Abstract
The effects of creatine (Cr) absence in skeletal muscle caused by a deletion of guanidinoacetate methyltransferase (GAMT) were studied in a knockout mouse model by in vivo 31P magnetic resonance (MR) spectroscopy. 31P MR spectra of hindleg muscle of GAMT-deficient (GAMT–/–) mice showed no phosphocreatine (PCr) signal and instead showed the signal for phosphorylated guanidinoacetate (PGua), the immediate precursor of Cr, which is not normally present. Tissue pH did not differ between wild-type (WT) and GAMT–/– mice, while relative inorganic phosphate (Pi) levels were increased in the latter. During ischaemia, PGua was metabolically active in GAMT–/– mice and decreased at a rate comparable to the decrease of PCr in WT mice. However, the recovery rate of PGua in GAMT–/– mice after ischaemia was reduced compared to PCr in WT mice. Saturation transfer measurements revealed no detectable flux from PGua to γ-ATP, indicating severely reduced enzyme kinetics. Supplementation of Cr resulted in a rapid increase in PCr signal intensity until only this resonance was visible, along with a reduction in relative Pi values. However, the PGua recovery rate after ischaemia did not change. Our results show that despite the absence of Cr, GAMT–/– mice can cope with mild ischaemic stress by using PGua for high energy phosphoryl transfer. The reduced affinity of creatine kinase (CK) for (P)Gua only becomes apparent during recovery from ischaemia. It is argued that absence of Cr causes the higher relative Pi concentration also observed in animals lacking muscle CK, indicating an important role of the CK system in Pi homeostasis.
To maintain homeostasis and carry out mechanical work, skeletal muscle cells require a constant input of free energy derived from high energy phosphoryl transfer. Hydrolysis of ATP serves as an instantaneous donor of free energy but its concentration in the muscle cell is limited. The phosphorylated form of creatine (Cr), phosphocreatine (PCr), however, is available in much higher concentrations and can regenerate ATP through the transfer of its high energy phosphoryl group to ADP in an equilibrium reaction catalysed by one of the three isoforms of creatine kinase (CK) found in muscle (Wallimann et al. 1992; Wyss & Kaddurah-Daouk, 2000):
One of the main functions attributed to the PCr–CK system is that of a temporal energy buffer for high energy phosphates by keeping ATP/ADP ratios balanced (Wallimann et al. 1992). Another function that has been ascribed to the PCr–CK system is that of a spatial energy buffer, or energy shuttle, linking sites of high energy phosphate production (mitochondria) to utilization sites (e.g. myofibrils, sarcoplasmatic reticulum) (Bessman & Geiger, 1981). However, the mechanism and importance of this transport in muscle are topics of ongoing debate (e.g. Meyer et al. 1986; Wallimann et al. 1992; Wyss & Kaddurah-Daouk, 2000; Dzeja & Terzic, 2003).
The importance of intact Cr metabolism in humans was recently highlighted by the identification of a Cr deficiency syndrome caused by a deficiency of guanidinoacetate methyltransferase (GAMT, EC 2.1.1.2) due to mutations in the human GAMT gene, which leads to severe symptoms including mental retardation and muscle hypotonia (Stöckler et al. 1994). GAMT is an essential enzyme in the biosynthesis of Cr where it catalyses the final step. The first step in this biosynthesis, catalysed by l-arginine: glycine amidinotransferase, consists of the transfer of the amidino group from arginine to glycine to yield l-ornithine and guanidinoacetic acid (Gua). Subsequently, the amidino group of Gua is methylated by GAMT to give Cr and, since it is assumed that in vertebrates this process mainly occurs in the pancreas and liver, Cr is exported to the blood and taken up by tissues such as muscle and brain (Wyss & Kaddurah-Daouk, 2000). Since a constant fraction of Cr is converted non-enzymatically to creatinine and excreted daily, biosynthesis and/or dietary Cr sources are needed to maintain a constant body pool of Cr (Walker, 1979).
In mice, studies of the physiological significance of the PCr–CK system have mainly focused on knockout mice lacking one or more of the CK isoforms (e.g. van Deursen et al. 1993; Steeghs et al. 1997; In 't Zandt et al. 1999, 2003) or mice fed creatine analogues (van Deursen et al. 1994; Boehm et al. 1996; among others). Recently, GAMT-deficient knockout mice (GAMT–/–) have become available (Schmidt et al. 2004) that completely lack the essential enzyme GAMT and thus cannot form Cr. This new mouse model provides an excellent opportunity to study the function of the PCr–CK system in energy metabolism from a different perspective.
Magnetic resonance spectroscopy (MRS) allows non-invasive assessment of various compounds central in the study of energy metabolism related to the PCr–CK system (Meyer et al. 1982) and is instrumental in the diagnosis of Cr deficiency syndromes in human patients. Our 31P and 1H MRS measurements with GAMT–/– mice validated their use as an animal model for creatine deficiency as they were in agreement with observations in patients (Renema et al. 2003). So far, in one patient only, 31P MRS of the calf muscle has been performed (Schulze et al. 2003) which showed a strongly reduced PCr signal along with a new signal that, in brain, had previously been assigned to phosphorylated Gua (PGua) (Frahm & Hanefeld, 1997). In addition to the measurement of steady-state compound levels using MRS, the method of saturation transfer (ST) (Forsén & Hoffman, 1963) provides a window on enzyme kinetics in vivo. During steady-state conditions, ST can be used to measure unidirectional rate constants of chemically exchanging compounds like PCr and ATP (Meyer et al. 1982). In ST measurements of the brain of a GAMT-deficient patient, flux between ATP and PGua was decreased below detection level (Frahm & Hanefeld, 1997).
Despite the absence of Cr biosynthesis, GAMT–/– mice are viable and show only minor overt abnormalities (Schmidt et al. 2004). Since possible adaptations and subtle deviations that result from this deficiency could elucidate the function of the Cr circuit in vivo, the aim of the present study was to investigate the consequences of GAMT deficiency on muscle energy metabolism. We examined muscle energy metabolism of GAMT–/– mice non-invasively during rest and ischaemia using 31P MRS and ST. To study the effects of Cr supplementation on enzyme kinetics, a separate group of GAMT–/– animals was supplemented with Cr and their muscles subjected to ischaemia. By comparing the results of the present investigation to the results from studies on CK-deficient mice, a distinct metabolic phenotype was uncovered for GAMT–/– mice.
Methods
Animals
GAMT–/– mice were generated by homologous recombination in embryonic stem cells (Schmidt et al. 2004). Homozygous and heterozygous wild-type (WT) littermates of the GAMT–/– animals were used as a reference (Schmidt et al. 2004). Adult animals (> 6 months old) were measured in the present study. Animals were given free access to standard chow based on vegetable protein (ssniff Spezialdiäten GmbH, Soest, the Netherlands, R/M-H) to eliminate possible Cr content.
