Abstract
Mitochondria play an important role in the homeostasis of intracellular Ca2+ and regulate its availability for exocytosis. Inhibitors of mitochondria Ca2+ uptake such as protonophore CCCP potentiate the secretory response to a depolarizing pulse of K+. Exposure of cells to agents that directly (cytochalasin D, latrunculin B) or indirectly (PMA) disrupt cortical F-actin networks also potentiate the secretory response to high K+. The effects of cytochalasin D and CCCP on secretion were additive whereas those of PMA and CCCP were not; this suggests different mechanisms for cytochalasin D and CCCP and a similar mechanism for PMA and CCCP. Mitochondria were the site of action of CCCP, because the potentiation of secretion by CCCP was observed even after depletion of Ca2+ from the endoplasmic reticulum. CCCP induced a small increase in the cytosolic Ca2+ concentration ([Ca2+]c) that was not modified by the protein kinase C (PKC) inhibitor chelerythrine. Both CCCP and PMA induced cortical F-actin disassembly, an effect abolished by chelerythrine. In addition, rotenone and oligomycin A, two other mitochondrial inhibitors, also evoked cortical F-actin disassembly and potentiated secretion; again, these effects were blocked by chelerythrine. CCCP also enhanced the phosphorylation of PKC and myristoylated alanine-rich C kinase substance (MARCKS), and these were also inhibited by chelerythrine. The results suggest that the rapid sequestration of Ca2+ by mitochondria would protect the cell from an enhanced PKC activation and cortical F-actin disassembly, thereby limiting the magnitude of the secretory response.
Chromaffin cells store their materials for export in membrane-bound organelles, the secretory vesicles (Trifaró & Poisner, 1982). Upon cell stimulation the vesicular content is extruded to the cell exterior by exocytosis (Trifaró & Poisner, 1982). This is a complex process of interaction between secretory vesicle components, plasma membranes and cytosolic factors leading to the fusion of vesicle and plasma membranes. Secretory vesicles are present in these cells in at least two compartments: (a) the release-ready vesicle pool and (b) the reserve pool (Heinemann et al. 1993; Neher & Zucker, 1993; Vitale et al. 1995). The traffic of vesicles between these compartments is subject to a fine regulation. Experimental evidence has demonstrated that the cortical F-actin network plays an important role in this regulation (Vitale et al. 1991, 1995).
Calcium ions play a pivotal role, acting at more than one level in the cascade of events leading to exocytosis. A rise in the cytosolic Ca2+ concentration ([Ca2+]c) triggers exocytosis and this increase in Ca2+ is, depending of the type of stimulus, due either to Ca2+ entering the cell through specific channels or to Ca2+ being released from intracellular stores such as the endoplasmic reticulum (Kuba, 2000). Another component of the intracellular buffering machinery is the mitochondria (Duchen, 1999), which have emerged as important players in the intracellular regulation of Ca2+ levels (Friel & Tsien, 1994; Park et al. 1996; Herrington et al. 1996; Babcock et al. 1997; Montero et al. 2000).
Activation of Ca2+ channels or Ca2+ release from the endoplasmic reticulum triggers fast millimolar mitochondrial Ca2+ transients that modulate chromaffin cell secretion (Giovannucci et al. 1999; Montero et al. 2000) as well as secretion from PC12 cells (Taylor et al. 2000). Exposure of chromaffin cells to protonophores abolished mitochondrial Ca2+ uptake and potentiated stimulated secretion; this led to the conclusion that mitochondria could regulate the availability of Ca2+ to the secretory machinery, and hence secretion (Montero et al. 2000). However, how this modulation is exerted is unknown. The purpose of the present investigation was to elucidate the mechanisms involved in the potentiation of secretion when the mitochondrial Ca2+ sequestration is interrupted by a protonophore. Here we demonstrate that, in chromaffin cells, the potentiation of the secretory response observed upon the collapse of the mitochondrial transmembrane electrochemical potential is accompanied by PKC and myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation and is mediated through cortical F-actin disassembly. These effects were inhibited by PKC blockers.
Methods
Materials
Phorbol 12-myristate, 13-acetate (PMA), rotenone, oligomycin A, chelerythrine chloride, and carbonyl cyanide m-chlorophenyl hydrazone (CCCP) were purchased from Sigma Chemical Co. (St Louis, MO, USA); latrunculin B and cytochalasin D were obtained from Calbiochem (San Diego, CA, USA); rhodamine phalloidin and fura-2 and 1,2-bis(2-amino-phenoxy)-ethane-N,N,N′N′-tetra-acetic acid tetrakis (acetoxymethyl ester) (BAPTA AM) were purchased from Molecular Probes Inc. (Eugene, Oregon, USA). PMA, CCCP and chelerythrine were prepared in DMSO and kept as 10−2 m stock solutions at −20°C. Dilutions were freshly prepared on the day of the experiment, and final concentrations of DMSO were 0.1% or less in each case. Rabbit polyclonal phospho-PKC (pan) and phospho-MARCKS (ser 152/156) antibodies were obtained from Cell Signalling Technology Inc. (Beverley, MA, USA) and mouse monoclonal antibody against tubulin was purchased from Sigma Chemical Co.
Isolation and culture of chromaffin cells
Bovine adrenal glands were obtained from a local slaughterhouse. Chromaffin cells were isolated by adrenal medulla digestion with collagenase, according to the procedure of Trifaró & Lee (1980) with some modifications (Moro et al. 1990). Our preparations were enriched in adrenaline-containing cells. Cells were suspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum, 10 μm cytosine arabinoside, 10 μm fluorodeoxyuridine, 50 IU ml−l penicillin, and 50 μg ml−l streptomycin. Cells (5 × 106 in 10 ml DMEM) were plated in 5 cm diameter Petri dishes and kept in a water-saturated incubator at 37°C, in a 5% CO2–95% air atmosphere, and used 3–5 days thereafter. The culture medium was replaced by serum-free DMEM 24 h later, and then every 2 days.
On-line measurement of catecholamine release from bovine chromaffin cells
Cells were scraped off carefully from the bottom of the Petri dish with a rubber policeman, and centrifuged at 800 g for 10 min. The cell sediment was resuspended in 200 μl of Krebs-Hepes (composition (mm): NaCl 144; KCl 5.9; MgCl2 1.2; glucose 11; Hepes 10; pH 7.4); the concentration of CaCl2 varied depending on the protocol. Cells were introduced to a jacketed microchamber for their superfusion at 37°C. The superfusion rate was 2 ml min−l. The liquid flowing from the superfusion chamber reached an electrochemical detector (model Metrohn AG CH-9100 Hersau) placed just at the outlet of the microchamber that monitored, ‘on line’ under the amperometric mode, the amount of catecholamines secreted (Borges et al. 1986). Cells were stimulated to secrete with short pulses (1–5 s) of high K+ Krebs-Hepes solution controlled by electrovalves. Depolarizing concentrations of K+ were applied in the presence or absence of different test compounds when cells were being superfused with Krebs-Hepes solution (see Results for further details).
