Abstract
We hypothesized that the effective inhibition of nitric oxide synthase (NOS), achieved via systemic infusion of NG-nitro-l-arginine methyl ester (l-NAME), would reduce the gas exchange threshold (GET) and the maximal oxygen uptake (V˙O2max) during incremental cycle exercise in man if NO is important in the regulation of muscle vasodilatation. Seven healthy males, aged 18–34 years, volunteered to participate in this ethically approved study. On two occasions, the subjects completed an incremental exercise test to exhaustion on an electrically braked cycle ergometer following the infusion of either l-NAME (4 mg kg−1 in 50 ml saline) or placebo (50 ml saline, CON). At rest, the infusion of l-NAME resulted in a significant increase in mean arterial pressure (MAP; CON vs. l-NAME, 89 ± 8 vs. 103 ± 11 mmHg (mean ± s.d.; P < 0.05)) and a significant reduction in heart rate (HR; CON vs. l-NAME, 60 ± 12 vs. 51 ± 8 beats min−1; P < 0.01). At submaximal work rates, there was no significant difference in V˙O2 between the conditions and no difference in the GET (CON vs. l-NAME, 1.94 ± 0.47 vs. 2.01 ± 0.41 l min−1). However, at higher work rates, differences in V˙O2 between the conditions became more pronounced such that V˙O2max was significantly lower with l-NAME (CON vs. l-NAME, 4.02 ± 0.41 vs. 3.80 ± 0.34 l min−1; P < 0.05). The reduction in V˙O2max was associated with a reduction in HRmax (CON vs. l-NAME, 186 ± 10 vs. 178 ± 7 beats min−1; P < 0.01). These results demonstrate that NOS inhibition with l-NAME has no effect on GET but reduces V˙O2max during large muscle group exercise in man, presumably by direct or indirect effects on cardiac output and muscle blood flow.
Nitric oxide (NO) is known to play an important role in a wide array of physiological processes (Moncada et al. 1991; Stamler & Meissner, 2001). Nitric oxide synthase (NOS), the enzyme responsible for the synthesis of NO, is present both within the vascular endothelium (known as eNOS) and within myocytes (known as nNOS). NO production increases during muscle contraction (Balon & Nadler, 1994) and it has been suggested that NO might be one of the factors contributing to skeletal muscle vasodilatation at the onset of exercise either by direct effects on the vascular endothelium (Moncada et al. 1991; Shepherd & Katusic, 1991) or by blunting sympathetically mediated vasoconstriction (Sander et al. 1995; Thomas & Victor, 1998). However, although it has been clearly demonstrated that NO contributes to the regulation of muscle blood flow at rest and following recovery from exercise (Dyke et al. 1995; Shoemaker et al. 1997; Radegran & Saltin, 1999; Frandsen et al. 2001), its importance during dynamic exercise is more controversial. Indeed, recent studies utilizing the one-legged knee extensor exercise model suggest that NO might not be essential for the regulation of muscle blood flow during steady-state exercise in man (Radegran & Saltin, 1999; Frandsen et al. 2001).
NOS activity can be inhibited with l-arginine analogues such as NG-monomethyl-l-arginine (l-NMMA) and the more potent NG-nitro-l-arginine methyl ester (l-NAME). l-NAME effectively inhibits both eNOS and nNOS (Sander et al. 1999; Frandsen et al. 2001). NOS inhibition results in a reduction in vascular conductance, an increase in mean arterial pressure (MAP), and a reduction in heart rate (HR) both before and during exercise (Sander et al. 1999; Frandsen et al. 2001). It has been suggested that the reduced HR with NOS inhibition results from baroreceptor-mediated sympathetic withdrawal (Sheriff et al. 2000; Joyner & Tschakovsky, 2003), although direct effects on cardiac function have also been reported (Sherman et al. 1997). Indeed, Kindig et al. (2000) reported that infusion of l-NAME caused a significant reduction in cardiac output (Q˙) during both submaximal and maximal treadmill running in the Thoroughbred horse. At running speeds corresponding to 50 and 80% V˙O2max, the reduction in muscle O2 delivery was compensated for by an increase in muscle O2 extraction such that pulmonary V˙O2 was unchanged. However, at a running speed corresponding to 100% V˙O2max, O2 extraction was unable to increase further, leading to a significant reduction in V˙O2max (and exercise tolerance) in the l-NAME compared to the control condition. In contrast, in one of the few studies to investigate the influence of NOS inhibition on cardio-respiratory responses to exercise in man, Frandsen et al. (2001) reported that l-NAME had no significant effect on leg blood flow or V˙O2 during either submaximal or maximal one-legged knee extension exercise. It is unclear whether the different results of Kindig et al. (2000) and Frandsen et al. (2001) are related to differences in the species studied (i.e. horse versus man), to the dose of l-NAME administered (20 mg kg−1 in the horse versus 4 mg kg−1 in man), or to differences in exercise modality (i.e. ‘whole-body’ versus small isolated muscle mass exercise). However, it is possible that NOS inhibition might impair muscle blood flow during maximal intensity exercise using large muscle groups (where maximal Q˙ is attained and the degree of sympatholysis has to be carefully regulated) but not during small muscle mass exercise (where the maximal Q˙ is not approached and muscle perfusion is very high).
To date, no studies have examined the influence of NOS inhibition on the V˙O2 response to incremental exercise using large muscle groups in man. During such exercise, it is known that both the gas exchange threshold (GET) and V˙O2max are sensitive to conditions which influence muscle O2 availability (e.g. Hogan et al. 1983; Koike et al. 1990; Saltin & Strange, 1992; Koga et al. 1999; Richardson et al. 1999; Wagner, 2000). If NO is important in the regulation of skeletal muscle blood flow during large muscle group exercise in man, then NOS inhibition would be expected to reduce both GET and V˙O2max during incremental exercise. The purpose of this study therefore was to investigate the effect of NOS inhibition with l-NAME on the cardio-respiratory response to incremental cycle exercise in man. We hypothesized that, relative to the control condition, NOS inhibition would significantly reduce both GET and V˙O2max.