During the MR experiments, all animals were anaesthetized with 1.5% isoflurane in a gas mixture of 50% O2 and 50% N2O delivered through a face mask. Rectal temperature was monitored using a fluoroptic thermometer (Luxtron 712, Santa Clara, CA, USA) and maintained at 37.0 ± 1°C using a warm water bed. Breathing frequency was monitored optically (Sirecust 401, Siemens). All experiments were approved by the animal ethics committee of the University Medical Centre Nijmegen and animals were killed by cervical dislocation after termination of the study.
MR experiments
MRS measurements were carried out on a 7.0 T, 120 mm horizontal bore, magnet (Magnex Scientific, Abingdon, UK) interfaced to a S.M.I.S. spectrometer (Surrey Medical Imaging Systems, Surrey, UK) operating at 121.53 MHz for 31P. A three-turn solenoid coil was used for 31P MR measurements, in combination with an Alderman-Grant type of proton coil for shimming of the magnetic field.
Ischaemia measurements were carried out on seven WT (3 males, 4 females) and seven GAMT–/– mice (4 males, 3 females). A diaphragm plate which allowed reversible and reproducible obstruction of blood flow through the hindlimb (Heerschap et al. 2004) was used to apply ischaemia. To assess basal metabolic levels a 31P MR spectrum with a high signal-to-noise ratio (SNR), employing a repetition time (TR) of 7000 ms at 76 averages, was recorded during resting conditions. Subsequently, the ischaemia protocol was started with the recording of 31P MR spectra with higher temporal resolution (TR = 1400 ms, 76 averages) for 7 min prior to ischaemia as control, during the 25 min of ischaemia and during 16 min of recovery. All experiments were carried out with a pulse-acquire sequence using a radio frequency (RF) pulse with a duration of 40 μs.
To assess the flux from γ-ATP to PCr or PGua, ST measurements were performed on six WT and six GAMT–/– mice (3 males and 3 females in both groups). After obtaining a 31P MR spectrum during resting conditions (TR = 7000 ms, 128 averages) the γ-ATP signal was selectively saturated for six different durations (500–5000 ms) prior to acquisition (TR = 7000 ms, 64 averages). To correct for any power spill-over of the selective RF pulse, control spectra were recorded with a selective saturation pulse applied at the ‘mirror frequency’ with respect to the PCr or PGua resonance frequencies.
Three groups of GAMT–/– animals were supplemented with 2 g (kg body weight)−1 day−1 Cr monohydrate (Sigma C0780) dissolved in the drinking water (Ipsiroglu et al. 2001). Saccharose (1.6 g (kg body weight)−1 day−1) was added to the drinking water of the supplemented animals to mask the bitter taste of the Cr monohydrate (Ipsiroglu et al. 2001). One group (n = 4, 3 females, 1 male) was supplemented for 1 day (24 h), the second group (n = 6, 5 females, 1 male) was supplemented for 2 days (48 h) and finally a third group (n = 4, 3 females, 1 male) was supplemented for 1 month. In the group that was supplemented for 48 h ischaemia measurements were carried out as described above.
Finally, since a repetition time of 1400 ms results in partly saturated phosphate spin systems, fully relaxed 31P MR spectra (TR = 25 s, 64 averages) were recorded from six WT and six GAMT–/– animals to obtain saturation correction factors and T1 relaxation times for PCr, PGua and γ-ATP.
Data analysis of MR spectra
Spectra of the 31P MR measurements were analysed using MRUI software (http://www.mrui.uab.es/mrui/mruiHomePage.html) and peak areas were obtained by fitting the signals to Lorentzian line shapes with fixed first-order phase correction.
31P MR spectra recorded during resting conditions were analysed with no fixed signal dampings except for the 31P MR spectra recorded after supplementing Cr where the damping of PGua was set equal to the damping of PCr.
For the signals obtained in the ischaemia experiments, the damping of the inorganic phosphate (Pi) signal was set at a fixed ratio of 1.3 times the PCr (WT) or PGua (GAMT–/–) peak (empirically determined). Due to a decreased SNR in GAMT–/– animals, the line width of the PGua peak was constrained for each individual mouse as the average line width per stage of the ischaemic protocol: prior to ischaemia, during ischaemia and after ischaemia. For the feeding experiment, the damping of the PGua peak was set equal to that of the PCr peak. All peak areas were normalized to the average β-ATP signal before ischaemia and corrected by using PCr/ATP, PGua/ATP and Pi/ATP ratios determined from fully relaxed spectra. Correction factors for PGua/ATP and Pi/ATP in GAMT–/– animals were assumed to be equal to PCr/ATP and Pi/ATP ratios in WT animals. The SNR of the β-ATP signal was calculated as the average signal intensity before ischaemia.
The rates of the PCr and PGua signal recovery after ischaemia were analysed by expressing the signal as a percentage of the initial value before ischaemia and fitting this normalized value to a mono-exponential function (Meyer, 1988) by the Levenberg-Marquardt least squares method using Graphpad software (GraphPad Prism, San Diego CA, USA):
where M(trec) is the amount of signal at trec seconds after the start of the recovery period, M0 is the intensity after recovery from ischaemia, Mcons is the difference between the PCr or PGua level at the end of ischaemia and M0, and τ is the time constant for signal recovery. The initial rate of signal recovery (Vi) was calculated as the first time derivative of this function (e.g. Foley & Meyer, 1993):
Pi signal recovery was fitted to a similar function as described in (1). Tissue pH was calculated from the shift in resonance position (S) of the Pi peak compared to the position of PCr (WT). In GAMT–/– animals, where a PCr signal is absent, the resonance position of the PGua signal was determined at 0.44 p.p.m. upfield of PCr. The following equation was used to calculate pH:
(Taylor et al. 1983) where S is the chemical shift difference between PCr and Pi.
The resting ATPase rate of the hindleg muscle complex was calculated from the slope of a linear regression line through the first four points during the ischaemic period of the PCr/ATP and PGua/ATP plots as pH did not decline during this period (Blei et al. 1993; Marcinek et al. 2004). As the increase of Pi/ATP during the ischaemic period could also elucidate changes in ATPase activity, the slope of this parameter was calculated in the same way.