Measurement of changes of [Ca2+]c in fura-2-loaded bovine chromaffin cells
Chromaffin cells were loaded with fura-2 by incubating them with fura-2 AM (4 μm) for 60 min at 37°C in Krebs-Hepes solution (pH 7.4) containing (mm): NaCl 144; KCl 5.9; MgCl2 1.2; CaCl2 2.4; sodium Hepes 10; glucose 10. The loading incubation was terminated by washing the coverslip containing the attached cells several times with Krebs-Hepes solution. The coverslip containing the fura-2-loaded cells was placed in a chamber mounted on the stage of a Nikon Diaphot inverted microscope. The chamber was continuously perfused at room temperature (22 ± 2°C) with Krebs-Hepes solution. Once selected, an individual cell was locally superfused with various solutions that were changed using electronically driven miniature solenoid valves coupled to a multibarrel concentration-clamp device, the common outlet of which was placed within 100 μm of the cell being explored. The flow rate was smaller than 1 ml min−1 and was regulated by gravity to achieve complete replacement of the cell surroundings within less than 1 s. This allowed the precise application of depolarizing pulses of K+ of 5 s duration.
The fluorescence of fura-2 in single cells was measured with the photomultiplier-based system described by Neher (1989), which produces a spatially averaged measure of the [Ca2+]c. Fura-2 was excited with light alternating between 360 and 390 nm, using a Nikon 40 × fluorite objective. Emitted light was transmitted through a 425 nm dichroic mirror and a 500–545 nm barrier filter before being detected by the photomultiplier. [Ca2+]c was calculated from the ratios of the light emitted when the dye was excited by the two alternating excitation wavelengths (Grynkiewicz et al. 1985).
Protein phosphorylation
A PKC phosphorylation assay was performed according to the manufacturer's instructions (Cell Signalling Technology Inc.). Briefly, 1 × 106 chromaffin cells were incubated with Krebs-Hepes buffer in the absence (control) or presence of PKC inhibitors and then 2 μm CCCP in Krebs-Hepes buffer was added for 90 s. When PKC inhibitors were used, cells were pre-exposed to 1 μm of RO31-8220, staurosporine or chelerythrine for 8.5 min and then incubated with 2 μm CCCP for 90 s. The reaction was stopped by addition of 1 × SDS Sample Buffer (62.5 mm Tris, pH 6.8, 2% w/v SDS, 20% glycerol, 50 mm DTT, 0.5 mm EDTA, 0.5 mm EGTA), supplemented with the protease inhibitors phenymethylsulphonyl fluoride (0.5 mm), leupeptin (10 μg ml−1) and aprotinin (10 μg ml−1), as well as phosphatase inhibitors sodium fluoride (1 mm) and sodium orthovanadate (0.1 mm), followed by boiling for 5 min. Samples, each containing an equal amount of protein, were separated by SDS-PAGE and then electrotransferred onto a nitrocellulose membrane for analysis of PKC phosphorylation by Western blotting. Primary antibodies were rabbit polyclonal phospho-PKC (pan) antibody (1 : 500), phospho-MARCKS (ser 152/156) rabbit polyclonal antibody (1 : 500) and mouse monoclonal anti-tubulin antibody (1 : 2000). Following incubation with the corresponding goat (anti-rabbit or anti-mouse) horseradish peroxidase conjugated IgG (1 : 3000) (Bio-Rad Laboratories, Hercules, CA, USA), the membranes were incubated in electrochemiluminescence (ECL) Western blotting detection reagents (Amersham, Oakville, Canada). Chemiluminescence-emitting signals were detected by Hyperfilm ECL. Multiple exposures of each set of samples were carried out and autoradiographs were scanned. The density and area of the bands were calculated with the Scion Image Beta-3b software (Scion Corporation, Frederick, MD, USA). Values obtained for each phospho-PKC band were normalized on the basis of the tubulin band intensity (loading control) to correct for minor variations in loading.
Fluorescence microscopy
Chromaffin cells were plated on collagen-coated coverslips contained within plastic Petri dishes at a density of 3 × 105 cells per 35 mm dish. Cells were rinsed with Krebs-Hepes solution. Cells were incubated for different periods of time with Krebs-Hepes solution and in the absence or presence of different compounds; then they were stimulated by high K+, fixed in 3.7% formaldehyde and processed for fluorescence microscopy (Lee & Trifaró, 1981). Chromaffin cells were stained with rhodamine phalloidin (a probe for filamentous actin; 1.25 U ml−1) and only changes in the cortical actin network of chromaffin cells were recorded and analysed. Coverslips were thoroughly washed with phosphate-buffered saline (PBS). Finally coverslips were rinsed with PBS and mounted in glycerol–PBS (1 : 1). Slides were viewed with a Leitz Ortholux fluorescence microscope and photographs were taken with Kodak-Tri-X pan films (400 ASA) (Vitale et al. 1991). To study the effect of several treatments on cortical F-actin disassembly, 100 single rounded chromaffin cells per coverslip (usually 6–8 coverslips per experimental condition) were examined. Each cell was classified as having either a ‘continuous’ or ‘discontinuous’ cortical rhodamine (F-actin) fluorescent ring (Vitale et al. 1991). The percentage of chromaffin cells showing cortical F-actin disassembly (discontinuous rhodamine fluorescent ring) was calculated for each experimental condition. To avoid personal bias, code numbers were given to each coverslip. The cells were examined and classified without knowing whether they were from control or treated preparations. The codes were revealed to identify the experimental conditions used only after all results were recorded (single-blind design).
Video-enhanced image processing
Quantitative analysis of cortical rhodamine fluorescence (F-actin) was performed by using a Hamamatsu Photonic KK Argus-50/CL Image Processor (Hamamatsu Photonic Systems, Bridgewater, NJ, USA). The fluorescence microscope was coupled to the video camera (Carl Zeiss, TV3M model), which was connected to the Argus 50-Image processor. Video camera control parameters (i.e. gain, offset and sensitivity) were set up to obtain a clear image of the cell on the monitor and a fluorescence intensity of 250 (arbitrary units) in the cortical region of the cell. The three-dimensional graphic analysis represents the coordinates of the equatorial plane of the cell as the X and Y axes and the fluorescence intensity of this plane as the Z axis (Vitale et al. 1995).
Data analyses and statistics
Data are presented as means ± standard errors of the mean (s.e.m.) and were analysed by one-way ANOVA. Scheffe's test was used to determine the level of significance of differences between groups. Results shown in Figs 4 and 6 were analysed by Student's t test.
