Abstract
Flecainide is a Class I antiarrhythmic drug and a potent inhibitor of the cardiac (Nav1.5) sodium channel. Although the flecainide inhibition of Nav1.5 is typically enhanced by depolarization, the contributions of the open and inactivated states to flecainide binding and inhibition remain controversial. We further investigated the state-dependent binding of flecainide by examining its inhibition of rapidly inactivating and non-inactivating mutants of Nav1.5 expressed in Xenopus oocytes. Applying flecainide while briefly depolarizing from a relatively negative holding potential resulted in a low-affinity inhibition of the channel (IC50 = 345 μm). Increasing the frequency of stimulation potentiated the flecainide inhibition (IC50 = 7.4 μm), which progressively increased over the range of voltages where Nav1.5 channels activated. This contrasts with sustained depolarizations that effectively stabilize the channels in inactivated states, which failed to promote significant flecainide inhibition. The voltage sensitivity and strong dependence of the flecainide inhibition on repetitive depolarization suggests that flecainide binding is facilitated by channel opening and that the drug does not directly bind to closed or inactivated channels. The binding of flecainide to open channels was further investigated in a non-inactivating mutant of Nav1.5. Flecainide produced a time-dependent decay in the current of the non-inactivating mutant that displayed kinetics consistent with a simple pore blocking mechanism (KD = 11 μm). At hyperpolarized voltages, flecainide slowed the recovery of both the rapidly inactivating (τ = 81 ± 3 s) and non-inactivating (τ = 42 ± 3 s) channels. Mutation of a conserved isoleucine of the D4S6 segment (I1756C) creates an alternative pathway that permits the rapid diffusion of anaesthetics out of the Nav1.5 channel. The I1756C mutation accelerated the recovery of both the rapidly inactivating (τ = 12.6 ± 0.4 s) and non-inactivating (τ = 7.4 ± 0.1 s) channels, suggesting that flecainide is trapped and not tightly bound within the pore when the channels are closed or inactivated. The data indicate that flecainide rapidly gains access to its binding site when the channel is open and inhibits Na+ current by a pore blocking mechanism. Closing of either the activation or the inactivation gate traps flecainide within the pore resulting in the slow recovery of the drug-modified channels at hyperpolarized voltages.
Flecainide is an orally administered Class I antiarrhythmic drug that is used in the management cardiac arrhythmias (Anderson et al. 1989; Henthorn et al. 1991). Flecainide produces a potent voltage- and frequency-dependent inhibition of cardiac Na+ channels, attributes that have generally proven to be beneficial in the treatment of cardiac arrhythmias (Hondeghem & Katzung, 1984).Unfortunately, the CAST antiarrhythmic drug trial discovered that the use of flecainide is associated with increased mortality in patients with cardiac arrhythmias resulting from myocardial infarction, which has significantly limited its general use as an antiarrhythmic agent (Cast Investigators, 1989). Despite the proarrhythmic potential associated with its use, flecainide has proved to be effective in treating atrial fibrillation, supraventricular arrhythmias, and long QT syndromes linked to naturally occurring genetic mutations of the cardiac Na+ channel (Brugada et al. 2000; Benhorin et al. 2000; Windle et al. 2001). In the later case the efficacy of flecainide has been attributed to the preferential block of the enhanced steady-state current characteristic of patients carrying the LQT3 mutations (Nagatomo et al. 2000).
Although the clinical use of flecainide has been limited by the results of the CAST trials, its strong preference for binding to activated states (i.e. open, inactivated) makes flecainide a valuable tool for studying the state-dependent binding of anaesthetics. The flecainide inhibition of Na+ channels is enhanced by rapid repetitive depolarization and increases over the range of voltages where the channels activate (Ragsdale et al. 1994, 1996; Qu et al. 1995; Liu et al. 2002). For these reasons flecainide is generally considered to be an activated or open state inhibitor of the Nav1.5 channel (Anno & Hondeghem, 1990; Nitta et al. 1992; Nagatomo et al. 2000). Preferential interaction and inhibition of open channels is further supported by studies showing that flecainide selectively reduces the open times of single Na+ channels (Grant et al. 2000). By contrast, prolonged depolarizations that stabilize Na+ channels in inactivated states fail to promote flecainide inhibition, indicating that direct binding to inactivated channels may not significantly contribute to the Na+ channel inhibition (Nitta et al. 1992; Nagatomo et al. 2000; Liu et al. 2002; Wang et al. 2003). This contrasts with studies of anaesthetics such as the Class IB antiarrhythmic drug lidocaine in which Na+ channel inhibition progressively increases with sustained depolarization and is consistent with high affinity binding to inactivated states of the channel (Bean et al. 1983; Sanchez-Chapula et al. 1983; Clarkson et al. 1988). Recent studies suggest that although flecainide inhibition is facilitated by channel opening, the high-affinity binding and subsequent slow recovery at hyperpolarized voltages is more closely linked to rapid inactivation (Liu et al. 2002, 2003). The relative contribution of flecainide block of open channels and inactivated-state binding to Na+ channel inhibition remains controversial.
In this study, we investigated the state dependence of flecainide binding and inhibition of rapidly inactivating and non-inactivating mutants of the Nav1.5 channel. Our studies of the wild-type channel are consistent with previous work showing that channel opening is required for flecainide binding and inhibition. Flecainide induces a characteristic time-dependent decay in the current of the non-inactivating mutant that displays properties consistent with an open-channel blocking mechanism. The data indicate that flecainide gains access to the cytoplasmic binding site by entering through the open channel and inhibits Na+ current by a pore blocking mechanism. Either deactivation or inactivation appears to trap flecainide within the channel resulting in the slow recovery at hyperpolarized voltages. The rapid flecainide block of open channels at depolarized voltages and the slow untrapping at hyperpolarized voltages appears to underlie the potent and long-duration inhibition of Nav1.5 channels.
Methods
Expression of Nav1.5 in oocytes
The cDNA encoding the human cardiac (Nav1.5) Na+ channel inserted into pcDNA plasmid (Invitrogen) was linearized with Xba I and full length capped mRNA transcribed using the T7 promoter (mMessage mMachine, Ambion, Austin TX, USA). Oocytes were harvested from mature female Xenopus laevis (Xenopus I, Ann Arbor, MI, USA). The animals were anaesthetized by immersion in tricaine (1.5 mg ml−1) and several ovarian lobes surgically removed under sterile conditions. The adhering follicle cell layer was removed by incubating oocytes with 1 mg ml−1 collagenase (Sigma Chemical, St Louis, MO, USA) in calcium-free OR2 (82.5 mm NaCl, 2.5 mm KCl, 1 mm MgCl2, 5 mm Hepes, pH 7.4) solution for 2 h. The oocytes were washed with calcium-free OR2 and transferred to OR3 (70% Leibovitz L-15 medium, Life Technologies) supplemented with 15 mm Hepes (pH 7.4), 5 mm l-glutamine, and 10 mg ml−1 gentamycin. Stage IV–V oocytes were microinjected with 50 nl of cRNA (1–2 μg μl−1) and incubated for 24–48 h at 18°C. After four surgeries, the frogs were deeply anaesthetized in tricaine and killed by pithing. These methods of animal handling are in accordance with the NIH guidelines and the protocol was approved by the Animal Use and Care Committee of Thomas Jefferson University.