In the analysis of the ST experiment, signal intensities of PGua and PCr after irradiation of γ-ATP were normalized to the signal intensity without irradiation. After subtraction of the control spectra, PCr and PGua signal intensities were fitted to a mono-exponential function (Brindle, 1988; Meyer et al. 1982) by the Levenberg-Marquardt least squares method using Graphpad software (GraphPad Prism):
where, after different saturation times (t), the signal intensity at this time (Mt) is divided by the signal intensity without saturation (M0), kfor is the pseudo-first-order unidirectional rate constant for the CK reaction from PCr or PGua to γ-ATP and T1i is the spin-lattice relaxation time measured in the presence of saturation of γ-ATP. Tissue concentrations of PCr and PGua were calculated with an assumed resting ATP concentration of 7.8 mm (In 't Zandt et al. 2003) and the PCr/ATP and PGua/ATP ratios obtained from the spectra with high SNR (TR = 7000 ms, 76 averages) corrected for partial saturation effects.
Chemical determination of ATP levels
Since possible differences in SNR could be due to alterations in ATP levels between mouse types, resting ATP levels of the calf were determined chemically in five WT and six GAMT–/– mice. As normalization to total Cr to correct for variations caused by the variable presence of non-muscle elements in the dry tissue powder is not possible in GAMT–/– animals due to the absence of Cr, chemically determined ATP levels were only used for the comparison of SNR values.
Animals were anaesthetized with dormicum–hypnorm–H2O (1: 1: 2, 0.01 ml (g body weight)−1). Left and right gastrocnemius, plantaris, soleus (GPS) muscle complexes were exposed and immediately clamp-frozen in brass tongs pre-cooled with liquid nitrogen and stored at −80°C. Muscles frozen in nitrogen were pulverized in a mortar under constant addition of liquid nitrogen, freeze-dried overnight and stored in liquid nitrogen until further analysis. Metabolites were separated and quantified using a high-performance liquid chromatography (HPLC) system using RP-18 columns (Hewlett Packard) as described previously (Karatzaferi et al. 1999). The HPLC system consisted of a Binary LC pump (Model 250, Perkin-Elmer, USA), an auto sampler with cooling tray and automatic injector (Basic Marathon, Spark Holland, the Netherlands) and a variable wavelength ultraviolet (UV) spectrophotometric detector (Model 795A, Applied Biosystems, The Netherlands). The size of the injection loop was 20 μl and the UV absorption was measured at 254 nm. ATP peaks were identified and quantified by a chromatography data system (model 717; Axxiom Chromatography, CA, USA) by comparing the peak heights of samples with those of external standards (Karatzaferi et al. 1999) and expressed in micromole per gram dry weight (μmol (g dry weight)−1).
Statistics
Normalized signal intensities of 31P MR spectra during rest and ischaemia, SNR and Vi values were compared between GAMT–/– and WT mice with a Students t test. Pi/ATP ratios before and after 1 month of Cr supplementation were compared with a paired t test. The τ values derived from the mono-exponential curves of the recovery after ischaemia and the slopes derived from the linear regression were compared with an F test (GraphPad Prism). Unless stated otherwise, all results were considered significantly different when P < 0.05 and are presented as means ± s.e.m.
Results
Resting conditions
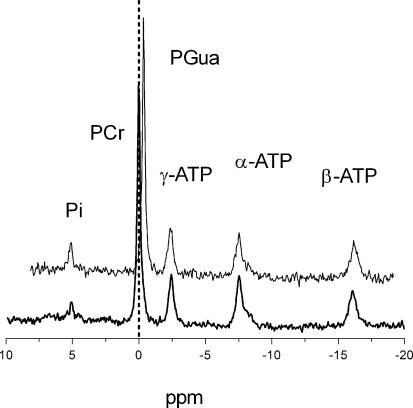
A comparison of 31P MR spectra of GAMT–/– mice and WT mice under resting conditions revealed a negligible PCr signal for GAMT–/– mice along with a new signal at 0.44 p.p.m. which was assigned to PGua, the immediate precursor of Cr (Fig. 1). The resting metabolite level of PGua in GAMT–/– mice, determined from 31P MR spectra and normalized to the β-ATP peak, did not differ significantly from the PCr/ATP ratio in WT mice (Table 1). Furthermore, resting tissue pH was not significantly different between WT and GAMT–/– animals (7.20 ± 0.02 and 7.19 ± 0.04, respectively). Relative Pi levels, however, were significantly higher in GAMT–/– animals (Table 1). The SNR of the β-ATP signal was almost 2-fold lower in GAMT–/– animals compared to WT animals (46.0 ± 2.5 and 83.9 ± 2.3, respectively) (P < 0.01). T1 relaxation times were 4.0 ± 0.9 s for PGua and 3.0 ± 0.5 s for PCr and did not differ significantly. The T1 relaxation time of γ-ATP was 1.5 ± 0.3 s.
Figure 1. In vivo 31P MR spectra of the mouse hindleg at rest.
Spectra are of a GAMT–/– mouse (top) and WT mouse (bottom), TR = 7000 ms, 128 averages. A vertical dashed line is positioned at 0 p.p.m. to show that the large resonance in the top spectrum is indeed PGua at 0.44 p.p.m. upfield from PCr. Peaks for inorganic phosphate (Pi), phosphocreatine (PCr), phosphorylated guanidinoacetate (PGua) and the three resonance positions of ATP (α,β,γ) are visible.
Table 1.
High energy phosphate ratios in GAMT–/– and WT hindleg muscle before and after Cr supplementation
| Pi/ATP | PCr/ATP | PGua/ATP | |
|---|---|---|---|
| WT | 0.40 ± 0.04 | 3.16 ± 0.10 | ud |
| GAMT–/– | 0.65 ± 0.04§ | ud | 3.04 ± 0.06 |
| GAMT–/– (sup 1 day) | 0.81 ± 0.06§ | 1.93 ± 0.11§ | 2.89 ± 0.20 |
| GAMT–/– (sup 2 days) | 0.60 ± 0.04§ | 2.18 ± 0.13§ | 1.91 ± 0.15* |
| GAMT–/– (sup 1 month) | 0.26 ± 0.03* | 2.89 ± 0.03 | ud |
Significantly different from WT animals
significantly different from GAMT–/– animals before supplementation.
Abbreviations: WT, WT animals; GAMT–/–, GAMT–/– animals; GAMT–/– (sup 1 day), GAMT–/– animals supplemented with Cr for 24 h; GAMT–/– (sup 2 days), GAMT–/– animals supplemented with Cr for 48 h; GAMT–/– (sup 1 month), GAMT–/– animals supplemented with Cr for 1 month; PCr, phosphocreatine; Pi, inorganic phosphate; ud, undetectable.
A small but significant difference in the chemically determined ATP concentrations was found between GAMT-deficient and WT mice (11.1 ± 1.2 µmol (g dry weight)−1 and 15.6 ± 1.1 µmol (g dry weight)−1, respectively).
