Abstract
The effects of cholinergic drugs on the quantal contents of the nerve-evoked endplate currents (EPCs) and the parameters of the time course of quantal release (minimal synaptic latency, main modal value of latency histogram and variability of synaptic latencies) were studied at proximal, central and distal regions of the frog neuromuscular synapse. Acetylcholine (ACh, 5 × 10−4 m), carbachol (CCh, 1 × 10−5 m) or nicotine (5 × 10−6 m) increased the numbers of EPCs with long release latencies mainly in the distal region of the endplate (90–120 μm from the last node of Ranvier), where the synchronization of transmitter release was the most pronounced. The parameters of focally recorded motor nerve action potentials were not changed by either ACh or CCh. The effects of CCh and nicotine on quantal dispersion were reduced substantially by 5 × 10−7 m (+)tubocurarine (TC). The muscarinic agonists, oxotremorine and the propargyl ester of arecaidine, as well as antagonists such as pirenzepine, AF-DX 116 and methoctramine, alone or in combination, did not affect the dispersion of the release. Muscarinic antagonists did not block the dispersion action of CCh. Cholinergic drugs either decreased the quantal content mo (muscarinic agonist, oxotremorine M, and nicotinic antagonist, TC), or decreased mo and dispersed the release (ACh, CCh and nicotine). The effects on mo were not related either to the endplate region or to the initial level of release dispersion. It follows that the mechanisms regulating the amount and the time course of transmitter release are different and that, among other factors, they are altered by presynaptic nicotinic receptors.
Different receptors located on the presynaptic membrane may modulate transmitter release (Ciani & Edwards, 1963; Miyamoto, 1977; Steinbach & Stevens, 1979; Starke, 1989; Bowman et al. 1990; Miller, 1998; MacDermott et al. 1999). One intriguing problem is the action of acetylcholine (ACh) released from cholinergic nerve endings on the presynaptic autoreceptors. A study of the effects of various nicotinic and muscarinic drugs might reveal the mechanisms of this modulation and its possible physiological significance. The ability of cholinergic drugs to change the quantal content of the evoked endplate current (EPC) has been shown by many investigators (Ciani & Edwards, 1963; Nikolsky & Giniatullin, 1979; Dunant & Walker, 1982; Vizi & Somogyi, 1989; Doležal & Tuèek, 1993; Re et al. 1993; Van der Kloot et al. 1997; Slutsky et al. 1999; Minic et al. 2002; Santafe et al. 2003). However, it has been demonstrated experimentally that the EPC amplitude is also sensitive to changes in the time course of quantal release due to dispersion of the synaptic latencies of individual quanta forming the EPC (Katz & Miledi, 1965; Magazanik & Minenko, 1986; Minenko & Magazanik, 1986). This has also been analysed by modelling studies (Soucek, 1971; Giniatullin et al. 1995). Recently, cholinergic drugs have been shown to affect not only the quantal content but also the time course of quanta release. In our preliminary studies both ACh and its non-hydrolysable analogue carbachol (CCh) decreased the quantal content and increased the dispersion of the uniquantal EPC synaptic delays. The pronounced dispersion of the quantal release resulted in a decrease of the multiquantal EPC amplitude (Nikolsky et al. 1995; Samigullin et al. 2003a). ACh and CCh activate both nicotinic and muscarinic cholinoreceptors. Therefore the type of receptor involved in their presynaptic effects remains to be determined. Moreover, it is not clear whether the same type of receptor is involved in the regulation of the quantal content and in the time course of the secretion. Slutsky et al. (2001) found out that the muscarinic antagonist methoctramine and exogenous acetylcholinesterase not only increased the quantal content but also slowed the exponential decay of the synaptic delay histograms. It was suggested that presynaptic muscarinic receptors of MR2 type are involved in the process determining the time course of transmitter secretion.
It is noteworthy that the distributions of the synaptic delays of quantal release, which reflect the kinetics of quantal secretion, in the proximal and distal regions of the frog motor nerve terminal are different (Bukharaeva et al. 2002; Nikolsky et al. 2002; Samigullin et al. 2003b). In the proximal region the dispersion of quantal release is more pronounced than in the distal region. The actions of drugs may depend on the initial degree of synchronization of quantal release. It has been found that noradrenaline synchronizes the release in the proximal regions where the release is originally the most dispersed, but is much less effective in the distal regions which are characterized by synchronous release (Bukharaeva et al. 2002). Thus the frog endplate offers the unique possibility of comparing the effects of drugs in regions of the same synapse where the initial degree of quanta release dispersion may differ. This motivated us to study the action of ACh, CCh and several more selective nicotinic and muscarinic drugs on the basic parameters of synaptic activity (presynaptic nerve conduction velocity, quantal content, amplitude and shape of uniquantal postsynaptic currents, time course of quantal release) in the proximal, central and distal regions of the frog endplate.
Evidence is presented that under the experimental conditions used the presynaptic actions of ACh and CCh are mediated predominantly by nicotinic receptors and consist of a decrease of quantal content and a slowing of the kinetics of release. However, we did not observe effects of muscarinic agonists and antagonists on the time course of transmitter release.
Methods
Animals and drugs
Experiments were performed on isolated m. cutaneous pectoris neuromuscular preparations from the frog Rana ridibunda during the winter period (October–March). Animals were anaesthetized with ether before being stunned and pithed in accordance with the European Communities Council Directive (24th November 1986; 86/609/EEC). The preparations were pinned to the bottom of a 3.5 ml translucent chamber, and superfused with the following solution (mm): NaCl 113.0, KCl 2.5, CaCl2 0.4, NaHCO3 3.0, MgCl2 4.0. pH was adjusted to 7.3. The solution flowed through the muscle chamber at the rate of 5 ml min−1. Monitoring of the bath solution throughout the experiment did not reveal any changes in pH after passing through the muscle chamber. The temperature was controlled by a Peltier semiconductor device. The experiments were performed at 20.0 ± 0.3°C.
The following drugs were used (all from Sigma, St Louis, MO, USA): acetylcholine chloride, carbachol chloride, oxotremorine M, propargyl ester of arecaidine (APET), pirenzepine, methoctramine (+)tubocurarine and nicotine. AF-DX 116 (11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H[2,3-b] [1,4]benzo-diazepine-6-one) was from Tocris Cookson (Bristol, UK). The drugs were added to the superfusing solution. The measurements were started 20 min after drug application unless otherwise stated. In most cases, the drugs were washed out for another 30 min and the EPCs were recorded again. Acetylcholine (ACh) and carbachol (CCh) were added to the superfusing solutions in concentrations of 5 × 10−4 and 1 × 10−5 m. The optimal concentrations that were still effective but did not desensitize the postsynaptic receptors below the recognition threshold during the EPC recording were found in preliminary tests to range from 1 × 10−7 to 1 × 10−3 m.
