of thin filaments to the elastic laminae (Qian et al. 2003b).
Evidence of a direct link of TRPPs to smooth muscle cationic channels comes partly from murine TRPP1 gene disruption experiments in which the homozygotes die at embryonic day 15.5 from massive haemorrhage, which is suggested to be explained by abnormality in the functions of vascular endothelial and smooth muscle cells (Kim et al. 2000). There are, however, more data focused specifically on smooth muscle: TRPP2+/− mice have abnormalities of Ca2+ handling in smooth muscle cells freshly isolated from the aorta. Resting [Ca2+]i, Ca2+ release evoked by caffeine and thapsigargin, and Ca2+ re-entry in store-depleted cells were suppressed by 13–28% with statistical P values < 0.008 (Qian et al. 2003a). Qian et al. (2003a) suggest resting [Ca2+]i and Ca2+ release are suppressed because store-operated Ca2+ entry is compromised. TRPP2 is a protein partner of TRPC1 (Tsiokas et al. 1999) (Figs 5 and 6) and, although the functional consequence of this interaction is unknown, it is notable that both proteins have impact on the store-operated Ca2+ channel signal of vascular smooth muscle cells. There is an apparent conflict between the intracellular localization of TRPP2 and plasma membrane localization of TRPC1 (Xu & Beech, 2001; Qian et al. 2003b). However, the cellular localization of these proteins is actively debated, there is TRPC1 inside cells as well as the membrane, and TRP proteins may shuttle to the membrane in response to stimuli or associated proteins (Hanaoka et al. 2000; Koulen et al. 2002; Beech et al. 2003; Cayouette et al. 2004). Further evidence of a link between TRPP2 and native cationic channels comes from a study of visceral smooth muscle in Drosophila in which loss-of-function TRPP2 mutations severely reduced smooth muscle contractile function (Gao et al. 2004). Contractility was restored by expressing wild-type TRPP2.
The precise properties of heterologously expressed TRPP proteins are unclear in part because of differences between several published studies. However, there is agreement that they are non-selective cationic channels that pass Ca2+. They are weakly selective with evidence of currents carried by the large cation NMDG+, magnesium, and even anions. Hanaoka et al. (2000) only found membrane currents when TRPP1 and TRPP2 were coexpressed. The current reversed near 0 mV and the current–voltage relationship was near-linear, or slightly outwardly rectifying. There was about 50% block by 50 μm La3+ and no effect of 50 μm niflumic acid. Ion channel function of TRPP2 was observed in lipid bilayers and when expressed in insect sf9 cells (Gonzalez-Perrett et al. 2001). There were multiple conductance states and amiloride was inhibitory (IC50 79 μm). Heterologous expression of TRPP2 alone seems not to lead to ion channel function in the plasma membrane of mammalian cells but function has been observed in recordings from porcine kidney cell endoplasmic reticulum-derived vesicles (Koulen et al. 2002). There was marked inward rectification, unitary conductance of 90–114 pS, permeability to Ca2+, Ba2+ and Mg2+, and activation by cytosolic Ca2+ (0.1–100 μm). Ca2+ release evoked by vasopressin was markedly enhanced by TRPP2, consistent with TRPP2 acting as a Ca2+-activated Ca2+ release channel. This effect was inhibited by 50–100 μm 2-APB. The C-terminal element of TRPP1 functions in the plasma membrane when over-expressed on its own in Xenopus oocytes (Vandorpe et al. 2001). It is sensitive to block by lanthanum, gadolinium, zinc, SKF96365 or amiloride, but not the chloride channel inhibitors DIDS or niflumic acid. Therefore, TRPP proteins are Ca2+-permeable cationic channels with a general pharmacology similar to other TRP proteins. They may function in intracellular and plasma membranes.
TRPC6
There is TRPC6 gene expression in smooth muscle as mRNA and protein (Fig. 3) (Inoue et al. 2001; Dalrymple et al. 2002; Welsh et al. 2002; Yu et al. 2003). The protein is localized near to the plasma membrane in freshly isolated portal vein cells but is more perinuclear in cultured cells (Inoue et al. 2001). Consistent with plasma membrane localization there is N-linked glycosylation. Deglycosylation shifted the apparent molecular mass from about 130–110 kDa (Yu et al. 2003), close to the predicted molecular mass of full-length TRPC6 and the mass of over-expressed full-length TRPC6 (Zhang & Saffen, 2001). Two splice variants of TRPC6 have been described, one of which appears non-functional as a protein (Zhang & Saffen, 2001; Jung et al. 2003). The splicing events result in deletions and a reduction in mass by about 10 or 20 kDa. Other deletion splices are evident in the sequence databases. Translation of these RNAs to form smaller TRPC6 proteins has not been evident in studies of smooth muscle.
Three studies provide evidence of a direct link between TRPC6 and cationic channels of vascular smooth muscle. In each case, isolated cells (Inoue et al. 2001; Yu et al. 2003) or tissues (Welsh et al. 2002) were incubated with antisense DNA targeted to TRPC6 mRNA for 12–24 h (Yu et al. 2003) or 3–5 days (Inoue et al. 2001; Welsh et al. 2002). In each case, TRPC6 protein levels were shown to be decreased either by immunostaining or Western blot and evidence of specificity was provided. Strikingly, however, the functional properties of the TRPC6-related signal were different in each study. Inoue et al. (2001) described activation of cationic channels by the α1-adrenoceptor agonist phenylephrine in rabbit portal vein smooth muscle cells. Welsh et al. (2002) described activation of cationic channels by the hypo-osmotic stress in rat cerebral artery smooth muscle cells. Yu et al. (2002) described activation of cationic channels by a store-depletion protocol in rat pulmonary artery smooth muscle cells at passage 3–6. One conclusion from this could be that receptor-, stretch- and store-operated properties are encoded by a single gene (Fig. 5). There may be multiple activators of TRPC6 or each stimulus may elevate a common second messenger substance. A candidate second messenger is diacylglycerol because it activates TRPC6 and is elevated by α1-adrenoceptor activation or membrane stretch. Protein kinase C activation by diacylglycerol is also proposed as a stimulus for activation of store-operated channels (Albert & Large, 2002a). If a common messenger is involved, it is unlikely to be the whole story. Each TRPC6-related signal had a distinct current–voltage relationship – from outwardly rectifying, to double rectifying, to inwardly rectifying. Such differences may be explained in part by heteromultimerization of TRPC6 with other TRPC proteins. Strong evidence exists for association of TRPC6 with TRPC3 or TRPC7, and both may be expressed in smooth muscle (Fig. 3). We also cannot exclude that there are differences between TRPC6 from different species. Human and mouse TRPC6 diverge at 70 amino acids – almost exclusively in the crucial N- and C-termini.