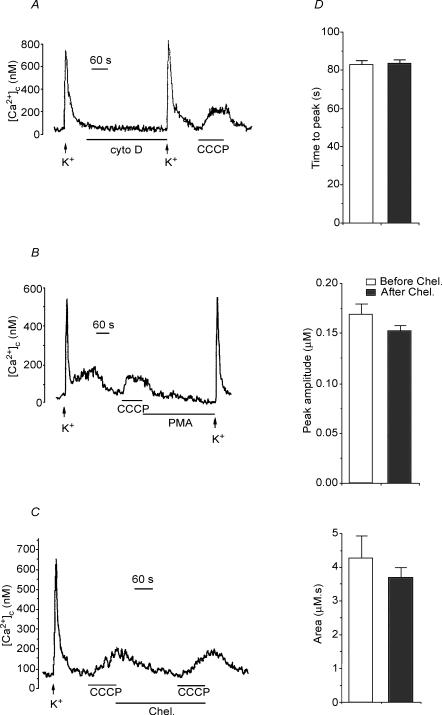
Figure 4. Ca2+ transients during exposure to high K+ or CCCP.
Chromaffin cells cultured on coverslips were loaded with fura-2 and [Ca2+]i was measured at a single cell level as described in Methods. Two 5 s pulses of high K+ (70 mm K+, lower Na+ solution) (arrows) were given to each cell, at the beginning and end of each experiment. Cytochalasin D (2 μm), CCCP (2 μm), PMA (100 nm) and chelerythrine (1 μm) were applied during the time indicated by the horizontal bars in A–C. D, comparison of kinetic parameters of [Ca2+]i transients evoked by CCCP before and after chelerythrine exposure. The top histogram shows the time to peak in seconds, the middle histogram shows the amplitude of the plateau of [Ca2+]c, in the presence of CCCP, and the lower histogram shows the area of the [Ca2+]c curve, expressed in μm s. Bars represent mean ± s.e.m. of 4 experiments.
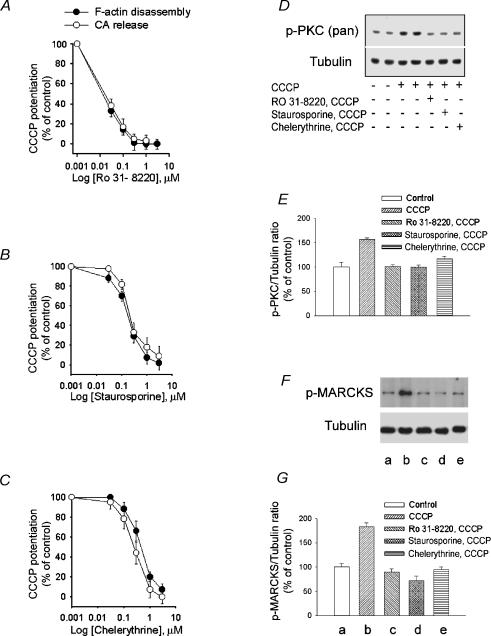
Figure 6. Effects of PCK inhibitors on the potentiation by CCCP of cortical F-actin disassembly, catecholamine secretion and PKC and MARCKS phosphorylation.
A–C, CCCP potentiation of high K+ depolarization-induced F-actin disassembly and catecholamine release was inhibited by RO31-8220, staurosporine and chelerythrine in a concentration-dependent manner. D, Western blot analysis of extracts from CCCP-treated bovine chromaffin cells in the presence or absence of PKC inhibitors. Chromaffin cells were pre-exposed to a Krebs-Hepes solution (control) or to the same solution containing either 1 μm of each of the PKC inhibitors RO31-8220, staurosporine or chelerythrine during 8.5 min. This was followed by incubation with Hepes solution (control) or 2 μm CCCP in Hepes solution for 90 s. The reaction was stopped by addition of ‘Sample buffer’ as described in Methods. Proteins in whole cell lysates were detected by Western blotting with phospho-PKC (pan), and tubulin antibodies. Tubulin was used as the gel-loading control. E, PKC phosphorylation expressed as percentage increase in phospho-PKC content relative to unstimulated (control) cells. Bars represent mean ± s.e.m. of data obtained from 3 different experiments. *P < 0.01 versus control. F, CCCP-induced phosphorylation of MARCKS. Chromaffin cells were incubated for 90 s with either Krebs-Hepes solution or the solution containing 2 μm CCCP. Cell extracts were prepared and proteins were separated by SDS-PAGE followed by Western blotting with phospho-MARCKS and tubulin antibodies. Tubulin was used as the gel-loading control. The effect of PKC inhibitors on CCCP-induced MARCKS phosphorylation is also shown. G, MARCKS phosphorylation expressed as percentage increase in phospho-MARCKS content relative to un-stimulated (control) cells. Letters a to e below the bars correspond to the equivalently labelled lanes in F. Bars represent mean ± s.e.m. of data obtained from 8 different experiments. *P < 0.01 versus control.
Results
Effect of collapsing mitochondrial transmembrane H+ gradient on secretion
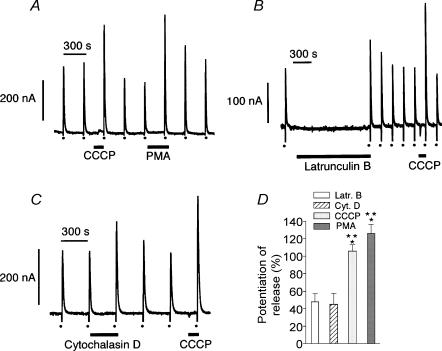
Recent published work has demonstrated that short exposure (90 s) of chromaffin cells to 2 μm of the protonophore cyanide m-chlorophenyl hydrazone (CCCP) potentiated the secretory response to a short depolarizing pulse of K+ (Montero et al. 2000). The strong potentiating effect on K+ depolarization-evoked amine release observed with CCCP treatment was similar to that detected when cells were exposed to 100 nm phorbol ester (PMA; Fig. 1A and D). It is known that potentiation of the chromaffin cell secretory response by PMA is mainly due to a disruption of the cortical F-actin networks allowing the movements of chromaffin vesicles to release sites on the plasma membrane (Vitale et al. 1995). Another way to disrupt cortical F-actin networks is through direct interactions with actin filaments, as was the case for latrunculin B or cytochalasin D treatments (Fig. 1B–D). However, in this situation, the potentiation of high K+-evoked amine release was smaller than that observed with either CCCP or PMA.
Figure 1. CCCP, PMA, latrunculin B and cytochalasin D enhance secretory responses induced by depolarizing concentrations of K+.
Chromaffin cells were superfused with Krebs-Hepes medium at 37°C. After a 10 min equilibration period, cells were stimulated at 5 min intervals with 5 s pulses of medium containing high K+ (150 mm with isosmotic reduction of Na+). High K+ challenges are represented by dots at the bottom of the panels. A, in this and the following experiments, 2 μm CCCP was applied 90 s prior to the high K+ pulse. PMA (100 nm) was applied 5 min prior to the depolarizing K+ pulse. Latrunculin B (5 μm) and cytochalasin D (2 μm) were applied 20 (B) and 5 (C) min, respectively, prior to the high K+ pulse. Amplitudes of secretion peaks are expressed as oxidation currents in nA. D, potentiation of high K+-evoked amine release by latrunculin B (n = 11), cytochalasin D (n = 6), CCCP (n = 34) and PMA (n = 10). Bars represent means ± s.e.m. *P < 0.01 versus latrunculin B; **P < 0.01 versus cytochalasin D.