Ischaemia
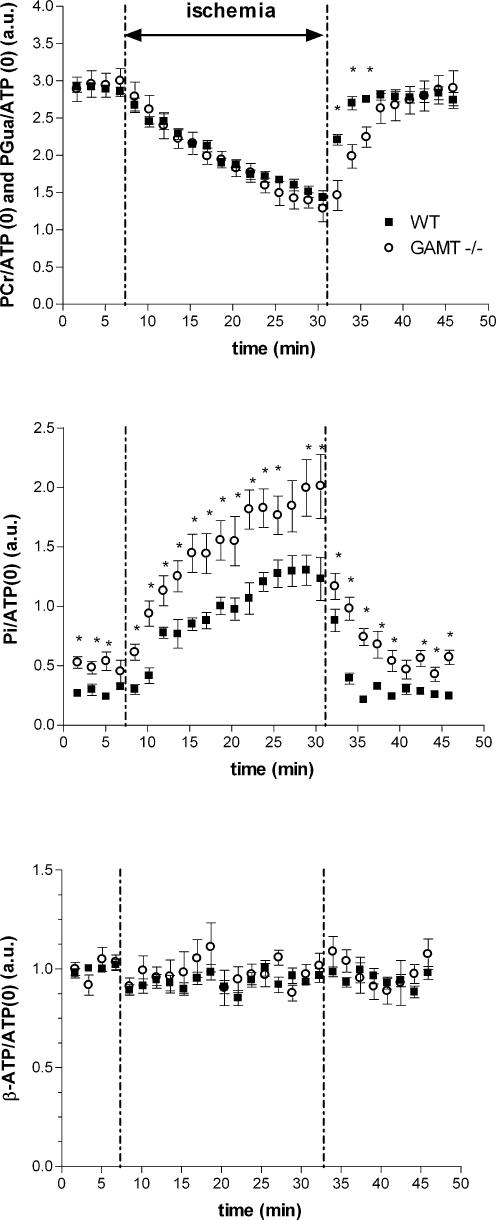
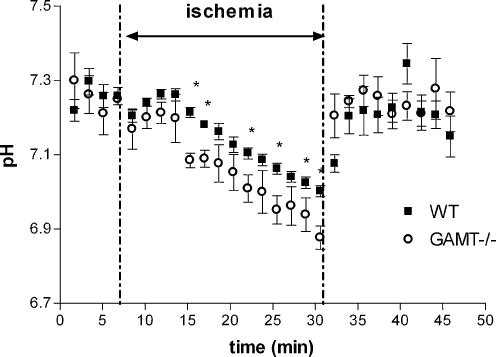
As the PCr–CK system is important under ischaemic conditions when oxidative phosphorylation is shut down, we subjected GAMT–/– animals and WT animals to 25 min of ischaemia and monitored changes in the compounds detectable by 31P MRS during ischaemia and recovery thereafter. During ischaemia, changes in normalized signal intensities of PCr and Pi in WT mice and PGua and Pi in GAMT–/– mice were observed (Fig. 2A and B). Interestingly, during the ischaemic period PGua in GAMT–/– animals declined at a rate similar to PCr in WT animals. The resting ATPase rate, determined from the slope of the decline of PCr and PGua, did not differ significantly between WT and GAMT–/– mice (Table 2). A similar calculation from the slopes of the increase in Pi/ATP values in both mouse types also showed no significant difference. In GAMT–/– mice, Pi levels were significantly elevated prior to ischaemia and remained significantly higher during and after ischaemia. Relative muscle ATP levels of both mouse types remained constant throughout the ischaemic period (Fig. 2C). Tissue pH did not differ between mouse types at the start of the ischaemic period, while pH was slightly lower in GAMT–/– mice towards the end of the ischaemic period (6.97 ± 0.01 for WT and 6.85 ± 0.03 for GAMT–/– mice) (Fig. 3).
Figure 2. Time plots of signal intensities normalized to the β-ATP signal before, during and after ischaemia.
Time courses of changes in PCr (WT) and PGua (GAMT−/−) (A), Pi (B) and ATP (C) are shown as means ± s.e.m. for WT (n = 7, ▪) and GAMT–/– (n = 7, ○). * Significantly different from WT at P < 0.05. a.u., arbitrary units.
Table 2.
Recovery kinetics and flux parameters of WT and GAMT-/- hindleg muscle
| WT | GAMT–/– | GAMT–/– (sup 2 days) | |
|---|---|---|---|
| τPCr (s) | 107 ± 14 | n.a. | 31 ± 40§ |
| τPGua (s) | n.a. | 383 ± 83** | 294 ± 131* |
| τPi (s) | 145 ± 29 | 168 ± 35 | n.d. |
| ViPCr (mm s−1) | 0.117 ± 0.01 | n.a. | 0.38 ± 0.05§§ |
| ViPGua (mm s−1) | n.a. | 0.034 ± 0.003** | 0.031 ± 0.003** |
| Forward flux (mm s−1) | 9.7 ± 0.5 | < 0.5† | n.d. |
| ATPase rate (mm s−1) | 0.027 ± 0.003 | 0.043 ± 0.006 | n.d. |
P < 0.05
P < 0.01: significantly different from WT animals
P < 0.05
P < 0.01: significantly different from PCr values in WT animals.
Calculated upper value. Abbreviations: n.a. = not applicable; n.d. = not determined; WT, WT animals; GAMT–/–, GAMT–/– animals; GAMT–/– (sup 2 days), GAMT–/– animals supplemented with Cr for 48 h; τ, time constants of recovery after ischaemia of phosphocreatine (PCr), phosphorylated guanidinoacetate (PGua) and inorganic phosphate (Pi); Vi, initial rates of recovery.
Figure 3. Time curves of the pH changes before, during and after ischaemia.
Tissue pH in hindleg muscle of WT (n = 7, ▪) and GAMT–/– (n = 7, ○) mice. Data are shown as means ± s.e.m. * Significantly different from WT at P < 0.05.
It has been stated that measurements of PCr recovery after depletion can be used as an index of relative oxidative capacity or mitochondrial content in muscle (e.g. Kemp et al. 1993). To assess these recovery kinetics, a mono-exponential function was fitted to the recovery of PCr, PGua and Pi and the resulting τ values revealed interesting differences (Table 2). The recovery of the PGua signal in GAMT–/– animals was significantly slower than the recovery of PCr in WT animals (P < 0.01), with τ values of PCr and PGua of 107 ± 14 and 383 ± 83 s, respectively. In contrast to the recovery of PGua, τ values of Pi/ATP recovery did not differ significantly between mouse types. By calculating Vi from the time constants, flux rates from γ-ATP to PCr and PGua can be compared. Vi values for PCr in WT and PGua in GAMT–/– animals were 0.117 ± 0.01 and 0.034 ± 0.003 mm s−1, respectively, and differed significantly (P < 0.01) (Table 2).