Two-electrode voltage clamp
The whole-cell Na+ current of oocytes expressing Nav1.5 were recorded using a standard two-electrode voltage clamp. Oocytes are impaled with microelectrodes (< 1 MΩ) filled with 3 m KCl and currents recorded using an OC-725C voltage clamp (Warner Instruments, Hamden, CT, USA). Oocytes were held at −100 mV and pulses generated using pCLAMP software (Version 7, Axon Instruments, Foster City CA, USA). Oocytes were incubated and currents recorded in frog Ringer solution containing (mm): 116 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 Hepes, pH 7.4. All recordings were performed at room temperature (21–23°C). Data are presented as the mean ± s.e.m. Curve fitting and plotting was performed using Sigmaplot (SPSS Science, Chicago, IL, USA). The recovery time courses were routinely fitted with models having between 1 and 3 exponential components. The model that provided the best approximation of the data with the fewest number of free parameters was reported. In all cases, the recovery time course of the wild-type and mutant channels were fitted with the same model. Flecainide was purchased from Sigma Chemical.
Site-directed mutagenesis
Single amino acid substitutions were made using the QuickChange Site-Directed Mutagenesis Kit (Stratagene Inc., La Jolla, CA, USA). A fragment (1–2 kb) encompassing the mutation site was excised and subcloned into pcDNA (Invitrogen). Complementary pairs of mutagenic oligonucleotides (22–29 bp) containing the appropriate nucleotide substitutions were prepared at the Nucleic Acid Facility, Kimmel Cancer Center (Jefferson Medical College, Philadelphia, PA, USA). These oligonucleotides were subsequently used as primers for synthesis of both strands of the plasmid. We used 20 ng of cDNA plasmid as template, 5 U Pfu DNA polymerase (Stratagene), primers, and free nucleotides in a total volume of 100 μl. After strand synthesis (≈20 cycles), 10 U of Dpn I was added to the reaction mixture to selectively digest the original template (37°C, 1–2 h). The restriction endonuclease was heat inactivated (65°C, 15 min), and the mixture used to transform DH5α bacteria by electroporation. Base substitutions were confirmed by automated DNA sequencing. DNA fragments (1–2 kb) carrying the mutation were then subcloned into wild-type Nav1.5 or non-inactivating (QQQ) mutant background.
Results
Flecainide inhibition of the Nav1.5 channel
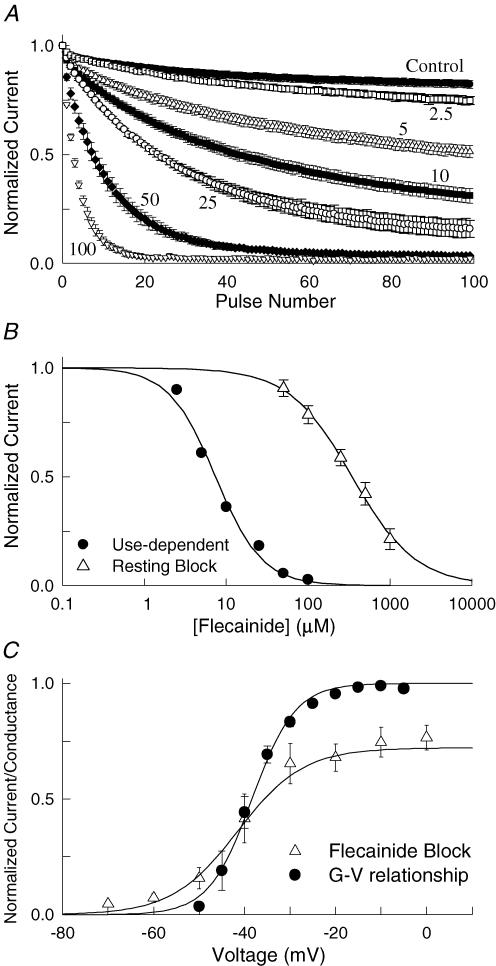
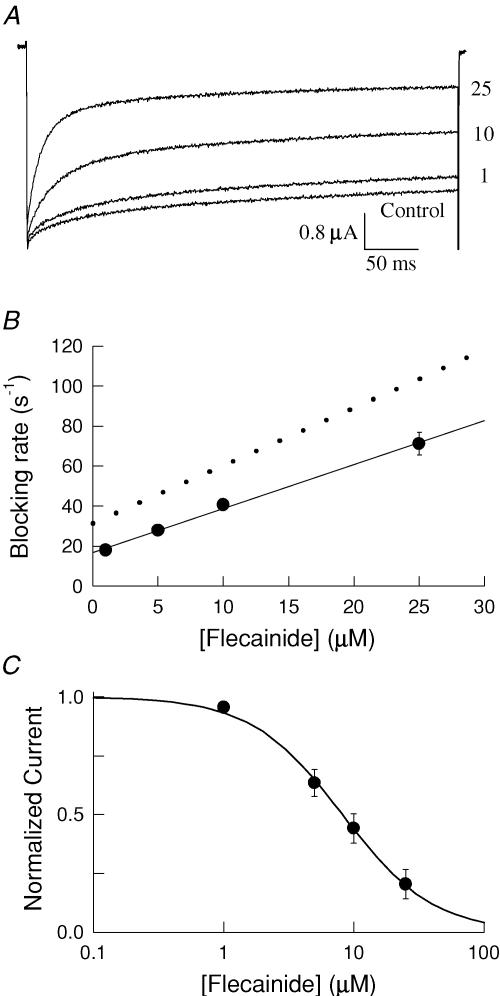
Nav1.5 channels were expressed in Xenopus oocytes and current recorded using two-electrode voltage clamp. Applying flecainide (10 μm) while stimulating at a low frequency (60 s intervals) resulted in a negligible (∼2%) reduction in the peak current amplitude. Because the currents were elicited by brief depolarizations from a negative holding potential suggests that flecainide does not appreciably bind to the channels under resting conditions. Increasing the frequency of stimulation typically enhances the inhibition produced by local anaesthetics, which appear to preferentially bind to the open or inactivated states of the channel. In the absence of drug, the peak current amplitudes of Nav1.5 are only slightly reduced by a train of depolarizing pulses applied at a frequency of 10 Hz (Fig. 1A). Following treatment with flecainide (5–100 μm), the same repetitive pulsing resulted in a progressive decrease in the amplitude of the test currents reflecting the use-dependent inhibition of the channels. The amplitude of the current measured after 100 pulses was normalized to that of the drug-free control and plotted versus the flecainide concentration (Fig. 1B). The continuous curve is fit of the data to the Hill equation with an IC50 of 7.4 μm. Also plotted is the resting flecainide block measured by briefly depolarizing at 60 s intervals, which had an IC50 of 345 μm. The relatively weak inhibition of resting channels and the significant increase induced by rapid repetitive depolarization are consistent with the preferential binding of flecainide to activated (i.e. open or inactivated) states of the channel.
Figure 1. Flecainide preferentially inhibits activated states of Nav1.5.
A, use-dependent inhibition was induced by applying a train of 100 depolarizing pulses (−10 mV, 20 ms) at a frequency of 10 Hz. The currents were measured before (Control) and 5 min after application of flecainide (2.5–100 μm). B, the steady-state current measured after 100 pulses (I100/I1) in the presence of flecainide was normalized to the drug-free control and plotted versus the flecainide concentration (Use-dependent). Also plotted is the resting block determined from single depolarizing pulses (−10 mV, 20 ms) applied at 60 s intervals from a holding potential of −120 mV (Resting Block). The continuous curves are a fit to the Hill equation [(I/Io = (1 + ([Flec]/IC50)(n)−1] where I and Io are the control and drug-modified current amplitudes and n is the Hill coefficient. The IC50 and n values are 7.4 ± 0.6 μm and 1.5 ± 0.2 for the use-dependent block (n = 5) and 345 ± 15 μm and 1.1 ± 0.06 for the resting block (n = 6). C, the voltage dependence of the flecainide inhibition (25 μm) was determined by varying the voltage of the pulses applied during repetitive stimulation (100 pulses, 10 Hz). The normalized steady-state inhibition after 100 pulses was determined and the fractional inhibition (1 − IFlec/ICont) plotted versus the test voltage. Also plotted is the normalized conductance versus voltage (G–V) relationship determined by briefly depolarizing (20 ms) to voltages between −70 and −5 mV. The conductance at each voltage (GV) was calculated (GV = INa/(V − Vr), where Vr = reversal potential), normalized to the conductance measured at −10 mV (Go) and plotted versus the test voltage (V). The continuous curves are fits to the Boltzmann equation (G/Go = Max/(1 + exp(V0.5 − V)/k))) with a midpoint (V0.5) of −42 ± 1.0 mV and maximal inhibition (Max) of 75 ± 2% for the flecainide block (n = 5) and V0.5 for the current–voltage relationship measured in the presence of flecainide (25 μm) of −36 ± 0.9 mV (n = 4).