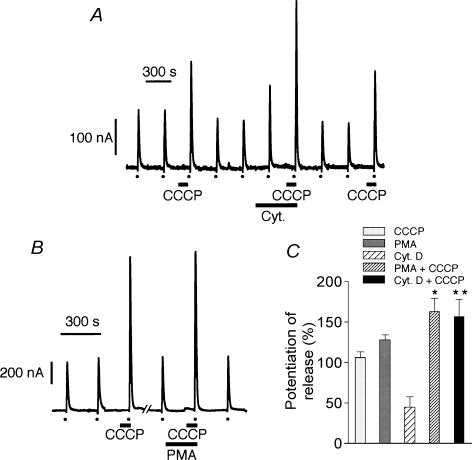
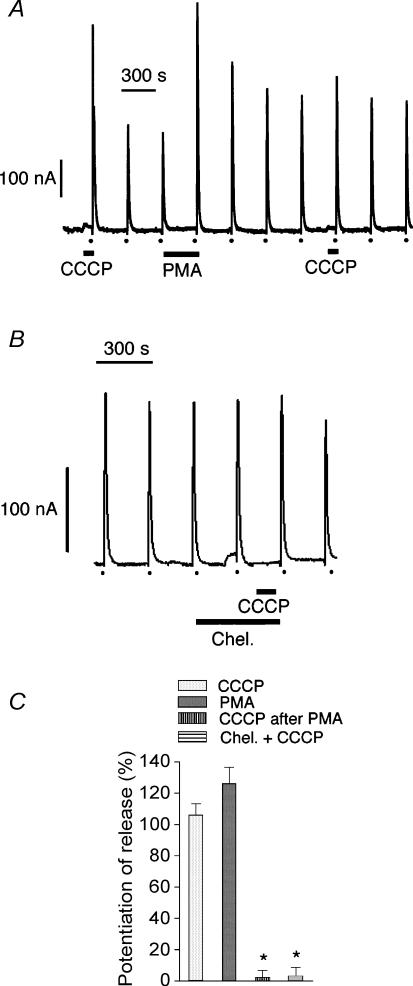
When cytochalasin D and CCCP were applied together, the potentiation of the high K+-evoked response was even larger, showing summation of effects (Fig. 2A and C). This would suggest that two different mechanisms might be involved in the potentiation of the secretory response to depolarization. On the other hand, there was no summation of effects during simultaneous perfusion with CCCP and PMA, although the secretory response to high K+ was a little bit larger than that obtained with either one of the compounds (Fig. 2B and C). It seems therefore possible that the same transduction pathway might be involved in the response to treatment with either CCCP or PMA. When cells were perfused with 100 nm PMA followed by 2 μm CCCP, the secretory response to high K+ was only potentiated during perfusion with PMA; no potentiation was observed in the presence of CCCP (Fig. 3A and C), suggesting, again, that the same transduction pathway was utilized in the potentiation of amine release produced by either of the two compounds. It is known that the effects of PMA on chromaffin cells are mediated through activation of protein kinase C (PKC) (Vitale et al. 1992, 1995). In the present experiments, 1 μm chelerythrine (an inhibitor of PKC), when present prior to and during perfusion with 2 μm CCCP, abolished the potentiating effects of CCCP on high K+ responses (Fig. 3B and C).
Figure 2. Effects of cytochalasin D and PMA on the potentiation of K+-evoked amine release produced by CCCP.
A, secretion peaks in nA obtained during 5 s high K+ (150 mm) pulses (dots at the bottom of panels) in the absence or presence (90 s prior to stimulation) of CCCP. Cytochalasin D (2 μm) was present in the medium 5 min prior to and during the second 90 s perfusion period with CCCP. B, similarly, 100 nm PMA was present in the medium 3.5 min prior to and during the 90 s perfusion with CCCP. C, potentiation of high K+-induced amine release by CCCP (n = 29), PMA (n = 6), cytochalasin D (n = 6), PMA + CCCP (n = 8), and cytochalasin D + CCCP (n = 8). Bars represent mean ± s.e.m. *P < 0.01 versus CCCP or PMA; **P < 0.01 versus cytochalasin D. Other conditions were as described in legend to Fig. 1.
Figure 3. Effect of PMA and chelerythrine on the potentiation of K+-induced amine release produced by CCCP.
Secretion peaks in nA obtained during 5 s pulses of depolarizing concentrations (150 mm) of K+ (dots at the bottom of the panels). A, PMA (100 nm) and CCCP (2 μm) were perfused 5 min and 90 s, respectively, prior to the high K+ challenges. B, chelerythrine (1 μm) was present in the medium 8.5 min prior to and during the 90 s period of perfusion with CCCP. C, potentiation of high K+-evoked amine release by CCCP (n = 36), PMA (n = 6), CCCP after PMA (n = 5) and CCCP in the presence of chelerythrine (n = 6). Bars represent mean ± s.e.m. *P < 0.01 versus CCCP. Other conditions were as described in legend to Fig. 1.
Changes of the [Ca2+]c evoked by CCCP
These experiments were performed in fura-2-loaded, single chromaffin cells, at room temperature. Figure 4A shows the original [Ca2+]c traces obtained in an example cell. After the [Ca2+]c reached a stable baseline (at about 100 nm concentration) a K+ pulse (70 mm K+, 5 s duration) caused a sharp rise of [Ca2+]c that declined quickly to reach basal levels in about 1 min. The cell was subsequently superfused with cytochalasin D (2 μm), which did not change the basal [Ca2+]c. Then a second pulse of K+ was added, followed by the application of CCCP (2 μm), which produced a slow rise of [Ca2+]c that reached a plateau at around 200 nm and returned quickly to the basal level upon washing out CCCP.
Similar experiments were performed in other cells to test the effect of PMA. For instance, Fig. 4B shows a cell that was first challenged with K+ and subsequently with CCCP. Note that, again, CCCP caused a mild and sustained elevation of the [Ca2+]c; note also that PMA (100 nm) did not change the basal levels of [Ca2+]c. An example of a third protocol is shown in Fig. 4C. Note that CCCP produced its typical [Ca2+]c elevation effect. When the cell was superfused with chelerythrine (100 μm), there was no change in the basal levels of Ca2+. When applied in the presence of chelerythrine, CCCP produced an elevation of the [Ca2+]c that was slightly higher. However, averaged results of four experiments show no changes in the kinetic parameters observed for the CCCP-evoked changes in [Ca2+]c, i.e. peak amplitude, time to peak and area of the Ca2+ curve, which represents the total Ca2+ accumulated in the cytosol during the CCCP challenge. Note that chelerythrine did not modify either the basal [Ca2+]c or the kinetic parameters of the [Ca2+]c elevation elicited by CCCP.