Methods
Subjects
Seven healthy, recreationally active but not highly trained, male subjects (mean ± s.d. age 25 ± 4 years, body mass 78.5 ± 9.2 kg) volunteered to participate in this study which had been approved by the Manchester Metropolitan University Research Ethics Committee. The subjects provided written informed consent after the possible benefits and risks of participation had been explained to them. All subjects had extensive prior experience of performing incremental exercise tests to fatigue in our laboratory. All procedures conformed to the standards set by the Declaration of Helsinki.
Procedures
The subjects attended the laboratory to perform an incremental exercise test to volitional exhaustion on an electrically braked cycle ergometer (Jaeger Ergoline E800, Germany) on two occasions separated by 7 days. They were instructed to arrive at the laboratory rested and fully hydrated, having avoided the consumption of food and caffeine in the preceding 2 h. For each subject, the two exercise tests were conducted in the morning at approximately the same time of day (± 1 h).
Following arrival at the laboratory, the subjects lay supine and a cannula was placed in a hand vein. The subjects then rested for 20 min before either l-NAME (Merck Biosciences AG, Nottingham, UK; 4 mg (kg body mass)−1 in 50 ml saline) or an equivalent volume of saline (as a placebo) was infused over 60 min. Our protocol for the l-NAME infusion was based on that described by Frandsen et al. (2001), who demonstrated that this resulted in a 67% reduction in NOS activity in skeletal muscle. Throughout the infusions, blood pressure was measured with an automated sphygmanometer and heart rate was monitored continuously by telemetry (Polar Electro, Finland). The subjects were blind to the nature of the infusate. However, because of the profound effects of l-NAME on heart rate and blood pressure (Sander et al. 1999; see Results), it was not possible to blind the principal investigators to whether subjects had received l-NAME or a placebo infusion. Four subjects received l-NAME on their first visit to the laboratory and the placebo infusion on their second visit to the laboratory. This order was reversed in the other three subjects. Following the l-NAME or saline infusion, the subjects rested for 60 min before performing an incremental cycle ergometer test to exhaustion (see below).
After mounting the cycle ergometer, the subjects accelerated the flywheel and performed 3 min of baseline pedalling at the lowest work rate available on the ergometer (20 W). Subsequently, the work rate was then increased by 5 W every 10 s (i.e. 30 W min−1) until the subject was unable to continue. The subjects cycled at the same self-selected pedal rate throughout the test (this varied between 70 and 90 r.p.m. across the subjects) and the pedal rate, along with the saddle and handlebar height and configuration, were recorded for each subject and reproduced in the second test. During the test, the subjects were verbally encouraged to continue for as long as possible. The subjects voluntarily terminated the test when their pedal rate fell more than 5 r.p.m. below their selected pedal rate. In all cases, this fall was precipitous. The exact time to volitional exhaustion from the onset of the ramp test was measured with a stopwatch and recorded to the nearest second.
Pulmonary gas exchange was measured breath-by-breath and HR was recorded every 5 s throughout the exercise tests. The subjects wore a nose-clip and breathed through a low dead space, low resistance mouthpiece and volume sensor assembly. Pulmonary gas exchange was measured with a mass spectrometer and volume turbine system (Morgan EX670, Morgan Medical Limited, Gillingham, Kent, UK). The system was calibrated prior to each test using gases of known concentration and a precision 3 l calibration syringe. Blood pressure was measured immediately before the onset of exercise and within 30 s of the termination of exercise. Mean arterial pressure was calculated as the diastolic pressure plus one-third of the pulse pressure.
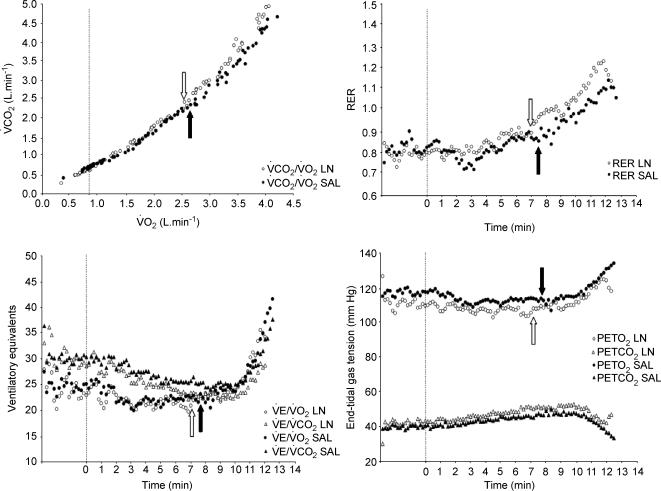
The breath-by-breath V˙O2 data for all tests were interpolated to give second-by-second values and then averaged over consecutive 10 s periods. The ‘baseline’ O2 cost of cycling in the incremental tests was taken as the average V˙O2 measured during the final minute of the 3 min baseline period. The gas exchange threshold (GET) was determined from a cluster of gas exchange indices as the V˙O2 at which there was: (i) a disproportionate increase in V˙CO2 from visual inspection of individual plots of V˙CO2 versus V˙O2 (Beaver et al. 1986); (ii) an increase in V˙E/V˙O2 (V˙E, expiratory ventilation) with no increase in V˙E/V˙CO2; and (iii) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension (Wasserman et al. 1999). We also examined the respiratory exchange ratio (RER) profiles to ensure that our identification of the GET was not confounded by the existence of ‘false positives’ (cf. Ozcelik et al. 1999). V˙O2max was determined as the average value recorded over the final 30 s of the each of the tests. The O2 pulse for each work rate and at exhaustion was calculated by dividing V˙O2 by HR (V˙O2/HR = stroke volume × arterial—venous O2 content difference).