Numerous studies include descriptions of the properties of heterologously expressed mouse, rat or human TRPC6, and there are few discrepancies between the results (Boulay et al. 1997; Hofmann et al. 1999, 2002; Inoue et al. 2001; Zhang & Saffen, 2001; Boulay, 2002; Basora et al. 2003; Dietrich et al. 2003; Jung et al. 2003; Lee et al. 2003; Estacion et al. 2004; Hisatsune et al. 2004). The unitary conductance of the channel is between 28 and 46 pS and the single channel unitary current–voltage relationship is linear. The reversal potential is close to 0 mV, there is no permeability to the large cation NMDG+, and the estimated ratio of Ca2+ to Na+ permeability is 4.5. Stimulation of the channels has occurred in response to G-protein-coupled receptor activation, intracellular GTP-γ-S or aluminium fluoride, relatively high concentrations of diacylglycerol and related compounds (EC50 for activation by 1-oleoyl-2-acetyl-sn-glycerol, 117 μm; Hofmann et al. 1999), the arachidonic acid metabolite 20-HETE, extracellular 1 mm Ca2+, or 100 μm flufenamate, but not to intracellular IP3, thapsigargin, phorbol esters, intracellular PIP2, ionomycin or intracellular alkalinization. Basora et al. (2003) found no effect of flufenamate. TRPC6 signals have been inhibited by 10 μm U73122, 2 μm calmidazolium, 250 μm cadmium, 4–6 μm lanthanum, 2 μm gadolinium, 4 μm SKF96365 or 130 μm amiloride. Rat TRPC6A was inhibited by phorbol ester, an effect prevented by protein kinase C inhibition (Zhang & Saffen, 2001). Boulay (2002) found an inhibitory effect of coexpressing a Ca2+-insensitive mutant of calmodulin. Glycosylation at the putative second extracellular loop suppresses constitutive activity of TRPC6 (Dietrich et al. 2003). Fyn-mediated tyrosine phosphorylation stimulates TRPC6 (Hisatsune et al. 2004). Most often there is a double rectifying current–voltage relationship characteristic of TRPC channels, but two studies show only mild outward or inward rectification (Boulay et al. 1997; Basora et al. 2003). Both of the latter studies were on the same stable cell-line expressing mouse TRPC6 with an HA tag. The commonly observed outward rectification may result from an increase in open probability at positive voltages, perhaps reflecting a mild intrinsic voltage-sensing mechanism akin to that in voltage-gated potassium channels. Single channel opening events of TRPC6 are very brief (Hofmann et al. 1999; Jung et al. 2003).
Inoue et al. (2001) provided, in addition to antisense DNA results, an exemplary comparison of the properties of mouse TRPC6 and the α1-adrenoceptor-linked cationic channel of rabbit portal vein smooth muscle cells. In all comparisons there were marked similarities – further strengthening the conclusion that TRPC6 is a critical element of this endogenous cationic channel and that it may operate in portal vein myocytes as a homomultimeric assembly. There are no reports of stretch- or store-operated properties of over-expressed TRPC6.
TRPV2
TRPV2, a member of the vanilloid receptor TRP subfamily, is mainly expressed in sensory organs, but is also widely distributed as mRNA and protein in non-sensory organs including vascular smooth muscles (Fig. 3) (O'Neil & Brown, 2003). Although intracellular localization of the protein is evident, TRPV2 has been suggested to localize to the plasma membrane in the presence of growth factors (Kanzaki et al. 1999). Glycosylation of TRPV2 has been suggested (Kanzaki et al. 1999), although the molecular mass of TRPV2 in cardiac and aortic smooth muscle is 85–90 kDa – close to the predicted mass of non-glycosylated full-length TRPV2 (∼86 kDa) (Iwata et al. 2003; K. Muraki & Y. Imaizumi, unpublished observation).
Striking new evidence has emerged for a role of TRPV2 in vascular smooth muscle (Fig. 4) (Muraki et al. 2003). In mouse aortic smooth muscle cells, cell-swelling caused by hypotonic solution activated non-selective cationic channels and elevated [Ca2+]i. These responses were inhibited by ruthenium red, a blocker of TRPV channels. Removal of external Ca2+ abolished elevation of [Ca2+]i evoked by hypotonic solution, suggesting stimulation of Ca2+ entry rather than Ca2+ release. TRPC6 expression was weak, but TRPV2 mRNA was detected and TRPV2 immunoreactivity was evident in single mouse aortic, mesenteric and basilar arterial smooth muscle cells. Treatment of mouse aorta with TRPV2 antisense DNA reduced the amount of TRPV2 protein and suppressed hypotonic stimulation-induced activation of cationic channels or the associated elevation of [Ca2+]i. Therefore, it would seem that TRPV2, as well as TRPC6, can function as a stretch-activated channel in vascular smooth muscle. Studies of intact arteries will be required for an understanding of the relative physiological significance of different TRP channels in responses to pressure, stretch and cell-swelling.
There are few studies on the properties of over-expressed TRPV2. However, consistent with the above studies on native smooth muscle cells, CHO-K1 (Chinese hamster ovary) cells over-expressing mouse TRPV2 exhibited non-selective channels that were stimulated by membrane stretch through the recording pipette or superfusion with hypotonic solution (Muraki et al. 2003). Moreover, stretch of the cells on an elastic silicone membrane elevated [Ca2+]i. Mechanisms involved in opening of TRPV2 by membrane-stretch have not been determined. However, deletion of ankyrin repeats, which are present in the N-terminal region of TRPV, abolished heat-activation of TRPV1 and TRPV4 (Schumacher et al. 2000; Watanabe et al. 2002). Since the ankyrin repeats interact with certain cytoskeletal proteins, this region of TRPV2 might be important for acceptance of applied mechanical stimuli. However, compared with the delay to opening of fly mechanoreceptor channels, TRPV2, TRPV4 and TRPC6 are slowly activating, suggesting ‘mechano-modulation’ rather than direct ‘mechano-gating’ (Gillespie & Walker, 2001). One hypothesis to explain this is that cell swelling or membrane stretch produce an endogenous ligand – perhaps a fatty acid – which activates TRPV2. Metabolites of arachidonic acid and diacylglycerol might modulate TRPV2, like they do TRPV4 and TRPC6 (Hofmann et al. 1999; Watanabe et al. 2003). Micro-domains comprising channels, receptors, lipids, enzymes and dynamically controlled lipids could be sensors of local membrane deformation.
Molecular basis of functional heterogeneity
A review of the many studies of non-selective cationic channels in smooth muscle reveals differences between pharmacological, regulatory and biophysical properties within or across smooth muscle types (Fig. 4). What is the basis of such diversity? Some ideas are summarized in Figs 5 and 6.
One explanation might be that there is expression of many TRP genes. Indeed, we already know that over half of the TRP genes are expressed as mRNA (Fig. 3), and protein evidence exists for some of them (see above). Added diversity may arise from heteromultimeric assembles, which are evident for TRPC1, {C3}, C4, C5 and P2, and TRPC3, C6 and C7 (Tsiokas et al. 1999; Hofmann et al. 2002; Strübing et al. 2003) (Fig. 5). Different smooth muscles may have different quantities of TRP proteins, leading to different stoichiometries of heteromultimers and tendencies to homomultimerization. For example, enhanced expression of TRPC3 may confer greater constitutive activity on a heteromultimer involving TRPC6, generating background cationic channels (Dietrich et al. 2003; Albert et al. 2003b). There is some evidence for differential TRP mRNA expression in smooth muscle (Fig. 3 and references therein).