Saturation transfer
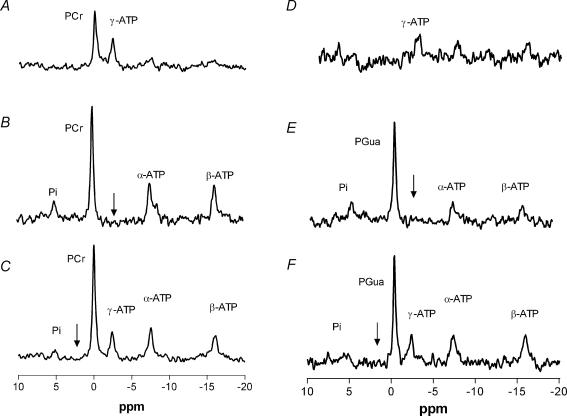
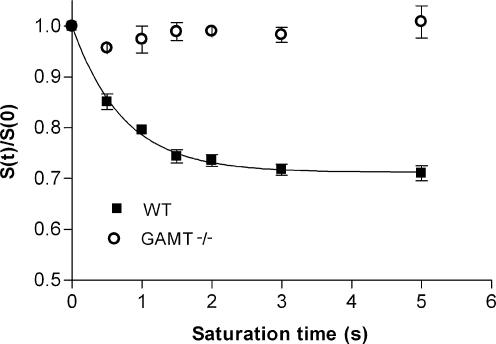
As the difference in recovery rate after ischaemia could be due to a difference in enzyme kinetics, ST experiments were performed (Fig. 4). With increasing irradiation times, no decrease in signal intensity of PGua was observed (Fig. 5). The kfor value calculated for PCr was 0.39 ± 0.03 s−1, resulting in a forward flux through the CK reaction of 9.7 ± 0.5 mm s−1 (Table 2). The absence of a detectable decrease in the PGua signal with increasing irradiation times shows that no chemical exchange could be detected in this period. As was shown previously for mice lacking the cytosolic form of CK (van Deursen et al. 1993), the absence of a detectable decrease in a signal in this type of ST experiment indicates at least a 20-fold reduction in reaction rates compared to CK in WT mice.
Figure 4. Typical saturation transfer spectra of a WT and a GAMT–/– mouse hindleg.
The appropriate control spectra (C and F), a spectrum with a 5000 ms (B) or a 3000 ms (E) saturation pulse applied at the frequency of γ-ATP and the corresponding difference spectra (A and D). Arrows denote the frequency of the selective saturation pulse. Spectra A–C are of a WT mouse, D–F of a GAMT–/– mouse.
Figure 5. Normalized signal intensities of PCr and PGua with increasing saturation times.
Signal intensities of PCr (WT; n = 6, ▪) and PGua (GAMT–/–; n = 6, ○) were normalized to the corresponding signal intensity in the absence of a saturation pulse. Data are shown as means ± s.e.m.
Cr supplementation
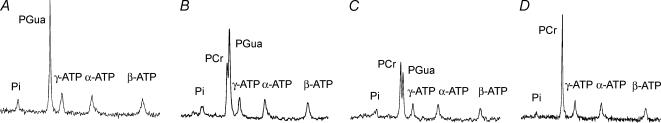
To observe the effects of Cr supplementation, 2 g (kg body weight)−1 day−1 Cr was added to the diet of GAMT–/– animals for three different time periods and this resulted in the appearance of a PCr signal in the 31P MR spectrum (Fig. 6). With increasing Cr supplementation durations, the ratio of PCr/PGua increased from 0.63 ± 0.08 to 1.19 ± 0.13 after 24 and 48 h, respectively, and differed significantly. Cr supplementation for 1 month resulted in a complete disappearance of the PGua peak (Fig. 6), a significant reduction in the Pi/ATP ratio compared to the pre-supplementation value and PCr/ATP levels comparable to values found in WT mice (Table 1).
Figure 6. Typical in vivo 31P MR spectra at rest of the hindleg of four GAMT–/– mice at different stages of Cr supplementation.
A, without supplementation. B, after 24 h of Cr supplementation. C, after 48 h of Cr supplementation. D, after 1 month of Cr supplementation. Peaks for inorganic phosphate (Pi), phosphocreatine (PCr), phosphorylated guanidinoacetate (PGua) and the three resonance positions of ATP (α,β,γ) are visible.
To assess whether Cr feeding had an effect on the recovery rate after ischaemia, the group of animals that was supplemented with Cr for 48 h was subjected to the same ischaemia protocol as the WT and non-supplemented GAMT–/– mice. After feeding of Cr, the τ and Vi values derived from changes in the PGua signal did not differ significantly from those of non-supplemented GAMT–/– mice. For the recovery of PCr, however, τ and Vi values were significantly increased in the supplemented GAMT–/– animals compared to WT animals (Table 2).
Discussion
This study demonstrates that despite inhibition of the final step in Cr biosynthesis, as shown by the absence of a PCr peak in the 31P MR spectrum, GAMT-deficient animals can cope with ischaemic stress. By using Gua, the immediate precursor of Cr, and thus PGua for high energy phosphoryl transfer they appear to compensate for the lack of Cr and PCr. Compared to WT animals they showed a higher relative Pi signal intensity, a slower recovery of PGua than of PCr after ischaemia and clearly reduced enzyme kinetics. Cr supplementation caused a rapid appearance of the PCr signal and a reduction of the Pi/ATP ratio to WT levels but did not alter PGua recovery kinetics after ischaemia.
Resting conditions
PCr is virtually absent in skeletal muscle of GAMT–/– mice. In a previous study by our group on these mice (Renema et al. 2003), the 31P MR spectra of skeletal muscle still showed a signal for PCr which was probably caused by trace amounts of Cr in the food. Recent observations have shown that the mice practice coprophagia, which could also account for residual PCr if GAMT–/– mice are housed with mixed-genotype groups. Consequently, mice used for the experiments reported here were housed according to genotype and fed Cr-free food. Our earlier hypothesis that residual PCr signals could result from oral intake is confirmed by the observation that after controlled Cr supplementation, a PCr signal appeared in GAMT–/– muscle. This PCr peak was visible after only 24 h of supplementation, despite the fact that Gua partially inhibits Cr uptake in skeletal muscle (Fitch & Chevli, 1980; Fitch et al. 1968). Longer supplementation of GAMT–/– mice with Cr caused a progressive decrease in the PGua signal intensity as the PCr signal increased. Since Gua uptake in skeletal muscle occurs through the Cr transporter (Wyss & Kaddurah-Daouk, 2000), the decrease in PGua is most likely caused by a competitive inhibition of Gua uptake by Cr (Fitch et al. 1968).