Statistical analysis
The results are presented as mean ± s.d. Paired t tests were used to determine the significance of any differences between the physiological responses at rest and during exercise following the l-NAME and placebo infusions.
Results
Efficacy of NOS inhibition
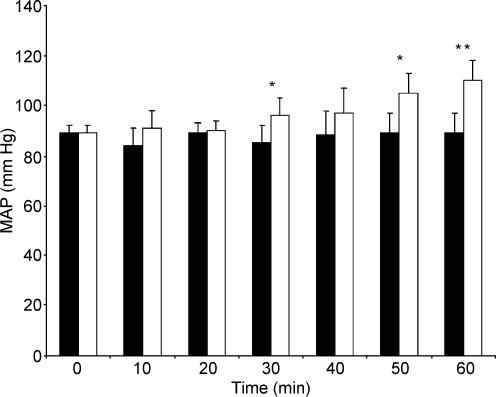
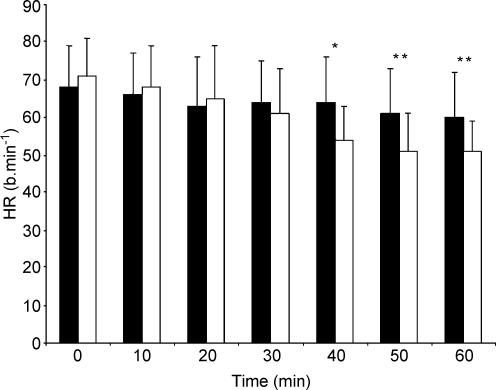
Infusion of l-NAME resulted in a significant increase in MAP (Fig. 1) and a significant reduction in HR (Fig. 2) beyond 30–40 min when compared to the control condition, consistent with successful inhibition of NOS. At the onset of exercise, MAP remained significantly elevated (CON vs. l-NAME, 97 ± 8 vs. 108 ± 10 mmHg; P < 0.05) and HR remained reduced (CON vs. l-NAME, 84 ± 10 vs. 78 ± 8 beats min−1; P = 0.09) compared to the control condition.
Figure 1. Influence of the infusion of saline (control) and of a NOS-inhibitor (l-NAME) on mean arterial blood pressure over 60 min.
Filled bars, control; open bars, l-NAME. *P < 0.05; **P < 0.01.
Figure 2. Influence of the infusion of saline (control) and of a NOS-inhibitor (l-NAME) on heart rate over 60 min.
Filled bars, control; open bars, l-NAME. *P < 0.05; **P < 0.01.
Although our subjects experienced no serious side-effects of receiving l-NAME, most were subdued and felt sleepy and lethargic in the afternoon and evening following l-NAME treatment.
Submaximal responses
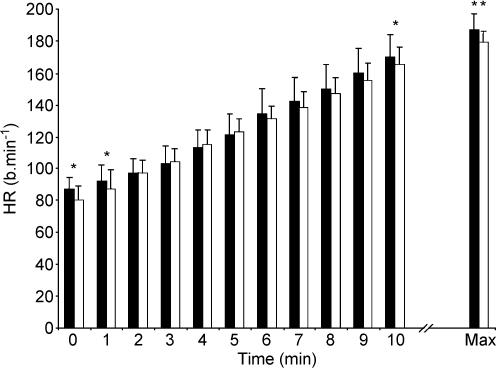
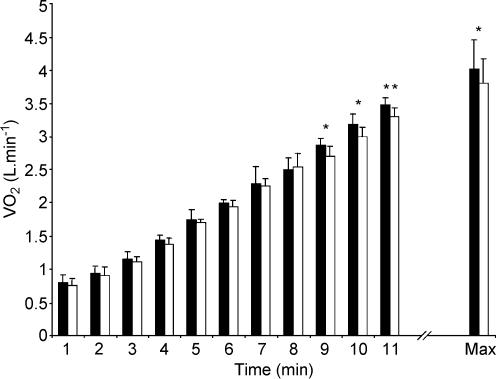
HR tended to be lower throughout the ramp test in the l-NAME compared to the control condition and this difference was significant at 20, 50, 320 and 350 W (Fig. 3). V˙O2 also tended to be lower in the l-NAME compared to control condition and this attained significance at 260, 290 and 320 W (Fig. 4). There was no significant difference in O2 pulse between the conditions. GET was not significantly different between conditions (CON vs. l-NAME, 1.94 ± 0.47 vs. 2.01 ± 0.41 l min−1; Table 1 and Fig. 5).
Figure 3. Heart rate response to incremental exercise in the control and l-NAME conditions.
Filled bars, control; open bars, l-NAME. Values are reported at the onset of the test (time 0), for every minute of the test up to minute 10, and at exhaustion (Max). *P < 0.05; **P < 0.01.
Figure 4. Pulmonary oxygen uptake response to incremental exercise in the control and l-NAME conditions.
Filled bars, control; open bars, l-NAME. Values are reported at the onset of the test (time 0), for every minute of the test up to minute 10, and at exhaustion (Max). *P < 0.05; **P < 0.01.
Table 1.
