Abstract
Neuromedin U (NMU) is a brain–gut peptide first isolated from the spinal cord. Recent studies on NMU and its receptors have suggested a role of NMU in sensory transmission. Here we report on the localization of NMU in sensory neurones, and the actions of NMU in the substantia gelatinosa (SG) and the deep layer of the dorsal horn (laminae III–V) in adult rat spinal cord slices using the patch-clamp technique. An immunohistochemical study revealed that NMU peptide was present in most of the dorsal root ganglion neurones. In the spinal cord, NMU-immunoreactive neurones were located in the deep layer (laminae III–V), but not in the SG. However, NMU-positive axon terminals were observed in the SG as well as the deep layer. Bath-applied NMU (10 μm) increased the frequency, but not amplitude, of miniature excitatory postsynaptic currents (mEPSCs) in the SG and deep layer neurones by 146 ± 14% (P < 0.01, n = 17) and 174 ± 21% (P < 0.01, n = 6), respectively, without inducing any postsynaptic membrane currents recorded in tetrodotoxin. On the other hand, NMU did not affect miniature inhibitory postsynaptic currents recorded in tetrodotoxin. These findings, taken together, suggest that NMU acts on the presynaptic terminals of the primary afferent fibres working as an autocrine/paracrine neuromodulator to increase mEPSC frequency of the SG and deep layer neurones. This may account for the spinal mechanisms of the NMU-induced hyperalgesia reported previously.
Neuromedin U (NMU), named for its potent contractile activity on the uterus, was first isolated from porcine spinal cord (Minamino et al. 1985) and later from brain, spinal cord, and intestine of other species (Honzawa et al. 1987). NMU is known to regulate not only smooth muscle contraction, but also ion transport in the gut (Brown & Quito, 1988), and adrenocortical function in the peripheral organs (Malendowicz et al. 1993). Although the peripheral actions of NMU are well understood, its central actions have only been described recently. Intracerebroventicular administration of NMU has been demonstrated to decrease food intake (Nakazato et al. 2000) and produce a stress response (Hanada et al. 2001). The recent discovery of two types of NMU receptors, NMU-R1 and NMU-R2, has promoted further investigations into its roles in the central nervous system (Howard et al. 2000).
A more recent study has shown that mRNAs for NMU-R1 and -R2 are expressed in the dorsal root ganglion (DRG) and the substantia gelatinosa (SG, laminae II), and that an intrathecal administration of NMU decreased both the mechanical threshold to von Frey hair stimulation and the withdrawal latency to a noxious thermal stimulus in rats (Yu et al. 2003). In addition, a systemic perfusion of NMU increased both background activity and noxious pinch-evoked responses of the dorsal horn neurones in adult mice (Cao et al. 2003), suggesting a role of NMU in nociception in the spinal cord.
The aim of the present study was to identify the cellular mechanisms underlying NMU-induced modulation of spinal nociceptive transmission. To address this issue, we first sought to identify the localization of peptide NMU in the sensory pathway, and then investigated the effects of NMU on synaptic transmission of the SG as well as laminae III–V neurones using the patch-clamp technique in rat spinal cord slices.
Methods
All experimental protocols were approved by committee of the Ethics on Animal Experiments, Kyushu University, and in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines.
Identification of NMU-containing neurones
Under deep anaesthesia with sodium pentobarbital (40 mg kg−1, i.p.), Wister rats (7–8 weeks old) were transcardially perfused with 4% paraformaldehyde. The tissue blocks of the spinal cord (L3–L6) and the DRG were removed for overnight post fixation, and then placed in 20% sucrose for 2 days at 4°C. Transverse slices (40 μm in thickness) were incubated overnight with rabbit anti-rat NMU antiserum (Nakahara et al. 2004) at 4°C, then with Alexa Fluor 488-labelled goat anti-rabbit IgG antibody (Molecular Probes, diluted 1: 200). Fluorescent images were observed with a laser scanning confocal microscope (Micro Radiance, Biorad, USA). Control slides stained with primary antibodies that had been treated with NMU peptide (1 μm) or with secondary antibodies only were examined in parallel.
