Abstract
Electrical resonance is a mechanism used by birds and many vertebrates to discriminate between frequencies of sound, and occurs when the intrinsic oscillation in the membrane potential of a specific hair cell corresponds to a specific stimulus sound frequency. This intrinsic oscillation results from an interplay between an inward Ca2+ current and the resultant activation of a hyperpolarizing Ca2+-activated K+ current. These channels are predicted to lie in close proximity owing to the fast oscillation in membrane potential. The interplay of these channels is widespread in the nervous system, where they perform numerous roles including the control of synaptic release, burst frequency and circadian rhythm generation. Here, we used confocal microscopy to show that these two ion channels are clustered and colocalized in the chick hair cell membrane. The majority of Ca2+ channels were colocalized while the proportion of colocalized BK channels was markedly less. In addition, we report both an apical–basal gradient of these clusters in individual hair cells, as well as a gradient in the number of clusters between hair cells along the tonotopic axis. These results give physical confirmation of previous predictions. Since the proportion of colocalized channels was a constant function of Ca2+ channels, and not of BK channels, these results suggest that their colocalization is determined by the former. The molecular mechanisms underpinning their clustering and colocalization are likely to be common to other neuronal cells.
Hair cells of the inner ear are stimulated by sound-induced mechanical movement of their sensory hair bundles. In many vertebrates the hair cells are arranged on the sensory epithelium in a tonotopic manner, meaning that from one end to the other, each hair cell responds to a stimulus of a particular frequency. Different mechanisms, some acting in concert, contribute to this frequency selectivity (Fettiplace & Fuchs, 1999). Electrical tuning (resonance) is one such mechanism that determines frequency selectivity in hair cells of the auditory epithelium of a number of vertebrates. Crawford and Fettiplace initially observed that the membrane potential of turtle auditory hair cells had a dampened oscillatory response to a small injected current and that the frequency of this oscillation corresponded to the frequency of sound that the hair cell best responded to (characteristic frequency) (Crawford & Fettiplace, 1981). Therefore the sensitivity and selectivity of the hair cell response is greatest when the frequency of the mechanical input to the hair bundle matches the oscillation frequency (electrical resonance) of the membrane (Fettiplace & Fuchs, 1999). The initial observation of electrical tuning in turtle hair cells has been extended to chick auditory hair cells, and alligator auditory and frog saccular hair cells (Ashmore, 1983; Fuchs et al. 1988; Fuchs & Evans, 1988). Oscillation of membrane potential in these hair cells is brought about by an inward Ca2+ current (L-type DHP-sensitive and possibly atypical N-type Ca2+ current) and an outward Ca2+-gated K+ current (BK) (Art & Fettiplace, 1987; Hudspeth & Lewis, 1988; Rodriguez-Contreras & Yamoah, 2001). Increasing channel numbers, decreasing cell capacitances, and increasingly faster kinetics of the BK channels, all contribute to the increasing frequency of oscillation in membrane potential of high frequency hair cells (Art & Fettiplace, 1987; Art et al. 1995). The molecular identity of these currents has been established with the cloning of these ion channels (Jiang et al. 1997; Kollmar et al. 1997; Navaratnam et al. 1997; Jones et al. 1998). The importance of the interaction between these channels has been demonstrated in other systems. For instance, their interplay is implicated in regulating Purkinje cell burst frequency (Swensen & Bean, 2003), transmitter release at the neuromuscular junction (NMJ; Augustine et al. 1988; Robitaille et al. 1993) and the circadian rhythm of spiking frequency in the suprachiasmatic nucleus (Cloues & Sather, 2003).
