Abstract
Background potassium channels control the resting membrane potential of neurones and regulate their excitability. Two-pore-domain potassium (2-PK) channels have been shown to underlie a number of such neuronal background currents. Currents through human TASK-1, TASK-2 and TASK-3 channels expressed in Xenopus oocytes were inhibited by extracellular acidification. For TASK-3, mutation of histidine 98 to aspartate or alanine considerably reduced this effect of pH. Zinc was found to be a selective blocker of TASK-3 with virtually no effect on TASK-1 or TASK-2. Zinc had an IC50 of 19.8 μm for TASK-3, at +80 mV, with little voltage dependence associated with this inhibition. TASK-3 H98A had a much reduced sensitivity to zinc suggesting this site is important for zinc block. Surprisingly, TASK-1 also has histidine in position 98 but is insensitive to zinc block. TASK-3 and TASK-1 differ at position 70 with glutamate for TASK-3 and lysine for TASK-1. TASK-3 E70K also had a much reduced sensitivity to zinc while the corresponding reverse mutation in TASK-1, K70E, induced zinc sensitivity. A TASK-3–TASK-1 concatamer channel was comparatively zinc insensitive. For TASK-3, it is concluded that positions E70 and H98 are both critical for zinc block. The native cerebellar granule neurone (CGN) leak current, IKSO, is sensitive to block by zinc, with current reduced to 0.58 of control values in the presence of 100 μm zinc. This suggests that TASK-3 channels underlie a major component of IKSO. It has recently been suggested that zinc is released from inhibitory synapses onto CGNs. Therefore it is possible that inhibition of IKSO in cerebellar granule cells by synaptically released zinc may have important physiological consequences.
When members of the superfamily of two-pore-domain potassium channels (2-PK channels) are functionally expressed, they give rise to K+-selective currents that are open at all voltages. 2-PK channels have been proposed to underlie background K+ channels in many native neurones. These channels control the resting membrane potential of neurones and are fundamental regulators of neuronal excitability. Currently there are known to be at least 15 mammalian channels in the 2-PK channel superfamily (see Lesage & Lazdunski, 2000; Goldstein et al. 2001; O'Connell et al. 2002; Lesage, 2003; also Sano et al. 2003).
A number of groups have described cell types where native background K+ channels display many features of the 2-PK channel TASK-1 (Buckler et al. 2000; Czirják et al. 2000; Millar et al. 2000; Talley et al. 2000) including the native background current in cerebellar granule neurones (CGNs), which we termed IKSO (Watkins & Mathie, 1996).
Because of their abundance and their interactions with other cell types in the cerebellum, CGNs play a crucial transduction role in cerebellar function (Herrup & Kuemerle, 1997). We have demonstrated, previously, the importance of IKSO in regulating CGN excitability (Watkins & Mathie, 1996). Recently, it has been suggested that the increase in frequency of spontaneous EPSCs in cerebellar Purkinje cells following synaptic release of acetylcholine occurs as a result of block of IKSO in CGNs (Takayasu et al. 2003).
CGNs, at the mRNA level at least, express a number of different 2-PK channels (Talley et al. 2001; Mathie et al. 2003). One of these, the TASK-3 channel, is functionally very similar to TASK-1. Recent studies have added support to our hypothesis that TASK-1 channels underlie IKSO and are crucial to CGN excitability (Brickley et al. 2001; Maingret et al. 2001) but TASK-3 channels either on their own or expressed as heterodimers with TASK-1 channels are also thought to be of physiological importance in CGNs (Han et al. 2002; Lauritzen et al. 2003; Kang et al. 2004). At present, there are few methods for distinguishing currents through TASK-1 and TASK-3 channels from each other.
Zinc serves structural, chemical and regulatory roles in biological systems and can be sequestered in specialized neurones called ‘zinc-containing neurones’ (Frederickson & Moncrieff, 1994; Takeda, 2000). The release of zinc from zinc-containing vesicles in these neurones has been demonstrated in the brain, and the concentration of zinc in the synaptic cleft has been suggested to reach levels in the tens of micromolar range (Li et al. 2001). In this study, we have considered the effects of extracellular zinc on TASK-1, TASK-2 and TASK-3 channels and on the native IKSO current in granule cells. With the recent finding of novel inhibitory zinc-enriched terminals in the cerebellum that form synapses with CGNs (Wang et al. 2002), it is possible that any zinc sensitivity of the channels underlying IKSO may be of considerable physiological importance.