To further investigate the state dependence of flecainide binding we determined the steady-state use-dependent inhibition for voltages between −70 and 0 mV. The flecainide inhibition after 100 depolarizing pulses was determined and the fractional inhibition plotted versus the test voltage (Fig. 1C). The flecainide inhibition progressively increased with depolarization reaching a maximal of ∼75% at voltages more depolarized than −10 mV. The continuous curve is a fit of the data to a Boltzmann function with a midpoint (V0.5) for the flecainide inhibition of −42 mV. Also plotted is the normalized conductance versus voltage relationship of Nav1.5 measured in the presence of flecainide (V0.5 = −36 ± 0.9 mV, n = 4), which was not significantly different from that of the drug-free controls (V0.5 = −38 ± 1.3 mV, n = 6, data not shown). The extensive overlap of voltage-dependent activation with the development of flecainide inhibition is consistent with preferentially binding of the drug to activated states of the channel.
Recovery from flecainide inhibition
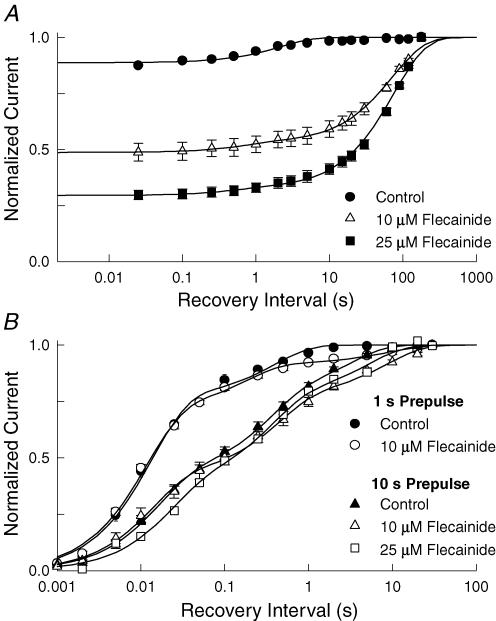
The progressive decrease in the amplitude of the test currents observed during rapid repetitive depolarization indicates that channels that bind flecainide during the 20 ms depolarizations do not fully recover during the 80 ms hyperpolarization between pulses (Fig. 1A). This was not observed in the absence of the flecainide, suggesting that drug binding may slow the recovery of Nav1.5 at hyperpolarized voltages. We investigated the underlying mechanism by directly measuring the time course of recovery from flecainide inhibition. Flecainide inhibition was induced by applying a train of 100 depolarizing prepulses at a frequency of 10 Hz. The voltage was then returned to −100 mV for a variable duration (0.025–180 s) before applying a standard test pulse to assay the extent of the recovery. The test currents were normalized to those measured after a prolonged rest at the holding voltage (> 180 s) and plotted versus the recovery interval (Fig. 2A). In the absence of drug, a small reduction in the test current was observed that recovered with a time constant of 2.5 s. This appears to reflect the recovery of a small fraction of the channels (11%) that had entered slow inactivated states during repetitive depolarization. After application of flecainide (25 μm), the steady-state inhibition induced by the depolarizing prepulses increased by 7-fold and the recovery time course was found to be biexponential with time constants of 0.4 s and 81 s, respectively. The slow component constitutes the majority (97%) of the time course and reflects the recovery of the drug-modified channels. This slow recovery appears to underlie the potent use-dependent inhibition produced by flecainide (Fig. 1A).
Figure 2. Flecainide slows the recovery of Nav1.5 channels.
A, flecainide inhibition was induced by applying 100 depolarizing pulses (−10 mV, 20 ms) at a frequency of 10 Hz. The voltage was then returned to −100 mV for a variable duration (0.025–180 s) before applying a standard test pulse (−10 mV, 20 ms). The peak current amplitudes (I) elicited by test pulses were normalized to the current measured after a prolonged rest (180 s) at −100 mV (Io) and plotted versus the recovery interval. The continuous curves are fits to either a single or biexponential function [I/Io = 1 − (A1(t/τ1) + A2(t/τ2)] where τ and A are the time constants and the corresponding relative amplitudes. The data had time constants (relative amplitude) of 2.5 ± 0.3 s (A = 0.11 ± 0.01) for the control (n = 12), τ1 = 0.7 ± 0.2 s (A1 = 0.04 ± 0.01) and τ2 = 75.3 ± 4.5 s (A2 = 0.47 ± 0.01) for 10 μm (n = 5), and τ1 = 0.4 ± 0.1 s (A1 = 0.02 ± 0.02) and τ2 = 81.3 ± 3.2 s (A2 = 0.75 ± 0.01) for 25 μm flecainide (n = 6). B, channels were inactivated by applying 1 or 10 s depolarizing pulses to −10 mV before returning to −100 mV for a variable duration (1 ms–30 s). A standard test pulse (−10 mV, 20 ms) was used to assay the fractional current (I), which was normalized to the current measured after holding at −100 mV for 120 s (Io) and plotted versus the recovery interval (t). The continuous curves are fits of the data to either the sum of three exponential components [I/Io = 1 − (A1 exp(− t/τ1) + A2 exp(− t/τ2) + A3 exp(− t/τ3))] where τ1 − τ3 are the recovery time constants and A1 – A3 are the corresponding relative amplitudes. The fitted parameters are listed in Table 1.
To further investigate the state dependence of the flecainide inhibition we examined the recovery of the control and drug-modified channels after applying single long depolarizing conditioning pulse (−10 mV, 1 s) rather than the brief repetitive pulses used to induce the use-dependent inhibition. Although the channels briefly open at the beginning of these prolonged depolarizations, the channels rapidly inactivate (τ ≈ 5 ms) and do not reopen during the remainder of the pulse. The majority of the inhibition observed in these experiments therefore reflects flecainide binding to inactivated channels. Although flecainide (10 μm) caused a small fraction (< 10%) of the channels to slowly recover (τ = 9 s), the majority of the channels recovered with time constants and relative amplitudes that are similar to those of drug-free controls (Fig. 2B). We considered the possibility that flecainide may bind with low affinity or that the onset of the drug inhibition may be slow when the channels are inactivated. However, increasing the prepulse duration to 10 s or the applied concentration of flecainide from 10 to 25 μm resulted in little change in the recovery time course by comparison to the drug-free controls (Table 1). The data indicate that simply stabilizing the channels in inactivated conformations fails to promote significant flecainide inhibition. This sharply contrasts with rapid repetitive pulsing, which produces a comparatively large inhibition at lower concentrations of the drug (Fig. 1) and dramatically slows the recovery when the channels are returned to a hyperpolarized potential (Fig. 2A). Overall, the data indicate that opening of the channels is an important determinant of flecainide binding and that at low concentrations (≤ 25 μm) the drug does not appreciably interact with the closed (Fig. 1B) or inactivated (Fig. 2B) states of the channel.
Table 1.