Both theory and evidence would place Ca2+ channels close to BK channels. The concept of clustering and colocalization, the empirical evidence of which has largely been indirect and inferred, is widely accepted. BK channels in hair cells are activated primarily by influx of Ca2+ at physiological membrane potentials (Art & Fettiplace, 1987; Roberts et al. 1990; Wu et al. 1995). The rapid oscillation of membrane potential (modelled to reach several thousand hertz in chick hair cells) in the presence of high concentrations of Ca2+ buffer (millimolar Calbindin in chick hair cells), which limit Ca2+ diffusion, would necessitate that the two channels reside in close approximation (Wu et al. 1995; Hackney et al. 2003). Furthermore, the rapid activation of Ca2+ channels is followed closely in time by activation of the BK channels, implying, based on diffusion limitations, that these channels are in close proximity (Fettiplace & Fuchs, 1999). Roberts et al. (1990) showed that patch recordings from the basolateral surface of frog saccular hair cells that contained Ca2+ currents also contained BK currents (Roberts et al. 1990). In addition, they inferred from the non-uniform distribution of randomly selected patches that evidenced current that the two channels existed in clusters (Roberts et al. 1990).
In this study we attempted to determine the distribution of these two channels within hair cells using antibody labelling of these channels visualized by confocal microscopy. We demonstrate here that both BK and Ca2+ channels exist in clusters in chick auditory hair cells. We also show that these two channels colocalize in these clusters. Furthermore, we illustrate that a number of these clusters have an apical-to-basal (vertical) density gradient within a single cell, and a gradient between cells from the low to high frequency regions of the sensory epithelium.
Methods
Confocal microscopy
Freshly isolated cochleas from 5-day-old white leghorn chicks killed by CO2 asphyxiation (in accordance with approved protocols from Yale University and the University of Pennsylvania animal use and care committees) were fixed for 30 min in 4% paraformaldehyde and the tegmentum vasculosum was removed to reveal the basilar papilla. Basilar papillae were washed in PBS and blocked with PBS, 10% goat serum, 2% BSA and 0.1% Tween 20, for a further 60 min at room temperature (RT). Tissue was then incubated with primary antibody (polyclonal rabbit anti-Ca2+ channel pan alpha; 5 μg ml−1 in blocking solution; Chemicon, Ltd) for an additional hour at RT. Secondary antibody (Alexa 488 highly cross-adsorbed goat anti-rabbit serum, 1 : 300 in blocking solution; Molecular Probes) was applied to the tissue for a further hour after 5 × washes in PBS, and 0.1% Tween 20. The tissue was then incubated with a primary antibody to the BK channel (monoclonal anti-Slo; 5 μg ml−1 in blocking solution; Transduction labs/BD) for 1 h at RT and washed 5 × with PBS, and 0.1% Tween 20. Rhodamine conjugated goat anti-mouse secondary antibody (BD labs) was applied to the tissue for a further 1 h, washed in PBS and 0.1% Tween 20 and mounted on a glass cover slip with Vectorshield. Fluorescing antibodies were observed with a Bio-Rad MRC6500 confocal microscope. Sections were obtained at 1 μm intervals. The specificity of the primary antibodies was established by pre-incubating them with the corresponding peptides (Ca2+ channel) and protein (Slo). The result was that no specific labelling was observed. In addition, Western blots of chick brain tissue established that the primary antibodies identified the respective proteins of the correct molecular mass. The specificity of the secondary antibody was established by removal of the corresponding primary antibody. In particular, we determined that the specificity of the rhodamine-conjugated anti-mouse antibody (applied in the second round of incubations) did not recognize the rabbit antibody by incubating tissue in the presence of the anti-Ca2+ channel polyclonal antibody and then incubating with the secondary anti-mouse antibody. No specific labelling was observed.
Image processing was done using ImageJ 1.30 for OS9 (rsb.info.nih.gov/ij/). Analysis was limited to three rows of cells from the neural edge to exclude short hair cells. Areas of clusters were defined by their intensity (2 × background within a cell) and location at the periphery of the cell, and the number of clusters in cells from three cochleas in a given locus was counted in all frames. Colocalization was established by using the colocalization function in Image J for each frame, after subtracting 2 × the background intensity. The number of colocalized clusters was counted. Furthermore, by increasing the gain in the rhodamine channel we were able to outline individual hair cells (owing to the high signal-to-noise ratio this did not affect our ability to identify BK clusters from background.). We established that this procedure defined hair cells by co-labelling with anti-myosin VII antibodies and Alexa 488 secondary antibody (data not shown). Each experimental dataset was obtained from three cochleas. Statistical analysis was done using Instat (Graphpad). One-way ANOVA with post hoc test comparison of all datasets was done for each set of experiments.