Individual values for gas exchange threshold (GET) and maximal oxygen uptake( V˙O2max)in the l-NAME and control conditions
| GET (ml kg−1 min−1) | V˙O2max (ml kg−1 min−1) | |||
|---|---|---|---|---|
| Subject | Control | l-NAME | Control | l-NAME |
| 1 | 16.7 | 19.1 | 44.5 | 43.6 |
| 2 | 24.6 | 25.0 | 50.0 | 50.9 |
| 3 | 31.8 | 32.5 | 65.2 | 61.9 |
| 4 | 19.7 | 19.3 | 45.0 | 42.0 |
| 5 | 23.5 | 24.5 | 55.5 | 48.5 |
| 6 | 32.7 | 30.7 | 48.4 | 47.1 |
| 7 | 25.7 | 29.2 | 53.7 | 48.4 |
| Mean | 25.0 | 25.8 | 51.8 | 48.9* |
| s.d. | 5.9 | 5.3 | 7.2 | 6.5 |
Significantly different (P < 0.05).
Figure 5. Determination of the gas exchange threshold (GET) using a ‘cluster’ of gas exchange indices.
Upper left panel illustrates the metabolic rate at which there is a disproportionate increase in CO2 output (‘V-slope’ method; Beaver et al. 1986); lower left panel shows the ventilatory equivalents for O2 and CO2; upper right panel shows the respiratory exchange ratio; and lower right panel shows the end-tidal gas tension responses to exercise following the inhibition of nitric oxide synthase (○) and following a placebo infusion (•) in a typical subject. See text for further information. GET in each condition is shown by the open and filled arrows, respectively. There was no significant difference in the group mean GET between conditions.
Maximal responses
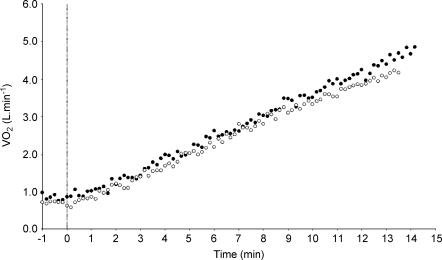
The time to exhaustion was reduced with l-NAME (CON vs. l-NAME, 12.3 ± 1.3 vs. 11.9 ± 1.1 min; P = 0.06). Maximal HR attained was significantly different between the two conditions (CON vs. l-NAME, 186 ± 10 vs. 178 ± 7 beats min−1; P < 0.01; Fig. 3) and MAP measured within 30 s of the termination of exercise tended to be greater with l-NAME (CON vs. l-NAME, 105 ± 15 vs. 122 ± 12 mmHg; P = 0.08). V˙O2max was significantly lower in the l-NAME condition (CON vs. l-NAME, 4.02 ± 0.41 vs. 3.80 ± 0.34 l min−1; P < 0.05; Fig. 4 and Table 1). There was no significant difference in the maximum O2 pulse between the conditions (CON vs. l-NAME, 22.0 ± 2.0 vs. 22.4 ± 2.1 ml O2 beat−1). The V˙O2 response to incremental exercise with and without NOS inhibition is shown for a typical subject in Fig. 6.
Figure 6. Oxygen uptake response to incremental exercise following the inhibition of nitric oxide synthase (○) and following a placebo infusion (•) in a typical subject.
Note the appreciable reduction in maximal oxygen uptake following nitric oxide synthase inhibition.
There was no significant difference between the conditions for V˙CO2max (CON vs. l-NAME, 4.67 ± 0.23 vs. 4.53 ± 0.33 l min−1; P = 0.07), V˙Emax (CON vs. l-NAME, 160 ± 30 vs. 150 ± 36 l min−1), V˙E/V˙O2max (CON vs. l-NAME, 39.8 ± 7.8 vs. 39.5 ± 7.3), V˙E/V˙CO2max (CON vs. l-NAME, 34.3 ± 5.8 vs. 33.1 ± 8.5), or RERmax (CON vs. l-NAME, 1.17 ± 0.08 vs. 1.20 ± 0.06).
Discussion
To our knowledge, this is the first study to investigate the effect of NOS inhibition (achieved via systemic infusion of l-NAME) on the cardiovascular and respiratory responses to incremental large muscle group exercise in man. The principal novel finding was the significant reduction in V˙O2max, with no change in submaximal V˙O2 or GET, following NOS inhibition. These results suggest that muscle O2 delivery was well maintained during submaximal cycle exercise following NOS inhibition. However, the reduction in V˙O2max, which was associated with a significant reduction in HRmax, strongly suggests that it was not possible to fully maintain blood flow to the contracting skeletal muscle mass during maximal intensity cycle exercise following NOS inhibition.
Comparison with previous studies
Our results are, in part, consistent with those of Kindig et al. (2000) who examined the effect of l-NAME on the cardiovascular and respiratory responses to incremental treadmill running in the Thoroughbred horse. These authors reported that l-NAME caused a significant increase in MAP and a significant reduction in HR and Q˙ throughout exercise compared to the control condition. During submaximal exercise at treadmill speeds requiring 50 and 80% of previously determined V˙O2max, they found that muscle O2 extraction increased to compensate for the reduction in muscle O2 delivery such that pulmonary V˙O2 was not significantly different between the l-NAME and control conditions. However, during maximal intensity exercise, increased O2 extraction could no longer compensate for the reduced muscle O2 delivery, and thus the V˙O2max in the l-NAME condition was significantly reduced compared to control. The results of our study are similar in that HR was reduced with l-NAME both during submaximal exercise (with no change in V˙O2) and during maximal exercise (where there was also a significant reduction in V˙O2max). However, the magnitude of the reduction in V˙O2max (∼11%) in the study of Kindig et al. (2000) was greater than the magnitude of the reduction in V˙O2max that we observed (∼6%). One possible explanation for this discrepancy is the difference in the dose of l-NAME administered (20 mg kg−1 in their study and 4 mg kg−1 in ours). This dose of 4 mg (kg body mass)−1 has been shown previously to result in a 67% reduction in nNOS activity (Frandsen et al. 2001). It is possible that complete NOS blockade with higher doses of l-NAME would have resulted in an even greater reduction in V˙O2max, but it is not known whether higher doses pose significant health risks to human subjects.