Recovery from flecainide inhibition
| τ1 | τ2 | τ3 | A1 | A2 | A3 | ||
|---|---|---|---|---|---|---|---|
| 1 s prepulse | Control | 0.014 ± 0.002 | 0.40 ± 0.10 | — | 0.77 ± 0.03 | 0.23 ± 0.03 | — |
| 10 μm flecainide | 0.011 ± 0.002 | 0.18 ± 0.08 | 9.2 ± 5.4 | 0.71 ± 0.04 | 0.20 ± 0.04 | 0.09 ± 0.02 | |
| 10 s prepulse | Control | 0.016 ± 0.002 | 0.33 ± 0.06 | 3.1 ± 0.6 | 0.42 ± 0.02 | 0.36 ± 0.03 | 0.22 ± 0.04 |
| 10 μm flecainide | 0.014 ± 0.002 | 0.43 ± 0.08 | 8.7 ± 1.7 | 0.42 ± 0.02 | 0.35 ± 0.03 | 0.22 ± 0.03 | |
| 25 μm flecainide | 0.022 ± 0.007 | 0.35 ± 0.13 | 5.3 ± 1.9 | 0.38 ± 0.05 | 0.39 ± 0.06 | 0.23 ± 0.05 |
Recovery time constants (τ1–τ3) and relative amplitudes (A1–A3) obtained from curve fits of data shown in Fig. 2B.
Flecainide binding to open channels
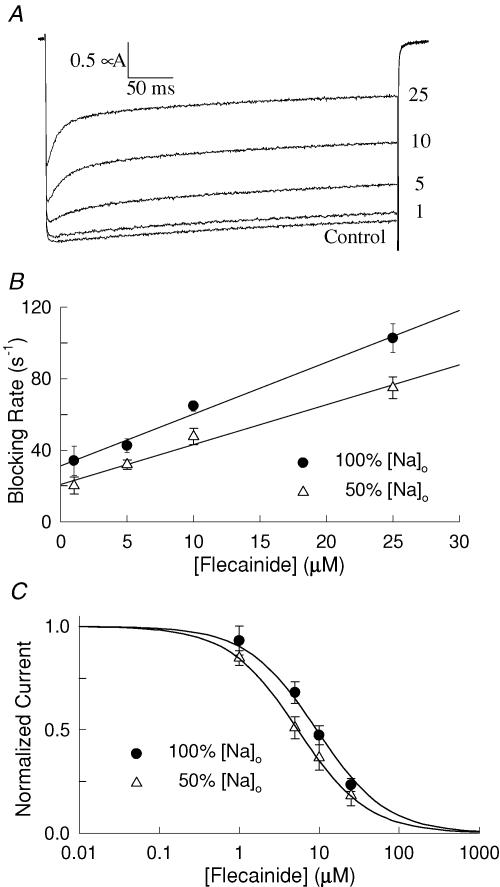
Opening of the Nav1.5 channel appears to play a prominent role in the use-dependent inhibition produced by flecainide. Unfortunately, at depolarizing voltages Nav1.5 channels only briefly open (< 1 ms) before inactivating, significantly complicating the characterization of flecainide interaction with the open states (O'Leary et al. 1995). We further investigated the role of the open state in flecainide binding using a non-inactivating mutant of Nav1.5. This mutant was constructed by replacing hydrophobic residues (I485, F1486, M1487) of the interdomain D3–D4 linker of the channel with glutamines (IFM→QQQ) (West et al. 1992). In the absence of drug, the QQQ mutant channels open normally at depolarized voltages and display minimal inactivation during 400 ms of depolarization (Fig. 3A). Flecainide (1–25 μm) induced a concentration-dependent increase in the rate of the current decay that was biexponential, with the rapid component (τf) reflecting the time course of the flecainide inhibition. Assuming a simple bimolecular interaction predicts that the blocking rate (1/τf) should increase linearly with the drug concentration where the slope and y-intercept represent the association (kon) and dissociation (koff) rate constants, respectively (O'Leary & Chahine, 2002). The plot of the blocking rate versus the flecainide concentration is well fitted by a straight line with an equilibrium binding constant (KD = koff/kon) of 10.8 μm (Fig. 3B and Table 2).
Figure 3. Flecainide inhibition of a non-inactivating mutant of Nav1.5A.
The non-inactivating (QQQ) mutant channels (see text) were expressed in oocytes and current elicited by depolarizing to −10 mV for 400 ms from a holding potential of −100 mV. Flecainide (1–25 μm) induced a time-dependent decay in the current in the otherwise slowly inactivating current. B, the flecainide-induced decay was fitted with either one (control) or the sum of two (flecainide) exponentials and the effective blocking rate (1/τf) plotted versus the flecainide concentration. The straight lines are consistent with a bimolecular interaction with slope (kon) and y-intercept (koff). Also plotted is the blocking rate measured after reducing the external concentration of Na+ by 50%. The association and dissociation rate constants and the blocking affinity (KD = koff/kon) are listed in Table 2. C, the steady-state current measured near the end of the depolarizing pulses was normalized to the current of drug-free controls and plotted versus flecainide concentration. The continuous curves are fits to a single-site binding model [(I/Io = (1 + [flecainide]/KD)−1] with equilibrium constants (KD) of 11.2 ± 1.3 μm (n = 7) and 4.1 ± 0.7 μm (n = 6) for 100 and 50% external Na+, respectively.
Table 2.
Kinetics of flecainide block of the QQQ and QQQ-I1756C channels
To further investigate the underlying mechanism, we examined the flecainide inhibition of the QQQ mutant after reducing the external concentration of Na+ to 50% by iso-osmotically replacing NaCl with choline chloride. The flecainide-induced current decay was fitted with exponentials and the blocking rate (1/τf) plotted versus the flecainide concentration (Fig. 3B). Lowering the external concentration of Na+ reduced both the association and dissociation rate constants of flecainide binding (Table 2). Also plotted is the normalized steady-state inhibition of the QQQ current measured near the end of the depolarizing pulses versus the flecainide concentration (Fig. 3C). The continuous curves are fits of the data to a single-site binding model with KD values of 11.2 and 4.1 μm for the 100% (116 mm) and 50% (58 mm) external Na+ Ringer solutions, respectively. Reducing the external concentration of Na+ significantly enhanced the steady-state flecainide inhibition of the QQQ mutant (Table 2). The simple bimolecular kinetics and the sensitivity of flecainide binding to changes in the external concentration of Na+ are consistent with an open-channel blocking mechanism.
Deactivation traps flecainide within the pore
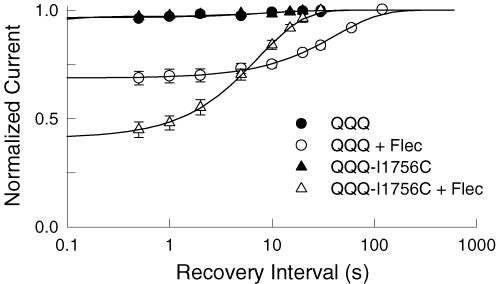
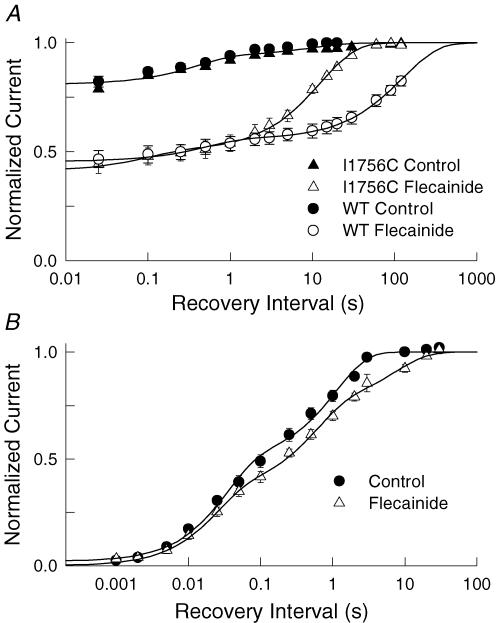
Flecainide produced a potent use-dependent inhibition during repetitive depolarization (Fig. 1A) and slows the recovery of Nav1.5 at hyperpolarized voltages (Fig. 2A). The slow dissociation of flecainide from either closed or inactivated states could account for the use-dependent inhibition of the channels. We further investigated the underlying mechanisms using the non-inactivating (QQQ) mutant, which enabled us to selectively examine the time course of recovery from closed states (O'Leary et al. 2003). Depolarizing prepulses (−10 mV, 100 ms) were used to induce the flecainide block of the open channels. The voltage was then returned to −100 mV for variable intervals before applying a standard test pulse to assay availability. The test currents were normalized to those measured after a prolonged rest (120 s) at −100 mV and plotted versus the recovery interval (Fig. 4). In the absence of drug, ∼3% of the QQQ current inactivated during the depolarizing prepulse and recovered with a time constant (τ) of 9.2 s. Flecainide (25 μm) induced a 10-fold increase in the fraction of current inhibited during the depolarizing prepulse and increased the recovery time constant (τ = 42.4 s). Because the QQQ mutant channels do not inactivate suggests that deactivation either traps flecainide within the pore or otherwise causes the drug to slowly dissociate from the channel.