There may be an additional mechanism for diversity. In Fig. 4 it is striking that single channel recordings have tended to reveal two primary unitary conductances: about 5 and 25 pS. Furthermore, one of the noticeable outcomes in reviewing TRPC6 in connection with smooth muscle is that three studies suggest three apparently separate functions. Also, single channel events just like those of the store-operated channel have been activated by noradrenaline in excised patches and thus seemingly independently of store-depletion (Albert & Large, 2002a). These are just a few emerging examples of the behaviour of TRP-like cationic channels. We suggest that such observations may be explained by different functional modalities of the same TRP protein. That is, the same TRP protein can have, for example, receptor- and stretch-operated properties, etc. (Fig. 5). Such versatility, or promiscuity, of gating is evident for over-expressed TRPC5 and TRPV4 (Vriens et al. 2004; Zeng et al. 2004). Cellular context may shift the emphasis from one gating mode to another.
Diversity in function can also result from differential expression of protein partners with a channel subunit. In the Drosophila TRP field the concept of a signalplex has emerged (Li & Montell, 2000). Similarly, a model is emerging for a signalplex associated with mammalian TRP channels. Figure 6 shows an example for TRPC1. Intriguingly, although many of the associated proteins have been proposed from studies outside the smooth muscle field, the majority are proteins already well-associated with smooth muscle function. Protein kinase C-α and caveolin-1 are examples fitting well with functional studies of smooth muscle SOCs (Albert & Large, 2002a; Bergdahl et al. 2003).
Roles of TRP proteins and non-selective cationic channels in tissues
Although we can state that a TRP protein forms a cationic channel in a heterologous system and may contribute, for example, to receptor-, stretch- and store-operated mechanisms in situ, these are not statements on the importance in whole physiological or pathological systems. However, information is emerging on the importance of these mechanisms: deletion of TRPP genes causes aneurysmal disease, perhaps suggesting a role in the secretory function of smooth muscle (Kim et al. 2000). Block of TRPC1 inhibits endothelin-evoked contraction in rat caudal artery (Bergdahl et al. 2003). Antisense DNA targeted to TRPC1 or TRPC6 inhibits proliferation of pulmonary artery smooth muscle cells in culture (Sweeney et al. 2002; Yu et al. 2003). Suppression of TRPC6 expression in cerebral artery inhibits myogenic tone (Welsh et al. 2002). The chemical agent LOE908, a commonly used inhibitor of receptor-operated channels, suppresses vasospasm and delays expansion of ischaemic damage in animal models of stroke (Hoehn-Berlage et al. 1997; Li et al. 1999; Tatlisumak et al. 2000; Kawanabe et al. 2003). Therefore, there would seem to be widespread roles of TRP proteins in the vascular and associated systems. Roles in other types of smooth muscle should be delineated soon.
TRPing along, or TRPing up?
If we are TRPing up and TRP genes do not explain the non-selective cationic channels of smooth muscle, where else might we look to solve these channels? CD20 protein is suggested to contribute to store-operated properties in myoblasts (Ju et al. 2003; Li et al. 2003). Although it is proposed as the first non-TRP candidate for SOCs, in predicted structure it shows a relationship to the S5–S6 region of TRPs. To our knowledge, CD20 is not expressed in smooth muscle. A few SOC signals are inhibited by classical Ca2+ antagonists (Fig. 4), leading some to propose the existence of truncated L-type voltage-gated Ca2+ channel α1C-subunit with SOC properties (Stokes et al. 2004). However, these would not be non-selective cationic channels. There are many other types of non-selective cationic channel, including IP3 receptors and P2X1 receptors, which have unitary conductance of about 75 and 25 pS, respectively. If we accept the concept of versatility of gating, should these channels be candidates? Signalling cascades of receptors and membrane deformation are complex and multifactorial, SERCA inhibition may profoundly impact on protein trafficking and local secretory mechanisms, leading to diverse effects on numerous proteins. A range of ion handling mechanisms may be influenced by such stimuli.
Nevertheless, in our opinion, the evidence is in favour of the conclusion that TRP proteins are the molecular basis of a significant number of the previously unsolved non-selective cationic channels of smooth muscle. The evidence is best for an association of specific TRP proteins with store-operated, receptor-operated and stretch-activated channels, but there are other candidates too, including background cationic channels and non-selective channels activated by lysophosphatidylcholine, Ca2+, and alkaline pH (Loirand et al. 1991; Zakharov et al. 1999, 2003; Jabr et al. 2000; Terasawa et al. 2002; Poteser et al. 2003) (Fig. 4). Since gating versatility is an emerging feature of TRP channels (Vriens et al. 2004; Zeng et al. 2004) these other effects may simply be features of TRP channels already known to be expressed and functional in smooth muscle. Versatility of gating is an interesting concept because it may enable TRP channels to act as physiological integrators or be a mechanism for increasing diversity of proteins without evolution of new genes. Alternatively, other TRP genes may be responsible for the diversity. For example, TRPM gene expression has been detected at mRNA level in smooth muscle (Fig. 3) and thus may underlie additional cationic channels. Recent reports suggest roles for TRPC3, TRPV4, TRPM2 and TRPM4 (Earley et al. 2004; Jackson et al. 2004; Jia et al. 2004; Waldron et al. 2004). TRPM7 is also a reasonable candidate given its proposed ubiquitous expression and our detection of mRNA in saphenous vein; it may have a role as a Mg2+ channel (Schmitz et al. 2003). A difficult but crucial element of any such study is proof of native protein expression at the plasma membrane. Once this is done it is a reasonable expectation that the protein will do something akin to that observed in heterologous expression systems, i.e. have cationic channel function. We need more TRP protein data, and more direct evidence of links with native cationic channels. Despite such hurdles, the future for studies of mammalian homologues of Drosophila TRP in smooth muscle looks promising. We might finally have found the genes that encode the calcium entry mechanisms initially brought to our attention a quarter of a century ago by the seminal works of Bolton, Casteels, Droogmans, van Breemen and their colleagues.
Acknowledgments
The work of the authors is supported by the Wellcome Trust, British Heart Foundation, Daiwa Foundation and Nakatomi Foundation.