Electrophysiology
Suprathreshold stimuli of 0.1 ms duration were applied to the nerves at 2 s intervals via a pair of platinum electrodes located in a small adjacent moist chamber. This arrangement minimized the stimulus artifact. Nerve action potentials and extracellular endplate currents were recorded using three focal Ringer solution-filled extracellular pipettes with tip diameters of 2–3 μm and 1–3 MΩ resistance. Extracellular pipettes were positioned under visual control (amplification × 256) in the proximal (P), central (C) and distal (D) regions of a long nerve terminal, 3–5 μm, 40–50 μm and 90–120 μm from the end of the myelinated segments of the axon, respectively. In these regions, three-component nerve action potentials (NAPs) were recorded simultaneously (Mallart, 1984; Shakiryanova et al. 1994). The velocities of NAP conduction from P to C and from C to D were measured. The recorded signals were filtered between 0.03 Hz and 10 kHz, digitized at 3 μs intervals by an analog–digital 9 bit converter, fed into the computer and processed by our application program package for amplitudes, rise times (between 20 and 80% of maximum amplitude) and the time constants (τ) of the exponential decay of the EPC. The amplitudes of the extracellular responses are expressed in millivolts. Each of three electrodes positioned in P, C and D regions recorded EPCs only in the immediate vicinity of the tip, and neighbouring electrodes never recorded the same signal simultaneously.
Estimating the time courses of the release of individual quanta from the value of the dispersions of the synaptic delays required knowing the uniquantal endplate currents (Katz & Miledi, 1965; Barrett & Stevens, 1972). Therefore, experiments were carried out in the presence of 0.4 mm Ca2+ and 4.0 mm Mg2+. The quantal contents (mo) of the low-quanta EPC were determined by measuring the EPCs during five or six stimulation periods (256 stimuli each) in the presence and absence of various drugs. The numbers of failures in the series of 250–400 uniquantal responses were measured and mo was calculated as equal to ln(N/no), where N is the total number of stimuli and no is the number of failures (Del Castillo & Katz, 1954; Martin, 1955). The numbers of stimuli were from 1280 to 1536 in each experiment.
Latency measurements
Latencies were measured as the time intervals between the peaks of the inward presynaptic Na+ currents and the times at which the rising phases of the quantal event reached 20% of maximum (Katz & Miledi, 1965; Bukcharaeva et al. 1999). Because the amplitudes of the Na+ currents decrease along the nerve terminal (Mallart, 1984), the distal recording sites were selected so that the Na+ peaks, from which the latencies were measured, were clearly seen on the averaged records from this part of the nerve terminal. The limit of the latency measurement was 6 ms. The stability of the recording electrode position next to the membrane region studied was crucial during long-lasting extracellular recordings. Therefore, we monitored the amplitudes of the NAP throughout each data set. Only experiments in which NAP changed by less than 10% during the drug application and washout were analysed (Bukcharaeva et al. 1999). Statistical analyses of pre- and postsynaptic events were performed using Student's t test for paired data.
Latency histograms of the selected uniquantal EPCs were constructed (for details, see Bukcharaeva et al. 1999). The quantitative characteristics of the time courses of evoked secretion can be obtained by cumulative curve analysis (Van der Kloot, 1991). The mean values of the shortest 5% of latencies in each series were taken as the minimal synaptic delay. For comparison of dispersion histograms in different regions of the terminal, the minimal synaptic delay was subtracted before the latency histograms were constructed. The intervals between the minimal synaptic delay and the time at which 90% of all measured uniquantal EPCs had occurred were designated as P90. The statistical significance of the differences between two cumulative curves was assessed by the Kolmogorov-Smirnov statistic; P < 0.05 was taken as significant (Bronstein & Semendjaev, 1986; Van der Kloot, 1991). The main modal values of the latency histogram were used as another parameter. They were corrected, i.e. mean values of the minimal synaptic delay were subtracted from each synaptic delay of a particular group of recordings. Statistical tests were performed with SigmaStat 0.1 (Jandel Corporation, 1992–1994). Analyses of variance of the experimental groups versus the control groups were performed by multiple comparisons using the ANOVA Bonferroni t test. The amplitudes, rise times of EPCs (from 20 to 80% of the maximal amplitude) and exponential decay constants τ of the responses are presented as means ± s.e.m. Differences between two groups were considered statistically significant at the probability level P = 0.05. The symbol n indicates the number of endplates measured in each group.
Results
The absence of effects of ACh and CCh on the motor nerve action potential and velocity of conduction along the nerve terminal
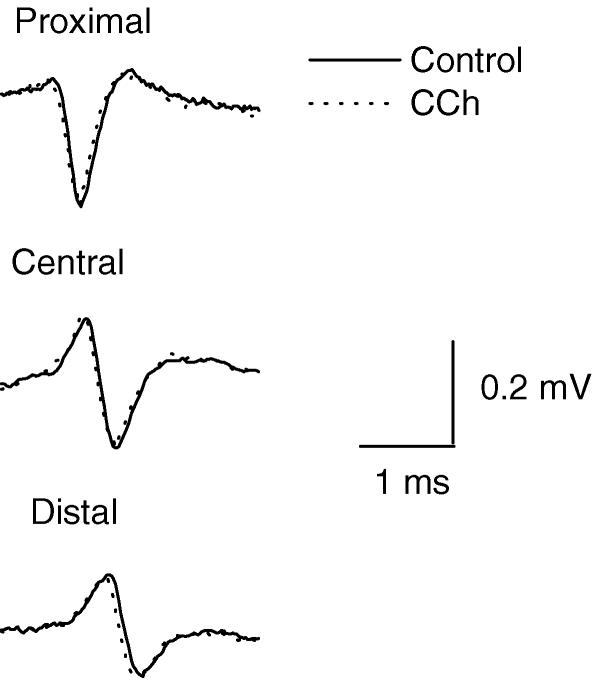
The main negative phase of the nerve action potentials (NAPs) recorded by each of three extracellular microelectrodes placed on the proximal (P), central (C) and distal (D) regions of endplate represents the initial inward Na+ current. Its amplitude diminished peripherally along the nerve ending (Mallart, 1984; Shakiryanova et al. 1994) (Fig. 1). Neither CCh (Fig. 1, Table 1) nor ACh changed the amplitude or time course of the NAP including the amplitude of its sodium current component. This indicates that there was no substantial depolarization of the presynaptic membrane by ACh or CCh (Giniatullin et al. 2002). The conduction velocities of the NAPs in the proximal (P–C) and distal (C–D) regions of the synapse were significantly different as reported earlier (Samigullin et al. 2003b) and not changed by 1 × 10−5 m CCh. The conduction velocities were 0.34 ± 0.02 m s−1 before and 0.33 ± 0.03 m s−1 after CCh in P–C and 0.27 ± 0.03 m s−1 and 0.26 ± 0.04 m s−1 in C–D regions. The same results were obtained in the presence of 5 × 10−4 ACh (data not shown).
Figure 1.