Figure 4. I1756C accelerates the recovery of the flecainide-blocked QQQ mutant.
Flecainide block was induced by applying a 100 ms depolarizing pulse to −10 mV before returning to −100 mV for a variable duration (0.5–120 s). A standard test pulse (−10 mV, 150 ms) was then used to assay availability. The test current amplitudes were normalized to control currents measured after 120 s (QQQ) or 30 s (QQQ-I1756C) of rest at −100 mV and plotted versus the recovery interval. The continuous curves are exponential fits with time constants (relative amplitudes) of τ = 9.2 ± 3.6 s (A = 0.03 ± 0.005) for the control (n = 14) and τ = 42.4 ± 2.6 s (A = 0.31 ± 0.005) after application of 25 μm flecainide (n = 7). Also plotted is the recovery of the QQQ-I1756C mutant which had time constants (relative amplitude) of τ = 11.9 ± 3.0 s (A = 0.03 ± 0.003) for the control (n = 14) and τ = 7.4 ± 0.1 s (A = 0.59 ± 0.005) for 25 μm flecainide (n = 9).
Mutations near the N-terminus of the D4S6 have been shown to facilitate the binding of externally applied quaternary analogues of anaesthetics (Ragsdale et al. 1994; Qu et al. 1995; Lee et al. 2001; Sunami et al. 2001). Mutation of a highly conserved isoleucine (I1756) within this region appears to create an alternative diffusion pathway for these membrane-impermeant compounds that facilitates the interaction of the externally applied drug with the cytoplasmic D4S6 binding site (Ragsdale et al. 1994; Sunami et al. 2001). We further investigated the untrapping mechanism by transferring the I1756C mutation to the non-inactivating (QQQ) mutant background (Fig. 4). In the absence of drug, 3% of the mutant channels inactivated and recovered with a time constant of 11.9 s. Flecainide increased the inhibition produced by the depolarizing prepulse 20-fold, and the drug-modified channels recovered with a time constant of 7.4 s. Despite the large increase in the relative amplitude of the inhibited current, the subsequent recovery time course was similar to the drug-free control and 6-fold faster than the recovery of the QQQ channel (τ = 42 s). The rapid recovery of the QQQ-I1756C mutant suggests that the slow diffusion of flecainide out of the pore may be the rate-limiting step in the recovery when the channels are closed.
In addition to accelerating the kinetics of closed-state untrapping, the I1756C mutation increased the amplitude of the slowly recovering component (AQQQ-I1756C = 0.59 versus AQQQ = 0.31) (Fig. 4). We speculated that this increase in the fraction of I1756C mutant channels inhibited during the depolarizing prepulse might reflect differences in the tonic flecainide block of the channel under resting conditions. To test this we directly compared the peak current amplitudes of the QQQ mutant current by applying depolarizing test pulses (−10 mV, 400 ms) at 2 min intervals immediately before and after applying 25 μm flecainide. Flecainide inhibited the QQQ and QQQ-I1756C channels by 27 ± 0.5% (n = 8) and 8.4 ± 1.6% (n = 11), respectively. The I1756C mutation significantly reduced the tonic block of the QQQ mutant. Assuming a single common flecainide binding site for both the closed and open channels, predicts that a larger fraction of the I1756C channels will be unoccupied at rest and therefore available for flecainide block as the channels open at depolarized voltages. The data suggest that closed-state untrapping may be an important determinant of both the recovery kinetics and the steady-state flecainide block of the channels under resting conditions.
An alternative explanation for the rapid recovery and weak resting block of the QQQ-I1756C channel is that this mutation may weaken flecainide binding thereby causing the drug to rapidly dissociate at hyperpolarized voltages. To investigate this possibility we directly measured the affinity of flecainide binding to the QQQ-I1756C mutant. Flecainide induced a concentration-dependent increase in the kinetics of the flecainide block of the QQQ-I1756C mutant (Fig. 5A) similar to what was observed for QQQ channel. The time course of the current decay was fitted with the sum of two exponentials and the blocking rate (1/τf) was plotted versus the flecainide concentration (Fig. 5B). The I1756C mutation reduced both kon and koff by comparison to the control QQQ channel (Table 2). Also plotted is the steady-state inhibition measured near the end of the 400 ms depolarizing pulses, which was well fitted by a single site model with a KD of 8.5 μm (Fig. 5C). If flecainide binding is an important determinant of the recovery from closed states then the reduced koff predicts slower recovery of the QQQ-I1756C mutant by comparison to the QQQ channel. This is opposite of what we observed experimentally (Fig. 4A) and is therefore inconsistent with an important role for flecainide binding in the recovery of the QQQ mutant. Rather the I1756C mutation appears to accelerate the recovery by permitting flecainide to rapidly escape from the pore when the channel is closed, similar to what we recently described for cocaethylene (O'Leary et al. 2003).
Figure 5. Flecainide block of the QQQ-I1756C mutant.
A, current of the QQQ-I1756C mutant channels before and after bath application of flecainide (1–25 μm). Currents were elicited by depolarizing to −10 mV for 400 ms from a holding potential of −100 mV. The decay of the current was fitted with either one (control) or the sum of two exponential components (flecainide). B, the effective blocking rates (1/τf) were plotted versus the flecainide concentration. The association and dissociation rate constants are listed in Table 2. The dotted line is a regression fit describing the blocking kinetics of the QQQ mutant measured in 100% Na+ redrawn from Fig. 3B. C, the current measured at the end of the 400 ms depolarizing pulses (A) was normalized to drug-free controls and plotted versus the flecainide concentration. The continuous curve is a fit of the data to a single site model with KD of 8.5 ± 1.5 μm (n = 8).
I1756C accelerates the recovery of rapidly inactivating channels
The I1756C mutation facilitated the closed-state untrapping of flecainide from the non-inactivating mutant (Fig. 4). We were therefore interested in determining if the I1756C mutation similarly altered the recovery of rapidly inactivating channels. The recovery time course of the wild type and I1756C mutant was determined by first applying a series of 100 depolarizing pulses at a frequency of 10 Hz to promote flecainide inhibition. The voltage was returned to the holding potential for a variable interval before applying a standard test pulse to assay availability. In the absence of drug, 19% of the I1756C mutant channels inactivated and recovered with fast (τf) and slow (τs) time constants of τf = 0.4 s (Af = 0.13) and τs = 10.3 s (As = 0.06) (Fig. 6A). Flecainide increased the fraction of slowly recovering channels to 59% by selectively increasing the relative amplitude (As = 0.50) and time constant (τs = 12.6 s) of the slow component. This contrasts with the recovery of the drug-modified wild type measured under identical conditions, which had time constants of τf = 1.2 s and τs = 128 s, respectively (Fig. 6A). Although the recovery of the I1756C mutant is slow by comparison to the drug-free controls, it is 10-fold faster than that of the wild-type channel. The I1756C mutation accelerated the recovery of drug-modified channels similar to what was observed for the non-inactivating QQQ mutant (Fig. 4). Untrapping of flecainide appears to be the rate-limiting step in the recovery of both closed and inactivated channels.