A preliminary report of some of these results has been published (Clarke et al. 2003).
Methods
Recombinant TASK channels
The two-electrode voltage clamp technique was used to record whole cell currents from Xenopus laevis oocytes injected with complementary DNA (cDNA) encoding various 2-PK channels. Wild-type human TASK-1, TASK-2 and TASK-3 DNAs were supplied by GlaxoSmithKline (for TASK-3 see, for example, Chapman et al. 2000; Meadows & Randall, 2001). All clones were in PCDNA3.1 vector. Rat TASK-1 was a kind gift from Dr C.S. Yost (University of California, San Francisco).
Site-directed mutagenesis of DNA constructs
Site-specific changes were made using the Quikchange kit (Stratagene, Westburg, the Netherlands) based on the Pfu polymerase chain reaction. Complementary primers (25–45 bp) containing the desired mutation were designed. High purity salt-free oligonucleotide primers were synthesized by MWG Biotech (Milton Keynes, UK). For the TASK-3–TASK-1 concatamer, primers were designed for the 3′ and 5′ ends of TASK-1 and TASK-3 ensuring that the stop codon of TASK-3 and the start codon of TASK-1 were removed. The TASK-3–TASK-1 concatamer had a non-conservative mutation (phenylalanine to serine) in transmembrane domain 4 of the TASK-1 segment. A TASK-1 construct with this mutation had normal biophysical characteristics and a similar lack of sensitivity to zinc as wild-type TASK-1. Mutant clones were analysed by restriction digests of restriction sites introduced/excluded using silent mutations. Sequences of selected mutants were confirmed with DNA sequencing (Eurogentec, Winchester, UK).
Oocyte preparation and channel expression
Female Xenopus laevis (African clawed toads) were anaesthetized by immersion in 0.2% (w/v) ice cold Tricaine (3-aminobenzoic acid ethyl ester methanosulphate salt solution, pH 7.4 with NaOH) for 30 min. When unresponsive to a pinch applied to the forearm or leg, toads were killed by severing the cervical spinal cord. Upon removal, oocytes were placed into sterile modified Barth's solution (MBS, see below). The tissue was disaggregated into small clumps manually with forceps, and agitated on a shaker in calcium-free Barth's solution, containing 1–2% collagenase A (Boehringer Mannheim) for 2–3 h. Healthy stage V–VI oocytes were then chosen for injection.
Injections of cDNA for human TASK-1, TASK-2, TASK-3, TASK-3 mutants, the TASK-3–TASK-1 concatamer or rat TASK-1 were made into the nuclei of the defolliculated oocytes (1.5 ng cDNA per oocyte). The oocytes were then incubated in filtered MBS supplemented with 0.1 mg ml−1 gentamycin at 19–22°C.
Two-electrode voltage clamp recording
Recordings were made using the two-electrode voltage clamp technique. Oocytes were placed in a recording chamber 1–3 days after injection and continuously perfused with MBS solution adjusted to pH 7.4 with 1 m NaOH. All the data presented were recorded at room temperature (21–23°C). Microelectrodes were pulled to tip resistances of 0.5–1.5 MΩ.
Resting membrane potentials were recorded and any unhealthy oocytes rejected. Uninjected oocytes were used as controls. Currents present in uninjected oocytes were so small (approximately 0.5 nA) compared to those in injected oocytes that no compensation was deemed necessary. Standard test ramps were then run from a holding potential of −80 mV. Oocytes were briefly stepped to +30 mV to deactivate any endogenous voltage-dependent channels that might be present. A ramp of 160 ms duration, from −120 mV to +80 mV was run, with the initial step to −120 mV being held for 8 ms to ensure capacity currents had returned to baseline. Step current–voltage relationships were obtained from recordings that consisted of 200 ms duration voltage steps, at 10 mV increments, from −100 mV to +80 mV with 2 second intervals between test pulses. Currents obtained at the end of each step (the average current present between 180 and 190 ms after the step) were measured and plotted against voltage. Current responses, digitized at 2 kHz and filtered at 1 kHz using a Geneclamp 500B (Axon instruments) and pCLAMP (Clampex 8) software (Axon Instruments, USA), were stored for later analysis.