Presynaptic nerve action potentials (NAPs) recorded extracellularly from the proximal, central and distal regions of a synapse before (Control, continuous line) and in the presence of 1 × 10−5 m carbachol (CCh, dashed line). Selected NAPs not followed by the endplate currents (failures) were superimposed (35 in each case). The amplitudes of the three-phase nerve spike decreased along the nerve ending but were not affected by CCh.
Table 1.
Amplitude (mV) of the downward Na+ component of the nerve action potential in proximal, central and distal regions of the nerve terminal before and after 1 × 10−5 m carbachol (CCh) application
| Proximal | Central | Distal | |
|---|---|---|---|
| Control | 0.21 ± 0.02 | 0.13 ± 0.01 | 0.07 ± 0.01 |
| CCh | 0.19 ± 0.03 | 0.15 ± 0.03 | 0.08 ± 0.03 |
Values are means ± s.e.m. of 6 measurements, P > 0.05.
Effects of ACh and CCh on parameters of uniquantal EPC
The control uniquantal EPC amplitudes, rise times and decay time constants (τ) recorded in P, C and D regions of synapse were the same (Table 2). After 30 min in the presence of ACh or CCh the amplitudes of the uniquantal EPCs were decreased and the time courses shortened. These effects of each drug were quite similar in all regions of the synapse (Table 2). There were only small differences in the postsynaptic actions of ACh and CCh: ACh, unlike CCh, did not influence the rise time of the EPC (Table 2). In general the data indicate that the quantum sizes and receptor densities were identical in all regions of the frog neuromuscular synapse and that there was no fundamental difference between the ACh and CCh-induced changes in the action of a single quantum.
Table 2.
Amplitude, rise time and decay time constant (τ) of uniquantal endplate currents (EPCs) in proximal (P), central (C) and distal (D) regions of the neuromuscular junction in control solution and after application of 5 × 10−4 m acetylcholine (ACh) and 1 × 10−5 m carbachol (CCh)
| Region of synapse | EPC amplitude (mV) | EPC rise time (ms) | EPC τ (ms) | |
|---|---|---|---|---|
| Control | P | 0.36 ± 0.09 | 0.13 ± 0.01 | 1.23 ± 0.09 |
| (n = 14) | C | 0.35 ± 0.10 | 0.12 ± 0.01 | 1.33 ± 0.10 |
| D | 0.37 ± 0.08 | 0.14 ± 0.03 | 1.24 ± 0.08 | |
| ACh | P | 0.29 ± 0.06* | 0.13 ± 0.01 | 1.08 ± 0.09* |
| (n = 5) | C | 0.28 ± 0.09* | 0.11 ± 0.01 | 1.09 ± 0.07* |
| D | 0.27 ± 0.07* | 0.13 ± 0.02 | 0.90 ± 0.06* | |
| CCh | P | 0.28 ± 0.05* | 0.15 ± 0.01* | 0.95 ± 0.09* |
| (n = 6) | C | 0.24 ± 0.07* | 0.15 ± 0.01* | 1.02 ± 0.08* |
| D | 0.23 ± 0.08* | 0.18 ± 0.02* | 0.93 ± 0.09* |
Values are means ± s.e.m.
Significant difference relative to control (P < 0.05).
Dispersion of synaptic delay and effects of ACh and CCh
The time course of quantal release is manifested by the variability of the synaptic delay, i.e. the time between the depolarization of the nerve ending and the generation of the EPC (Katz & Miledi, 1965; Minenko & Magazanik, 1986). In agreement with data obtained earlier (Bukharaeva et al. 2002; Samigullin et al. 2003a,b), the synaptic delays of the uniquantal responses were different in parts P, C and D (Fig. 2, control records). The results of quantitative analysis of the time courses of release are presented in synaptic delay histograms (Fig. 3, left) and the cumulative plots (Fig. 3, right). The minimal synaptic delays in regions P, C and D differed significantly (0.54 ± 0.04 ms, 0.43 ± 0.04 ms and 0.34 ± 0.03 ms, respectively, P < 0.05, n = 14). To compare the dispersion histograms in different regions of the terminal, the minimal synaptic delay was subtracted from the individual synaptic delays of each recorded uniquantal EPC before the latency histograms were constructed. The main modal values of these corrected histograms were similar in P, C and D (0.22 ± 0.06 ms, 0.18 ± 0.07 ms and 0.19 ± 0.05 ms, P > 0.05, n = 14 each). The histograms were asymmetrical due to the large numbers of long synaptic delays in all regions of the ending but their proportion decreased from proximal to distal regions of the synapse. A comparison of the delay dispersions, expressed as P90, corrected for minimal synaptic delay (Fig. 2, right, open circles) showed it to be the greatest in the proximal region P (0.88 ± 0.06 ms) and to decrease to 0.67 ± 0.04 ms in C and to 0.50 ± 0.05 ms (57% of the proximal P-region) in D; all the differences were significant at P < 0.05 (n = 14). These observations demonstrate that the quantal release in the distal region of the nerve terminal was highly synchronized.
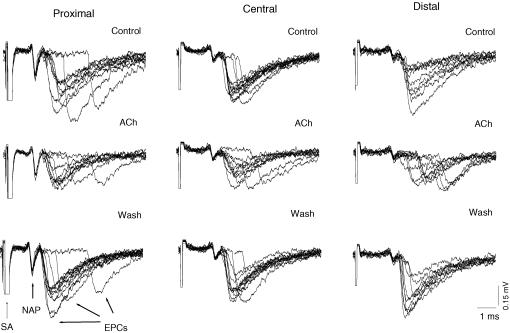
Figure 2.
Latencies of quantal release in proximal, central and distal regions of the synapse before (Control), in the presence of 5 × 10−4 m acetylcholine (ACh) and 60 min after washout (Wash). Recordings (9–11) were superimposed, showing extracellularly recorded presynaptic nerve action potentials (NAPs), individual endplate currents (EPCs) and stimulus artifacts (SA). The time intervals between the peak of the inward presynaptic Na+ currents of the nerve spike (downward deflection) and the times at which the rising phases of each EPC reached 20% of maximum was defined as the release latency. Note the most pronounced increase in latency dispersion in the distal region induced by ACh.
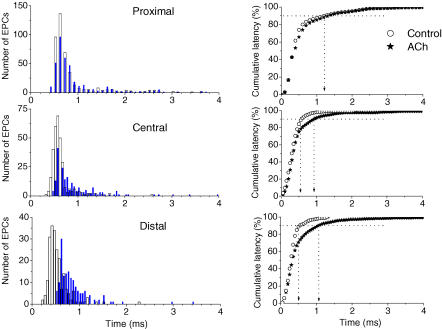
Figure 3.
Latency histograms and cumulative plots of latencies of the uniquantal EPCs recorded simultaneously by three microelectrodes located in proximal, central and distal regions of the same endplate before ACh (Control: open circles and columns) and after ACh application (filled columns and asterisks). Left, non-corrected latency histograms (values of minimal synaptic latencies were not subtracted) of the uniquantal EPCs (data from a single experiment). The bin size was 0.05 ms. Right, the cumulative plots of latencies for the same data, corrected for minimal synaptic delays. The vertical dotted lines indicate the times when 90% of the quanta have been released (P90) in the proximal, central and distal regions.