Preparation of the spinal cord slice
The methods used to prepare a slice and to analyse synaptic transmission were similar to those described elsewhere (Yang et al. 2000). In brief, following anaesthesia of 6- to 7-week-old (200–270 g) Sprague-Dawley male rats with urethane (1.2 g kg−1, i.p.), lumbosacral laminectomy was performed, and then the spinal cord was excised. After removing the dura, arachnoid and pia mater, the spinal cord was mounted on a vibratome, and transverse slices (L3–L6 level, 500 μm thickness) were made. The spinal cord slice was then placed in a recording chamber and perfused continuously with Krebs solution (10–20 ml min−1) at 37 ± 1°C. The composition of Krebs solution was (in mm); 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose.
Blind whole-cell voltage-clamp technique
Whole-cell patch-clamp recordings were made from neurones located in the SG and deep layer of the dorsal horn with patch pipette electrodes having a resistance of 5–10 MΩ. The spontaneous excitatory and inhibitory postsynaptic currents (sEPSCs and sIPSCs) were recorded at holding potentials (VH) of −70 and 0 mV, respectively. The composition of the pipette solutions used for recording was (mm); potassium gluconate, 135; KCl, 5; CaCl2, 0.5; MgCl2, 2; EGTA, 5; Hepes, 5; and Mg-ATP, 5. Currents obtained with an Axopatch 200B (Axon Instruments, Foster City, CA, USA) were low pass-filtered at 5 kHz, recorded continuously on a pen recorder, and stored on a microcomputer for the analysis with pCLAMP v8.0 and AxoGraph 4.0 (Axon Instruments). The recorded neurones were identified as SG neurones by the depth from the surface of the spinal cord and in some instance further confirmed by intracellular injection of neurobiotin through a patch pipette (0.3% in electrode solution, Vector Laboratories). After fixation, transverse sections in 100 μm thickness were incubated overnight with 0.3% Triton X-100 containing streptavidine–Texas Red (diluted 1: 500, Jackson ImmunoResearch Laboratories) and FITC-labelled Isolectin B4 (IB4) (diluted 1: 200, Sigma). The sections were viewed and photographed with an LSM 510 laser scanning confocal microscope (Carl Zeiss, Oberkochen, German).
Drugs
Drugs used in this study were neuromedin U-23 (NMU-23; Peptide Institute, Japan) and tetrodotoxin (TTX; Wako, Japan). NMU was first dissolved in saline at a concentration of 100 μm and then diluted to the final concentration in external solution immediately before use.
Statistics
Average values are presented as mean ±s.e.m. Statistical significance was determined as P < 0.05. Student's paired t test was used for statistics except for analysis of cumulative distribution by Kolmogorov-Smirnov's test, and the dose dependency by one-way analysis of variance (ANOVA) followed by Scheffe's test.
Results
NMU in sensory pathway
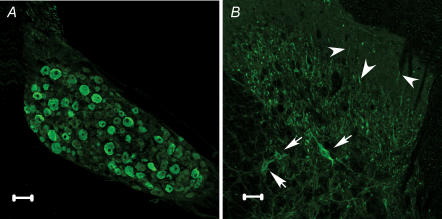
As shown in Fig. 1A, most of the DRG neurones were NMU immunoreactive, although large- and medium-sized neurones seemed to have stronger staining than small ones. Within the spinal cord, NMU-immunoreactive neurones (arrows) were seen in the deep layer (laminae III–V), but not in the SG. In addition, axon terminals in the SG and deep layer (arrowheads) were also labelled (Fig. 1B). Control slices demonstrated no immunoreactivity except for background staining (not shown).
Figure 1. NMU immunoreactivity in the DRG and the dorsal horn of the spinal cord.
A, NMU immunoreactivity is observed in small- to large-sized DRG neurones. B, NMU-immunoreactive neurones (arrows) are located in the deep layer of the dorsal horn (laminae III–V), but not in the SG. NMU-positive axon terminals are densely observed in the deep layer and sparsely in the SG (arrowheads). Scale bars, 50 μm.