Control solution in each experiment was modified Barth's solution (MBS) containing (mm): NaCl 88, KCl 1, NaHCO3 2.4, Hepes 1.5, MgSO4 0.82, Ca(NO3)2 0.33, CaCl2 0.41; buffered to pH 7.4 with NaOH. For the pH experiments, various test solutions were made from modified Barth's solution, altered to the required pH with either NaOH or HCl.
In zinc block experiments, a stock solution of 10 mm ZnCl2 was made in slightly acidified MBS. For experiments, zinc solutions were rebuffered to pH 7.4 with NaOH. Electrodes were filled with 3 m KCl.
IKSO in cerebellar granule neurones
The native leak K+ current, IKSO, was recorded from cerebellar granule neurones in culture using the whole-cell patch-clamp technique.
Tissue culture of granule neurones
Granule neurones were isolated using previously described methods (Huston et al. 1993; Watkins & Mathie, 1996) from the cerebella of 6- to 8-day-old Sprague-Dawley rats of either sex, which had been killed by decapitation. Following dispersion, cells were plated onto 13 mm glass coverslips coated with poly-ornithine or poly-l-lysine and allowed to adhere. They were then covered with a minimum essential medium (MEM) comprising Earle's balanced salt solution supplemented with 10% fetal calf serum, 50 i.u. ml−1 chick embryo extract, 39 mm glucose and 2 mm glucamine, and maintained in 5% CO2 at 37°C. The medium also contained 23 mm potassium, which has been shown to enhance the viability of cerebellar granule neurones in culture (Gallo et al. 1987).
Whole-cell patch-clamp recordings from granule neurones
Whole-cell patch-clamp recordings were made from cultures of CGNs aged from 8 to 14 days in a physiological saline solution containing (mm): NaCl 120, KCl 2.5, CaCl2 0.5, MgCl2 2, glucose 5, Hepes 10 adjusted to pH 7.4. The neurones were whole-cell voltage clamped using amphotericin B-permeablized patches to minimize disruption of intracellular composition. The pipette solution contained (mm): KCl 125, MgCl2 5, Hepes 5, BAPTA 0.1, amphotericin B 240 μg ml−1 adjusted to pH 7.4. All experiments were performed using the amphotericin perforated patch technique since under whole-cell recording IKSO has been observed to experience considerable run down (see also Watkins & Mathie, 1996). External solutions were applied by bath perfusion at a rate of 4–5 ml min−1 and complete exchange of the bath solution occurred within 20–40 s. Solutions were applied at room temperature 20–23°C.
Neurones were voltage clamped using an Axopatch 1D amplifier (Axon Instruments, USA) and low pass filtered at 5 kHz before sampling (usually at 10 kHz) and capture on-line with a Digidata 1200 interface (Axon Instruments). Neurones had a zero current level (equivalent to their resting membrane potential) of −78 ± 4 mV (n = 22). Data acquisition was carried out using pCLAMP software (Axon Instruments). CGNs were typically held at −30 mV and stepped to −80 mV for 100 ms before returning to the holding potential; this protocol was repeated every 5 s. IKSO was measured as the outward current at −30 mV after the CGN has been at this potential for over 4.8 s.
Data analysis
Data were analysed using Clampfit software (Axon Instruments), Microsoft Excel 97 and Origin 5.0 (OriginLab Corp., USA). Mean data at different zinc concentrations were fitted using a logistic equation:
| (1) |
where A1 is the maximum effect of zinc, A2 is the minimum effect, k is the IC50 and n is the slope factor. IC50 values, when quoted, refer to the concentration at which 50% of the maximum observed effect was seen. The error associated with these values represents the error in the fit to the mean data at each concentration.
The relative permeability of TASK channels to Na+ ions compared to K+ ions was calculated using a modified Goldman-Hodgkin-Katz (GHK) voltage equation:
 |
(2) |
where 1 refers to the first solution, 2 refers to second solution, P is the permeability of the ion and R, T, F and z have their usual meanings.
Statistical comparisons were carried out using Student's t test and P-values are given in the text. Results are given as means ± standard error of the mean with n as the number of experiments.