After 1 month of Cr supplementation in GAMT–/– mice, no PGua peak was visible and PCr/ATP levels were comparable to WT animals. Furthermore, these animals showed a significant reduction in Pi/ATP levels compared to the level before supplementation. Interestingly, the Pi/ATP levels in these animals were similar to WT littermates, indicating a change to the WT muscle phenotype.
The observed effect of Cr on the Pi/ATP ratio could explain the absence of a difference in Pi/ATP levels between WT and GAMT–/– mice where (P)Cr was present continuously (Renema et al. 2003). However, as only the mice supplemented with Cr for 1 month showed a significant decrease in their relative Pi content, this reduction apparently needs some time to take effect.
The almost 2-fold lower SNR of the β-ATP peak in GAMT–/– muscles could result from a number of factors including a lower ATP level and decreased muscle mass. As the chemically determined ATP values of GAMT–/– hindleg muscle only differed slightly from WT muscle, it is unlikely that a decrease in resting ATP level alone causes the decreased SNR. Recently we observed (Kan et al. 2004) that GAMT–/– medial gastrocnemius muscle weight was significantly lower than in WT mice, indicating that a reduced muscle mass contributes to this decreased SNR.
Comparison to creatine kinase knockout mice
PCr and PGua
Since it has been shown that the cytosolic isoform of CK phosphorylates Cr analogues (e.g. Fitch & Chevli, 1980; van Deursen et al. 1994; Boehm et al. 1996), it is likely that CK activity is responsible for the phosphorylation of Gua. Therefore, it is interesting to compare the present results with those from studies on mice lacking muscle CK.
The constant ATP levels throughout the entire ischaemic period indicate that, despite the absence of Cr, GAMT–/– animals can sustain the stress of short-term ischaemia well. A constant ATP concentration in skeletal muscle during ischaemia has also been found in mice lacking the mitochondrial isoform of CK (ScCKmit–/–) and in mice lacking the cytosolic isoform of CK (M-CK–/–) but not in mice lacking both isoforms (M-CK/ScCKmit–/–) (In 't Zandt et al. 1999). This indicates that the absence of Cr due to GAMT deficiency does not compromise ischaemic skeletal muscle energy metabolism to the same extent as a complete absence of CK. The rate of the decrease of PGua during ischaemia shows that the compound is metabolically active and that GAMT–/– mice can use Gua for high energy phosphoryl transfer at a similar rate as PCr is used by WT animals. Therefore, despite the severe reduction in forward flux as indicated in the ST experiments, the reaction rate of PGua is still sufficient to meet energy demand during ischaemia as ATP levels remained constant. Apparently the resting ATPase rate, determined by the decrease of PCr and PGua at constant pH, can still be matched by the flux of PGua to γ-ATP (Table 2). This was also shown in M-CK–/– animals, where despite a considerably lower flux rate (van Deursen et al. 1993), PCr levels decreased at a similar rate as in WT animals (In 't Zandt et al. 1999).
Pi and pH
During rest and ischaemia relative Pi values and kinetics differ between GAMT–/– mice and CK knockout mice. The higher resting relative Pi concentration found in GAMT–/– mice was absent in both ScCKmit–/– and M-CK–/– mice (In 't Zandt et al. 1999), while M-CK/ScCKmit–/– animals displayed a 2- to 3-fold higher Pi/ATP ratio compared to WT mice (Steeghs et al. 1998). However, in contrast to GAMT–/– mice, the increase in Pi during ischaemia is severely compromised in the M-CK/ScCKmit–/– mice (In 't Zandt et al. 1999).
Since, among other energy signalling molecules (Bose et al. 2003), Cr is thought to play an important role in stimulating mitochondrial respiration in concert with the mitochondrial isoform of CK (e.g. Boehm et al. 1998; Wallimann et al. 1998; Kay et al. 2000), the absence of Cr might be crucial here. As ScCKmit is unable to phosphorylate Gua (Boehm et al. 1996), Gua cannot substitute for Cr in stimulating mitochondrial respiration in GAMT–/– animals. M-CK/ScCKmit–/– mice also lack Cr stimulated respiration due to the absence of ScCKmit and M-CK, and the function of ScCKmit cannot be taken over by M-CK as in ScCKmit–/– animals (Boehm et al. 1998). Since both mutants display elevated Pi concentrations, it is conceivable that lack of Cr or the inability to phosphorylate this compound can lead to compensatory mechanisms resulting in a shift in Pi homeostasis. This is supported by the fact that in the GAMT–/– animals where (P)Cr was present continuously, no higher resting Pi concentrations were found (Renema et al. 2003). In contrast to M-CK/ScCKmit–/– animals, in GAMT–/– animals high energy phosphate transfer from PGua to ATP is still possible through M-CK, thereby producing a higher Pi level during ischaemia.
At the beginning of the ischaemic period, tissue pH remained fairly constant in the WT mice. This is in agreement with other studies on ischaemia (In 't Zandt et al. 1999; Marcinek et al. 2004) and studies on exercise (e.g. Adams et al. 1990; Houtman et al. 2001; Crowther et al. 2002) and is attributed to proton consumption by PCr hydrolysis. No differences were observed between GAMT–/– and WT mice at the first time points during ischaemia, suggesting that PGua supports proton buffering as well. Similar tissue pH values at the beginning of the ischaemic period were also found in M-CK–/– and ScCKmit–/– mice but not in M-CK/ScCKmit–/– mice where tissue pH immediately started to decline at the onset of ischaemia due to proton production, e.g. by ATP hydrolysis (In 't Zandt et al. 1999).
After about 10 min of the ischaemic period a small but significant pH difference is observed between GAMT–/– and WT mice. Since in ischaemic conditions pH will fall due to an increased proton production by glycolysis compared to proton consumption by PCr breakdown (Kemp et al. 2001), the earlier decrease in pH observed in GAMT–/– mice could be ascribed to an earlier start of glycolysis caused by higher levels of Pi in GAMT–/– animals (Crowther et al. 2002).
Recovery rate after ischaemia and enzyme kinetics
Ischaemia is a relatively slow process and it has been shown that during brief periods of ischaemia, the ATPase rate does not differ from aerobic conditions (Conley et al. 1998; Kemper et al. 2001). Recovery after ischaemia, however, is a faster process that uncovered some interesting differences between GAMT–/– and WT mice have been uncovered.