Only one other study has given l-NAME systemically and examined the effect on V˙O2 during incremental exercise in man. In this study, Frandsen et al. (2001) measured limb blood flow and oxygen extraction, and therefore V˙O2, during incremental one-legged knee extension exercise in five subjects. They reported a significant reduction in leg vascular conductance and HR during submaximal exercise with l-NAME, but no significant differences in leg blood flow, O2 extraction or V˙O2. These data imply that Q˙ was reduced but the perfusion of working muscle was maintained with NOS inhibition. At rest and during recovery from exercise in man, there is clear evidence that NOS inhibition reduces tissue blood flow, with V˙O2 being maintained by an increased O2 extraction (Dyke et al. 1995; Shoemaker et al. 1997; Radegran & Saltin, 1999; Frandsen et al. 2001). However, several studies have shown that NO is not obligatory for normal muscle vasodilatation during exercise, at least in small muscle groups, indicating the existence of some redundancy in the system (Shoemaker et al. 1997; Radegran & Saltin, 1999; Boushel et al. 2002; Hillig et al. 2003). It seems unlikely that the maintained skeletal muscle blood flow with l-NAME despite evidence for a reduced vascular conductance in the study of Frandsen et al. (2001) can be fully explained by the greater perfusion pressure. Rather, it appears that the reduced vascular conductance arises from vasoconstriction in the vascular beds of other organs including non-active skeletal muscle (Hirai et al. 1994), with blood flow to contracting skeletal muscle being well preserved. Local factors which might facilitate vasodilatation in the face of NOS inhibition include adenosine, potassium, prostaglandins (PG) and endothelial-derived hyperpolarizing factors (EDHF), although it is likely that the integration of a number of factors that interact determines blood flow (Frandsen et al. 2000; Boushel et al. 2002; Hillig et al. 2003). For example, Boushel et al. (2002) have recently reported that the combined inhibition of NOS and PG resulted in a ∼30% reduction in skeletal muscle blood flow during exercise. Furthermore, Hillig et al. (2003) have reported that combined inhibition of NOS and cytochrome P450 (to block EDHF formation) resulted in a ∼16% reduction in muscle blood flow. These results suggest a synergy between NO, PG and EDHF in the regulation of muscle vasodilatation and exercise hyperaemia. While there is apparently no compensatory increase in interstitial adenosine, potassium or prostacyclin following NOS inhibition (Frandsen et al. 2000), it is presently unknown whether NOS inhibition alters the EDHF or bradykinin responses to exercise. However, irrespective of the mechanism involved, our results, in concert with those of Frandsen et al. (2001), demonstrate that during exercise where only a relatively small amount of muscle is recruited (for example, during single leg knee extension exercise and submaximal cycle exercise), the system is able to maintain a close matching of blood flow to metabolic rate (i.e. Q˙/V˙O2) in working muscle, even when NO production is markedly attenuated.
Influence of NOS inhibition on the gas exchange threshold and maximal oxygen uptake
To our knowledge, ours is the first study to assess the influence of NOS inhibition on GET. The physiological mechanisms responsible for GET (and the simultaneous increase in blood [lactate] which typically accompanies it) have been extensively debated (cf. Jones & Ehrsam, 1982; Walsh & Banister, 1988; Wasserman et al. 1999). However, it is known that GET is sensitive to reductions in O2 availability. For example, interventions which might be expected to reduce muscle O2 availability such as the inspiration of hypoxic gas (Hogan et al. 1983; Hughson & Kowalchuk, 1995; Walsh & Banister, 1995) or gas with increased carbon monoxide content (Koike et al. 1990), or the performance of supine exercise (Koga et al. 1999) all result in a greater accumulation of blood lactate and therefore an earlier appearance of GET. Furthermore, GET is lower in patients with diseases of the central or peripheral circulation (Wasserman et al. 1999). The fact that GET was not significantly altered with l-NAME in our study therefore further suggests that Q˙/V˙O2 of active skeletal muscle was preserved despite effective NOS inhibition during submaximal cycle exercise (at least up to ∼50% of whole-body V˙O2max), consistent with previous findings (Frandsen et al. 2001). However, unlike in the study of Frandsen et al. we found that V˙O2max was significantly reduced following NOS inhibition.
One important difference between our study and that of Frandsen et al. (2001) is the exercise modality (upright two-legged cycling versus one-legged knee extension exercise). Although exercise performance during knee extension exercise is sensitive to O2 availability (Richardson et al. 1999), the volume of muscle recruited to perform exercise of this type is so small that fatigue is believed to result principally from peripheral rather than central factors. Indeed, relative to cycle ergometry (where peak muscle blood flow is ∼150 ml kg−1 min−1), there is a much higher blood flow per unit muscle mass during knee extension exercise (peak muscle blood flow of ∼400 ml kg−1 min−1) (Knight et al. 1992; Richardson et al. 1993). Indeed, in the control condition in the study of Frandsen et al. (2001), subjects attained maximal HRs of ∼155 beats min−1 and V˙O2max values of ∼0.85 l min−1, on average, at exhaustion (appreciably less than the corresponding values in the present study). These values indicate that subjects became fatigued before maximal Q˙ was attained. As mentioned above, the similar leg blood flow and V˙O2, despite a significant reduction in HRmax to ∼125 beats min−1 with l-NAME in the study of Frandsen et al. (2001) could presumably have resulted from a compensatory increase in stroke volume and/or a redistribution of blood flow away from other vascular beds.