Results
Ca2+ channels and BK channels are clustered and colocalized in hair cells
In order to identify Ca2+ channels and BK channels we used antibodies for these two proteins. Freshly fixed chick cochleae labelled with these two antibodies and imaged by confocal microscopy are shown in Fig. 1. As is evident from this figure, hair cells contain clusters of both Ca2+ channels and BK channels. Both these channel clusters are found circumferentially on the surface of these cells. The number of BK channel clusters exceeded the number of Ca2+ channel clusters. Substantial numbers (80%) of the Ca2+ channel clusters were found to colocalize with BK channels (see below). Analysis of isolated single hair cells showed the same pattern of channel clustering (data not shown). In addition, increasing the focal depth revealed no channel clustering in the afferent nerve fibres innervating these cells. We were thus able to exclude the possibility that channel clustering in afferent nerves significantly affected the identification/enumeration of channel clusters in hair cells.
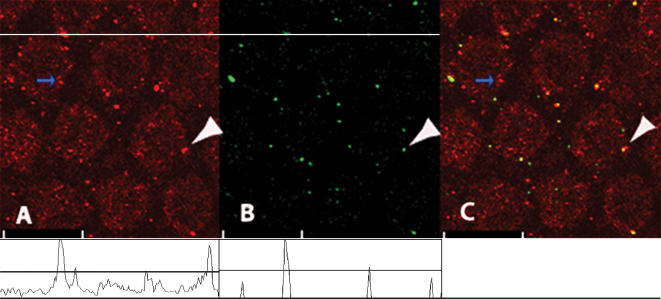
Figure 1. Clustering and colocalization of Ca2+ and BK channels in hair cells.
The figure shows a confocal image of hair cells from the chick approximately 30% fractional distance from the low frequency end of the papilla. A shows cells that have been labelled with an antibody to the BK channels (Slo) that cluster on the peripheral surface of the cell. B is the identical section showing labelling of clustered Ca2+ channels, also on the peripheral surface of the cell, identified by their size and intense fluorescence, with some clusters colocalizing with BK channel clusters. C is the merged image showing colocalized channel clusters (white arrows). A number of Slo channel clusters do not colocalize with Ca2+ channels (blue arrows). Line scan maps of the white line passing through the upper part of A and B are shown beneath each panel. The threshold used to demarcate cluster intensity from background is shown in the line scan map (black line). The scale bar is 10 μm.
BK channels and Ca2+ channels are preferentially clustered at the basal pole of hair cells
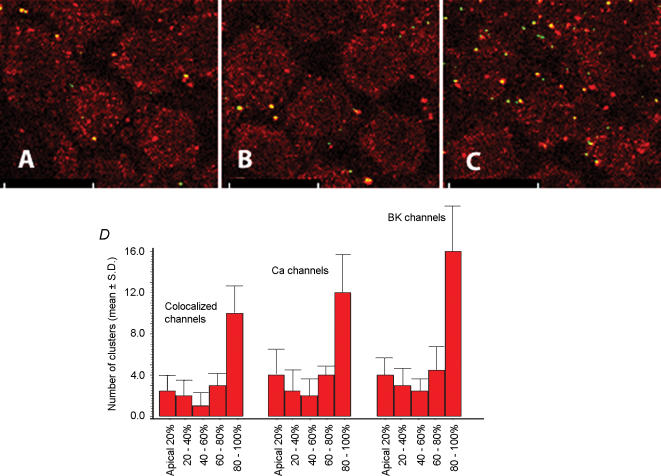
Hair cells have well-defined apical and basal segments that subserve different functions. The apical-most surface contains the sensory hair bundle while the basal half contains the synaptic region of the cell. Figure 2 demonstrates confocal sections from the apical, middle and basal regions of several hair cells positioned approximately 60% fractional distance from the low frequency end of the basilar papilla. As is evident from this figure the majority of both Ca2+ channels and BK channels are clustered at the basal portions of these hair cells. We next quantified the distribution of these channel clusters along the apical–basal axis of the cell by determining the number of each of these channel clusters in five segments along the vertical axis of the cell. For this analysis we identified 14 cells from three cochleas within a limited tonotopic location, 75% fractional distance from the low frequency end of the basilar papilla, and quantified the number of channel clusters. These cells were chosen since the greater number of ion channel clusters within these cells