Results
Oocytes expressing 2-PK channels developed a characteristic hyperpolarized resting membrane potential. Resting membrane potentials for human (h) TASK-1, TASK-2 and TASK-3 expressing oocytes were −70 ± 3 mV (n = 7), −72 ± 4 mV (n = 7) and −78 ± 3 mV (n = 9), respectively, compared to −23 ± 2 mV for uninjected oocytes (n = 9). Currents evoked in 1 mm external K+ (K+o) were outwardly rectifying for all three channels (Fig. 1A–C) and measured 2.18 ± 0.3 μA (n = 10) for TASK-1, 3.64 ± 0.7 μA (n = 9) for TASK-2 and 10.7 ± 1.0 μA (n = 13) for TASK-3 at +80 mV.
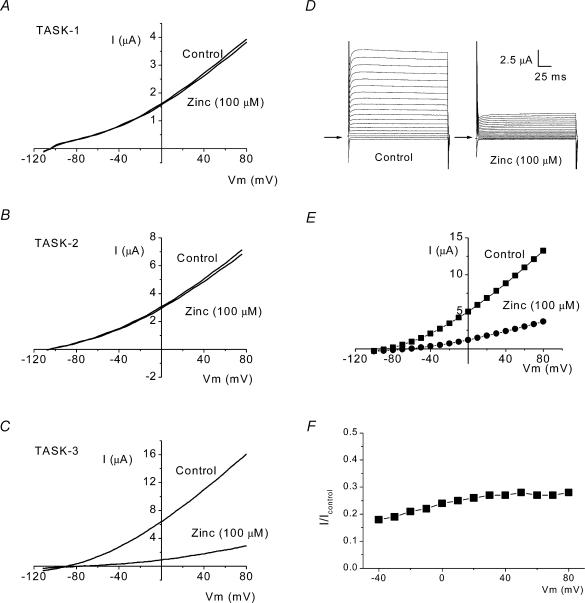
Figure 1. Zinc sensitivity of TASK potassium channels.
A–C, representative current–voltage relationships for TASK-1, TASK-2 and TASK-3 channels in control solutions and solutions containing 100 μm zinc. Currents were evoked by a 160 ms ramp from −120 to +80 mV. D and E, representative current–voltage plot of TASK-3 control currents and currents in 100 μm zinc following 200 ms step changes in voltage, in 10 mV increments, from −100 to +80 mV. Arrows in D indicate zero current. F, percentage inhibition of TASK-3 current by zinc shows little voltage dependence.
Reversal potentials in 1 mm external K+ were −107 ± 7, −109 ± 7 and −105 ± 5 mV for TASK-1, TASK-2 and TASK-3, respectively. A 25-fold increase in K+o led to a +59.8 ± 4.5 mV (n = 5), +64.9 ± 5.1 mV (n = 5) and +64.8 ± 4.5 mV (n = 7) shift in reversal potential for TASK-1, TASK-2 and TASK-3 currents, respectively.
According to the GHK voltage equation, if the channels were solely permeable to K+ ions then changing from 1 to 25 mm K+ would give a +81.3 mV change in reversal potential. Assuming that the deviations from this value are due to a finite Na+ permeability, a modified GHK voltage equation (see Methods) was used and the permeability of Na+ through the channels was calculated, assuming that K+ has a permeability of 1. From these data, the calculated Na+/K+ permeability ratio was 0.02 for TASK-1, 0.01 for TASK-2 and 0.01 for TASK-3, confirming that TASK channels are highly selectively permeable to K+ ions.
As has been shown previously, TASK channels expressed in oocytes were substantially blocked by changing the extracellular pH from 7.4 to 6. Full pH–response curves showed that TASK-1 was more pH sensitive than TASK-3, with pKa values of 7.3 ± 0.08 and 6.6 ± 0.04, respectively. TASK-2 was, in fact, significantly blocked even at pH 7, but was potentiated by more alkaline extracellular solutions.
Zinc is a selective blocker of TASK-3
Zinc is a selective blocker of hTASK-3. The currents recorded in 100 μm zinc compared to control currents at +80 mV were 1.04 ± 0.1 (n = 5) for TASK-1, 0.95 ± 0.03 (n = 5) for TASK-2 and 0.31 ± 0.1 (n = 7) for TASK-3 (Fig. 1A–C). The effect of zinc was also measured on rat (r) TASK-1. Zinc at 100 μm caused a reduction in rTASK-1 current to just 0.90 ± 0.08 (n = 5) of control currents.