Effects of NMU on SG neurones
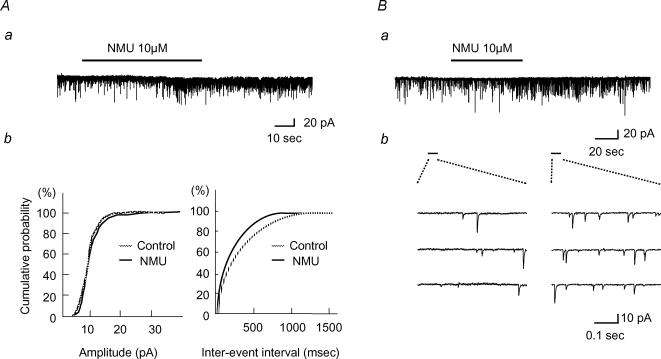
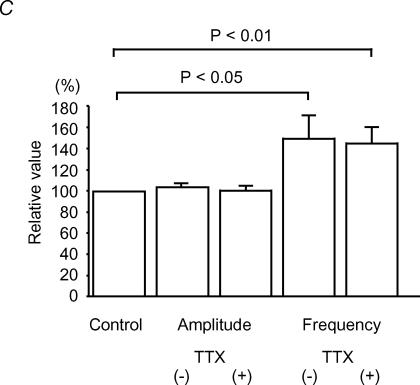
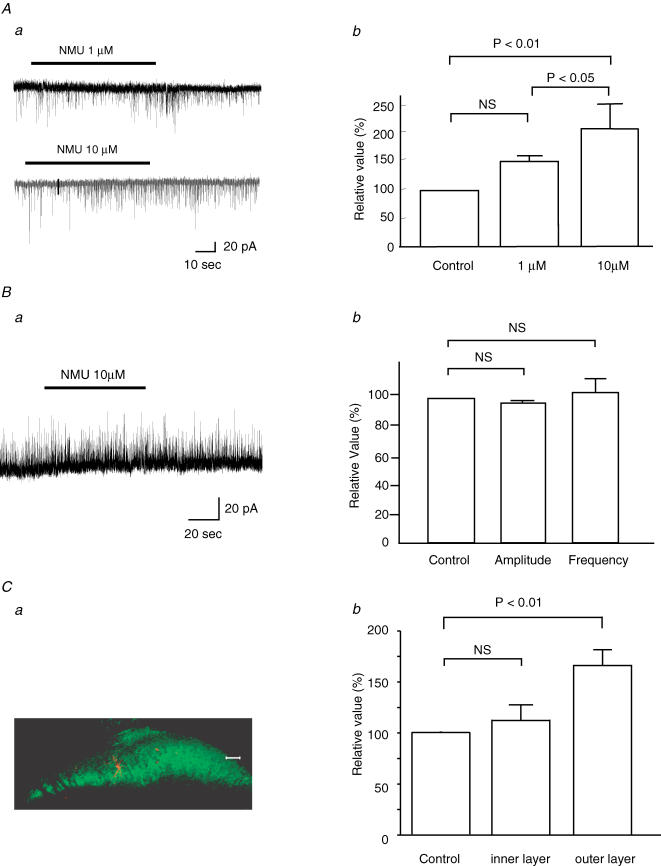
The SG was easily identified as a translucent band across the dorsal horn under a binocular microscope with light transmitted from below. All neurones tested had membrane potentials more negative than −55 mV. As shown in Fig. 2Aa, bath application of NMU (10 μm, 60 s) enhanced the frequency, but not the amplitude, of sEPSCs in the SG neurones without inducing any postsynaptic membrane currents at −70 mV. When estimated for 30 s immediately after the end of NMU application, the frequency and amplitude were 150 ± 20% (paired t test, P < 0.01, n = 23) and 103 ± 4.0% (P > 0.05, n = 23) of the controls (9.5 ± 1.2 Hz and 14.7 ± 1.0 pA), respectively, which were sampled for 30 s before NMU application. Figure 2Ab shows the effects of NMU on the cumulative distributions of the interevent interval and amplitude of sEPSCs. While NMU shifted the distribution of the interevent interval to the left (Kolmogorov-Smirnov's test, P < 0.05), it had no consistent effect on the amplitude distribution (P > 0.05). When NMU was perfused in the presence of TTX (1 μm), the frequency of miniature EPSCs (mEPSCs) also increased to 146 ± 14% (P < 0.01, n = 17) of the controls (11.4 ± 2.0 Hz), as seen in sEPSCs at −70 mV (Fig. 2B). No significant changes were observed in the amplitude of mEPSCs (100 ± 3%, n = 17, P > 0.05) of the controls (15.4 ± 1.3 pA) (Fig. 2C). When present at concentrations of 1 and 10 μm, NMU increased mEPSC frequency dose-dependently (Fig. 3Aa and b). On the other hand, mIPSCs recorded from the SG neurones were not affected by NMU (10 μm): frequency was 102 ± 8% (P > 0.05, n = 6) of the control value measured before NMU (15.7 ± 2.3 Hz), and amplitude was 94 ± 4% (P > 0.05, n = 6) of control (13.1 ± 1.3 pA) (Fig. 3B).