References
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase C-α phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Albert AP, Aromolaran AS, Large WA. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. J Physiol. 2001;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J Physiol. 2002a;544:113–125. doi: 10.1113/jphysiol.2002.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002b;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J Physiol. 2003a;552:789–795. doi: 10.1113/jphysiol.2003.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Piper AS, Large WA. Properties of a constitutively active Ca2+-permeable non-selective cation channel in rabbit ear artery myocytes. J Physiol. 2003b;549:143–156. doi: 10.1113/jphysiol.2002.038190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels. Br J Pharmacol. (1) 2004;141(suppl. 1):S1–126. doi: 10.1038/sj.bjp.0705672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédee T, Benham CD, Bolton TB, Byrne NG, Large WA. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–C173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- Aromolaran AS, Large WA. Comparison of the effects of divalent cations on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. J Physiol. 1999;520:771–782. doi: 10.1111/j.1469-7793.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- Bae YM, Park MK, Lee SH, Ho WK, Earm YE. Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit. J Physiol. 1999;514:747–758. doi: 10.1111/j.1469-7793.1999.747ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhramov A. Effects of high-energy phosphates on carbachol-evoked cationic current in single smooth muscle cells from guinea-pig ileum. J Physiol. 1995;485:659–669. doi: 10.1113/jphysiol.1995.sp020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem. 2003;278:31709–31716. doi: 10.1074/jbc.M304437200. [DOI] [PubMed] [Google Scholar]
- Bayguinov O, Hagen B, Sanders KM. Muscarinic stimulation increases basal Ca2+ and inhibits spontaneous Ca2+ transients in murine colonic myocytes. Am J Physiol Cell Physiol. 2001;280:C689–C700. doi: 10.1152/ajpcell.2001.280.3.C689. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Zeng F, Mair L, Sivaprasadarao A. Antibody to the predicted outer pore of TRPC5 ablates calcium entry evoked by store-depletion in isolated rabbit arterioles. J Physiol. 2004;557.P:C78. [Google Scholar]
- Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Yidirim E, Abramowitz J. A comparison of the genes coding for canonical TRP channels and their M, V and P relatives. Cell Calcium. 2003;33:419–432. doi: 10.1016/s0143-4160(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Bolton TB. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Boulay G. Ca2+-calmodulin regulates receptor-operated Ca2+ entry activity of TRPC6 in HEK-293 cells. Cell Calcium. 2002;32:201–207. doi: 10.1016/s0143416002001550. [DOI] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad LM, Cannon TR, Taylor CW. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J Physiol. 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad LM, Powis DA, Taylor CW. Differentiation of BC3H1 smooth muscle cells changes the bivalent cation selectivity of the capacitative Ca2+ entry pathway. Biochem J. 1996;316:759–764. doi: 10.1042/bj3160759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne NG, Large WA. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–7246. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- Chen S, Inoue R, Ito Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br J Pharmacol. 1993;109:793–801. doi: 10.1111/j.1476-5381.1993.tb13644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi DL, Wu S, Stevens T. On the endothelial cell I (SOC) Cell Calcium. 2003;33:323–336. doi: 10.1016/s0143-4160(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cloutier M, Campbell S, Basora N, Proteau S, Payet MD, Rousseau E. 20-HETE inotropic effects involve the activation of a nonselective cationic current in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2003;285:L560–L568. doi: 10.1152/ajplung.00381.2002. [DOI] [PubMed] [Google Scholar]
- Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol. 2004;30:145–154. doi: 10.1165/rcmb.2003-0134OC. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Major EH, Trimble ER, Scholfield CN. Diabetes-induced activation of protein kinase C inhibits store-operated Ca2+ uptake in rat retinal microvascular smooth muscle. Diabetologia. 2003;46:1252–1259. doi: 10.1007/s00125-003-1178-5. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J Physiol. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Beech D, Poston L, Tribe RM. Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod. 2002;8:946–951. doi: 10.1093/molehr/8.10.946. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992;262:C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos Y, Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- Dohke Y, Oh YS, Ambudkar IS, Turner RJ. Biogenesis and topology of the transient receptor potential Ca2+ channel TRPC1. J Biol Chem. 2004;279:12242–12248. doi: 10.1074/jbc.M312456200. [DOI] [PubMed] [Google Scholar]
- Doi S, Damron DS, Horibe M, Murray PA. Capacitative Ca2+ entry and tyrosine kinase activation in canine pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L118–L130. doi: 10.1152/ajplung.2000.278.1.L118. [DOI] [PubMed] [Google Scholar]
- Dreja K, Bergdahl A, Hellstrand P. Increased store-operated Ca2+ entry into contractile vascular smooth muscle following organ culture. J Vasc Res. 2001;38:324–331. doi: 10.1159/000051063. [DOI] [PubMed] [Google Scholar]
- Earley S, Walderon BJ, Patlak J, Brayden JE. Transient receptor potential channel TRPM4 contributes to myogenic vasoconstriction of cerebral arteries. FASEB J. 2004 doi: 10.1161/01.RES.0000147311.54833.03. abstract Experimental Biology Meeting 2004. [DOI] [PubMed] [Google Scholar]
- Estacion M, Li S, Sinkins WG, Gosling M, Bahra P, Poll C, Westwick J, Schilling WP. Activation of Human TRPC6 Channels by Receptor Stimulation. J Biol Chem. 2004;279:22047–22056. doi: 10.1074/jbc.M402320200. [DOI] [PubMed] [Google Scholar]
- Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286:F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- Fellner SK, Arendshorst WJ. Store-operated Ca2+ entry is exaggerated in fresh preglomerular vascular smooth muscle cells of SHR. Kidney Int. 2002;61:2132–2141. doi: 10.1046/j.1523-1755.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Flemming R, Cheong A, Dedman AM, Beech DJ. Discrete store-operated calcium influx into an intracellular compartment in rabbit arteriolar smooth muscle. J Physiol. 2002;543:455–464. doi: 10.1113/jphysiol.2002.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming R, Xu SZ, Beech DJ. Pharmacological profile of store-operated channels in cerebral arteriolar smooth muscle cells. Br J Pharmacol. 2003;139:955–965. doi: 10.1038/sj.bjp.0705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Joseph E, Ruden DM, Lu X. Drosophila Pkd2 is haploid-insufficient for mediating optimal smooth muscle contractility. J Biol Chem. 2004;279:14225–14231. doi: 10.1074/jbc.M312223200. [DOI] [PubMed] [Google Scholar]