Current block recorded at 0 mV and +80 mV showed that the effect of 100 μm zinc on TASK-3 channels had little voltage dependence (at least at voltages of 0 mV and above) with 0.36 ± 0.1 (n = 5) of control current remaining at 0 mV (in the presence of 100 μm zinc compared to control) and 0.31 ± 0.1 (n = 7) at +80 mV (P > 0.2, see also, Fig. 1D–F). The zinc concentration–response curve for TASK-3 indicated an IC50 of 19.8 ± 2.7 μm (n = 6, Fig. 2E and F).
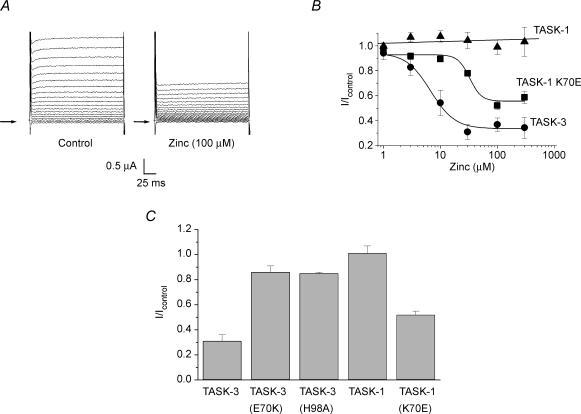
Figure 2. TASK-3 mutants H98A and E70K have reduced zinc sensitivity.
A, representative current–voltage relationships for TASK-3 channels at pH 7.4 and pH 6. Currents evoked by a 160 ms ramp from −120 to +80 mV. B, representative current–voltage relationships for mutated TASK-3 potassium channel H98A at pH 7.4 and pH 6. C, representative current–voltage relationships for TASK-3 H98A channels in control solution and in 100 μm zinc. D, representative current–voltage relationships for TASK-3 E70K channels in control solution and in 100 μm zinc. E, zinc concentration–response curves for WT TASK-3 (▪) and TASK-3 H98A (▴) shows the reduction in zinc efficacy. F, zinc concentration–response curves for WT TASK-3 (▪) and TASK-3 E70K (•).
H98 is important for zinc sensitivity
It has been established that histidine (H) 98 is an important residue in the first pore region of TASK-1 and TASK-3 channels when considering pH sensitivity (Kim et al. 2000; Lopes et al. 2001; Morton et al. 2003). However, the equivalent residue on TASK-2 (N103) is not involved in that channel's pH sensitivity (see Discussion and Morton et al. 2003). The TASK-3 mutant H98A, with the histidine replaced by alanine (A), was created using site-directed mutagenesis and studied for the expected change in pH sensitivity. Current remaining at pH 6 (compared to pH 7.4) at +30 mV was 0.98 ± 0.04 (n = 8), and 0.34 ± 0.06 (n = 5) for H98A and WT TASK-3, respectively (Figs 2A and B).
Oocytes expressing hTASK-3 H98A were tested for the effects of zinc (Fig. 2C). Mutation H98A led to a substantial reduction of zinc block. The TASK-3 H98A currents, at +80 mV, were reduced to 0.85 ± 0.01 (n = 5) of control currents by 100 μm zinc (Fig. 2E). This effect of zinc is significantly smaller than that seen for WT TASK-3 currents (P < 0.01). Furthermore, the zinc IC50 was shifted from 19.8 ± 2.7 μm (n ≥ 5) in WT TASK-3 channels to 95.8 ± 14.1 μm (n = 5) in the H98A mutant.
The equivalent histidine residue is present in hTASK-1 and, as for TASK-3, has been shown to be involved in H+ ion binding (Lopes et al. 2001; Morton et al. 2003); however, hTASK-1 in this study was not sensitive to block by zinc. This, combined with the fact that mutation H98A is still slightly sensitive to extracellular zinc, seemed to suggest that although H98 is important for zinc block, it is not the only residue involved.