Figure 2. Effects of NMU on EPSCs of the SG neurones.
Aa, continuous chart recording shows an increase in the frequency of spontaneous EPSCs (sEPSCs) induced by bath application of NMU (10 μm, bar). Ab, cumulative distributions of the amplitude (left; P > 0.05, Kolmogorov-Smirnov's test) and the interevent interval (right; P ^lt; 0.01) of sEPSCs in the absence (chequered line) and presence (continuous line) of NMU. Aa and b were obtained from the same neurones. Ba, continuous chart recording in the presence of TTX indicates an increase in mEPSC frequency by NMU (10 μm, bar). Bb, consecutive traces of mEPSCs before NMU (left) and after NMU (right). C, summary of effects of NMU on amplitude and frequency of EPSCs. Control value was obtained before NMU application and each bar is expressed relative to the control. TTX(−), n = 23; TTX(+), n = 16. Student's paired t test. VH=−70 mV.
Figure 3. Dose-dependent NMU effects on mEPSCs and lack of effects on mIPSCs, and difference in the effects on mEPSCs between inner and outer layer neurones.
Aa, continuous chart recording of mEPSCs at concentrations of 1 (upper) and 10 μm (lower) NMU. These were obtained from the same neurone. Ab, normalized frequency of mEPSCs at 1 μm and 10 μm NMU (n = 3). ANOVA followed by Scheffe's post hoc test. Ba, continuous chart recording indicates no change in mIPSC following bath application of NMU (10 μm, bar). Bb, summary of effects of NMU on the amplitude and frequency of mIPSCs (n = 6). Student's paired t test. VH= 0 mV. Ca, fluorescent immunohistochemical labelling of a neurone with neurobiotin (red) and the inner layer of lamina II with IB4 (green). Scale bar, 50 μm. Cb, neurones in the outer layer, but not in the inner layer, show an increase in mEPSC frequency (n = 11).
Difference in the effects of NMU between neurones in outer and inner layers of SG
The SG neurones were further divided into two groups, those in the inner and outer layers of the SG, based on the IB4-positive terminals in the inner layer of the SG. An example of a neurone located in the inner layer of the SG is shown in Fig. 3Ca. In the outer layer of the SG, NMU (10 μm) increased sEPSC frequency to 166 ± 17% (P < 0.01, n = 11) of the control value (9.6 ± 1.6 Hz). Although the application of NMU (10 μm) also produced a slight increase in sEPSC frequency of the inner layer neurones (113 ± 15%, P > 0.05, n = 11) compared with the controls (13.4 ± 2.3 Hz), it was not statistically significant (Fig. 3Cb), suggesting that the NMU-induced increase in the frequency of the sEPSC was mainly confined to the outer layer neurones.