The application of ACh increased the number of long-latency EPCs (Fig. 2, middle row) and shifted the histograms of the synaptic latencies to the right (Fig. 3, left), but the changes differed along the synapse. In fact, no parameters, i.e. minimal synaptic delay, main modal value of the histogram or P90, were affected in region P, where the dispersion was originally high, but all were increased in region D (Fig. 4). The minimal synaptic latency, the main modal values of the histograms corrected by subtracting the minimal synaptic delay and the corrected value of P90 (after subtraction of the minimal synaptic delay) were significantly changed in the presence of ACh only in region D (P < 0.05, n = 5). All of the changes in the release dispersion observed were reversible and eliminated by ACh washout. On the other hand, the changes of P90 in region C were marginal and absent in region P which was characterized, as already mentioned, by the most dispersed release (Figs 3 and 4).
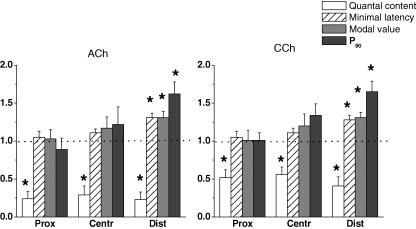
Figure 4.
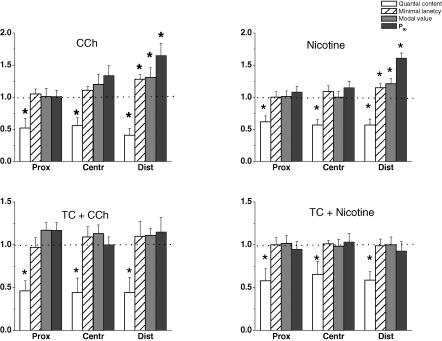
Effects of 5 × 10−4 m acetylcholine (ACh) and 1 × 10−5 m carbachol (CCh) on the quantal contents, minimal latencies, mean modal values of the latency histograms and the P90 parameter in the proximal, central and distal regions of the synapse. The values (means ± s.e.m.) from 5–6 endplates are normalized to the controls before application of the drugs (1.0). Asterisks indicate statistically significant differences at P < 0.05.
CCh prolonged the minimal synaptic delays, increased the main modal values of the latency histogram and the parameter P90. The minimal synaptic delay and main modal value of the latency histogram increased from 0.34 ± 0.03 ms to 0.43 ± 0.04 ms and from 0.19 ± 0.03 ms to 0.25 ± 0.05 ms (n = 6 each, P < 0.05) (Fig. 4, region D). The control value of corrected P90 was 0.50 ± 0.05 ms and it was increased significantly to 0.82 ± 0.04 ms (n = 6, P < 0.05) in the presence of 1 × 10−5 m CCh. On the other hand, the changes in region C were marginal and those in region P were not significant just as in the presence of ACh. The effects of CCh were also completely reversible.
Effects on EPC quantal content (mo)
A proximo-distal gradient of mo was observed in simultaneous recordings by three extracellular microelectrodes under identical conditions. mo was maximal in the proximal (P) region (0.92 ± 0.09), smaller in the central (C) (0.75 ± 0.11) and was the smallest (0.51 ± 0.12) in the distal (D) region. The presence of 5 × 10−4 m ACh or 1 × 10−5 m CCh reduced mo in all regions in a similar way. This indicates that the sensitivities of all regions of the terminal to ACh as well as to CCh were similar, but in the distal region of synapse the decrease of quantal content was accompanied by the greater release dispersion (Fig. 4).
Nicotinic regulation of quantal release
Since ACh and CCh can activate both muscarinic and nicotinic types of cholinoreceptors, experiments were performed with selective nicotinic drugs.
Nicotine
We found that the specific agonist, nicotine, closely mimicked the presynaptic effects of ACh and CCh on mo, and the kinetics of release. Nicotine, 5 × 10−6 m decreased mo (Fig. 5). The amplitudes, time courses and conduction velocities of the presynaptic spike were not changed by nicotine. The postsynaptic EPCs were affected similarly as in the presence of ACh or CCh. The EPC amplitudes were diminished in all regions to 80% and the EPC decay time constants were shortened to 70% of the control. In region D, nicotine significantly increased the minimal synaptic delay, the main modal value of the corrected synaptic delay histogram and P90 similarly to the dispersive actions of ACh and CCh (cf. Figs 4 and 5). The effects of nicotine on the time course of transmitter release in regions C and P were insignificant.
Figure 5.
Effects of 1 × 10−5 m carbachol (CCh) and 5 × 10−6 m nicotine before (upper row) and after (lower row) application of 5 × 10−7 m (+)tubocurarine (TC) on the quantal contents, minimal latencies, mean modal values of the latency histograms and the P90 parameter in proximal, central and distal regions of the synapse. Values (means ± s.e.m.) from 5–7 endplates were normalized to controls before application of the drugs (1.0). Asterisks indicate statistically significant differences at P < 0.05.
(+)Tubocurarine (TC)
In the presence of 5 × 10−7 m TC the amplitudes of the uniquantal EPC were reduced to 70% and the time constants of decay were shortened to 70% of the controls in all regions of the synapse, due to competition of TC with ACh for the postsynaptic receptors and its ability to block the open channels of the receptor (Beránek & Vyskočil, 1967, 1968; Katz & Miledi, 1978; Mallart & Molgo, 1978; Colquhoun et al. 1979; Shaker et al. 1982). Concomitantly 5 × 10−7 m TC decreased mo to 55% as described earlier (Matzner et al. 1988). The decrease of quantal content was observed in all regions of the synapse without any effect on the presynaptic nerve spike. In contrast to mo, TC did not affect the time course of release; the minimal synaptic delays, the main modal values of the histogram and P90 were unchanged in all regions. After pretreatment of the muscles with 5 × 10−7 m TC for 40 min, 1 × 10−5 m CCh was added. As expected, the postsynaptic effects of CCh, i.e. decrease of amplitude and decay time constant of uniquantal responses, were abolished by TC (data not shown). However, TC also effectively depressed the presynaptic action of CCh on the time course of transmitter release, in particular at the distal region of the endplate (Fig. 5). The minimal synaptic delay, mean modal value of the histogram and parameter P90 were not significantly different (P > 0.05) from the control values. A tendency to increase these parameters after CCh application in the presence of TC was observed in 2 out of 5 experiments. There was no way to increase the TC concentration further due to the progressive fall in unitary EPC amplitude. Unexpectedly, despite the presence of TC, CCh was still able to decrease the quantal content: mo, initially decreased to 50% by TC, was decreased further to 26–34% of control by CCh (1 × 10−5 m) before application of both drugs. This effect was observed in all regions of the endplate. Thus the effects of CCh and TC on quantal content were found to be additive. Similar results were obtained in another series of experiments where nicotine was used instead of CCh. Nicotine (5 × 10−6 m) was applied to the neuromuscular preparation treated for 40 min with 5 × 10−7 m TC. TC effectively blocked the effects of nicotine on minimal synaptic latency, the main modal value of latency histogram and P90 at the distal region (Fig. 5), but, again, the prominent fall of mo was present in all regions of the endplate.