Effect of CCCP on chromaffin cell cortical F-actin networks
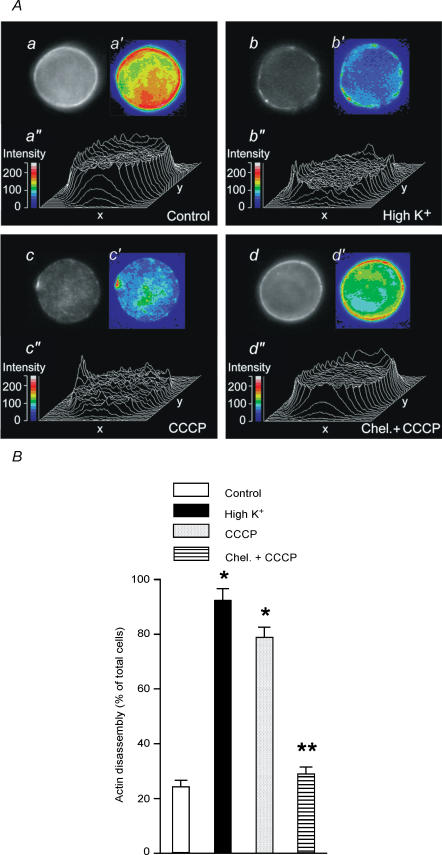
The chromaffin cell possesses a cortical network of filamentous actin underneath the plasma membrane which is disrupted by cholinergic receptor stimulation, cell depolarization or PMA treatment (Vitale et al. 1991, 1995). Therefore, one of the effects of PKC activation responsible for the potentiation of the secretory response is the disassembly of cortical filamentous-actin networks (Vitale et al. 1995), an effect mediated through the phosphorylation of MARCKS (Rosé et al. 2001). Treatment of chromaffin cells for 90 s with 2 μm CCCP produced cortical F-actin disassembly as evaluated by fluorescence microscopy of rhodamine–phalloidin (a probe for filamentous actin) stained cells (Fig. 5A and B). The fluorescence images of cortical F-actin disassembly obtained were quite similar to those observed after 100 nm PMA or high K+ treatment of chromaffin cells (Vitale et al. 1995). Moreover, the disruption in the cortical F-actin fluorescence ring produced by CCCP was inhibited by treatment with 1 μm chelerythrine (Fig. 5A and B), suggesting the involvement of PKC in this process. Similarly to PKC stimulation, CCCP-induced cortical F-actin disassembly was not dependent on extracellular Ca2+. CCCP-induced F-actin disassembly was observed in 76.9 ± 3.2% (n = 400) of the cells in the presence of extracellular Ca2+, and in 74.1 ± 1.8% (n = 600) of the cells in Ca2+-free medium. Moreover, F-actin disassembly in Ca2+-free medium in response to CCCP was inhibited by 98.8 ± 6.6% (n = 600) by preincubation of cells for 30 min with 50 μm BAPTA AM.
Figure 5. Effect of CCCP on chromaffin cell cortical F-actin networks.
Cells cultured on coverslips were stimulated by either a 5 s pulse of high K+ or a 90 s exposure to 2 μm CCCP. In some experiments, 1 μm chelerythrine was present 8.5 min prior to and during the 90 s exposure to CCCP. A, fluorescence microscopy and video-enhanced image analysis of F-actin fluorescent profiles of a single control (a, a′, a″) cell or cells stimulated with high K+ (b, b′, b″), CCCP (c, c′, c″) or CCCP in the presence of chelerythrine (d, d′, d″). Image a shows a resting chromaffin cell after rhodamine–phalloidin treatment. A weak cytoplasmic staining and a continuous and bright cortical fluorescent ring is observed. Stimulation of cells with either high K+ (b) or CCCP (c) caused a disruption of the cortical fluorescent ring. In the presence of chelerythrine, CCCP was unable to induce disruption of the cortical fluorescent ring (d). Images a′, b′, c′ and d′ and three-dimensional plots of fluorescence intensities (a″, b″, c″ and d″) of the same cells shown in a′, b′, c′ and d′. Fluorescence intensities of a′, b′, c′ and d′ are depicted in pseudo-colours according with the scales shown in arbitrary units. B, under the fluorescence microscope the rhodamine cortical staining was analysed and classified as being ‘continuous’, as in Aa and a′ and Ad and d′, or ‘discontinuous’, as in Ab and b′ and Ac and c′, and the percentage of cells displaying cortical F-actin disassembly (disrupted cortical rhodamine staining) in control and treated preparations was calculated as indicated in Methods. For each experimental condition, 400 cells from at least 3 different cultures were examined. Bars represent mean ± s.e.m. *P < 0.01 versus control; **P < 0.01 versus CCCP.
Correlation between cortical F-actin disassembly and catecholamine output and their concentration-dependent inhibition by PKC inhibitors
Potentiating by CCCP of high K+ -evoked catecholamine output and cortical F-actin disassembly was measured in the presence or absence of increasing concentrations of chelerythrine, staurosporine or RO31-8220 (Fig. 6A–C), three PKC inhibitors. The three blockers produced a concentration-dependent inhibition of both responses, suggesting the involvement of PKC in CCCP potentiation of these two high K+-evoked effects. Moreover each blocker produced the same degree of inhibition for both catecholamine release and F-actin disassembly (Fig. 6A–C). Thus, as expected each inhibitor showed similar IC50 values for catecholamine output and F-actin disassembly. IC50 values were (μm): chelerythrine, 0.35 and 0.46, staurosporine, 0.22 and 0.20, RO31-8220, 0.013 and 0.016 for catecholamine output and F-actin disassembly, respectively.
Effect of CCCP on PKC and MARCKS phosphorylation
Phosphorylation of PKC is an indication of its activation (Keranen et al. 1995). Therefore, the effect of 90 s CCCP exposure on chromaffin cell PKC phosphorylation was measured by Western blotting using an antibody specific for phosphorylated PKC. Under these experimental conditions, a 50% increase in PKC phosphorylation was observed (Fig. 6D and E). As expected, CCCP-evoked PKC phosphorylation was inhibited by the three PKC blockers.
Furthermore, experiments from our laboratory have shown that cortical F-actin disassembly induced by PKC activation is mediated by phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) (Rosé et al. 2001), a PKC substrate. Unphosphorylated MARCKS cross-links actin filaments whereas phosphorylated MARCKS decreases F-actin cross-linking and promotes F-actin disassembly (Hartwig et al. 1992; Rosé et al. 2001). An increase in MARCKS phosphorylation was observed 90 s following CCCP exposure (Fig. 6F and G) and, again, this effect was abolished by PKC inhibitors. MARCKS phosphorylation was similar in the presence (70 ± 9.5% increase in phosphorylation, n = 8) or absence (61 ± 8.2%, n = 6) of extracellular Ca2+. Moreover, the increase in MARCKS phosphorylation produced by CCCP in Ca2+-free medium was inhibited 100 ± 8% (n = 6) by 30 min preincubation with 50 μm BAPTA AM.
Specificity of CCCP effects: Is mitochondria the only target for CCCP?