Effects of NMU on deep layer neurones
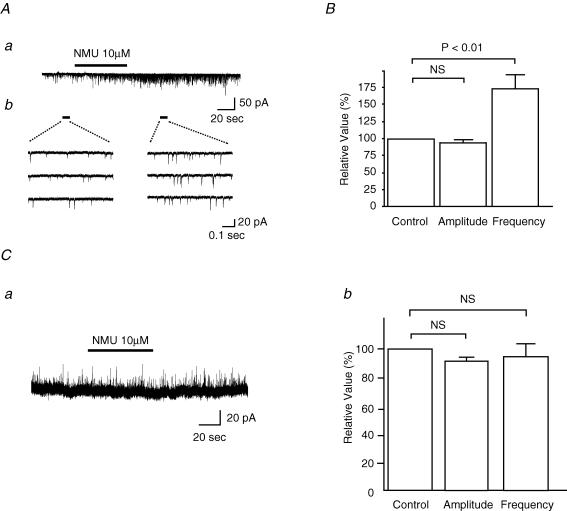
As observed in the SG, application of NMU at 10 μm for 60 s also enhanced the frequency of mEPSCs to 174 ± 21% (P < 0.01, n = 6) of the controls (9.9 ± 4.7 Hz) at −70 mV in the deep layer of the dorsal horn (laminae III–V). No significant change was observed in the amplitude of mEPSCs (94 ± 5% of the controls (21.2 ± 4.2 pA), P > 0.05, n = 6) (Fig. 4A and B). NMU (10 μm) did not affect the mIPSC frequency (95 ± 12% of the controls (15.8 ± 5.3 Hz), P > 0.05, n = 6) or amplitude (93 ± 3% of the controls (11.5 ± 0.9 pA), P > 0.05, n = 6) of the neurones in the deep layer of the dorsal horn (Fig. 4C).
Figure 4. Effects of NMU on mEPSCs and mIPSCs of the deep layer neurones.
Aa, continuous chart recording shows an increase in the frequency of mEPSCs induced by bath application of NMU (10 μm, bar). Ab, consecutive traces of mEPSCs before NMU (left) and after NMU (right). B, summary of effects of NMU on the amplitude and frequency of mEPSCs (n = 6). Student's paired t test. VH=−70 mV. Ca, continuous chart recording indicates no change in mIPSC following bath application of NMU (10 μm, bar). Cb, summary of effects of NMU on the amplitude and frequency of mIPSCs (n = 6). Student's paired t test. VH= 0 mV.
Discussion
The present study demonstrated that NMU dose-dependently enhances the frequency, but not the amplitude, of mEPSCs of the SG and deep layer (laminae III–V) neurones in the spinal dorsal horn, due to its direct action on the presynaptic terminals. Cao et al. (2003) reported that NMU-23 increased the background activity of nociceptive specific and wild dynamic range neurones in the dorsal horn in mice. The present finding that NMU increased the frequency of mEPSCs might well explain the increase in the spontaneous firing rate of the dorsal horn neurones following administration of NMU.On the other hand, mIPSCs were not affected by NMU application.
It has been shown that NMU-R1 mRNA is expressed in small- and medium-sized DRG neurones (Yu et al. 2003). Since [125I]NMU-23 binding sites are detected in lamina I and the outer layer of the SG, but not in the DRG (Yu et al. 2003), it is thought that NMU-R1 protein is expressed in the presynaptic terminals of the primary afferent fibres. Our electrophysiological results suggesting that NMU acted on the presynaptic terminals to increase mEPSC frequency were thus compatible with the morphological studies. The finding that NMU was more effective in the outer layer of the SG than in the inner layer (Fig. 3B) was also consistent with the result of [I]-NMU23 receptor autoradiography (Yu et al. 2003). Since a strong expression of NMU is observed in the DRG neurones and axons in the spinal cord (Fig. 1), it is possible that one of the origins of NMU in the spinal cord is the primary afferent nerves. If this is the case, NMU may act on the nerve terminals in an autocrine and/or paracrine fashion to increase mEPSC frequency. However, it is also possible that NMU is derived from neurones in the spinal cord, e.g. NMU-positive deep layer neurones (Fig. 1B). Furthermore, since NMU-R2 mRNA has been shown to be expressed in neurones in lamina I and the SG (Yu et al. 2003), the possibility that NMU acts on the presynaptic terminals of interneurones cannot be completely excluded.