E70 on TASK-3 is critical for zinc sensitivity
Recent work by Derst et al. (2002) and Czirják & Enyedi, 2003) has identified glutamate (E) at position 70 of TASK-3 as an important determinant of sensitivity to extracellular divalent cations and ruthenium red. TASK-1 has a lysine (K) in this position. Mutation of TASK-3 E70 to K leads to a functional, TASK-like channel, which retains K+ selectivity (Fig. 2D).
Oocytes expressing hTASK-3 E70K mutants were tested for the effects of zinc at pH 7.4 (Fig. 2D). This mutation led to a reduction in efficacy of zinc block, the divalent ion reducing current maximally to 0.86 ± 0.05 of control levels (P < 0.01 compared to WT TASK-3 currents) with an IC50 of 105 ± 24 μm (n = 6, Fig. 2F).
The converse mutation (K70E) was made for TASK-1 channels. If the residue at position 70 is indeed critical for zinc sensitivity, one would predict that this mutation should induce zinc sensitivity in TASK-1. This was found to be the case. K70E TASK-1 currents were 0.52 ± 0.03 (n = 6) of control values in 100 μm with an IC50 of 32 ± 11 μm (Fig. 3). This inhibition by zinc was significantly larger than that seen for WT TASK-1 currents (P < 0.01) and not significantly different from that seen for WT TASK-3 currents (0.1 > P > 0.05).
Figure 3. TASK-1 mutant K70E has increased zinc sensitivity.
A, representative TASK-1 K70E control currents and currents in 100 μm zinc following 200 ms step changes in voltage, in 10 mV increments, from −100 to +80 mV. Arrows indicate zero current. B, zinc concentration–response curves for WT TASK-1 (▴), WT TASK-3 (•) and TASK-1 K70E (▪) shows the increase in zinc efficacy in TASK-1 K70E compared to WT TASK-1. C, bar chart of relative effect of 100 μm zinc on WT TASK-3, TASK-3 E70K, WT TASK-1 and TASK-1 K70E currents.
TASK-1–TASK-3 concatamer has a low sensitivity to zinc
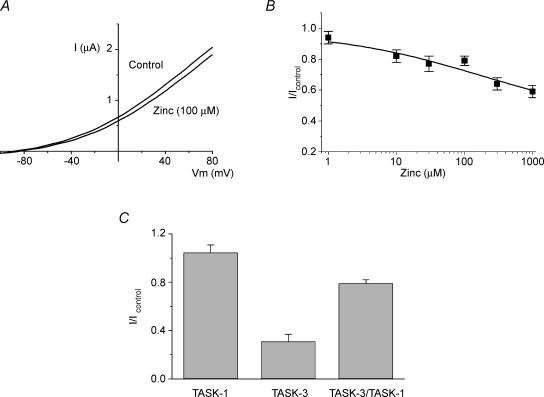
It is possible that TASK-1 and TASK-3 can form heterodimers in vivo. However, the evidence for this is equivocal at present (see Karschin et al. 2001; Czirják & Enyedi, 2002; Lauritzen et al. 2003; Pei et al. 2003; Kang et al. 2004). To test the effect of zinc on TASK-1–TASK-3 heterodimers, we constructed a concatamer of TASK-3–TASK-1 which, when expressed in oocytes, gave outwardly rectifying TASK-like K+ currents (Fig. 4). This concatamer was found to be relatively zinc insensitive, with 100 μm zinc reducing the current to 0.79 ± 0.03 (n = 10) of control levels (significantly different from that seen for WT TASK-3, P < 0.01) with an apparent IC50 of around 190 μm (Fig. 4B).
Figure 4. TASK-3–TASK-1 concatamer has low zinc sensitivity.
A, representative current-voltage relationships for TASK-3–TASK-1 concatamer channels in control solution and in 100 μm zinc. B, zinc concentration–response curve for TASK-3–TASK-1 concatamer channels. C, bar chart of relative effect of 100 μm zinc on WT TASK-3, WT TASK-1 and TASK-3–TASK-1 concatamer currents.
IKSO is blocked by zinc
Previously, we have described and characterized the native leak current in cerebellar granule cells, termed IKSO (Watkins & Mathie, 1996). We suggested that this current most likely arose from expression of 2-PK channels of the TASK family and initially, on the basis of information available at the time, attributed it to TASK-1 (Millar et al. 2000; see also Brickley et al. 2001; Maingret et al. 2001). More recently, however, TASK-3 channels have also been described (Kim et al. 2000; Rajan et al. 2000) and have been shown to be expressed in cerebellar granule cells (Talley et al. 2001). TASK-3 has been suggested to be a major component of IKSO (Han et al. 2002; Lauritzen et al. 2003). It is of considerable interest therefore to determine the effect of zinc on IKSO.