Muscarinic regulation of quantal release
It was recently reported that M1R and M2R receptors could affect quantal release at the frog endplate. Activation of M1R receptors by muscarine facilitated the multiquantal release whereas activation of M2R inhibited it (Slutsky et al. 1999). M2R receptors were also considered to regulate the time course of the release (Slutsky et al. 2001). To reveal the types of cholinergic receptors mediating the action of CCh on evoked uniquantal secretion, the effects of several muscarinic drugs on secretion dispersion in the absence and presence of CCh were compared. The following experiments were done only with CCh as there were no principal differences between the effects of ACh and CCh.
Oxotremorine M
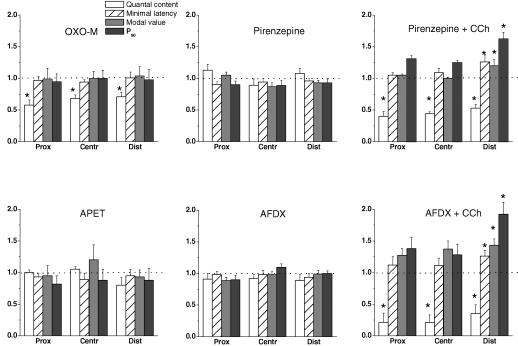
Oxotremorine M is generally considered to be a muscarinic receptor agonist with some preference for the M2 subtype (Caulfield, 1993). The absence of nicotinic postsynaptic effects was evident as there were no significant changes of the amplitudes and time courses of the uniquantal EPC when this drug was applied at a concentration of 1 × 10−6 m. Similar to ACh and CCh, oxotremorine M had no effect on nerve conduction velocity or on the shape and size of the presynaptic spike indicating no substantial changes of Na+ currents (data not shown). On the other hand, it decreased the quantum content, mo, by 30–40% in all regions of the endplate (Fig. 6). But in contrast to ACh and CCh, oxotremorine M did not change the distribution of synaptic delay in any region of the endplate when applied in the concentrations of 1 × 10−6, 1 × 10−5 and 5 × 10−5 m, respectively.
Figure 6.
Effects of 1 × 10−6 m oxotremorine M (OXO-M), 1 × 10−6 m propargyl ester of arecaidine (APET), 1 × 10−5 m pirenzepine alone and in combination with 1 × 10−5 m carbachol (CCh), 5 × 10−6 m AF-DX 116 (AFDX) alone and in combination with 1 × 10−5 m carbachol on the quantal contents, minimal latencies, mean modal values of the latency histograms and the P90 parameter in proximal, central and distal regions of the synapse. Values (means ± s.e.m.) from 4–6 endplates were normalized to controls before application of the drugs (1.0). Asterisks indicate the statistically significant differences at P < 0.05.
Propargyl ester of arecaidine (APET)
APET is another muscarinic agonist with a slight preference for the M1R receptor over the M2R receptor (Barlow & Weston-Smith, 1985; Moser, 1989). At concentrations of 1 × 10−6, 1 × 10−5 and 5 × 10−5 m this drug failed to change the time course of release and – in contrast to CCh and oxotremorine M – it did not depress the quantum content of the EPC (Fig. 6). The amplitude and time course of the postsynaptic currents were also unchanged.
Pirenzepine and AF-DX 116
Pirenzepine is a highly specific antagonist of the M1R muscarinic receptors. In the present experiments, 1 × 10−5 m pirenzepine did not affect the nerve conduction velocity, shape and size of the presynaptic spike as well as the uniquantal EPC parameters. The minimal synaptic delay was also not changed. We did not find any effect of the drug on the quantum content, mo, unlike the report of Slutsky et al. (1999). Application of CCh to muscle preparation pretreated for 30–60 min with 1 × 10−5 m pirenzepine induced the same changes of parameters of the time course of release and uniquantal EPCs as when CCh was applied alone (Fig. 6). In the distal regions of the endplate, pirenzepine did not alter the effect of CCh on minimal synaptic delay and the parameter P90, both of which were still increased (P < 0.05, n = 6, for each) (Fig. 6).
AF-DX 116 is considered to be an M2R-specific antagonist (Eglen et al. 1996). Similar to the M1R antagonist pirenzepine, 5 × 10−6 m AF-DX 116 also had no significant pre- or postsynaptic effects and did not eliminate the actions of CCh on quantal content, minimal synaptic delay and P90 in the distal region (Fig. 6) or on the amplitude and time course of the EPC.
Methoctramine
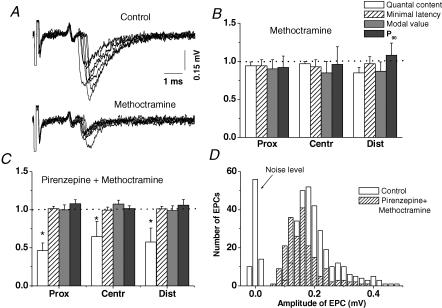
The M2R/M4R inhibitor methoctramine was tested in view of recent reports about its potency to increase mo 2.5 times and to prolong the synaptic delay of release at the frog neuromuscular junction pretreated with pirenzepine (Slutsky et al. 2001). In our experiments 5 × 10−6 m methoctramine did not increase mo (Fig. 7). The time course of release, the minimal synaptic delay, the modal value of the histogram and the parameter P90 were close to the controls in all regions of the synapse (Fig. 7B). As reported by Slutsky et al. (2001) there were significant postsynaptic effects (Fig. 7A). Several minutes after application of the drug, the uniquantal EPC declined in all regions of the synapse, and within 20 min the amplitudes were stable at 69 ± 5%, 77 ± 6% and 67 ± 7% of the control (P < 0.05, n = 6 for each) in regions P, C and D. The rise times of these EPCs were shortened on average to 87% of the control in all regions of the synapse (P < 0.05, n = 6). The decreases of the EPC decay time constant were also similar along the synapse (51–58% of the control values, P < 0.05). The slight shortening of the rise time and the pronounced effects on the decay time constant of uniquantal EPCs indicate that methoctramine may act as an open channel blocker. A high affinity of methoctramine to the open channel of the nicotinic cholinoreceptor has been described (Bixel et al. 2000).
Figure 7.