In contrast to one-legged knee extension exercise, incremental two-legged cycle exercise involves the recruitment of a substantially greater muscle mass and provides a greater challenge to the cardiovascular system such that it is generally accepted that V˙O2max is limited by a failure of O2 supply to meet muscle O2 demand (Knight et al. 1992; Saltin & Strange, 1992; Wagner, 2000; but see also Noakes, 1997 for an alternative perspective). In our study, it is very unlikely that an increase in stroke volume could have compensated for the reduction of maximal HR with l-NAME (from 186 to 178 beats min−1, on average) because stroke volume reaches a maximum at around 40–50% V˙O2max in normal healthy subjects (Rowell, 1974; Stringer et al. 1997). It is also unlikely that muscle blood flow could be maintained by a redistribution of blood flow away from other organs when the exercising muscles already receive a very high fraction of the maximal Q˙ during high-intensity exercise with large muscle groups (Knight et al. 1992). Although muscle O2 extraction could be increased to compensate for a reduced O2 delivery, this might be insufficient to prevent a reduction in V˙O2max during maximal intensity cycle exercise where the arterial–venous O2 difference might be approaching a ceiling (Knight et al. 1992; Kindig et al. 2000).
Mechanisms
For the reasons given above, we believe that a likely explanation for the significantly lower V˙O2max we observed with l-NAME is the significant reduction in HR (and therefore, presumably, Q˙ and muscle blood flow) at maximal work rates. In support of this, it is interesting to note that the magnitude of the reduction in HRmax and V˙O2max with l-NAME were similar (4.3 versus 5.5%, respectively). Indeed, the maximum O2 pulse was not significantly different between the conditions (∼22 ml O2 beat−1), suggesting that the reduction in V˙O2max with l-NAME was proportional to the reduction in HRmax. It has been suggested that the reduced HR with l-NAME might be mediated by a baroreflex caused by the elevated blood pressure which reduces sympathetic efferent activity to the heart (Hirai et al. 1994). It should be mentioned here that this inhibition of sympathetic outflow with l-NAME might also increase vascular conductance and mask any reduction in muscle blood flow during submaximal exercise that would otherwise have resulted from a local reduction in NO-mediated vasodilatation (Sheriff et al. 2000). It is interesting to note, however, that MAP was not significantly different between the l-NAME and control conditions early in the recovery period following the termination of exercise. This suggests that the lower maximal HR with l-NAME might, at least in part, result from central effects of NOS inhibition on the heart. In addition to the direct or indirect effects on HR, it is also possible that l-NAME reduces stroke volume during exercise. For example, the reduced vascular conductance with l-NAME will increase myocardial afterload and reduce venous return. Indeed, Kindig et al. (2000) found that while the reduced Q˙ they observed at a work rate equivalent to 50% V˙O2max could be attributed equally to reductions in HR and stroke volume, the lower Q˙ at work rates equivalent to 80 and 100% V˙O2max was principally caused by a reduced stroke volume. In this context, it is interesting to note that Sherman et al. (1997) have reported that intracoronary infusion of l-NAME in intact dogs reduced segment shortening and myocardial O2 consumption. A reduction in cardiac myocyte contractility with l-NAME might therefore also have the potential to reduce Q˙ during exercise, although direct measurements have not been made in humans.
As exercise intensity (and therefore the mass of recruited muscle and the supporting Q˙) increases during incremental exercise, there is an increase in sympathetic activity in order to maintain blood pressure (Rowell, 1993). Our results might therefore also be interpreted to suggest that locally mediated vasodilatation is unable to completely overcome the sympathetically mediated increase in vascular tone during near-maximal intensity cycle exercise following NOS inhibition such that muscle blood flow is reduced compared to the control condition. In this case, the reduced HR we observed would be a consequence rather than a cause of the reduced muscle blood flow and V˙O2. If true, this implies that NO might be important in the regulation of muscle blood flow during very high intensity exercise in man either through local effects on the vascular endothelium or by opposing sympathetic vasoconstriction (Moncada et al. 1991; Shepherd & Katusic, 1991; Sander et al. 1995; Thomas & Victor, 1998). One other possibility for the reduction in V˙O2max with l-NAME is the development of a (more pronounced) Q˙/V˙O2 mismatch at maximal work rates. Again, it might be expected that any effect of NOS inhibition on regional Q˙/V˙O2 would be greater during exercise requiring a large compared to a small muscle mass due to the differences in muscle perfusion noted earlier. Consequently, a more pronounced regional perfusion heterogeneity invoked by NOS inhibition might impact upon V˙O2max measured during ‘whole body’ exercise (present study) but not during isolated small muscle group exercise (Frandsen et al. 2001).
It should be pointed out that the reduction in V˙O2max that we observed cannot be attributed to an impairment of muscle contraction or mitochondrial function. On the contrary, in vitro studies indicate that NO might also play an important role in the regulation of muscle contraction and/or energy turnover. For example, NO has been shown (i) to reduce sarcoplasmic reticulum Ca2+ release, Ca2+ sensitivity and cross-bridge cycling kinetics, muscle force and actomyosin ATPase activity (Perkins et al. 1997; Heunks et al. 2001a,b); (ii) to inhibit key metabolic enzymes such as creatine kinase, aconitase, oxoglutarate dehydrogenase, glyceraldehydes-3-phosphate dehydrogenase, and respiratory complexes I–IV (Zhang & Snyder, 1995; Cassina & Radi, 1996; Andersson et al. 1998; Brown, 1999; Kaasik et al. 1999); and (iii) to modulate the respiratory rate by competing with O2 for the binding site at cytochrome oxidase (Shen et al. 1994; Giulivi, 1998; Brown, 2000; Elfering et al. 2002). Therefore, NOS inhibition theoretically has the potential to enhance mitochondrial respiration. Indeed, several recent studies have shown that NOS inhibition with l-NAME accelerates the adaptation of V˙O2 towards the steady-state V˙O2 requirement upon the sudden transition to a moderate (∼40–50% V˙ O2max) or heavy (∼70–80% V˙O2max) work rate both in the Thoroughbred horse (Kindig et al. 2001, 2002) and in humans (Jones et al. 2003, 2004). The similar absolute V˙O2 for the same work rate between the l-NAME and control conditions during submaximal exercise that we observed in the present study is consistent with the similar ‘steady-state’ V˙O2 attained during submaximal constant work rate exercise in these previous studies (Kindig et al. 2001, 2002; Jones et al. 2003, 2004). It is pertinent to emphasize therefore that the lower V˙O2max attained with l-NAME in the present study occurred despite a probable enhancement of mitochondrial and contractile function.