Figure 6. I1756C accelerates the recovery of rapidly inactivating channels.
A, the recovery time course of the rapidly inactivating wild type and I1756C mutant were measured by applying 100 depolarizing pulses at a frequency of 10 Hz. The voltage was then returned to −120 mV for a variable interval (0.02–90 s) before applying a standard test pulse (−10 mV, 20 ms). The amplitude of the test current was normalized to controls measured after 180 s rest at the holding potential and plotted versus the recovery interval. In the absence of drug the recovery of the wild type (n = 9) and I1756C mutant (n = 5) was biexponential with time constants (relative amplitude) of τf = 0.39 ± 0.10 s (Af = 0.13 ± 0.01) and τs = 10.3 ± 4.2 s (As = 0.06 ± 0.01). After application of flecainide (10 μm) the wild type had τf = 1.2 ± 0.1 s (Af = 0.10 ± 0.005) and τs = 127.6 ± 8.8 s (As = 0.44 ± 0.004) (n = 7). For the I1756C mutant the time constants were τf = 0.10 ± 0.03 s (Af = 0.09 ± 0.01) and τs = 12.6 ± 0.4 s (As = 0.50 ± 0.01) after application of flecainide (n = 5). B, recovery time course of the I1756C mutation. The recovery was determined by first applying a 10 s depolarizing pulse to −10 mV. The recovery time course (1 ms–60 s) was assessed using a standard test pulse. Test currents were normalized to controls measured after 120 s of rest at −120 mV. The continuous curves are biexponential curve fits with time constants (relative amplitude) of τf = 0.03 ± 0.005 s (Af = 0.47 ± 0.03) and τs = 1.1 ± 0.1 s (As = 0.50 ± 0.03) for controls (n = 3); τf = 0.05 ± 0.009 s (Af = 0.45 ± 0.03) and τs = 2.2 ± 0.3 s (As = 0.52 ± 0.03) after application of 25 μm flecainide (n = 3).
A recent study indicated that flecainide binding to inactivated states of Na+ channels is slow (τ = 8 s) (Wang et al. 2003), which may explain the relatively weak flecainide inhibition produced by long depolarizing pulses (Fig. 2B). Binding of the native inactivation gate to its receptor located near the internal mouth of the channel is believed to occlude the pore and may prevent flecainide from rapidly accessing its binding site. The finding that the I1756C mutant creates an alternative pathway for flecainide diffusion that accelerates closed- and inactivated-state untrapping, raises the possibility that this mutation may also facilitate the direct binding of flecainide to inactivated channels. To test this we applied long depolarizing conditioning pulses (−10 mV, 10 s) to stabilize the I1756C mutant in inactivated conformations and to promote flecainide binding. The voltage was then returned to the holding potential and the recovery time course was measured using a standard test pulse. The recovery of the I1756C mutant measured in the presence of flecainide was not substantially different from the drug-free control (Fig. 6B). Despite creating a pathway that enhances drug access to the cytoplasmic binding site, flecainide did not appear to appreciably bind at depolarized voltages where the channels are inactivated. Reduced access to the cytoplasmic binding site induced by rapid inactivation cannot account for the weak flecainide inhibition of the I1756C mutant. Rather these findings suggest that the flecainide inhibition rapidly dissipates when the voltage is returned to a hyperpolarized potential, a result that is inconsistent with high-affinity binding to inactivated states.
Discussion
In this study, we investigated the state-dependent binding of flecainide to human cardiac (Nav1.5) Na+ channels expressed in Xenopus oocytes. While briefly depolarizing from a negative holding potential, flecainide produced a relatively low affinity inhibition (IC50 = 345 μm) indicating that the drug weakly binds under resting conditions where the channels are predominately closed (Fig. 1B). Increasing the frequency of stimulation enhanced the flecainide inhibition (IC50 = 7.4 μm), which progressively increased over the range of voltages where the channels activated (Fig. 1C). These data suggest that flecainide preferentially interacts with the open or inactivated states of the channel. Unfortunately, the repetitive pulsing protocols necessary to induce the flecainide inhibition causes the channels to rapidly cycle through the open and inactivated conformations complicating attempts to directly identify the state(s) important for drug binding. We further investigated the state dependence of binding by applying depolarizing pulses that effectively stabilized the channels in inactivated conformations (Fig. 2B). These long depolarizations failed to promote significant flecainide inhibition, suggesting that direct binding to inactivated states is not an important determinant of the flecainide inhibition. Rather the potent use-dependent inhibition induced by repetitive depolarization, the voltage sensitivity of the inhibition, and the relatively weak inhibition of closed (Fig. 1B) and inactivated channels (Fig. 2B) indicates that channel opening plays a prominent role in drug binding (Liu et al. 2002; Wang et al. 2003).
Flecainide binding to the open state was further investigated using a non-inactivating (QQQ) mutant of Nav1.5. Flecainide produced a rapid time-dependent decay in the otherwise slow inactivating current of the QQQ mutant (Fig. 3A). The majority of the flecainide inhibition occurred after the channels had open and displayed concentration dependence consistent with a simple bimolecular interaction of the drug with its binding site (Grant et al. 2000; O'Leary & Chahine, 2002). Lowering the external concentration of Na+ altered the association and dissociation rate constants of flecainide binding (Fig. 3B) and potentiated the steady-state inhibition (Fig. 3C). The reduction in koff suggests that Na+ and flecainide compete for distinct but functionally overlapping binding sites within the pore (O'Leary & Chahine, 2002). The strong dependence of the flecainide inhibition on channel opening, bimolecular binding kinetics, and competition with Na+ ions for pore binding sites are consistent with an open-channel blocking mechanism. Flecainide appears to preferentially gain access to its cytoplasmic binding site located within the pore by entering through the internal aqueous pathway created by the opening of the channel. Occupancy of this cytoplasmic site by flecainide blocks the pore resulting in the inhibition of Na+ current.
Deactivation and inactivation slows the recovery from flecainide inhibition
Flecainide substantially slows the recovery of Nav1.5 channels (τ = 81 s) by comparison to drug-free controls (τ = 2.5 s), which appears to account for the potent use-dependent inhibition produced by this drug (Fig. 1A). Our data indicate that during repetitive depolarization, flecainide rapidly binds to the open channels, and that subsequent gating transitions further potentiate the inhibition and slow the recovery at hyperpolarized voltages. Several mechanisms have been proposed to account for the slow recovery of Na+ channels that are modified by local anaesthetics. Inactivation is believed to induce conformational changes near the cytoplasmic mouth of Na+ channels that increases the affinity of anaesthetic binding (Hille, 1977; Hondeghem & Katzung, 1977). The slow dissociation of anaesthetics from a high-affinity binding site could be the rate-limiting step in the recovery of the drug-modified channels. Alternatively, anaesthetics could become trapped within the channel as the activation or inactivation gates close (Starmer et al. 1984). Drug that rapidly accesses the cytoplasmic binding site when the channel is open may escape via an inherently slower pathway when the cytoplasmic aqueous pathway is occluded by the activation or inactivation gates. In this case, the recovery of drug-modified channels may be more closed linked to the diffusion of anaesthetics out of the channel rather than the slow dissociation from a high-affinity binding site.