allowed for a more robust statistical analysis (however, a similar pattern of ion channel cluster distribution was observed from cells at the low frequency location too). At this location (75% fractional distance) the mean numbers of coclustered ion channels were 2.5, 2, 1, 3 and 10 in the apical-most to basal-most quintile, respectively. Similarly, the mean numbers of Ca2+ and BK channel clusters were 4, 2.5, 2, 4 and 12, and 4, 3, 2.5, 4.5, and 16, respectively. These data are represented in bar graph form in Fig. 2D, and show the marked increase in each channel cluster in the basal-most segment. The numbers of each channel cluster in the lowest segment were significantly different from the numbers in each of the remaining four segments (P < 0.001 ANOVA). Similarly, the number of coclustered channels in the lowest segment was also significantly different from the number of coclustered channels in the four segments above it (P < 0.001 ANOVA). Interestingly, although the small numbers made statistical significance difficult to establish in the upper segments, the number of colocalized channel clusters as a percentage of total BK and separately total Ca2+ channel clusters remained more or less constant across the vertical axis. If hair cells were perfect cylinders, you might expect that basal (and apical) segments would demonstrate higher numbers of ion channel clusters that the intermediate segments simply due to their relatively larger membrane surface areas. However hair cells are not perfect cylinders, but rather contain a tapering lower portion with a progressively smaller surface area. Thus, it is unlikely that the greater number of ion channel clusters in the lowest segment is due to an increased surface area, which however, is also difficult to quantify. Furthermore, even when we corrected for surface area, assuming hair cells to be perfect cylinders, the number of ion channel clusters in the most basal segment was still significantly greater than the segments above (P < 0.05 ANOVA).
Figure 2. There is an apical–basal gradient of ion channel clusters within a hair cell.
The figure shows confocal images taken at varying depths in a number of hair cells (60% fractional distance from the low frequency end of the papilla) that have been labelled with antibodies to the BK (Rhodamine red) and Ca2+ (Fluorescein green) channels. Colocalized channels of these merged images are yellow. A is from the upper apical portion of these cells, B is from the middle and C is from the basal end. As is evident, the number of BK channels (red), Ca2+ channels (green) and colocalized channels (yellow) increase from the apical to basal end. The scale bar is 10 μm. D shows a bar graph of the mean number of BK channel clusters, Ca2+ channel clusters, and colocalized channel clusters in 5 segments of hair cells divided in an apical-to-basal direction. These 14 cells were obtained from three cochleas, and were located approximately 75% fractional distance from the low frequency end of the papilla. As is evident, the number of channel clusters is most concentrated at the base of the cell (P < 0.001 for both channels and colocalized channel clusters compared with any of the upper segments). Approximately half of a cell's ion channel clusters are located in the lowest segment.