As mentioned above, measurements of PCr recovery after depletion can be used as an index of relative oxidative capacity or mitochondrial content in muscle. After ischaemia, oxygen is available again and recovery of high energy phosphates will be mainly due to ATP production in the mitochondria via oxidative phosphorylation. Since the recovery of PGua in GAMT–/– mice was significantly slower than PCr recovery in WT mice, this delay could be explained by a reduced oxidative capacity. However, since no reduction was found in citrate synthase activity of GAMT–/– mice compared to WT mice (Schmidt et al. 2004), it is unlikely that a reduced oxidative capacity caused the delayed recovery of PGua in GAMT–/– mice.
Another factor that could explain the reduced recovery rate of PGua after ischaemia is the absence of Cr stimulated respiration. In WT animals, Cr increases and PCr decreases during ischaemia, which leads to an increased stimulation of mitochondrial respiration (Walsh et al. 2001) as soon as oxygen is available again. In GAMT–/– animals (P)Cr is absent and, as mentioned above, Gua cannot take over the proposed role of Cr since ScCKmit is unable to phosphorylate the compound. This could lead to a less effective stimulation of mitochondrial respiration and thereby a slowing of the PGua recovery rate.
A third potential factor that might influence the recovery rate is the affinity of CK for Gua. Since Gua is 100-fold less reactive with CK compared to Cr in vitro (Boehm et al. 1996), and as the ST experiments showed a severe reduction of flux through the CK reaction in vivo, enzyme kinetics could be reduced to such an extent that they become rate limiting. Furthermore, the initial rate of recovery of PGua (Vi) (Table 2) indicates that the actual flux rate is well below the detection limit of the ST experiment.
Finally, tissue pH has also been shown to influence recovery kinetics (Iotti et al. 1993, 2004; Paganini et al. 1997). However, in the GAMT–/– animals that were supplemented with 2 g kg−1 Cr for 2 days, tissue pH was intrinsically the same for PCr and PGua recovery, since they are in the same compartment. Therefore, since the difference in recovery rate of PCr and PGua persisted, it is unlikely that pH alone accounts for the difference.
If the absence of the stimulatory effect of Cr was the major factor influencing the recovery of PGua, we would have expected the recovery rate of PGua to increase after Cr supplementation. However, the recovery of the PGua signal after ischaemia was not significantly elevated in the Cr-supplemented GAMT–/– animals compared to the non-fed GAMT–/– group. The recovery of the PCr signal in the Cr-supplemented GAMT–/– mice was significantly faster than in WT animals. Apparently, Cr is still able to stimulate mitochondrial respiration in GAMT–/– animals without leading to an elevation in the recovery rate of PGua. This strongly suggests that the lowered affinity of CK for Gua limits the recovery of PGua after ischaemia.
In contrast to the significant difference in τ values for the recovery of PGua and PCr, no such difference was found between τ values for Pi of WT and GAMT–/– mice. This could be explained by the fact that Pi has more than one fate after the ischaemic period, whereas PCr and PGua can only be generated from ATP through the CK reaction. Furthermore, at pH values below 6.95, Pi recovery is faster than at higher pH values, possibly due to an increased pH gradient over the mitochondrial membrane (Iotti et al. 1993). Since in GAMT–/– animals the pH value at the end of the ischaemic period is below this value, an effect of pH cannot be excluded.
In summary, our results indicate that GAMT–/– mice can use the immediate precursor of Cr Gua for high energy phosphoryl transfer despite the fact that the corresponding enzyme kinetics are severely reduced. These reduced enzyme kinetics cause a significant delay in the recovery of the PGua signal after ischaemia compared to the PCr recovery in WT mice. The effect of Cr supplementation on relative Pi levels in GAMT–/– mice reveals that the absence of Cr or PCr results in a shift in skeletal muscle Pi values. Future experiments involving a more severe metabolic challenge, such as exercise, will provide further details on the use of PGua as an energy donor and the consequences of GAMT deficiency.
Acknowledgments
The authors would like to thank Professor Dr A. de Haan (Institute for Fundamental and Clinical Human Movement Sciences, VU, Amsterdam) for obtaining HPLC measurements and Andor Veltien and Dennis Klomp for technical assistance and fruitful discussions. This study was supported by the Deutsche Forschungsgemeinschaft (SFB 545, Project A3 to D.I.) and the Netherlands Organization for Scientific Research (NWO/ZONMW to B. Wieringa and A.H.);
References
- Adams GR, Foley JM, Meyer RA. Muscle buffer capacity estimated from pH changes during rest-to-work transitions. J Appl Physiol. 1990;69:968–972. doi: 10.1152/jappl.1990.69.3.968. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol. 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EA, Radda GK, Tomlin H, Clark JF. The utilisation of creatine and its analogues by cytosolic and mitochondrial creatine kinase. Biochim Biophys Acta. 1996;1274:119–128. doi: 10.1016/0005-2728(96)00018-7. [DOI] [PubMed] [Google Scholar]
- Boehm E, Veksler V, Mateo P, Lenoble C, Wieringa B, Ventura-Clapier R. Maintained coupling of oxidative phosphorylation to creatine kinase activity in sarcomeric mitochondrial creatine kinase-deficient mice. J Mol Cell Cardiol. 1998;30:901–912. doi: 10.1006/jmcc.1998.0692. [DOI] [PubMed] [Google Scholar]
- Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem. 2003;278:39155–39165. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- Brindle KM. NMR methods for measuring enzyme kinetics in vivo. Prog NMR Spectroscopy. 1988;20:257–293. [Google Scholar]
- Conley KE, Kushmerick MJ, Jubrias SA. Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol. 1998;511:935–945. doi: 10.1111/j.1469-7793.1998.935bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting skeletal muscle. I. Turning it on. Am J Physiol Endocrinol Metab. 2002;282:E67–E73. doi: 10.1152/ajpendo.2002.282.1.E67. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- Fitch CD, Chevli R. Inhibition of creatine and phosphocreatine accumulation in skeletal muscle and heart. Metabolism. 1980;29:686–690. doi: 10.1016/0026-0495(80)90115-8. [DOI] [PubMed] [Google Scholar]
- Fitch CD, Shields RP, Payne WF, Dacus JM. Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J Biol Chem. 1968;243:2024–2027. [PubMed] [Google Scholar]
- Foley JM, Meyer RA. Energy cost of twitch and tetanic contractions of rat muscle estimated in situ by gated 31P NMR. NMR Biomed. 1993;6:32–38. doi: 10.1002/nbm.1940060106. [DOI] [PubMed] [Google Scholar]