We further investigated these potential mechanisms by examining the recovery of the non-inactivating (QQQ) mutant channels (Fig. 4). In the presence of flecainide, the recovery of the QQQ mutant at hyperpolarized voltages was slow (τ = 42 s) by comparison to the drug-free controls (τ = 9 s). Assuming that at low concentrations (≤ 25 μm) flecainide does not bind to closed channels provides an estimate of the rate of flecainide dissociation from the closed channel (1/τrec = 0.02 s−1) that is three orders of magnitude slower than the dissociation rate constant directly determined from open-channel blocking experiments (Fig. 3, koff = 31 s−1). Conformational changes that occur as the QQQ channels are returned to a hyperpolarized potential stabilize the flecainide block (Grant et al. 2000; Wang et al. 2003). Because fast inactivation has been disabled by the QQQ mutations suggests that closing of the activation gate at hyperpolarized voltages trapped flecainide within the pore.
We used the I1756C mutation to further investigate the mechanism(s) underlying the slow recovery from flecainide inhibition. Homologous mutations in neuronal and skeletal muscle Na+ channels potentiate the inhibition produced by externally applied quaternary analogues of anaesthetics by creating an alternative access pathway that facilitates the binding of these membrane-impermeant compounds when the channels are closed (Ragsdale et al. 1994; Qu et al. 1995; Lee et al. 2001; Sunami et al. 2001). We found that the recovery of the drug-modified QQQ-I1756C mutant (τ = 7.4 s) was 6-fold faster than that of the QQQ channel (τ = 42 s). When expressed in the non-inactivating background, the I1756C mutation accelerated the recovery from flecainide inhibition. The more rapid recovery of the QQQ-I1756C mutant cannot be attributed to a simple weakening of flecainide binding because the affinity is not altered by the I1756C mutation (Table 2). Rather the I1756C mutation appears to facilitate flecainide escape from the pore when the channel is closed. Slow untrapping of flecainide from within the pore appears to be the rate-limiting step in the recovery of the non-inactivating mutant channels. Channel opening enhances flecainide binding and deactivation traps the drug within the channel. These data are consistent with a model in which the activation gate acts as an effective barrier that regulates flecainide access to and escape from its cytoplasmic binding site.
At physiological pH (7.4), flecainide is positively charged (99%), which may account for its strong preference for accessing the cytoplasmic binding site via the hydrophilic aqueous pathway of open channels rather than the hydrophobic routes used by uncharged anaesthetics (Liu et al. 2003). The interdomain D3–D4 linker of Na+ channels is believed to act as a ‘hinged lid’ that binds to the internal mouth of the channel during inactivation and inhibits Na+ permeation (West et al. 1992). The weak flecainide inhibition induced by long depolarizing conditioning pulses (Fig. 2B) suggests that when closed the inactivation gate prevents flecainide from readily access its binding site via the internal aqueous pathway (Liu et al. 2002; Wang et al. 2003). Although rapid inactivation does not directly promote flecainide binding, inactivation significantly contributes to the slow recovery of drug-modified channels at hyperpolarized voltages. For example, the closed-state untrapping of flecainide from the non-inactivating QQQ mutant (τ = 42 s) is more rapid then the recovery of the inactivating wild type (τ = 81 s). This raises the possibility that rapid inactivation may slow the recovery by trapping flecainide within the channel (Courtney, 1975). Alternatively, high-affinity binding of flecainide could bias the gating equilibrium toward stable inactivated states thereby contributing to the slow recovery of the channels (Liu et al. 2002; Wang et al. 2003).
We found that the recovery of the drug-modified I1756C mutant (τI1756C = 12.6 s) was rapid by comparison to the wild type (τ = 81 s) and displayed kinetics surprisingly similar to the closed-state untrapping from the QQQ-I1756C mutant (τQQQ-I1756C = 7.4 s). Creating a pathway that enables the rapid escape of flecainide from the channel accelerated the recovery from both closed and inactivated states. This is substantially different from what we previously observed for the inhibition of Nav1.5 by cocaethylene, a metabolite of cocaine and ethanol (O'Leary et al. 2003). In that study, the I1756C mutation selectively facilitated the recovery from the closed but not the inactivated state. The data indicated that despite enabling rapid untrapping, the recovery of the I1756C mutant remained slow because of enhanced cocaethylene binding to inactivated states, a result that is consistent with the predictions of the modulated receptor hypothesis (Hille, 1977; Hondeghem & Katzung, 1977). Our current finding that the I1756C mutation caused the flecainide inhibition to rapidly dissipate in both the inactivating and non-inactivating mutants argues against a mechanism in which flecainide is tightly bound when the channels are inactivated. This is further supported by data showing that prolonged depolarizations that stabilize channels in inactivated states failed to promote the flecainide inhibition of the I1756C mutant (Fig. 6B). Unlike the wild type, reduced access of flecainide to the binding site when the channels are inactivated cannot account for the weak inhibition of the I1756C mutant. These data support the conclusion that flecainide inhibits Nav1.5 by a mechanism that does not require high-affinity binding to inactivated states of the channel. Rather the data suggest that deactivation and rapid inactivation potentiate the inhibition and slow the recovery by trapping flecainide within the pore. These findings are consistent with a ‘guarded receptor’ mechanism in which the channel gates regulate flecainide access to the cytoplasmic binding site (Starmer et al. 1984).
Mechanisms of state-dependent flecainide binding and inhibition
Previous studies of the state-dependent binding of flecainide to Na+ channels generally support a mechanism in which channel opening is an important step in flecainide binding (Anno & Hondeghem, 1990; Nitta et al. 1992; Qu et al. 1995; Ragsdale et al. 1996; Nagatomo et al. 2000; Liu et al. 2002; Wang et al. 2003). This is consistent with our data showing that flecainide preferentially accesses the cytoplasmic binding site of Nav1.5 by entering through the aqueous pathway of open channels. However, recent work suggests that flecainide may inhibit Na+ channels by directly binding to inactivated states (Viswanathan et al. 2001; Desaphy et al. 2004). We found that at low concentrations (≤ 25 μm), flecainide failed to inhibit during sustained depolarization where the channels are predominately inactivated (Fig. 2B). This contrasts with repetitive pulsing protocols that significantly inhibited the current (75%) under identical conditions (Fig. 1). These findings are inconsistent with an important role for the direct binding of flecainide to inactivated states of the channel. At high concentrations (100 μm), flecainide binding to inactivated channels has been reported but the onset of this inhibition is slow and therefore deemed unlikely to significantly contribute to the inhibition produced by short depolarizing pulses (Wang et al. 2003). Overall, our data support the conclusions of previous studies indicating that flecainide inhibits Na+ channels by preferentially binding to the open state.
Although Na+ channel opening clearly promotes flecainide binding, the mechanism of inhibition remains controversial. A recent study reported that although channel opening contributes to flecainide binding, the drug does not appear to act by a pore blocking mechanism (Liu et al. 2002). This contrasts with studies showing that flecainide reduces single-channel open times (Grant et al. 2000) and causes a time-dependent decay in the current of non-inactivating mutant channels (Wang et al. 2003), both of which are consistent with the flecainide block of open channels. The voltage sensitivity of the flecainide inhibition (Fig. 1C), simple binding kinetics (Fig. 3B) and sensitivity to changes in the concentration of external Na+ (Fig. 3B,C) are in good agreement with the predictions of a pore blocking mechanism. In addition, the steady-state inhibition of Nav1.5 (Fig. 1B) and the QQQ mutant (Fig. 3C) display similar concentration dependence, suggesting that open-channel block may underlie the flecainide inhibition of both the inactivating and the non-inactivating channels.