There is a tonotopic gradient in the number of ion channel clusters
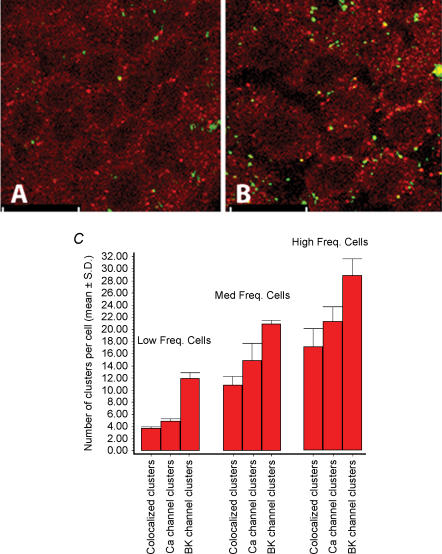
We then determined the distribution of ion channel clusters along the tonotopic axis. Figure 3 shows confocal sections from the lower half of hair cells from the low and high frequency regions of the basilar papilla. This figure demonstrates that the number of Ca2+ and BK channel clusters increases as cells are sampled from the low frequency to the high frequency region of the sensory sheet. We quantified the number of ion channel clusters by counting the number of clusters in randomly selected cells each from the low, middle and high frequency regions of the basilar papilla. Data from cells of three cochleas located at fractional distances of approximately 15% (28 cells), 65% (28 cells) and 80% (24 cells) from the low frequency end of three cochleas are shown in graph form in Fig. 3D. The mean numbers of coclusters, Ca2+ channel clusters and BK channel clusters per cell in these three loci were 4, 5.5 and 12 in the low frequency region, 11, 15 and 22 in the mid-frequency region, and 16, 20, and 29 in the high frequency region, respectively. On average there was a 4-fold increase in Ca2+ channel clusters, and a 2.5-fold increase in BK channel clusters in the higher frequency hair cells compared to those at the lower frequency tonotopic location. It should also be noted that the number of colocalized channel clusters increased as a percentage of the Slo channel clusters with increasing frequency (35% in low frequency cells, 50% in mid-frequency cells and 55% in high frequency cells). In contrast, the number of colocalized channel clusters as a percentage of Ca2+ channel clusters remained more or less unchanged (80%). The ratio of ion channels to coclustered ion channels across the apical–basal axis of a hair cell remained constant for that particular tonotopic location.
Figure 3. There is a tonotopic gradient in ion channel clusters.
Confocal images of hair cells from the low frequency (A) and high frequency (B) end of the basilar papilla that have been labelled with antibodies to the BK (red) and Ca2+ (green) channels is shown. The figure shows that the number of both channel clusters and colocalized channel clusters (yellow) is increased in cells in the high frequency location in the basilar papilla. The sections shown were taken across the basal fifth of the cells. The scale bar is 10 μm. C shows the mean number of ion channel clusters per cell (Colocalized/Ca2+/BK) from low frequency (15% fractional distance, 28 cells), mid-frequency (65% fractional distance, 28 cells) and high frequency (80% fractional distance, 23 cells) hair cells in bar graph form. The cells were from three cochleas. The numbers of each ion channel and colocalized channel clusters were significantly different in the cells from each of the three different frequency locations (P < 0.05). While the proportion of colocalized Ca2+ channels remained constant (∼80%), the proportion of colocalized Slo channels increased from 35% in low frequency hair cells to 58% in high frequency hair cells.
Discussion
We present here data confirming clustering and colocalization of Ca2+ and BK channels in chick auditory hair cells. We also show that these channels are congregated in the lower fifth of hair cells. Our data confirmed that the numbers of each ion channel cluster increase in high frequency cells, with an increasing proportion of BK channel clusters colocalizing with Ca2+ channel clusters. Moreover, the proportion of colocalized clusters remained constant along the vertical axis of any given hair cell.
Previous investigations have predicted the clustering of BK and Ca2+ channels in the hair cell (Roberts et al. 1990). Patch recordings of hair cell membranes revealed that the distribution of these ion channels was not even, with some patches containing Ca2+ and BK currents and others not (Roberts et al. 1990). The demonstration of ion channel clusters in this study confirms this previous prediction. The clustering of these channels further suggests that there may be microdomains with specific physiological function within hair cells. For example both these ionic currents occur around active synaptic zones in hair cells (Roberts et al. 1990; Issa & Hudspeth, 1994; Zenisek et al. 2003). We were unable to demonstrate that ion channels colocalized with the hair cell synaptic ribbons (called dense bodies) owing to an absence of antibodies for the dense body in chick hair cells. There is experimental evidence in other systems linking the muscle L-type, P/Q-type and N-type Ca2+ channels to the synaptic complex (reviewed by Atlas, 2001). Furthermore, in a recent paper reporting the use of the hair cell L-type Ca2+ channel (Ca v1.3) as bait in a yeast two-hybrid screen of chick cochlea cDNA library, Rim binding proteins, a component of ribbon synapses, were identified as interacting with the Ca2+ channel (Hibino et al. 2002). Our data, demonstrating the concentrated clustering of these ion channels around the lower half of hair cells, where synaptic bodies are focused, is possibly related to their importance in synaptic function. Martinez-Dunst et al. (1997) found most synaptic bodies to lie below the nucleus. Although they (Martinez-Dunst et al. 1997) did not quantify the distribution below the nucleus, a superficial analysis of their figures suggests a distribution of dense bodies that is similar to that of ion channel clusters reported in this paper. In this context, these authors also note that synaptic bodies within a hair cell were often ‘polarized within a neural cell’. We observed a similar polarization of ion channel clusters within hair cells in successive horizontal sections.