- Forsén S, Hoffman RA. Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys. 1963;39:2892–2901. [Google Scholar]
- Frahm J, Hanefeld F. Localized proton magnetic resonance spectroscopy of brain disorders in childhood. In: Bachelard H, editor. Magnetic Resonance Spectroscopy and Imaging in Neurochemistry – Advances in Neurochemistry. Vol. 8. New York: Plenum Press; 1997. pp. 329–403. [Google Scholar]
- Heerschap A, Sommers MG, In't Zandt HJA, Renema WKJ, Veltien A, Klomp DWJ. Nuclear magnetic resonance in laboratory animals. Meth Enzymol. 2004;385:41–63. doi: 10.1016/S0076-6879(04)85003-1. [DOI] [PubMed] [Google Scholar]
- Houtman CJ, Heerschap A, Zwarts MJ, Stegeman DF. pH heterogeneity in tibial anterior muscle during isometric activity studied by (31)P-NMR spectroscopy. J Appl Physiol. 2001;91:191–200. doi: 10.1152/jappl.2001.91.1.191. [DOI] [PubMed] [Google Scholar]
- In't Zandt HJ, Groof AJ, Renema WK, Oerlemans FT, Klomp DW, Wieringa B, et al. Presence of (phospho) creatine in developing and adult skeletal muscle of mice without mitochondrial and cytosolic muscle creatine kinase isoforms. J Physiol. 2003;548:847–858. doi: 10.1113/jphysiol.2002.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In't Zandt HJ, Oerlemans F, Wieringa B, Heerschap A. Effects of ischemia on skeletal muscle energy metabolism in mice lacking creatine kinase monitored by in vivo 31P nuclear magnetic resonance spectroscopy. NMR Biomed. 1999;12:327–334. doi: 10.1002/(sici)1099-1492(199910)12:6<327::aid-nbm570>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Iotti S, Gottardi G, Clementi V, Barbiroli B. The mono-exponential pattern of phosphocreatine recovery after muscle exercise is a particular case of a more complex behaviour. Biochim Biophys Acta. 2004;1608:131–139. doi: 10.1016/j.bbabio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Iotti S, Lodi R, Frassineti C, Zaniol P, Barbiroli B. vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed. 1993;6:248–253. doi: 10.1002/nbm.1940060404. [DOI] [PubMed] [Google Scholar]
- Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stockler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine monohydrate in various animal species. Life Sci. 2001;69:1805–1815. doi: 10.1016/s0024-3205(01)01268-1. [DOI] [PubMed] [Google Scholar]
- Kan HE, Renema WKJ, de Haan A, Isbrandt D, Heerschap A. Phosphorylated Guanidinoacetate in muscle of GAMT deficient mice only partly compensates for PCr as assessed by 31P MRS and functional measurements; Proceedings of the ISMRM 12th scientific meeting. kyoto, Japan. 2004. p. 795. abstract. [Google Scholar]
- Karatzaferi C, De Haan A, Offringa C, Sargeant AJ. Improved high-performance liquid chromatographic assay for the determination of ‘high-energy’ phosphates in mammalian skeletal muscle. Application to a single-fibre study in man. J Chromatogr B Biomed Sci Appl. 1999;730:183–191. doi: 10.1016/s0378-4347(99)00221-2. [DOI] [PubMed] [Google Scholar]
- Kay L, Nicolay K, Wieringa B, Saks V, Wallimann T. Direct evidence for the control of mitochondrial respiration by mitochondrial creatine kinase in oxidative muscle cells in situ. J Biol Chem. 2000;275:6937–6944. doi: 10.1074/jbc.275.10.6937. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Roussel M, Bendahan D, Le Fur Y, Cozzone PJ. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J Physiol. 2001;535:901–928. doi: 10.1111/j.1469-7793.2001.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993;6:66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- Kemper WF, Lindstedt SL, Hartzler LK, Hicks JW, Conley KE. Shaking up glycolysis: Sustained, high lactate flux during aerobic rattling. Proc Natl Acad Sci U S A. 2001;98:723–728. doi: 10.1073/pnas.011387598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinek DJ, Schenkman KA, Ciesielski WA, Conley KE. Mitochondrial coupling in vivo in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;286:C457–C463. doi: 10.1152/ajpcell.00237.2003. [DOI] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Brown TR, Krilowicz BL, Kushmerick MJ. Phosphagen and intracellular pH changes during contraction of creatine-depleted rat muscle. Am J Physiol. 1986;250:C264–C274. doi: 10.1152/ajpcell.1986.250.2.C264. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Kushmerick MJ, Brown TR. Application of 31P-NMR spectroscopy to the study of striated muscle metabolism. Am J Physiol. 1982;242:C1–C11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272:C501–C510. doi: 10.1152/ajpcell.1997.272.2.C501. [DOI] [PubMed] [Google Scholar]
- Renema WK, Schmidt A, van Asten JJ, Oerlemans F, Ullrich K, Wieringa B, et al. MR spectroscopy of muscle and brain in guanidinoacetate methyltransferase (GAMT) -deficient mice: validation of an animal model to study creatine deficiency. Magn Reson Med. 2003;50:936–943. doi: 10.1002/mrm.10627. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Marescau B, Boehm EA, Renema WK, Peco R, Das A, et al. Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum Mol Genet. 2004;13:905–921. doi: 10.1093/hmg/ddh112. [DOI] [PubMed] [Google Scholar]
- Schulze A, Bachert P, Schlemmer H, Harting I, Polster T, Salomons GS, et al. Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann Neurol. 2003;53:248–251. doi: 10.1002/ana.10455. [DOI] [PubMed] [Google Scholar]
- Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, et al. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Steeghs K, Oerlemans F, de Haan A, Heerschap A, Verdoodt L, de Bie M, et al. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem. 1998;184:183–194. [PubMed] [Google Scholar]
- Stöckler S, Holzbach U, Hanefeld F, Marquardt I, Helms G, Requart M, et al. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res. 1994;36:409–413. doi: 10.1203/00006450-199409000-00023. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- van Deursen J, Heerschap A, Oerlemans F, Ruitenbeek W, Jap P, Terlaak H, et al. Skeletal-muscles of mice deficient in muscle creatine-kinase lack burst activity. Cell. 1993;74:621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]
- van Deursen J, Jap P, Heerschap A, ter Laak H, Ruitenbeek W, Wieringa B. Effects of the creatine analogue beta-guanidinopropionic acid on skeletal muscles of mice deficient in muscle creatine kinase. Biochim Biophys Acta. 1994;1185:327–335. doi: 10.1016/0005-2728(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Dolder M, Schlattner U, Eder M, Hornemann T, O'Gorman E, et al. Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors. 1998;8:229–234. doi: 10.1002/biof.5520080310. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the phosphocreatine circuit for cellular energy homeostatis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]