- Gibson A, Fernandes F, Wallace P, McFadzean I. Selective inhibition of thapsigargin-induced contraction and capacitative calcium entry in mouse anococcygeus by trifluoromethylphenylimidazole (TRIM) Br J Pharmacol. 2001;134:233–236. doi: 10.1038/sj.bjp.0704286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Golovina VA. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Prestwich SA. Characteristics of hyperpolarization-activated cation currents in portal vein smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C744–C753. doi: 10.1152/ajpcell.00393.2001. [DOI] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Torres VE, Grande JP, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997;8:616–626. doi: 10.1681/ASN.V84616. [DOI] [PubMed] [Google Scholar]
- Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. J Physiol. 1999;514:843–856. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert C, Flemming R, Beech DJ. Prevention of a hypoxic Ca2+i response by SERCA inhibitors in cerebral arterioles. Br J Pharmacol. 2002;135:927–934. doi: 10.1038/sj.bjp.0704547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert C, Marthan R, Savineau JP. 5-HT induces an arachidonic acid-sensitive calcium influx in rat small intrapulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1228–L1236. doi: 10.1152/ajplung.00265.2003. [DOI] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and – 2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Alpha 1-adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J Physiol. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Facilitatory effect of Ca2+ on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. J Physiol. 1998;512:731–741. doi: 10.1111/j.1469-7793.1998.731bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- Hoehn-Berlage M, Hossmann KA, Busch E, Eis M, Schmitz B, Gyngell ML. Inhibition of nonselective cation channels reduces focal ischemic injury of rat brain. J Cereb Blood Flow Metab. 1997;17:534–542. doi: 10.1097/00004647-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AD, Schachter M. Multiple pathways for entry of calcium and other divalent cations in a vascular smooth muscle cell line (A7r5) Cell Calcium. 1994;15:317–330. doi: 10.1016/0143-4160(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Inoue R, Brading AF. Human, pig and guinea-pig bladder smooth muscle cells generate similar inward currents in response to purinoceptor activation. Br J Pharmacol. 1991;103:1840–1841. doi: 10.1111/j.1476-5381.1991.tb12338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990a;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol. 1990b;258:C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- Inoue R, Kitamura K, Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987;410:69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Inoue R, Kuriyama H. Dual regulation of cation selective channels by muscarinic and alpha 1-adrenergic receptors in the rabbit portal vein. J Physiol. 1993;465:427–448. doi: 10.1113/jphysiol.1993.sp019685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Morita H, Yanagida H, Ito Y. Potentiating actions of lanthanum on ACh-induced cation current in guinea-pig ileal smooth muscle cells. J Smooth Muscle Res. 1998;34:69–81. doi: 10.1540/jsmr.34.69. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Inoue R, Waniishi Y, Ito Y. Extracellular H+ modulates acetylcholine-activated nonselective cation channels in guinea pig ileum. Am J Physiol. 1995;268:C162–C170. doi: 10.1152/ajpcell.1995.268.1.C162. [DOI] [PubMed] [Google Scholar]
- Inoue R, Waniishi Y, Yamada K, Ito Y. A possible role of tyrosine kinases in the regulation of muscarinic receptor-activated cation channels in guinea pig ileum. Biochem Biophys Res Commun. 1994;203:1392–1397. doi: 10.1006/bbrc.1994.2339. [DOI] [PubMed] [Google Scholar]
- Ito S, Kume H, Yamaki K, Katoh H, Honjo H, Kodama I, Hayashi H. Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by rho-kinase in tracheal smooth muscle. Am J Respir Cell Mol Biol. 2002;26:491–498. doi: 10.1165/ajrcmb.26.4.4701. [DOI] [PubMed] [Google Scholar]
- Iwamuro Y, Miwa S, Minowa T, Enoki T, Zhang XF, Ishikawa M, Hashimoto N, Masaki T. Activation of two types of Ca2+-permeable nonselective cation channel by endothelin-1 in A7r5 cells. Br J Pharmacol. 1998;124:1541–1549. doi: 10.1038/sj.bjp.0701984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamuro Y, Miwa S, Zhang XF, Minowa T, Enoki T, Okamoto Y, Hasegawa H, Furutani H, Okazawa M, Ishikawa M, Hashimoto N, Masaki T. Activation of three types of voltage-independent Ca2+ channel in A7r5 cells by endothelin-1 as revealed by a novel Ca2+ channel blocker LOE 908. Br J Pharmacol. 1999;126:1107–1114. doi: 10.1038/sj.bjp.0702416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa K, Nakajima T, Hazama H, Goto A, Shin WS, Toyo-Oka T, Omata M. Effects of extracellular pH on receptor-mediated Ca2+ influx in A7r5 rat smooth muscle cells: involvement of two different types of channel. J Physiol. 1997;503:237–251. doi: 10.1111/j.1469-7793.1997.237bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabr RI, Yamazaki J, Hume JR. Lysophosphatidylcholine triggers intracellular calcium release and activation of non-selective cation channels in renal arterial smooth muscle cells. Pflugers Arch. 2000;439:495–500. doi: 10.1007/s004249900206. [DOI] [PubMed] [Google Scholar]
- Jackson PK, Muraki K, Zeng F, McHugh D, Xu SZ, Flemming R, Cheong A, Miller P, Kemp PJ, Kumar B, Munsch C, Benham CD, Beech DJ. Lysophosphatidylcholine and arachidonic acid activated ion channels expressed in vascular smooth muscle. J Physiol. 2004 abstract Glasgow meeting. [Google Scholar]
- Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–278. doi: 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- Ju YK, Wu MJ, Chaulet H, Marciniec T, Graham RM, Allen DG. IGF-1 enhances a store-operated Ca2+ channel in skeletal muscle myoblasts: involvement of a CD20-like protein. J Cell Physiol. 2003;197:53–60. doi: 10.1002/jcp.10347. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Kang TM, Kim YC, Sim JH, Rhee JC, Kim SJ, Uhm DY, So I, Kim KW. The properties of carbachol-activated nonselective cation channels at the single channel level in guinea pig gastric myocytes. Jpn J Pharmacol. 2001;85:291–298. doi: 10.1254/jjp.85.291. [DOI] [PubMed] [Google Scholar]
- Kang TM, Park MK, Uhm DY. Effects of hypoxia and mitochondrial inhibition on the capacitative calcium entry in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2003;72:1467–1479. doi: 10.1016/s0024-3205(02)02441-4. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Karaki H, Kubota H, Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979;56:237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Hashimoto N, Masaki T. Characterization of Ca2+ channels involved in endothelin-1-induced contraction of rabbit basilar artery. J Cardiovasc Pharmacol. 2002;40:438–447. doi: 10.1097/00005344-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Masaki T, Hashimoto N. Effects of the Ca++-permeable nonselective cation channel blocker LOE 908 on subarachnoid hemorrhage-induced vasospasm in the basilar artery in rabbits. J Neurosurg. 2003;98:561–564. doi: 10.3171/jns.2003.98.3.0561. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Okamoto Y, Hashimoto N, Masaki T. Characterization of Ca2+ channels involved in endothelin-1-induced mitogenic responses in vascular smooth muscle cells. Eur J Pharmacol. 2001;422:15–21. doi: 10.1016/s0014-2999(01)01052-4. [DOI] [PubMed] [Google Scholar]