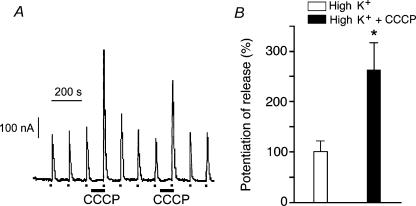
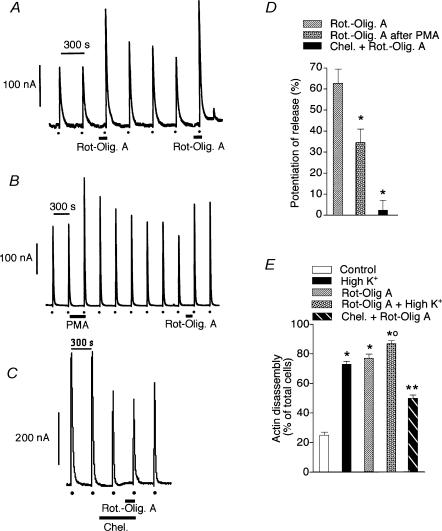
It has been suggested that CCCP may have other intracellular targets such as the endoplasmic reticulum (ER) and therefore the possibility exists that the effects observed here with CCCP were the result of Ca2+ being released from the ER. To test this possibility, the ER Ca2+ store was depleted by 30 min treatment with a combination of 10 mm caffeine, 10 μm ryanodine and 1 μm thapsigargin. This treatment releases ER Ca2+, leaves the ryanodine receptor channel of the ER in a low conductance open state (Alonso et al. 1999) and, in addition, blocks Ca2+ uptake into the ER. Under these conditions, the CCCP potentiating effect on high K+-evoked secretory responses was maintained (Fig. 7) whereas the secretory response to caffeine was abolished (data not shown). Furthermore, when mitochondrial respiration and ATP production were blocked by treatment with a combination of 4 μm rotenone and 3 μm oligomycin A, a potentiation of the high K+-evoked secretion was also observed (Fig. 8A and D). As with the CCCP-evoked potentiation of depolarization-evoked amine release, pretreatment with 100 nm PMA significantly reduced the rotenone and oligomycin A-induced potentiation (Fig. 8B and D) and, in addition, 1 μm chelerythrine produced a complete blockade (Fig. 8C and D), suggesting the involvement of PKC in the process. Cortical F-actin cytoskeleton disassembly was also observed with this combined treatment (Fig. 8E). The fluorescence images of cortical F-actin disassembly obtained following rotenone–oligomycin A treatment were quite similar to those observed upon CCCP treatment. Moreover, the effects of rotenone–oligomycin A on the cytoskeleton were blocked by 1 μm chelerythrine (Fig. 8E).
Figure 7. The potentiating effect of CCCP on high K+-evoked amine release remains after depletion of endoplasmic reticulum (ER) Ca2+ stores.
Chromaffin cell ER Ca2+ stores were depleted by 30 min treatment with a combination of 10 mm caffeine, 10 μm ryanodine and 1 μm thapsigargin in Ca2+-free medium as previously described (Alonso et al. 1999). Cells were then superfused with regular Krebs-Hepes solution and stimulated with 5 s pulses of high K+ (dots at the bottom of the panel). A, when cells were perfused with 2 μm CCCP for 90 s prior to the high K+ pulse, the secretory response to high K+ was significantly potentiated. B, bars represent mean ± s.e.m. (n = 11) of amine secretion during high K+ pulses (control; the secretory response to high K+ was considered to be 100%) or the pulses preceded by 90 s exposure to 2 μm CCCP. Secretion was calculated by integration of the areas of the secretory peaks and then expressed as percentages. *P < 0.01 versus high K+.
Figure 8. Rotenone and oligomycin A potentiate high K+-evoked amine release.
A–C, amine secretion peaks in nA obtained during 5 s pulses of depolarizing K+ (dots at the bottom of panels). A combination of 4 μm rotenone and 3 μm oligomycin A was superfused, when indicated by horizontal bars, prior to the high K+ pulse. Chelerythrine (1 μm) was superfused 8.5 min prior to and during the rotenone–oligomycin A exposure. D, potentiation of high K+-induced amine release by rotenone–oligomycin A (n = 7), rotenone–oligomycin A following PMA exposure (n = 5), and rotenone–oligomycin A in the presence of chelerythrine (n = 6). Bars represent mean ± s.e.m. *P < 0.01 versus rotenone–oligomycin A. E, chromaffin cell cortical F-actin disassembly in control (n = 5) and in cells stimulated for 5 s with high K+ (n = 4), 90 s exposure to rotenone–oligomycin A in the absence (n = 5) or presence of high K+ (n = 4) during the last 5 s exposure to the compounds, and rotenone–oligomycin A in the presence of 1 μm chelerythrine (n = 4). The rhodamine cortical staining was analysed as described in the legend to Fig. 5. Bars represent the mean ± s.e.m. *P < 0.01 versus control; **P < 0.01 versus rotenone–oligomycin A; †P < 0.01 versus high K+ or rotenone–oligomycin A.
Discussion
Several publications (Giovannucci et al. 1999; Montero et al. 2000; Taylor et al. 2000), including the present work, have shown that treatment with mitochondrial inhibitors, such as the protonophore CCCP, potentiates the secretory response to chromaffin and PC12 cell stimulation. In the present experiments, the exposure of chromaffin cells to 2 μm CCCP was for 90 s prior to the depolarizing pulse of K+ (3.5 min after the previous stimulus). Under these conditions, the secretory response was potentiated 2–2.5 times (Fig. 1). It seems that during the exposure to CCCP, enough Ca2+ was accumulated to potentiate the secretory response to high K+. Indeed, exposure of chromaffin cells to 2 μm CCCP for 90 s increased cytosolic Ca2+ even when total cytosolic Ca2+ had apparently reached resting levels (Fig. 4C). This would suggest that, under resting conditions, there would be cellular microenvironments where the level of intracellular Ca2+ would be in the range of at least 2–3 μm (Montero et al. 2000), a concentration which is enough to evoke uptake into mitochondria and, consequently, observe the effect of CCCP.
As demonstrated here, other treatments such as exposure to cytochalasin D, latrunculin B or phorbol esters (PMA) also potentiated the secretory response to a depolarizing pulse of K+. However, exposure to these substances did not modify resting intracellular Ca2+ levels (Fig. 4). It is known that PMA potentiates chromaffin cell responses to different secretagogues (Burgoyne & Norman, 1984; Pocotte et al. 1985; Brocklehurst et al. 1985; Burgoyne et al. 1988; Knight et al. 1988; Terbush et al. 1988; Bittner & Holz, 1990; Tachikawa et al. 1990; Vitale et al. 1992, 1995; Smith et al. 1998; Rosé et al. 2001). It is also known that PMA treatment does not induce secretion but disrupts chromaffin cell cortical F-actin networks and increases both the number of secretory vesicles at the subplasmalemmal zone (release-ready vesicle pool) and the initial rate of exocytosis in response to stimulation (Vitale et al. 1995). Indeed, membrane capacitance studies showed, in PMA-treated cells, an increased number of vesicles fusing with the plasma membrane during cell depolarization (Vitale et al. 1995; Smith et al. 1998). The chromaffin cell cortical F-actin network acts as a barrier to the secretory vesicles by blocking their movement toward the plasma membrane (Trifaró et al. 1982, 1985; Cheek & Burgoyne, 1986; Burgoyne & Cheek, 1987; Sontag et al. 1988; Burgoyne et al. 1989). Cell stimulation is accompanied by a focal transient disassembly of the cortical F-actin network (Cheek & Burgoyne, 1986; Vitale et al. 1991). This allows the movement of additional vesicles from the reserve pool to release sites at the plasma membrane (Vitale et al. 1995).