Our data are consistent with previous studies indicating that flecainide preferentially gains access to its binding site when the channels are open and that inhibition of the channel is further potentiated by rapid inactivation (Liu et al. 2002; Wang et al. 2003). These findings have been interpreted within the framework of the modulated receptor hypothesis in which anaesthetics are believed to bind with high affinity to the inactivated states of the channel (Hille, 1977; Hondeghem & Katzung, 1977). In this model, the recovery of drug-modified channels is determined by the interplay between the slow dissociation of flecainide from its high-affinity binding site and the recovery from inactivation. Although our data are consistent with an important role for rapid inactivation in the slow recovery of drug-modified channels, it is inconsistent with high-affinity binding to inactivated states. The slow dissociation of flecainide from a high-affinity binding site does not appear to be the rate-limiting step in the recovery of drug-modified channels. Rather our data suggest that when shut the activation and inactivation gates act as effective barriers that prevent the rapid escape of the positively charged flecainide from its binding site located within the pore. Cooperative interaction between these gates in the drug-modified channel appears to potentiate flecainide trapping within the pore resulting in the slow recovery of the Nav1.5 channel at hyperpolarized voltages. It is known that Na+ channels must first deactivate prior to recovering from inactivation (Kuo & Bean, 1994). Flecainide may alter deactivation or disrupt the coupling between closing and recovery resulting in channels that remain persistently blocked and inactivated at hyperpolarized voltages. Alternatively, flecainide occupancy of the pore may alter the voltage-dependent gating causing the channels to become locked in a non-conducting conformation (Sheets & Hanck, 2003). Additional studies of flecainide untrapping from closed and inactivated channels will provide new insight into the mechanisms of local anaesthetic inhibition.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (DA15192) and the American Heart Association.
References
- Anderson J, Gilbert E, Alpert B, Henthorn R, Waldo A, Bhandari A, Hawkinson R, Pritchett E. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circ. 1989;80:557–1570. doi: 10.1161/01.cir.80.6.1557. [DOI] [PubMed] [Google Scholar]
- Anno T, Hondeghem L. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ Res. 1990;66:89–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- Bean B, Cohen C, Tsien R. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhorin J, Taub R, Goldmit M, Kerem B, Kass R, Windman I, Medina A. Effects of flecainide in patients with new SCN5A mutation: mutation-specific therapy for long–QT syndrome? Circ. 2000;101:1698–1706. doi: 10.1161/01.cir.101.14.1698. [DOI] [PubMed] [Google Scholar]
- Brugada R, Brugada J, Antzelevitch C, Kirsch G, Potenza D, Towbin J, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circ. 2000;101:510–515. doi: 10.1161/01.cir.101.5.510. [DOI] [PubMed] [Google Scholar]
- Cast Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- Clarkson C, Follmer C, Ten Eick R, Hondeghem L, Yeh J. Evidence for two components of sodium channel block by lidocaine in isolated cardiac myocytes. Circ Res. 1988;63:869–878. doi: 10.1161/01.res.63.5.869. [DOI] [PubMed] [Google Scholar]
- Courtney K. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975;195:225–236. [PubMed] [Google Scholar]
- Desaphy J, Luca A, Didonna M, George A, Jr, Camerino D. Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block. J Physiol. 2004;554:321–334. doi: 10.1113/jphysiol.2003.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Chandra R, Keller C, Carboni M, Starmer C. Block of wild-type and inactivation-deficient cardiac sodium channels IFM/QQQ stably expressed in mammalian cells. Biophys J. 2000;79:3019–3035. doi: 10.1016/S0006-3495(00)76538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn R, Waldo A, Anderson J, Gilbert E, Alpert B, Bhandari A, Hawkinson R, Pritchett E. Flecainide acetate prevents recurrence of symptomatic paroxysmal supraventricular tachycardia. The Flecainide Supraventricular Tachycardia Study Group. Circ. 1991;83:119–125. doi: 10.1161/01.cir.83.1.119. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L, Katzung B. Time- and voltage–dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977;472:373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hondeghem L, Katzung B. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Kuo C, Bean B. Na+ channels must deactivate to recover from inactivation. Neuron. 1994;12:819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Lee P, Sunami A, Fozzard H. Cardiac-specific external paths for lidocaine, defined by isoform-specific residues, accelerate recovery from use-dependent block. Circ Res. 2001;89:1014–1021. doi: 10.1161/hh2301.100002. [DOI] [PubMed] [Google Scholar]
- Liu H, Atkins J, Kass R. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Tateyama M, Clancy C, Abriel H, Kass R. Channel openings are necessary but not sufficient for use-dependent block of cardiac Na+ channels by flecainide: evidence from the analysis of disease-linked mutations. J Gen Physiol. 2002;120:39–51. doi: 10.1085/jgp.20028558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo T, January C, Makielski J. Preferential block of late sodium current in the LQT3 ΔKPQ mutant by the class I(C) antiarrhythmic flecainide. Mol Pharmacol. 2000;57:101–107. [PubMed] [Google Scholar]
- Nitta J, Sunami A, Marumo F, Hiraoka M. States and sites of actions of flecainide on guinea-pig cardiac sodium channels. Eur J Pharmacol. 1992;214:191–197. doi: 10.1016/0014-2999(92)90118-n. [DOI] [PubMed] [Google Scholar]
- O'Leary M, Chahine M. Cocaine binds to a common site on open and inactivated human heart (Nav1.5) sodium channels. J Physiol. 2002;541:701–716. doi: 10.1113/jphysiol.2001.016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M, Chen L, Kallen R, Horn R. A molecular link between activation and inactivation of sodium chanels. J Gen Physiol. 1995;106:641–658. doi: 10.1085/jgp.106.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M, DiGregorio M, Chahine M. Closing and inactivation potentiate the cocaethylene inhibition of cardiac sodium channels by distinct mechanisms. Mol Pharmacol. 2003;64:1575–1585. doi: 10.1124/mol.64.6.1575. [DOI] [PubMed] [Google Scholar]
- Qu Y, Rogers J, Tanada T, Scheuer T, Catterall W. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel. Proc Natl Acad Sci U S A. 1995;92:11839–11843. doi: 10.1073/pnas.92.25.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale D, McPhee J, Scheuer T, Catterall W. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale D, McPhee J, Scheuer T, Catterall W. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Chapula J, Tsuda Y, Josephson I. Voltage- and use-dependent effects of lidocaine on sodium current in rat single ventricular cells. Circ Res. 1983;52:557–565. doi: 10.1161/01.res.52.5.557. [DOI] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Molecular action of lidocaine on the voltage sensors of sodium channels. J Gen Physiol. 2003;121:163–175. doi: 10.1085/jgp.20028651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C, Grant A, Strauss H. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984;46:15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A, Glaaser I, Fozzard H. Structural and gating changes of the sodium channel induced by mutation of a residue in the upper third of IVS6, creating an external access path for local anesthetics. Mol Pharmacol. 2001;59:684–691. doi: 10.1124/mol.59.4.684. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, Bezzina C, George A, Jr, Roden D, Wilde A, Balser J. Gating-dependent mechanisms for flecainide action in SCN5A–linked arrhythmia syndromes. Circ. 2001;104:1200–1205. doi: 10.1161/hc3501.093797. [DOI] [PubMed] [Google Scholar]
- Wang G, Russell C, Wang S. State-dependent block of wild-type and inactivation-deficient Na+ channels by flecainide. J Gen Physiol. 2003;122:365–374. doi: 10.1085/jgp.200308857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Patton D, Scheuer T, Wang Y, Goldin A, Catterall W. A cluster of hydrophobic amino acid residues required for fast Na+ channel inactivation. Proc Natl Acad Sci U S A. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle J, Geletka R, Moss A, Zareba W, Atkins D. Normalization of ventricular repolarization with flecainide in long QT syndrome patients with SCN5A: DeltaKPQ mutation. Ann Noninvasive Electrocardiol. 2001;6:153–158. doi: 10.1111/j.1542-474X.2001.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]