The number of active synaptic zones and ion channel clusters has been estimated in one study to be approximately 20 in saccular hair cells of the frog (Issa & Hudspeth, 1994). Our data in chick auditory hair cells approximated these numbers only in high frequency hair cells. The discrepancy may be methodological, with immunofluorescence detection being less sensitive than Ca2+-sensitive dyes in detecting Ca2+ channels. These differences may also be peculiar to the different hair cells used. Martinez-Dunst et al. (1997), comparing Ca2+ currents and synaptic release area, showed that chick hair cells contain significantly smaller Ca2+ currents (and release areas) than turtle and frog hair cells. Our data on the number of Ca2+ channel clusters approximates the number of pre synaptic bonds (PDBs) per hair cell described in this paper. These authors also demonstrated that the total surface area of PDBs increased 4-fold along the tonotopic axis, which is in agreement with the increase in Ca2+ channel clusters in our data. Taken together these data further support an intimate relationship between Ca2+ channel clusters and synaptic release. As noted earlier, others have predicted the colocalization of BK and Ca2+ channel clusters (Art & Fettiplace, 1987; Roberts et al. 1990; Art et al. 1995). This prediction is based, in part, on the need for micromolar concentrations of Ca2+ to open these BK channels and the high concentration of Ca2+ buffers that limit the temporal and spatial gradients of Ca2+ ions flowing in from Ca2+ channels (Roberts et al. 1990; Wu et al. 1995; Art et al. 1995). Thus, the high frequency oscillation (resonance) in the membrane potential requires the presence of these ion channels in close proximity to each other. It should be pointed out that our results give anatomical confirmation to previous electrophysiological observations by Roberts et al. (1990) that Ca2+ current clusters were associated with BK currents. Our data showing the presence of BK channel clusters without colocalized Ca2+ channel clusters also resolves one inconclusive aspect in this previous study. Roberts et al. (1990) found a number of patches that contained BK currents but no Ca2+ currents. Since the were small and transient, they were unable to conclude that the absence of Ca2+ currents in these patches was not due to their (technical) inability to detect them. Our present data establish the presence of BK channel clusters that are not associated with Ca2+ channel clusters (although it is still possible that Ca2+ channels in these locales were not clustered and therefore not detectable). In addition, we also show the presence of a minority of Ca2+ channel clusters in isolation without associated BK channels.