Cytochalasin D is a fungal toxin that depolymerizes F-actin and disrupts actin filaments (Brenner & Korn, 1979; Flanagan & Lin, 1980; Schliwa, 1982) and latrunculin B is a unique marine sponge toxin that also disrupts actin microfilaments, but in this case, by blocking actin polymerization (Spector et al. 1983). These two toxins did not cause secretion on their own but, as demonstrated here, they potentiated the secretory response. It is therefore quite possible that the potentiating effect of the toxins was due to the movement of secretory vesicles to the subplasmalemma area prior to the depolarizing stimulus; this being the result of the disruption of cortical F-actin (Gil et al. 2000). Perfusion of chromaffin cells with a combination of cytochalasin D and CCCP produced a summation of effects in the potentiation of high K+ secretory responses (Fig. 2), suggesting the involvement of different mechanisms. On the other hand, the combination of PMA and CCCP did not show summation of effects, although responses to stimulation were larger than those in the presence of either substance (Fig. 2). This observation suggested to us that PMA and CCCP elicited potentiation of the secretory response through the same final common pathway. Indeed, when the CCCP pulse was preceded by a PMA pulse the potentiating effect of CCCP was completely abolished (Fig. 3), suggesting, again, a common pathway for these effects. The effect of PMA on secretion is the result of PKC stimulation (Vitale et al. 1992, 1995) since the effect is blocked by PKC inhibitors (Vitale et al. 1992). Chelerythrine, staurosporine and RO31-8220, three known inhibitors of PKC, also blocked the potentiating effect of CCCP (Fig. 6), suggesting the involvement of PKC in the effects elicited by this compound. Indeed, exposure to CCCP for 90 s increased PKC and MARCKS phosphorylation, effects blocked by the three PKC inhibitors. Published work from our laboratory has also demonstrated that MARCKS, a PKC substrate, mediates, at least in part, the effect of PKC on secretion (Rosé et al. 2001). In view of these observations, immunocytochemical studies with rhodamine-labelled phalloidin, a probe for filamentous actin, indicated, as expected, that exposure of chromaffin cells to CCCP induced cortical F-actin disassembly which was inhibited by the three PKC blockers (Fig. 6), suggesting, again, the involvement of PKC in this process. Cortical F-actin disassembly was also observed during the exposure of resting cells to CCCP. Under these conditions, no increase in secretion was observed. These results are similar to those obtained with PMA, a substance which evoked cortical F-actin disassembly without inducing secretion (Vitale et al. 1995). The increase in cellular Ca2+ observed upon CCCP treatment was of such magnitude as to trigger the mechanism responsible for cortical F-actin disassembly. In this regard, it has been suggested that an increase in cytosolic Ca2+ might signal the mechanism involved in supplying vesicles to release sites (Von Rüden & Neher, 1993; Kamiya & Zucker, 1994). It can be concluded from the present results that CCCP potentiation of the secretory responses was, as with PMA treatment, the result of translocation of secretory vesicles from the reserve pool to the subplasmalemma area (release-ready vesicle pool) in preparation for exocytosis. As a site of action for these effects, mitochondria were demonstrated to be responsible for the potentiation of the secretory response observed in the presence of CCCP, even when the release of Ca2+ from the endoplasmic reticulum was blocked by previous treatment with a cocktail of caffeine, ryanodine and thapsigargin. In addition, exposure of chromaffin cells to a combination of rotenone and oligomycin A, two other mitochondrial inhibitors that do not affect Ca2+ mobilization from the endoplasmic reticulum, also caused a potentiation of secretion. The combination of rotenone and oligomycin A not only potentiated the secretory response to high K+, but also evoked chromaffin cell cortical F-actin disassembly, effects that were also inhibited by chelerythrine (Fig. 8). The inhibition by chelerythrine suggests, again, the involvement of PKC in this process. Two pathways are known to be involved in the control of chromaffin cell cortical F-actin networks during secretion (Trifaró et al. 2000). These are the Ca2+–scinderin and the PKC–MARCKS pathway. The Ca2+–scinderin pathway uses Ca2+ that enters the cell through voltage-dependent channels during depolarization induced by nicotinic receptor stimulation (Trifaró et al. 2000) whereas the PKC pathway seems to be activated during either the release of Ca2+ from the endoplasmic reticulum (Zhang et al. 1995; Trifaró et al. 2000; Rosé et al. 2001) or the increase in cell Ca2+ observed when mitochondria Ca2+ uptake is inhibited, as in this case with CCCP treatment.
In conclusion, our findings suggest that the clearance of cytosolic Ca2+ by mitochondria during chromaffin cell stimulation limits the extent of the exocytotic response by preventing excessive stimulation of PKC and enhanced cortical F-actin disassembly. Thus excessive PKC activation would increase the number of secretory vesicles at release sites followed by an increased and perhaps unnecessary secretory response.
Acknowledgments
We acknowledge Canadian Institutes of Health Research (CIHR) grants to J.-M.T and the following grants to A.G.G.: Grupos Estratégicos III PRICIT CAM/UAM, DGICYT PM99-0004, FIS N°01/0183, and Red CIEN ISCIII.