Effects of 5 × 10−6 m methoctramine on pre- and postsynaptic responses. A, seven superimposed extracellular recordings from the distal region of a single endplate before (Control) and after application of methoctramine. B, the normalized (to the control = 1.0) values (means ± s.e.m.) from 6 endplates of quantal contents, minimal latencies, mean modal values of the latency histograms and the P90 parameter in proximal, central and distal regions of the synapse in the presence of methoctramine. C, same as in B but in the presence of a combination of 1 × 10−5 m pirenzepine with 5 × 10−6 m methoctramine. D, histograms of uniquantal postsynaptic current amplitudes in control and in the presence of a combination of methoctramine and pirenzepine.
Pirenzepine and methoctramine
In view of the account of Slutsky et al. (2001) that methoctramine increased the quantal content and prolonged the time course of secretion when the M1R receptors were inhibited by pirenzepine, we tested the combination of methoctramine and pirenzepine. After 50 min perfusion with 1 × 10−5 m pirenzepine, 5 × 10−6 m methoctramine was added. There were no changes in the time course of transmitter release, similar to the presence of pirenzepine alone (Fig. 7C). However, the combination of pirenzepine plus methoctramine significantly reduced the EPC quantal content, a phenomenon that was not manifested when the drugs were applied separately (cf. Figs 6 and 7B). This could not be a result of the reduced resolution of the estimation of the quantal content because the postsynaptic inhibition induced by the combination of methoctramine and pirenzepine did not lead to the loss of the smallest uniquantal responses (Fig. 7D).
The level of decrease of mo did not differ significantly along the endplate, and the combined application of pirenzepine and methoctramine did not affect the time course of transmitter release either (Fig. 7C).
Discussion
The main result of the present study is the finding of the dispersive effects of ACh, CCh and nicotine on the time course of transmitter release at the neuromuscular junction. These drugs are able to increase reversibly the minimal synaptic delay of single quanta and to shift the main modal value of the synaptic delay histogram to the right due to a higher number of long-latency quanta. Thus both drugs could decrease the synaptic transmission safety by making the release of quanta less synchronous. This effect evidently requires the activation of presynaptic nicotinic receptors since it was induced not only by ACh and CCh, but also by nicotine. It could be blocked or diminished by the selective nicotinic antagonist (+)tubocurarine, and not by the muscarinic antagonists pirenzepine and AF-DX 116. Another noteworthy finding was a positive correlation between the degree of synchronization of transmitter release at a particular region of the long frog endplate and the effectiveness of cholinergic agonists to increase the release latencies. Of major importance is the demonstrated independence of two cholinergic effects – one on the release dispersion and the other on the release probability, i.e. quantal number.
It has been shown in the experiments performed on different preparations that an increase in action potential duration can lengthen the synaptic delay (Benoit & Mambrini, 1970; Datyner & Gage, 1980; Sabatini & Regehr, 1999; Taschenberger & von Gersdorff, 2000; Lin & Faber, 2002). Moreover, the value of the synaptic latency depends on the waveform of the imposed presynaptic stimulus distinguishing between the voltage step and the nerve action potential (Lin & Faber, 2002). In the present study only conventional nerve stimulation was used and ACh or CCh did not affect either the amplitude or the time course of the focally recorded presynaptic currents. Therefore an influence of these cholinergic drugs on the generation of the presynaptic spike can be excluded as a source of the alterations in release dispersion.
Although the involvement of presynaptic nicotinic receptors in the dispersive effect was obvious (nicotine action), it was necessary to check the involvement of presynaptic muscarinic receptors as well, because Slutsky et al. (2001) showed that the activation of frog presynaptic MR2 by endogenous ACh leads to synchronization of transmitter release as long as the MR2 antagonist, methoctramine, induces the opposite action. The muscarinic agonists (oxotremorine M and APET) and antagonists (pirenzepine, AF-DX 116 and methoctramine) were therefore tested. Surprisingly, we found no changes of the time course of release either with agonists or antagonists in contrast to data published recently (Slutsky et al. 2001; Santafe et al. 2003, 2004). Nevertheless the inhibitory effect of oxotremorine M on EPC quantal content was reproduced in accord with earlier observations (Michaelson et al. 1979; Abbs & Joseph, 1981; Dunant & Walker, 1982). The discrepancy between the published data and results presented here may be due mainly to the difference in the conditions required for the preferential effects of ACh on nicotinic (present study) or muscarinic (Slutsky et al. 1999, 2001) presynaptic autoreceptors. The effects of muscarinic agonist and antagonist drugs on transmitter release from frog or mice motor nerve terminals depend strongly on voltage (Slutsky et al. 1999, 2001, 2003), on the stage of endplate maturation (Santafe et al. 2003, 2004) and on the level of acetylcholinesterase expression (Minic et al. 2002, 2003). These data taken together are evidence that the conditions for reliable manifestation of muscarinic presynaptic effects are restricted, especially those on the time course of transmitter release. During physiological patterns of stimulation, the activation of muscarinic autoreceptors is apparently insufficient to modify release.
It has been suggested that different mechanisms may control the amounts and the time courses of transmitter release (Bukcharaeva et al. 1999; Parnas & Parnas, 1999; Zucker, 1999). There are examples of simultaneous actions on both processes as well (Miledi, 1966; Slutsky et al. 2001; Samigullin et al. 2003a). However, many experimental manoeuvres that are known to change the quantal contents of the postsynaptic responses do not affect the release latencies (Andreu & Barrett, 1980; Van der Kloot, 1988; Hochner et al. 1991; Samigullin et al. 2003b). For instance, noradrenaline shortens the time course of ACh release from the frog motor nerve endings but does not affect the quantal content of release (Bukcharaeva et al. 1999). Cholinergic drugs studied in the present work either decreased quantal content (mo) only (muscarinic agonist, oxotremorine M, and nicotinic antagonist, TC), or decreased mo and prolonged the release time (ACh, CCh and nicotine). The effects of TC and cholinergic agonists on the quantal content were synergistic but were antagonistic on the synchronization of release; TC effectively prevented the latency prolongation provoked by CCh and nicotine.
In the case of the nicotinic agonist action, the parallelism in the effects on the amount and time course of release was observed only in the distal region of the endplate where release is the most synchronized. In the proximal and central regions the dispersive actions of ACh, CCh and nicotine were virtually absent, although mo of the simultaneously recorded postsynaptic currents was decreased in the same way as in the distal region. This confirms not only the relative dissociation between mechanisms controlling the intensity and kinetics of transmitter release but also clearly indicates that the modulation of the release synchronization depends greatly on its basal level. Modulation is very pronounced at low levels of quantal release as shown by the present study. However, as the ACh release increases, CCh acquires the ability not only to decrease the amount of release but also to prolong the duration of the mean synaptic delay by acting at the proximal region of the frog endplate. In addition, this prolongation is still more pronounced at the distal region where synchronization was initially high (Giniatullin et al. 2002; Nikolsky et al. 2002). The enhancement of release prompts its synchronization and creates more favourable conditions for the effects of nicotinic agonists. The assumption is in accordance with recent findings at the nerve terminals of the calyx of Held that the time difference between the peak Ca2+ current and the peak of transmitter release becomes progressively shorter as the amplitude of the Ca2+ current increases. It has been suggested that the time course of the phasic transmitter release is not completely invariant to changes of release probability (Felmy et al. 2003).