- Kennedy BG, Mangini NJ. Plasma membrane calcium-ATPase in cultured human retinal pigment epithelium. Exp Eye Res. 1996;63:547–556. doi: 10.1006/exer.1996.0145. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Ahn SC, So I, Kim KW. Quinidine blockade of the carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Br J Pharmacol. 1995;115:1407–1414. doi: 10.1111/j.1476-5381.1995.tb16631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A. 2000;97:1731–1736. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Kim SJ, Kang TM, Suh SH, So I, Kim KW. Effects of myosin light chain kinase inhibitors on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflugers Arch. 1997;434:346–353. doi: 10.1007/s004240050407. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim SJ, Sim JH, Cho CH, Juhnn YS, Suh SH, So I, Kim KW. Suppression of the carbachol-activated nonselective cationic current by antibody against alpha subunit of Go protein in guinea-pig gastric myocytes. Pflugers Arch. 1998;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- Kirber MT, Walsh JV, JR, Singer JJ. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988;412:339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Xiong Z, Teramoto N, Kuriyama H. Roles of inositol trisphosphate and protein kinase C in the spontaneous outward current modulated by calcium release in rabbit portal vein. Pflugers Arch. 1992;421:539–551. doi: 10.1007/BF00375049. [DOI] [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2004 doi: 10.1152/ajplung.00452.2003. published on-line. [DOI] [PubMed] [Google Scholar]
- Kunzelmann-Marche C, Freyssinet JM, Martinez MC. Loss of plasma membrane phospholipid asymmetry requires raft integrity. Role of transient receptor potential channels and ERK pathway. J Biol Chem. 2002;277:19876–19881. doi: 10.1074/jbc.M200324200. [DOI] [PubMed] [Google Scholar]
- Kushida N, Kabuyama Y, Yamaguchi O, Homma Y. Essential role for extracellular Ca2+ in JNK activation by mechanical stretch in bladder smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C1165–C1172. doi: 10.1152/ajpcell.2001.281.4.C1165. [DOI] [PubMed] [Google Scholar]
- Large WA. Receptor-operated Ca2+-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol. 2002;13:493–501. doi: 10.1046/j.1540-8167.2002.00493.x. [DOI] [PubMed] [Google Scholar]
- Lee CH, Rahimian R, Szado T, Sandhu J, Poburko D, Behra T, Chan L, Van Breemen C. Sequential opening of IP3-sensitive Ca2+ channels and SOC during alpha-adrenergic activation of rabbit vena cava. Am J Physiol Heart Circ Physiol. 2002;282:H1768–H1777. doi: 10.1152/ajpheart.00637.2001. [DOI] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Store-operated calcium channels. Adv Second Messenger Phosphoprotein Res. 1999;33:279–307. doi: 10.1016/s1040-7952(99)80014-7. [DOI] [PubMed] [Google Scholar]
- Li F, Carano RA, Irie K, Tatlisumak T, Silva MD, Pschorni U, Sotak CH, Fisher M. Neuroprotective effects of a novel broad-spectrum cation channel blocker, LOE 908 MS, on experimental focal ischemia: a multispectral study. J Magn Reson Imaging. 1999;10:138–145. doi: 10.1002/(sici)1522-2586(199908)10:2<138::aid-jmri5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Li H, Ayer LM, Lytton J, Deans JP. Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem. 2003;278:42427–42434. doi: 10.1074/jbc.M308802200. [DOI] [PubMed] [Google Scholar]
- Li HS, Montell C. TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J Cell Biol. 2000;150:1411–1422. doi: 10.1083/jcb.150.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol Cell Biol. 2000;20:6686–6694. doi: 10.1128/mcb.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- Loirand G, Pacaud P, Baron A, Mironneau C, Mironneau J. Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. J Physiol. 1991;437:461–475. doi: 10.1113/jphysiol.1991.sp018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca2+ entry. Circ Res. 2000;87:551–557. doi: 10.1161/01.res.87.7.551. [DOI] [PubMed] [Google Scholar]
- Ma R, Rundle D, Jacks J, Koch M, Downs T, Tsiokas L. Inhibitor of myogenic family, a novel suppressor of store-operated currents through an interaction with TRPC1. J Biol Chem. 2003;278:52763–52772. doi: 10.1074/jbc.M309610200. [DOI] [PubMed] [Google Scholar]
- McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JX. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Nishizaki T, Nomura T. The voltage-dependent non-selective cation channel sensitive to the L-type calcium channel blocker efonidipine regulates Ca2+ influx in brain vascular smooth muscle cells. Biochem Biophys Res Commun. 1997;240:484–487. doi: 10.1006/bbrc.1997.7624. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Minowa T, Miwa S, Kobayashi S, Enoki T, Zhang XF, Komuro T, Iwamuro Y, Masaki T. Inhibitory effect of nitrovasodilators and cyclic GMP on ET-1-activated Ca2+-permeable nonselective cation channel in rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:1536–1544. doi: 10.1038/sj.bjp.0701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Yamaoka K, Garfield RE, Ohama K. Identification of a non-selective cation channel current in myometrial cells isolated from pregnant rats. Pflugers Arch. 2004;447:457–464. doi: 10.1007/s00424-003-1175-z. [DOI] [PubMed] [Google Scholar]
- Moneer Z, Dyer JL, Taylor CW. Nitric oxide co-ordinates the activities of the capacitative and non-capacitative Ca2+-entry pathways regulated by vasopressin. Biochem J. 2003;370:439–448. doi: 10.1042/BJ20021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneer Z, Taylor CW. Reciprocal regulation of capacitative and non-capacitative Ca2+ entry in A7r5 vascular smooth muscle cells: only the latter operates during receptor activation. Biochem J. 2002;362:13–21. doi: 10.1042/0264-6021:3620013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Hazama H, Hamada E, Wu SN, Igarashi K, Yamashita T, Seyama Y, Omata M, Kurachi Y. Endothelin-1 and vasopressin activate Ca2+-permeable non-selective cation channels in aortic smooth muscle cells: mechanism of receptor-mediated Ca2+ influx. J Mol Cell Cardiol. 1996;28:707–722. doi: 10.1006/jmcc.1996.0066. [DOI] [PubMed] [Google Scholar]
- Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- Ohta T, Yasuda W, Hasegawa A, Ito S, Nakazato Y. Effects of inhibitors for tyrosine kinase and non-selective cation channel on capacitative Ca2+ entry in rat ileal smooth muscle. Eur J Pharmacol. 2000;387:211–220. doi: 10.1016/s0014-2999(99)00814-6. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Adachi N, Nakamura Y, Setoguchi M, Abe I, Fujishima M. Stretch-activated channels in arterial smooth muscle of genetic hypertensive rats. Hypertension. 1998;31:254–258. doi: 10.1161/01.hyp.31.1.254. [DOI] [PubMed] [Google Scholar]
- Oike M, Kitamura K, Kuriyama H. Protein kinase C activates the non-selective cation channel in the rabbit portal vein. Pflugers Arch. 1993;424:159–164. doi: 10.1007/BF00374607. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Ong HL, Brereton HM, Harland ML, Barritt GJ. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology. 2003;8:23–32. doi: 10.1046/j.1440-1843.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Ong HL, Chen J, Chataway T, Brereton H, Zhang L, Downs T, Tsiokas L, Barritt G. Specific detection of the endogenous transient receptor potential (TRP)-1 protein in liver and airway smooth muscle cells using immunoprecipitation and Western-blot analysis. Biochem J. 2002;364:641–648. doi: 10.1042/BJ20020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil RG, Brown RC. The vanilloid receptor family of calcium-permeable channels: molecular integrators of microenvironmental stimuli. News Physiol Sci. 2003;18:226–231. doi: 10.1152/nips.01468.2003. [DOI] [PubMed] [Google Scholar]