The increased number of ion channel clusters along the tonotopic axis is in keeping with previous electrophysiological recordings from turtle hair cells that showed a 5-fold greater BK and Ca2+ current in high frequency hair cells compared to low frequency hair cells (Fettiplace & Fuchs, 1999). Martinez-Dunst et al. (1997), recording from chick hair cells from a more limited tonotopic span, observed a 2.5-fold increase in Ca2+ current in higher frequency hair cells. An increased number of ion channels in high frequency cells has also been implied by the previous observation that there is a tonotopic gradient in the amount of Ca2+ buffer in chick hair cells (Navaratnam et al. 1995; Hackney et al. 2003). The greater number of ion channels in higher frequency hair cells is one mechanism responsible for their faster rates of membrane oscillation, and may be important for sharp tuning (Wu et al. 1995; Fettiplace & Fuchs, 1999). While the data showing a higher number of ion channel clusters supports the electrophysiological data, we do not know if the higher number of clusters is also paralleled by a higher number of ion channels within a cluster. Immunofluorescence, the technique used to label ion channels in this study, has insufficient resolution to quantify the number of ion channels within the cluster. The demonstration of a 4-fold increase in the number of Ca2+ channel clusters in high frequency hair cells (versus low frequency hair cells) cells is consistent with the electrophysiological data in turtles where the highest frequency hair cells had a 5-fold excess of Ca2+ current (Fettiplace & Fuchs, 1999). However, our data showing only a 2.5-fold increase in BK channel clusters in high frequency hair cells do not correspond to the 4-fold increase in BK currents found in turtle hair cells (Fettiplace & Fuchs, 1999). There are several plausible explanations for this apparent discrepancy. Since the BK channels open in the presence of Ca2+, and since the proportion of BK channels that were colocalized with Ca2+ channels was less in low frequency cells, it is possible that the electrophysiological data underestimated the number of BK channels in these low frequency cells. Another possible explanation is that the number of BK channels within a cluster is less in low frequency hair cells. The alternative explanation that, for some unknown reason, a number of BK channel clusters in high frequency hair cells were undetectable using immunofluorescence is unlikely.
The physical clustering of BK and Ca2+ channels and their colocalization within hair cells generates several questions relating to the underlying molecular mechanisms of membrane targeting. Clustering of ion channels and receptors has been demonstrated in a number of systems, and it appears that interactions with other proteins is an important common mechanism (Sheng & Kim, 1996; Rasband et al. 2001). Unfortunately, the molecular mechanisms underlying Ca2+ and BK channel clustering and colocalization are poorly understood (Atlas, 2001). In myocytes, multiple regions of the skeletal L-type Ca2+ channel have been shown to be important for subcellular targeting and association with other ion channels (Nakai et al. 1998; Grabner et al. 1999; Flucher et al. 2000). Our data show that a constant proportion of Ca2+ channel clusters were colocalized along the tonotopic axis concomitant with increasing numbers of BK and Ca2+ channel clusters, and a changing stoichiometric ratio of Ca2+ channel to BK channel clusters. In contrast, the proportion of BK channel clusters that were colocalized varied along the tonotopic axis. Together these results suggest that the signals for colocalization are intrinsic to Ca2+ channel clusters. In addition, since there were proportionally greater numbers of ion channel clusters at the lower end of the cell, a feature of hair cells, even within mammals (Santos-Sacchi et al. 1997), there appear to be mechanisms for targeting these proteins to this region. Since the proportion of colocalized channel clusters was more or less invariant along the vertical axis of the cell, the mechanisms responsible for targeting to the lower surface and for colocalization would be predicted to be independent. We are presently exploring the possible mechanisms underlying Ca2+ and BK channel clustering and colocalization in hair cells. It is likely that some of these mechanisms are common to other tissues since these channels have been shown to exist in clusters in other cells as well (Robitaille et al. 1993).
Acknowledgments
We wish to thank Dr James Davies and the confocal facility at Yale University and the University of Pennsylvania for technical assistance and the Myosin VII antibodies. We also wish to thank Dr Joseph Santos-Sacchi for critical reading of the manuscript. D.S.N. was supported by grants from the NIH (K08 DC5352), Bumpus Foundation, and NOHR Foundation. J.C.S. was supported by an award from the NIDCD (DC000710).