It is also important to stress that our results cannot be explained by a reduced effort on the part of the subjects in the incremental test following l-NAME infusion. The subjects were blind to the nature of the infusate, were highly motivated, and had significant experience of performing exercise tests involving maximal effort in our laboratory. The actual V˙O2max attained during incremental exercise is, of course, effort dependent; however, in our study, V˙O2 was also significantly reduced at the same absolute high-intensity (but submaximal) work rates in the l-NAME condition (Fig. 4). HRmax is frequently used to judge the extent to which a maximal effort is given during incremental exercise tests. Although the HRmax was significantly lower in the l-NAME condition compared to control, this clearly resulted from the direct or indirect action of the drug, as evidenced by the lower HR for the same work rate throughout exercise (Fig. 3). Interestingly, although V˙O2max was reduced with l-NAME, there was no significant reduction in the maximal values of V˙CO2 and V˙E between the experimental and control conditions (although there was a statistical tendency for these to be reduced also; P = 0.07–0.15). This might be explained by a relatively greater contribution of anaerobic glycolysis to energy metabolism following l-NAME administration, with the greater V˙CO2 and V˙E (relative to V˙O2) resulting from increased bicarbonate buffering of the lactic acidosis. The necessity for an increased anaerobic ATP production to maintain ATP turnover during near-maximal exercise might also explain the reduction in exercise tolerance with l-NAME in comparison to the control condition. Unfortunately, we did not measure blood [lactate] in the present study and so are unable to confirm this possibility.
Limitations
A limitation to our study is the lack of direct measurements of muscle blood flow during exercise. Such measurements are technically challenging during upright cycle ergometer exercise. However, it has been shown that there is a strong linear relationship between muscle and pulmonary V˙O2 during incremental cycle exercise, with muscle O2 consumption contributing ∼83% to the pulmonary V˙O2 at maximal work rates (Knight et al. 1992). The remaining ∼17% has been attributed principally to the O2 cost associated with the work of breathing (Aaron et al. 1992). Because V˙Emax was not significantly different between the l-NAME and control conditions in the present study, the reduced pulmonary V˙O2 at near-maximal work rates was almost certainly a direct reflection of a reduced rate of O2 consumption in the leg muscles. Further studies with direct measurements of muscle blood flow are required to clarify the mechanisms underpinning the reduction in V˙O2max during whole-body exercise following NOS inhibition.
In conclusion, the systemic infusion of l-NAME (which caused a significant increase in MAP and a significant reduction in HR) did not significantly alter GET or V˙O2 at submaximal work rates. However, our results demonstrate for the first time that NOS inhibition causes a significant (albeit relatively small, i.e. ∼6%) reduction in V˙O2max during whole body exercise in humans. These findings suggest that muscle blood flow was well preserved during submaximal exercise following NOS inhibition, indicating multifactorial regulation of vascular tone to ensure precise matching of Q˙/V˙O2. However, at near-maximal work rates, there was a significant reduction in V˙O2 following NOS inhibition which was associated with a significantly lower HR. Because NOS inhibition theoretically has the potential to enhance mitochondrial respiration by several mechanisms, our results strongly suggest that the reduction in V˙O2max was caused by a reduced blood flow to the working muscles.
References
- Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol. 1992;72:1818–1825. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- Andersson U, Leighton B, Young ME, Blomstrand E, Newsholme EA. Inactivation of aconitase and oxoglutarate dehydrogenase in skeletal muscle in vitro by superoxide anions and/or nitric oxide. Biochem Biophys Res Comm. 1998;249:512–516. doi: 10.1006/bbrc.1998.9171. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;366:233–249. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide as a competitive inhibitor of oxygen consumption in the mitochondrial respiratory chain. Acta Physiol Scand. 2000;168:667–674. doi: 10.1046/j.1365-201x.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- Cassina A, Radi R. Different inhibitory actions of NO and peroxynitrite on mitochondrial electron transport. Arch Biophys Biochem. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfering SL, Sarkela TM, Giulivi C. Biocjhemistry of mitochondrial nitric-oxide synthase. J Biol Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Langberg H, Saltin B, Hellsten Y. Inhibition of nitric oxide synthesis by systemic N(G)-monomethyl-L-arginine administration in humans: Effects on interstitial adenosine, prostacyclin and potassium concentrations in resting and contracting skeletal muscle. J Vasc Res. 2000;37:297–302. doi: 10.1159/000025743. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivi C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem J. 1998;332:673–679. doi: 10.1042/bj3320673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heunks LMA, Cody MJ, Geiger PC, Dekhuijen PNR, Sieck GC. Nitric oxide impairs Ca2+ activation and slows cross-bridge cycling kinetics in skeletal muscle. J Appl Physiol. 2001a;91:2233–2239. doi: 10.1152/jappl.2001.91.5.2233. [DOI] [PubMed] [Google Scholar]