References
- Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo-Ibañez I, García AG, García-Sancho J, Montero M, Alvarez J. Ca2+-induced Ca2+ release from chromaffin cell seen from inside the ER with targeted aequorin. J Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner MA, Holz RW. Phorbol esters enhance exocytosis from chromaffin cells by two mechanisms. J Neurochem. 1990;54:205–210. doi: 10.1111/j.1471-4159.1990.tb13302.x. [DOI] [PubMed] [Google Scholar]
- Borges R, Sala F, García AG. Continuous monitoring of catecholamine release from perfused cat adrenals. J Neurosci Meth. 1986;16:389–400. doi: 10.1016/0165-0270(86)90054-3. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Korn ED. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. J Biol Chem. 1979;254:9982–9985. [PubMed] [Google Scholar]
- Brocklehurst KW, Morita K, Pollard HB. Characterization of protein kinase C and its role in catecholamine secretion from bovine adrenal medullary cells. Biochem J. 1985;228:35–42. doi: 10.1042/bj2280035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Cheek TR. Reorganization of peripheral actin filaments as a prelude to exocytosis. Biosci Rep. 1987;7:281–288. doi: 10.1007/BF01121449. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A, O'Sullivan AJ. A major role for protein kinase C in calcium activated exocytosis in permeabilized adrenal chromaffin cells. FEBS Lett. 1988;238:151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A, O'Sullivan AJ. The control of cytoskeletal actin and exocytosis in intact and permeabilized adrenal chromaffin cells: role of calcium and protein kinase C. Cell Signal. 1989;1:323–334. doi: 10.1016/0898-6568(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Norman K-M. Effect of calmidazolium and phorbol ester on catecholamine secretion from adrenal chromaffin cells. Biochim Biophys Acta. 1984;805:37–43. doi: 10.1016/0167-4889(84)90034-x. [DOI] [PubMed] [Google Scholar]
- Cheek TR, Burgoyne RD. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 1986;207:110–114. doi: 10.1016/0014-5793(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signaling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan MD, Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980;225:835–838. [PubMed] [Google Scholar]
- Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+] J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía L, García AG, Morad M. ATP modulation of calcium channels in bovine chromaffin cells. J Physiol. 1993;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Rueda J, Viniegra S, Gutiérrez LM. The F-actin cytoskeleton modulates slow secretory components rather than readily releasable vesicle pool in bovine chromaffin cells. Neurosci. 2000;98:605–614. doi: 10.1016/s0306-4522(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Giovannucci DR, Hlubek MD, Stuenkel EL. Mitochondria regulate the Ca2+-exocytosis relationship of bovine adrenal chromaffin cells. J Neurosci. 1999;19:9261–9270. doi: 10.1523/JNEUROSCI.19-21-09261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Peonie M, Tsien RY. A new generation of Ca 2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PH, Nairh AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Heinemann C, von Rüden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Knight DE, Sugden D, Baker PF. Evidence implicating protein kinase C in exocytosis from electro-permeabilized bovine chromaffin cells. J Memb Biol. 1988;104:21–34. doi: 10.1007/BF01871899. [DOI] [PubMed] [Google Scholar]
- Kuba K. Ca2+ dynamics and modulation. In: Kuba K, Higashida H, Brown DA, Yoshioka T, editors. Slow Synaptic Responses and Modulation. New York: Springer; 2000. pp. 163–181. [Google Scholar]
- Lee RWH, Trifaró J-M. Characterization of anti-actin antibodies and their use in immunocytochemical studies on the localization of actin in adrenal chromaffin cells. Neuroscience. 1981;6:2087–2108. doi: 10.1016/0306-4522(81)90048-8. [DOI] [PubMed] [Google Scholar]
- Montero M, Alonso MT, Carnicero E, Cuchillo-Ibañez I, Albillos A, García AG, García-Sancho J, Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nature Cell Biol. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- Moro MA, López MG, Michelena P, García AG. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990;185:243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Neher E. Combined Fura-2 and patch-clamp measurements in rat peritoneal mast cells. In: Sellin LC, Libelius R, Thesleff S, editors. Neuromuscular Junction 5. the Netherlands: Elsevier; 1989. pp. 65–76. [Google Scholar]
- Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Park YB, Herrington J, Babcock DF, Hille B. Ca2+ clearance mechanisms in isolated rat adrenal chromaffin cells. J Physiol. 1996;492:329–346. doi: 10.1113/jphysiol.1996.sp021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocotte SL, Freyre RA, Senter RA, TerBush DR, Lee SA, Holz RW. Effects of phorbol ester on catecholamine secretion and protein phosphorylation in adrenal medullary cell cultures. Proc Nat Acad Sci U S A. 1985;82:930–934. doi: 10.1073/pnas.82.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosé SD, Lejen T, Zhang Li, Trifaró J-M. Chromaffin cell F-actin disassembly and potentiation of catecholamine release in response to protein kinase C activation by phorbol esters is mediated through myristoylated alanine rich C kinase substrate phosphorylation. J Biol Chem. 2001;276:36757–36763. doi: 10.1074/jbc.M006518200. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeleton networks. J Cell Biol. 1982;92:79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- Sontag J-M, Aunis D, Bader MF. Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur J Cell Biol. 1988;46:316–326. [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Tachikawa E, Takahashi S, Kashimoto T, Kondo Y. Role of Ca2+/phospholipids dependent protein kinase in catecholamine secretion from bovine adrenal medullary chromaffin cells. Biochem Pharmacol. 1990;40:1505–1513. doi: 10.1016/0006-2952(90)90447-s. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Shaw SM, Peers C. Mitochondrial inhibitors evoke catecholamine release from phoechromocytoma cells. Biochem Biophys Res Commun. 2000;273:17–21. doi: 10.1006/bbrc.2000.2894. [DOI] [PubMed] [Google Scholar]
- Terbush DR, Bittner MA, Holz R. Ca2+ influx causes rapid translocation of protein kinase C to membranes. J Biol Chem. 1988;263:18873–18879. [PubMed] [Google Scholar]
- Trifaró J-M, Bader M-F, Doucet JP. Chromaffin cell cytoskeleton: its possible role in secretion. Can J Biochem Cell Biol. 1985;63:661–679. doi: 10.1139/o85-084. [DOI] [PubMed] [Google Scholar]
- Trifaró J-M, Lee RWH. Morphological characteristics and stimulus secretion coupling in bovine adrenal chromaffin cell cultures. Neuroscience. 1980;5:1533–1546. doi: 10.1016/0306-4522(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Trifaró J-M, Lee RWH, Kenigsberg RL, Côté A. Contractile proteins and chromaffin cell function. Adv Biosci. 1982;V:151–158. [Google Scholar]
- Trifaró J-M, Poisner AM. Common properties in the mechanisms of synthesis, processing and storage of secretory products, in the secretory process. In: Poisner A M, Trifaró J-M, editors. The Secretory Granule. I. the Netherlands: Elsevier/North Holland; 1982. pp. 387–407. [Google Scholar]
- Trifaró J-M, Rosé SD, Lejen T, Elzagallaai B. Two pathways control chromaffin cell cortical F-actin dynamics during exocytosis. Biochimie. 2000;82:339–352. doi: 10.1016/s0300-9084(00)00193-0. [DOI] [PubMed] [Google Scholar]
- Vitale ML, Rodríguez Del Castillo A, Tchakarov L, Trifaró J-M. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis: a phenomenon not exhibited by gelsolin. J Cell Biol. 1991;113:1057–1067. doi: 10.1083/jcb.113.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale ML, Rodríguez Del Castillo A, Trifaró J-M. Protein kinase C activation by phorbol esters induces chromaffin cell cortical filamentous actin disassembly and increases the initial rate of exocytosis in response to nicotine receptor stimulation. Neuroscience. 1992;51:463–474. doi: 10.1016/0306-4522(92)90330-5. [DOI] [PubMed] [Google Scholar]
- Vitale ML, Seward EP, Trifaró J-M. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Von Rüden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rodríguez Del Castillo A, Trifaró J-M. Histamine-evoked chromaffin cell scinderin redistribution, F-actin disassembly, and secretion: in the absence of cortical F-actin disassembly, an increase in intracellular Ca2+ fails to trigger exocytosis. J Neurochem. 1995;65:1297–1308. doi: 10.1046/j.1471-4159.1995.65031297.x. [DOI] [PubMed] [Google Scholar]