- Oonuma H, Nakajima T, Nagata T, Iwasawa K, Wang Y, Hazama H, Morita Y, Yamamoto K, Nagai R, Omata M. Endothelin-1 is a potent activator of nonselective cation currents in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2000;23:213–221. doi: 10.1165/ajrcmb.23.2.3868. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim Y, Lee YH, Earm YE, Ho WK. Mechanosensitive cation channels in arterial smooth muscle cells are activated by diacylglycerol and inhibited by phospholipase C inhibitor. Circ Res. 2003;93:557–564. doi: 10.1161/01.RES.0000093204.25499.83. [DOI] [PubMed] [Google Scholar]
- Patterson RL, Van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, Kurosaki T, Snyder SH, Gill DL. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- Patterson RL, Van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- Poteser M, Wakabayashi I, Rosker C, Teubl M, Schindl R, Soldatov NM, Romanin C, Groschner K. Crosstalk between voltage-independent Ca2+ channels and L-type Ca2+ channels in A7r5 vascular smooth muscle cells at elevated intracellular pH: evidence for functional coupling between L-type Ca2+ channels and a 2-APB-sensitive cation channel. Circ Res. 2003;92:888–896. doi: 10.1161/01.RES.0000069216.80612.66. [DOI] [PubMed] [Google Scholar]
- Potocnik SJ, Hill MA. Pharmacological evidence for capacitative Ca2+ entry in cannulated and pressurized skeletal muscle arterioles. Br J Pharmacol. 2001;134:247–256. doi: 10.1038/sj.bjp.0704270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., JR A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet. 2003a;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- Qian Q, Li M, Cai Y, Ward CJ, Somlo S, Harris PC, Torres VE. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003b;14:2280–2287. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Samain E, Bouillier H, Perret C, Safar M, Dagher G. ANG II-induced Ca2+ increase in smooth muscle cells from SHR is regulated by actin and microtubule networks. Am J Physiol. 1999;277:H834–H841. doi: 10.1152/ajpheart.1999.277.2.H834. [DOI] [PubMed] [Google Scholar]
- Sanders KM. Invited review: mechanisms of calcium handling in smooth muscles. J Appl Physiol. 2001;91:1438–1449. doi: 10.1152/jappl.2001.91.3.1438. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Moff I, Sudanagunta SP, Levine JD. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. J Biol Chem. 2000;275:2756–2762. doi: 10.1074/jbc.275.4.2756. [DOI] [PubMed] [Google Scholar]
- Shi J, Li J, Ito Y, Inoue R. Glycolytic ATP production regulates muscarinic cation currents in guinea-pig ileum. J Smooth Muscle Res. 2003;39:21–29. doi: 10.1540/jsmr.39.21. [DOI] [PubMed] [Google Scholar]
- Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod. 2003;69:647–655. doi: 10.1095/biolreprod.103.015396. [DOI] [PubMed] [Google Scholar]
- Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Mol Cell. 2002;9:739–750. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- Sinkins WG, Goel M, Estacion M, Schilling WP. Association of immunophilins with mammalian TRPC channels. J Biol Chem. 2004;279:34521–34529. doi: 10.1074/jbc.M401156200. [DOI] [PubMed] [Google Scholar]
- Slish DF, Welsh DG, Brayden JE. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2196–H2201. doi: 10.1152/ajpheart.00605.2002. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Leno E, Csutora P, Trepakova ES, Bolotina VM. Ca2+-independent phospholipase A2 is a novel determinant of store-operated Ca2+ entry. J Biol Chem. 2003;278:11909–11915. doi: 10.1074/jbc.M210878200. [DOI] [PubMed] [Google Scholar]
- Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So I, Yang DK, Kim HJ, Min KW, Kang TM, Kim SJ, Kim KW, Park KH, Jeon JH, Choi KH, Kim IG. Five subtypes of muscarinic receptors are expressed in gastric smooth muscles of guinea pig. Exp Mol Med. 2003;35:46–52. doi: 10.1038/emm.2003.7. [DOI] [PubMed] [Google Scholar]
- Stephenson LA, Haney LB, Hussaini IM, Karns LR, Glass WF. Regulation of smooth muscle α-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998;54:1175–1187. doi: 10.1046/j.1523-1755.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Stepien O, Marche P. Amlodipine inhibits thapsigargin-sensitive Ca2+ stores in thrombin-stimulated vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;279:H1220–H1227. doi: 10.1152/ajpheart.2000.279.3.H1220. [DOI] [PubMed] [Google Scholar]
- Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Imai T, Igarashi T, Takayanagi K, Otsuka K, Yamaki F, Tanaka H, Shigenobu K. Comparison of the Ca2+ entry channels responsible for mechanical responses of guinea-pig aorta to noradrenaline and thapsigargin using SK & F 96365 and LOE 908. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:160–168. doi: 10.1007/s002100000272. [DOI] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- Tatlisumak T, Carano RA, Takano K, Meiler MR, Li F, Sotak CH, Arndts D, Pschorn U, Fisher M. Broad-spectrum cation channel inhibition by LOE 908 MS reduces infarct Volume in vivo and postmortem in focal cerebral ischemia in the rat. J Neurol Sci. 2000;178:107–113. doi: 10.1016/s0022-510x(00)00380-4. [DOI] [PubMed] [Google Scholar]
- Terasawa K, Nakajima T, Iida H, Iwasawa K, Oonuma H, Jo T, Morita T, Nakamura F, Fujimori Y, Toyo-Oka T, Nagai R. Nonselective cation currents regulate membrane potential of rabbit coronary arterial cell: modulation by lysophosphatidylcholine. Circulation. 2002;106:3111–3119. doi: 10.1161/01.cir.0000039345.00481.1d. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT. Properties of a tonically active, sodium-permeable current in mouse urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2004;286:C1246–C1257. doi: 10.1152/ajpcell.00501.2003. [DOI] [PubMed] [Google Scholar]
- Torres VE, Cai Y, Chen X, Wu GQ, Geng L, Cleghorn KA, Johnson CM, Somlo S. Vascular expression of polycystin-2. J Am Soc Nephrol. 2001;12:1–9. doi: 10.1681/ASN.V1211. [DOI] [PubMed] [Google Scholar]
- Tosun M, Paul RJ, Rapoport RM. Coupling of store-operated Ca2+ entry to contraction in rat aorta. J Pharmacol Exp Ther. 1998;285:759–766. [PubMed] [Google Scholar]
- Trepakova ES, Csutora P, Hunton DL, Marchase RB, Cohen RA, Bolotina VM. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. J Biol Chem. 2000;275:26158–26163. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci U S A. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L, Sampieri A. Calmodulin modulates the delay period between release of calcium from internal stores and activation of calcium influx via endogenous TRP1 channels. J Biol Chem. 2002;277:42178–42187. doi: 10.1074/jbc.M204531200. [DOI] [PubMed] [Google Scholar]
- Van Breemen C, Aaronson P, Loutzenhiser R. Sodium–calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978;30:167–208. [PubMed] [Google Scholar]
- Van Renterghem C, Lazdunski M. Identification of the Ca2+ current activated by vasoconstrictors in vascular smooth muscle cells. Pflugers Arch. 1994;429:1–6. doi: 10.1007/BF02584023. [DOI] [PubMed] [Google Scholar]