References
- Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Wu YC, Fettiplace R. The calcium-activated potassium channels of turtle hair cells. J General Physiol. 1995;105:49–72. doi: 10.1085/jgp.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF. Frequency tuning in a frog vestibular organ. Nature. 1983;304:536–538. doi: 10.1038/304536a0. [DOI] [PubMed] [Google Scholar]
- Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J Neurochem. 2001;77:972–985. doi: 10.1046/j.1471-4159.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Horn R. Role of calcium-activated potassium channels in transmitter release at the squid giant synapse. J Physiol. 1988;398:149–164. doi: 10.1113/jphysiol.1988.sp017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Kasielle N, Grabner M. The triad targeting signal of the skeletal muscle calcium channel is localized in the COOH terminus of the a 1S subunit. J Cell Biol. 2000;151:467–477. doi: 10.1083/jcb.151.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Evans MG. Voltage oscillations and ionic conductances in hair cells from the alligator cochlea. J Comp Physiol. 1988;164:151–163. doi: 10.1007/BF00603947. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Nagai T, Evans MG. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci. 1988;8:2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Suda N, Beam KG. The II–III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J Biol Chem. 1999;274:21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- Hackney Carole, Mahendrasingham MS, Jones EMC, Fettiplace R. The distribution of calcium buffering proteins in the turtle cochlea. J Neurosci. 2003;23:4577–4589. doi: 10.1523/JNEUROSCI.23-11-04577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (rbps) couple rab3-interacting molecules (RIMs) to voltage-gated Ca channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Lewis RS. A model for electrical resonance and frequency tuning in saccular hair cells of the bullfrog, Rana catesbeiana. J Physiol. 1988;400:275–297. doi: 10.1113/jphysiol.1988.sp017120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci U S A. 1994;91:7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang GJ, Zidanic M, Michaels RL, Michael TH, Griguer C, Fuchs PA. CSlo encodes calcium-activated potassium channels in the chick's cochlea. Proc R Soc Lond B Biol Sci. 1997;264:731–737. doi: 10.1098/rspb.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Laus C, Fettiplace R. Identification of Ca2+-activated K+ channel splice variants and their distribution in the turtle cochlea. Proc R Soc Lond B Biol Sci. 1998;265:685–692. doi: 10.1098/rspb.1998.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmar R, Fak J, Montgomery LG, Hudspeth AJ. Hair cell-specific splicing of mRNA for the alpha1D subunit of voltage-gated Ca2+ channels in the chicken's cochlea. Proc Natl Acad Sci U S A. 1997;94:14889–14893. doi: 10.1073/pnas.94.26.14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Dunst C, Michaels RL, Fuchs PA. Release sites and calcium channels in hair cells of the chicks cochlea. J Neurosci. 1997;17:9133–9144. doi: 10.1523/JNEUROSCI.17-23-09133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Tanabe T, Konno T, Adams B, Beam KG. Localization in the II–III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J Biol Chem. 1998;273:24983–24986. doi: 10.1074/jbc.273.39.24983. [DOI] [PubMed] [Google Scholar]
- Navaratnam DS, Bell TJ, Tu TD, Cohen EL, Oberholtzer JC. Differential distribution of Ca2+-activated K+ channel splice variants among hair cells along the tonotopic axis of the chick cochlea. Neuron. 1997;19:1077–1085. doi: 10.1016/s0896-6273(00)80398-0. [DOI] [PubMed] [Google Scholar]
- Navaratnam DS, Escobar L, Covarrubias M, Oberholtzer JC. Permeation properties and differential expression across the auditory receptor epithelium of an inward rectifier K+ channel cloned from the chick inner ear. J Biol Chem. 1995;270:19238–19245. doi: 10.1074/jbc.270.33.19238. [DOI] [PubMed] [Google Scholar]
- Rasband M, Mathew N, Trimmer JST. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Yamoah EN. Direct measurement of single-channel Ca2+ currents in bullfrog hair cells reveals two distinct channel subtypes. J Physiol. 2001;534:669–689. doi: 10.1111/j.1469-7793.2001.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Huang GJ, Wu M. Mapping the distribution of outer hair cell voltage-dependent conductances by electrical amputation. Biophys J. 1997;73:1424–1429. doi: 10.1016/S0006-3495(97)78174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. Ion channel associated proteins. Current Opin Neurobiol. 1996;6:602–608. doi: 10.1016/s0959-4388(96)80091-2. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci. 2003;23:9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Art JJ, Goodman MB, Fettiplace R. A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog Biophys Mol Biol. 1995;63:131–158. doi: 10.1016/0079-6107(95)00002-5. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Davilla V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]