The frog endplate provides the unique opportunity for analysing the mechanisms involved in the number of quanta released and their time dispersion with the help of pharmacological tools (nicotinic agonists, noradrenaline) that act selectively on synaptic regions that have different quantal release characteristics.
Cholinergic modulation of presynaptic mechanisms is hardly significant for most vertebrate endplates where the safety margin is high (Wood & Slater, 2001). However, during extreme physiological states or under pathological conditions, during the development of synaptic transmission (Chuhma & Ohmori, 2002) or hibernation periods (Moravec et al. 1973), this kind of modulation should be taken into account, especially in the junctions where the probability of transmitter release is low. The physiological significance of feedback mechanisms could be more prominent in central cholinergic synapses, and it is possible that the receptor and molecular mechanisms of modulation in peripheral and central cholinergic synapses are similar.
Acknowledgments
Thanks are due to Dr Charles Edwards, Dr Clarke Slater and an anonymous referee for valuable suggestions. This work was supported by GAAVA5011411, GACR305/02/1333 and MSMT11310003 to F.V., RFBR 02-04-48901, Sci. Schools of Russia 1063.2003.4 to E.E.N., by the ‘Russian Science Support Foundation’ and MK-2153.2003.04. to D.S. and RFBR 02-04-49737, Sci. Schools of Russia 2222.2003.4 to L.G.M.
References
- Abbs ET, Joseph DN. The effects of atropine and oxotremorine on acetylcholine release in rat phrenic nerve-diaphragm preparations. Br J Pharmacol. 1981;73:481–483. doi: 10.1111/j.1476-5381.1981.tb10446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu R, Barrett EF. Calcium dependence of evoked transmitter release at very low quantal content at the frog neuromuscular junction. J Physiol. 1980;308:79–97. doi: 10.1113/jphysiol.1980.sp013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Weston-Smith P. The relative potencies of some agonists at M2 muscarinic receptors in guinea pig ileum, atria and bronchi. Br J Pharmacol. 1985;85:437–440. doi: 10.1111/j.1476-5381.1985.tb08879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Stevens CF. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972;227:691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P, Mambrini J. Modification of transmitter release by ions which prolong the presynaptic action. J Physiol. 1970;210:681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R, Vyskočil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967;188:53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R, Vyskočil F. The effect of atropine on the frog sartorius neuromuscular junction. J Physiol. 1968;195:493–503. doi: 10.1113/jphysiol.1968.sp008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixel MG, Krauss M, Liu Y, Bolognesi ML, Rosini M, Mellor IS, et al. Structure-activity relationship and site of binding of polyamine derivatives at the nicotinic acetylcholine receptor. Eur J Biochem. 2000;267:110–120. doi: 10.1046/j.1432-1327.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- Bowman WC, Prior C, Marshall I. Presynaptic receptors in the neuromuscular junction. Ann NY Acad Sci. 1990;604:69–81. doi: 10.1111/j.1749-6632.1990.tb31983.x. [DOI] [PubMed] [Google Scholar]
- Bronstein IN, Semendjaev KA. Handbook of Mathematics. Moscow: Science Publishing House; 1986. [Google Scholar]
- Bukcharaeva E, Kim K, Moravec J, Nikolsky E, Vyskočil F. Noradrenaline synchronizes evoked quantal release at frog neuromuscular junctions. J Physiol. 1999;517:879–888. doi: 10.1111/j.1469-7793.1999.0879s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukharaeva E, Samigullin D, Nikolsky E, Vyskočil F. Protein kinase A cascade regulates quantal release dispersion at frog muscle endplate. J Physiol. 2002;538:837–848. doi: 10.1113/jphysiol.2001.012752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors – characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Ohmori H. Role of Ca2+ in the synchronization of transmitter release at calyceal synapses in the auditory system of rat. J Neurophysiol. 2002;87:222–228. doi: 10.1152/jn.00235.2001. [DOI] [PubMed] [Google Scholar]
- Ciani S, Edwards C. The effect of acetylcholine on neuromuscular transmission in the frog. J Pharmacol Exp Ther. 1963;142:21–23. [PubMed] [Google Scholar]
- Colquhoun D, Dreyer F, Sheridan RE. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N, Gage P. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954;124:574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doležal V, Tuček S. Presynaptic muscarinic receptors and the release of acetylcholine from cerebrocortical prisms: role of Ca2+ and K+ concentrations. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:228–233. doi: 10.1007/BF00169149. [DOI] [PubMed] [Google Scholar]
- Dunant Y, Walker AI. Cholinergic inhibition of acetylcholine release in the electric organ of Torpedo. Eur J Pharmacol. 1982;78:201–212. doi: 10.1016/0014-2999(82)90237-0. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. The timing of phasic transmitter release is Ca2+-dependent and lacks a direct influence of presynaptic membrane potential. Proc Natl Acad Sci U S A. 2003;100:15200–15205. doi: 10.1073/pnas.2433276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Kheeroug LS, Vyskočil F. Modelling endplate current: dependence on quantum secretion probability and postsynaptic miniature current parameters. Eur Biophys J. 1995;23:443–446. doi: 10.1007/BF00196832. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Samigullin D, Grishin S, Bukharaeva E. The effects of carbachol on the proximal and distal parts of frog motor nerve endings. Neurosci Behav Physiol. 2002;32:589–593. doi: 10.1023/a:1020401509774. [DOI] [PubMed] [Google Scholar]
- Hochner B, Parnas H, Parnas I. Effects of intra-axonal injection of Ca2+ buffers on evoked release and on facilitation in the crayfish neuromuscular junction. Neurosci Lett. 1991;125:215–218. doi: 10.1016/0304-3940(91)90032-o. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction. Proc R Soc Lond B Biol Sci. 1965;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978;203:119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Lin JW, Faber DS. Modulation of synaptic delay during synaptic plasticity. Trends Neurosci. 2002;25:449–455. doi: 10.1016/s0166-2236(02)02212-9. [DOI] [PubMed] [Google Scholar]
- MacDermott A, Role L, Siegelbaum S. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Magazanik LG, Minenko ML. Polymodal distribution of synaptic delays in the frog neuro-muscular junction. Neurophysiology. 1986;18:748–763. [PubMed] [Google Scholar]
- Mallart A. Presynaptic currents in frog motor endings. Pflugers Arch. 1984;400:8–20. doi: 10.1007/BF00670529. [DOI] [PubMed] [Google Scholar]