- Vandorpe DH, Chernova MN, Jiang L, Sellin LK, Wilhelm S, Stuart-Tilley AK, Walz G, Alper SL. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J Biol Chem. 2001;276:4093–4101. doi: 10.1074/jbc.M006252200. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Sanders KM. Cholinergic stimulation activates a non-selective cation current in canine pyloric circular muscle cells. J Physiol. 1990;429:223–236. doi: 10.1113/jphysiol.1990.sp018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron BJ, Reading S, Earley S, Brayden JE. TRPC3 channels mediate agonist-induced depolarization of cerebral artery smooth muscle cells. FASEB J. 2004 abstract 4688, Experimental Biology Meeting 2004. [Google Scholar]
- Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wallace P, Ayman S, McFadzean I, Gibson A. Thapsigargin-induced tone and capacitative calcium influx in mouse anococcygeus smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:368–375. doi: 10.1007/s002109900100. [DOI] [PubMed] [Google Scholar]
- Walter M, Tepel M, Nofer JR, Neusser M, Assmann G, Zidek W. Involvement of phospholipase D in store-operated calcium influx in vascular smooth muscle cells. FEBS Lett. 2000;479:51–56. doi: 10.1016/s0014-5793(00)01880-9. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hogg RC, Large WA. A monovalent ion-selective cation current activated by noradrenaline in smooth muscle cells of rabbit ear artery. Pflugers Arch. 1993;423:28–33. doi: 10.1007/BF00374957. [DOI] [PubMed] [Google Scholar]
- Wang YX, Kotlikoff MI. Signalling pathway for histamine activation of non-selective cation channels in equine tracheal myocytes. J Physiol. 2000;523:131–138. doi: 10.1111/j.1469-7793.2000.t01-3-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Large WA. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Laurier LG, Sims SM, Preiksaitis HG. Enhanced capacitative calcium entry and TRPC channel gene expression in human LES smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1074–G1083. doi: 10.1152/ajpgi.00227.2002. [DOI] [PubMed] [Google Scholar]
- Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004;287:C357–364. doi: 10.1152/ajpcell.00068.2004. [DOI] [PubMed] [Google Scholar]
- Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L848–L858. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Wayman CP, Gibson A, McFadzean I. Depletion of either ryanodine- or IP3-sensitive calcium stores activates capacitative calcium entry in mouse anococcygeus smooth muscle cells. Pflugers Arch. 1998;435:231–239. doi: 10.1007/s004240050506. [DOI] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Inhibition by sodium nitroprusside of a calcium store depletion-activated non-selective cation current in smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996a;118:2001–2008. doi: 10.1111/j.1476-5381.1996.tb15636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996b;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, Wallace P, Gibson A, McFadzean I. Correlation between store-operated cation current and capacitative Ca2+ influx in smooth muscle cells from mouse anococcygeus. Eur J Pharmacol. 1999;376:325–329. doi: 10.1016/s0014-2999(99)00400-8. [DOI] [PubMed] [Google Scholar]
- Weirich J, Seiler L, Hug MJ, Fleckenstein-Grun G. Ca2+ entry into primary cultured pig coronary smooth muscle cells after previous store depletion by repetitive P2Y purinoceptor stimulation. Cell Calcium. 2001;29:359–367. doi: 10.1054/ceca.2001.0198. [DOI] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol. 1993a;466:213–227. [PMC free article] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Stretch-activated nonselective cation channels in urinary bladder myocytes: importance for pacemaker potentials and myogenic response. Exs. 1993b;66:93–99. doi: 10.1007/978-3-0348-7327-7_6. [DOI] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Stretch effects on whole-cell currents of guinea-pig urinary bladder myocytes. J Physiol. 1994;480:439–448. doi: 10.1113/jphysiol.1994.sp020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Brayden JE. Mechanisms of coronary artery depolarization by uridine triphosphate. Am J Physiol Heart Circ Physiol. 2001;280:H2545–H2553. doi: 10.1152/ajpheart.2001.280.6.H2545. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Nelson MT, Eckman DM, Brayden JE. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J Physiol. 2000;527:139–148. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Mason HS, Smith GD, Nicholson N, Johnston L, Janiak R, Hume JR. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol. 2002;543:917–931. doi: 10.1113/jphysiol.2002.021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]
- Wu SN, Lin PH, Hsieh KS, Liu YC, Chiang HT. Behavior of nonselective cation channels and large conductance Ca2+-activated K+ channels induced by dynamic changes in membrane stretch in cultured smooth muscle cells of human coronary artery. J Cardiovasc Electrophysiol. 2003;14:44–51. doi: 10.1046/j.1540-8167.2003.02040.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1751–H1761. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Beech DJ. Multiple trp genes expressed as mRNA and protein in rabbit and human arteries. J Physiol. 2001a;535:569. [Google Scholar]
- Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001b;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Xu SZ, McHugh D, Zeng F, Shah S, Munsch C, Sivaprasadarao A, Beech DJ. TRPC5 is a glycosylated protein that is expressed and associated with TRPC1 in human blood vessels. J Physiol. 2002;544:17P. [Google Scholar]
- Yan HD, Okamoto H, Unno T, Tsytsyura YD, Prestwich SA, Komori S, Zholos AV, Bolton TB. Effects of G-protein-specific antibodies and G beta gamma subunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br J Pharmacol. 2003;139:605–615. doi: 10.1038/sj.bjp.0705289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod. 2002;67:988–994. doi: 10.1095/biolreprod.102.004119. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zakharov SI, Mongayt DA, Cohen RA, Bolotina VM. Monovalent cation and L-type Ca2+ channels participate in calcium paradox-like phenomenon in rabbit aortic smooth muscle cells. J Physiol. 1999;514(1):71–81. doi: 10.1111/j.1469-7793.1999.071af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SI, Smani T, Leno E, MacIanskiene R, Mubagwa K, Bolotina VM. Monovalent cation (MC) current in cardiac and smooth muscle cells: regulation by intracellular Mg2+ and inhibition by polycations. Br J Pharmacol. 2003;138:234–244. doi: 10.1038/sj.bjp.0705074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Saffen D. Muscarinic acetylcholine receptor regulation of TRP6 Ca2+ channel isoforms. Molecular structures and functional characterization. J Biol Chem. 2001;276:13331–13339. doi: 10.1074/jbc.M008914200. [DOI] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. J Physiol. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. J Physiol. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. Effects of protons on muscarinic receptor cationic current in single visceral smooth muscle cells. Am J Physiol. 1997;272:G215–G223. doi: 10.1152/ajpgi.1997.272.2.G215. [DOI] [PubMed] [Google Scholar]
- Zholos AV, Tsytsyura YD, Gordienko DV, Tsvilovskyy VV, Bolton TB. Phospholipase C, but not InsP3 or DAG-dependent activation of the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br J Pharmacol. 2004;141:23–36. doi: 10.1038/sj.bjp.0705584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- Zitt C, Halaszovich CR, Luckhoff A. The TRP family of cation channels: probing and advancing the concepts on receptor-activated calcium entry. Prog Neurobiol. 2002;66:243–264. doi: 10.1016/s0301-0082(02)00002-3. [DOI] [PubMed] [Google Scholar]