- Heunks LMA, Machiels HA, Dekhuijen PNR, Prakask YS, Sieck GC. Nitric oxide affects sarcoplasmic calcium release in skeletal myotubes. J Appl Physiol. 2001b;91:2117–2124. doi: 10.1152/jappl.2001.91.5.2117. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Visnski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Cox RH, Welch HG. Lactate accumulation during incremental exercise with varied inspired oxygen fractions. J Appl Physiol. 1983;55:1134–1140. doi: 10.1152/jappl.1983.55.4.1134. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Kowalchuk JM. Kinetics of oxygen uptake for submaximal exercise in hyperoxia, normoxia, and hypoxia. Can J Appl Physiol. 1995;20:198–210. doi: 10.1139/h95-014. [DOI] [PubMed] [Google Scholar]
- Jones NL, Ehrsam RE. The anaerobic threshold. Ex Sport Sci Rev. 1982;10:49–83. [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Koppo K, Wilmshurst S, Campbell IT. Inhibition of nitric oxide synthase by L-NAME speeds V˙O2 kinetics in the transition to moderate intensity exercise in man. J Physiol. 2003;552:265–272. doi: 10.1113/jphysiol.2003.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Wilmshurst S, Campbell IT. Influence of L-NAME on pulmonary O2 uptake kinetics during heavy-intensity cycle exercise. J Appl Physiol. 2004;96:1033–1038. doi: 10.1152/japplphysiol.00381.2003. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Tschakovsky ME. Nitric oxide and physiologic vasodilation in human limbs: where do we go from here? Can J Appl Physiol. 2003;28:475–490. doi: 10.1139/h03-035. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Minajeva A, DeSousa E, Ventura-Clapier R, Veksler V. Nitric oxide inhibits cardiac energy production via inhibition of mitochondrial creatine kinase. FEBS Lett. 1999;444:75–77. doi: 10.1016/s0014-5793(99)00033-2. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Gallatin LL, Erickson HH, Fedde MR, Poole DC. Cardiorespiratory impact of the nitric oxide synthase inhibitor L-NAME in the exercising horse. Respir Physiol Neurobiol. 2000;120:151–166. doi: 10.1016/s0034-5687(00)00096-7. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of L-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol. 2001;91:891–896. doi: 10.1152/jappl.2001.91.2.891. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Nitric oxide synthase inhibition speeds oxygen uptake kinetics in horses during moderate domain running. Respir Physiol Neurobiol. 2002;132:169–178. doi: 10.1016/s1569-9048(02)00068-x. [DOI] [PubMed] [Google Scholar]
- Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD. Relationship between body and leg [Reinsert]during maximal cycle ergometry. J Appl Physiol. 1992;73:1114–1121. doi: 10.1152/jappl.1992.73.3.1114. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ. Kinetics of oxygen uptake during supine and upright heavy exercise. J Appl Physiol. 1999;87:253–260. doi: 10.1152/jappl.1999.87.1.253. [DOI] [PubMed] [Google Scholar]
- Koike A, Weiler-Ravell D, McKenzie DK, Zanconato S, Wasserman K. Evidence that the metabolic acidosis threshold is the anaerobic threshold. J Appl Physiol. 1990;68:2521–2526. doi: 10.1152/jappl.1990.68.6.2521. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Noakes TD. Challenging beliefs: ex Africa semper aliquid novi. Med Sci Sports Exerc. 1997;29:571–590. doi: 10.1097/00005768-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Ozcelik O, Ward SA, Whipp BJ. Effect of altered body CO2 stores on pulmonary gas exchange dynamics during incremental exercise in humans. Exp Physiol. 1999;84:999–1011. doi: 10.1111/j.1469-445x.1999.01868.x. [DOI] [PubMed] [Google Scholar]
- Perkins WJ, Han YS, Sieck GC. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol. 1997;83:1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent V̇O2max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Saltin B, Strange S. Maximal oxygen uptake: ‘old’ and ‘new’ arguments for a cardiovascular limitation. Med Sci Sports Exerc. 1992;24:30–37. [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Victor RG. A large blood pressure raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension. 1995;26:691–695. doi: 10.1161/01.hyp.26.4.691. [DOI] [PubMed] [Google Scholar]
- Shen W, Xu X, Ochoa M, Zhao G, Wolin MS, Hintze TH. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994;75:1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Shepherd JT, Katusic ZS. Endothelium-derived vasoactive factors. I. Endothelium-dependent relaxation. Hypertension. 1991;18:76–85. doi: 10.1161/01.hyp.18.5_suppl.iii76. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation? Am J Physiol. 2000;279:H726–H732. doi: 10.1152/ajpheart.2000.279.2.H726. [DOI] [PubMed] [Google Scholar]
- Sherman AJ, Davis CA, Klocke FJ, Harris KR, Srinivasan G, Yaacoub AS, Quinn DA, Ahlin KA, Jang JJ. Blockade of nitric oxide synthase reduces myocardial oxygen consumption in vivo. Circulation. 1997;95:3505–3512. doi: 10.1161/01.cir.95.5.1328. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwell JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol. 1997;82:908–912. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. New ideas on limitations to V̇O2max. Exerc Sport Sci Rev. 2000;28:10–14. [PubMed] [Google Scholar]
- Walsh ML, Banister EW. Possible mechanisms of the anaerobic threshold. A review. Sports Med. 1988;5:269–302. doi: 10.2165/00007256-198805050-00001. [DOI] [PubMed] [Google Scholar]
- Walsh ML, Banister EW. The influence of inspired oxygen on the oxygen uptake response to ramp exercise. Eur J Appl Physiol. 1995;72:71–75. doi: 10.1007/BF00964117. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Whipp BJ, Casaburi R. Principles of Exercise Testing and Interpretation. 3. Philadelphia: Lea & Febiger; 1999. [Google Scholar]
- Zhang J, Snyder SH. Nitric oxide and the nervous system. Annu Rev Pharmacol Toxicol. 1995;35:213–233. doi: 10.1146/annurev.pa.35.040195.001241. [DOI] [PubMed] [Google Scholar]