- Mallart A, Molgo J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition of the endplate potential. J Physiol. 1955;130:114–112. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner H, Parnas H, Parnas I. Presynaptic effects of d-tubocurarine on neurotransmitter release at the neuromuscular junction of the frog. J Physiol. 1988;398:109–121. doi: 10.1113/jphysiol.1988.sp017032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson DM, Avissar S, Kloog Y, Sokolovsky M. Mechanism of acetylcholine release: possible involvement of presynaptic muscarinic receptors in regulation of acetylcholine release and protein phosphorylation. Proc Natl Acad Sci U S A. 1979;76:6336–6340. doi: 10.1073/pnas.76.12.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Strontium as a substitute for calcium in the process of transmitter release at the neuromuscular junction. Nature. 1966;212:1233–1234. doi: 10.1038/2121233a0. [DOI] [PubMed] [Google Scholar]
- Miller R. Presynaptic receptors. Ann Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Minenko ML, Magazanik LG. Phenomena of asynchronous evoked transmitter release at the neuromuscular junction of the frog. Neurophysiology. 1986;18:346–354. [PubMed] [Google Scholar]
- Minic J, Chatonnet A, Krejci E, Molgo J. Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions. Br J Pharmacol. 2003;138:177–187. doi: 10.1038/sj.bjp.0705010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minic J, Molgo J, Karlson T, Krejci E. Regulation of acetylcholine release by muscarinic receptors at mouse neuromuscular junction depends on the activity of acetylcholinesterase. Eur J Neurosci. 2002;15:439–448. doi: 10.1046/j.0953-816x.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. The actions of cholinergic drugs on motor nerve terminals. Pharmacol Rev. 1977;29:221–247. [PubMed] [Google Scholar]
- Moravec J, Melichar I, Jansky L, Vyskočil F. Effect of hibernation and noradrenaline on the resting state of neuromuscular junction of golden hamster (Mesocricetus auratus) Pflugers Arch. 1973;345:93–106. doi: 10.1007/BF00585833. [DOI] [PubMed] [Google Scholar]
- Moser J. Structure-activity relationships of new analogues of arecaidine propargyl ester at muscarinic M1 and M2 receptor subtypes. Br J Pharmacol. 1989;96:319–324. doi: 10.1111/j.1476-5381.1989.tb11820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolsky E, Bukharaeva E, Kovyazina I, Gainulov R. The synchrony of the postsynaptic membrane activation by mediator quanta at the frog synapses is disrupted by carbachol. Ninth International Symposium on Cholinergic Mechanisms, Mainz. 1995:24. [Google Scholar]
- Nikolsky EE, Bukharaeva EA, Samigullin DV, Gainulov R. Characteristics of the time course of evoked secretion of transmitter quanta in different parts of the motor nerve ending in the frog. Neurosci Behav Physiol. 2002;32:265–274. doi: 10.1023/a:1015010307181. [DOI] [PubMed] [Google Scholar]
- Nikolsky E, Giniatullin R. Inhibition of presynaptic effect of carbachol by d-tubocurarine. Bull Eksp Biol Med. 1979;2:171–174. (in Russian) [PubMed] [Google Scholar]
- Parnas I, Parnas H. Different mechanisms control the amount and time course of neurotransmitter release. J Physiol. 1999;517:629. doi: 10.1111/j.1469-7793.1999.0629s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re L, Cola G, Fulgenzi F, Marinelli C, Concettoni C, Rossini L. Muscarinic modulation of neurotransmission: the effects of some agonists and antagonists. Gen Pharmacol. 1993;24:1447–1453. doi: 10.1016/0306-3623(93)90433-x. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of synaptic transmission. Annu Rev Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- Samigullin D, Bukharaeva EA, Nikolsky E, Adámek S, Vyskočil F. Long release latencies are increased by acetylcholine at frog endplate. Physiol Res. 2003a;52:475–480. [PubMed] [Google Scholar]
- Samigullin D, Bukharaeva EA, Nikolsky E, Vyskočil F. Temperature effect on proximal to distal gradient of quantal release of acetylcholine at frog endplate. Neurochem Res. 2003b;28:507–514. doi: 10.1023/a:1022817205814. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Salon I, Garcia N, Lanuza MA, Uchitel OD, Tomas J. Modulation of ACh release by presynaptic muscarinic autoreceptors in the neuromuscular junction of the newborn and adult rat. Eur J Neurosci. 2003;17:1–9. doi: 10.1046/j.1460-9568.2003.02428.x. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Salon I, Garcia N, Lanuza MA, Uchitel OD, Tomas J. Muscarinic autoreceptors related with calcium channels in the strong and weak inputs at polyinnervated developing rat neuromuscular junctions. Neuroscience. 2004;123:61–73. doi: 10.1016/j.neuroscience.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Shaker N, Eldefrawi AT, Aguayo LG, Warnick JE, Albuquerque EX, Eldefrawi ME. Interactions of d-tubocurarine with the nicotinic acetylcholine receptor/channel molecule. J Pharm Exp Ther. 1982;220:172–177. [PubMed] [Google Scholar]
- Shakiryanova DM, Zefirov AL, Nikolsky EE, Vyskočil F. The effect of acetylcholine and related drugs on currents at the frog motor nerve terminal. Eur J Pharmacol. 1994;263:107–114. doi: 10.1016/0014-2999(94)90530-4. [DOI] [PubMed] [Google Scholar]
- Slutsky I, Parnas H, Parnas I. Presynaptic effects of muscarine on ACh release at the frog neuromuscular junction. J Physiol. 1999;514:769–782. doi: 10.1111/j.1469-7793.1999.769ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky I, Silman I, Parnas I, Parnas H. Presynaptic M2 muscarinic receptors are involved in controlling the kinetics of ACh release at the frog neuromuscular junction. J Physiol. 2001;536:717–725. doi: 10.1111/j.1469-7793.2001.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky I, Wess J, Gomeza J, Dudel J, Parnas I, Parnas H. Use of knockout mice reveals involvement of M2-muscarinic receptors in control kinetics of acetylcholine release. J Neurophysiol. 2003;89:1954–1967. doi: 10.1152/jn.00668.2002. [DOI] [PubMed] [Google Scholar]
- Soucek B. Influence of latency fluctuations and the quantal process of transmitter release on the end-plate potential's amplitude distribution. Biophys J. 1971;11:127–139. doi: 10.1016/S0006-3495(71)86202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Steinbach J, Stevens C. Neuromuscular transmission. In: Llinas R, Precht W, editors. Frog Neurobiology. Berlin: Springer-Verlag; 1979. pp. 32–92. [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. The kinetics of quantal releases during end-plate currents at the frog neuromuscular junction. J Physiol. 1988;402:605–626. doi: 10.1113/jphysiol.1988.sp017225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Progr Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W, Molgó J, Naves LA. Cholinergic agonists decrease quantal output at the frog neuromuscular junction by targeting a calcium channel blocked by μ-conotoxin. Pflugers Arch. 1997;434:735–741. doi: 10.1007/s004240050459. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Somogyi GT. Prejunctional modulation of acetylcholine release from the skeletal neuromuscular junction: link between positive (nicotinic) – and negative (muscarinic) – feedback modulation. Br J Pharmacol. 1989;97:65–70. doi: 10.1111/j.1476-5381.1989.tb11924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Progr Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]