In addition to the SG neurones, those in the deep layer of the dorsal horn also demonstrated an increase in EPSC frequency after application of NMU (Fig. 4), although NMU-R mRNAs were not detected in the deep layer by in situ hybridization analysis (Yu et al. 2003). Since the density of neurones in the deep layer is low compared with the SG, NMU-R transcript labelling by in situ hybridization as well as [I]-NMU23 binding may be too weak to be detected. Alternatively, since neurones in the deep layer are known to extend their dendrites to the SG and form axo-dendritic synapses with the terminal of C-fibres (Ribeiro Da Silva & Coimbra, 1982), it is possible that an increase in EPSC frequency recorded in the deep layer neurones is attributed to the action of NMU on the terminals of the NMU-R positive C-fibre in the SG.
It is thought that the action of NMU on presynaptic terminals, leading to an increase in mEPSC frequency, contributes to an increase in excitability of the postsynaptic neurones in the dorsal horn, thereby inducing thermal and mechanical hyperalgesia after intrathecal administration of NMU in rats (Yu et al. 2003). The present study suggests that NMU may play a role in nociceptive transmission in the spinal cord, working presynaptically as an autocrine/paracrine neuromodulator. Among various neuropeptides known to be present in the DRG and the dorsal horn of the spinal cord (Todd & Spike, 1993), NMU seems to be unique with regard to its mechanism of action.
Acknowledgments
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), Brain Science Foundation, the Naito Foundation, the Mitsubishi Foundation, Grants-in Aid for Scientific Research (15029247, 16390271 and 15300135) to H.F., M.K and M.Y., and by 21st Century COE program to M.Y. from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government.
References
- Brown DR, Quito FL. Neuromedin U octapeptide alters ion transport in porcine jejunum. Eur J Pharmacol. 1988;155:159–162. doi: 10.1016/0014-2999(88)90415-3. [DOI] [PubMed] [Google Scholar]
- Cao CQ, Yu XH, Dray A, Filosa A, Perkins MN. A pro-nociceptive role of neuromedin U in adult mice. Pain. 2003;104:609–616. doi: 10.1016/S0304-3959(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Murakami N, Sakihara S, Yoshimatsu H, Toshinai K, et al. A role for neuromedin U in stress response. Biochem Biophys Res Commun. 2001;289:225–228. doi: 10.1006/bbrc.2001.5945. [DOI] [PubMed] [Google Scholar]
- Honzawa M, Sudoh T, Minamino N, Tohyama M, Atsuo H. Topographic localization of neuromedin U-like structures in the rat brain: an immunohistochemical study. Neuroscience. 1987;3:1103–1122. doi: 10.1016/0306-4522(87)90185-0. [DOI] [PubMed] [Google Scholar]
- Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406:70–74. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Nussdorfer GG, Nowak KW, Mazzocchi G. Effect of neuromedin U-8 on the rat pituitary adrenocortical axis. In Vivo. 1993;7:419–422. [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Matsuo H. Neuromedin U-8 and U-25: novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem Biophys Res Commun. 1985;130:1078–1085. doi: 10.1016/0006-291x(85)91726-7. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hanada R, Murakami N, Teranishi H, Ohgusu H, Fukushima N, et al. The gut-brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem Biophys Res Commun. 2004;318:156–161. doi: 10.1016/j.bbrc.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Hanada R, Murakami N, Date Y, Mondal MS, Kojima M, et al. Central effects of neuromedin U in the regulation of energy homeostasis. Biochem Biophys Res Commun. 2000;277:191–194. doi: 10.1006/bbrc.2000.3669. [DOI] [PubMed] [Google Scholar]
- Ribeiro Da Silva A, Coimbra A. Two types of synaptic glomeruli and their distribution in laminae I–III of the rat spinal cord. J Comp Neurol. 1982;209:176–186. doi: 10.1002/cne.902090205. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I–III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Hurue H, Li YQ, Yoshimura M. Capsaicin induces a slow inward current that is not mediated by substance P in substantia gelatinosa neurons of the rat spinal cord. Neuropharmacology. 2000;39:2185–2194. doi: 10.1016/s0028-3908(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Yu XH, Cao CQ, Mennicken F, Puma C, Dray A, O'Donnell D, et al. Pro-nociceptive effects of neuromedin U in rat. Neuroscience. 2003;120:467–474. doi: 10.1016/s0306-4522(03)00300-2. [DOI] [PubMed] [Google Scholar]