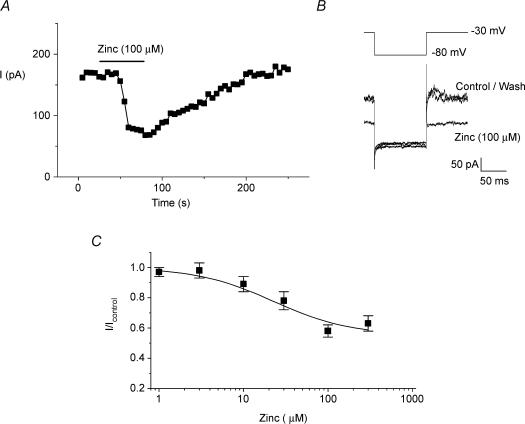
Zinc was found to partially block IKSO in CGNs. Zinc at 10 μm reduced IKSO to 0.89 ± 0.05 (n = 5) of control values, while a maximally effective concentration of zinc (100 μm) reduced IKSO to 0.58 ± 0.04 (n = 6) of control (Fig. 5). The concentration response–relationship for zinc gave an IC50 of 23 ± 6 μm (Fig. 5C), which is not significantly different from the IC50 for zinc block of TASK-3 channels (20 μm). Block by zinc also significantly increased (P < 0.05) the calculated CGN input resistance (measured between −30 and −80 mV) from 273 ± 85 MΩ in control solutions to 648 ± 132 MΩ in the presence of 100 μm zinc (n = 6). Taken together, these data suggest that a major component of IKSO is carried through TASK-3 channels and that the level of TASK-3 channel activity is important in regulating CGN excitability.
Figure 5. IKSO in cerebellar granule neurones is sensitive to block by zinc.
CGNs were held at −30 mV and stepped to −80 mV for 100 ms. This protocol was repeated once every 5 s to evoke IKSO. A, time course for zinc inhibition of IKSO. IKSO was measured at −30 mV after the CGNs had been at this potential for 4.8 s. B, representative current recordings in control solution, in the presence of 100 μm zinc and after wash out of zinc. C, zinc concentration–response curve for IKSO in cerebellar granule cells.
IKSO is blocked by extracellular Ca2+ ions
TASK-3 currents (unlike TASK-1 currents) have been shown to be enhanced by removing divalent cations (Ca2+ and Mg2+) from the extracellular solution and this effect is also attributed to these divalent cations, when present, acting on E70 (Derst et al. 2002). We have seen a similar enhancement of TASK-3 currents, when removing Ca2+ from the extracellular recording solution (data not shown). Removing Ca2+ from the extracellular solution also enhanced IKSO in cerebellar granule cells. Changing the extracellular Ca2+ concentration from 0.5 mm to, nominally, zero, increased the magnitude of IKSO, at −30 mV, to 1.33 ± 0.09 of control levels (n = 7). This provides further support for the hypothesis that TASK-3 channels underlie at least part of the IKSO current.
Discussion
Zinc is a selective blocker of TASK-3
In this study, 100 μm zinc blocked WT TASK-3 currents by around 70% whilst TASK-1 and TASK-2 currents were relatively unaffected by the same zinc concentration. Previously, TASK-1 has been shown to be weakly sensitive to block by zinc (Leonoudakis et al. 1998), while TASK-3 has been said to be unaffected by the ion (Kim et al. 2000). As a consequence, zinc has been used to distinguish between TASK-1 and TASK-3 currents in native tissues (Hartness et al. 2001; Barbuti et al. 2002) with zinc sensitivity said to be indicative of functional TASK-1 channels.
Our findings can be compared with those of Leonoudakis et al. (1998), who showed that TASK-1 was weakly sensitive to zinc with an IC50 of 175 μm. In this study the maximal block of TASK-1 current by zinc, even at the highest concentration tested (200 μm), was less than 10%. Nevertheless, both studies would suggest that the sensitivity of TASK-1 channels to zinc is much less that that found for TASK-3 in this study, which has an IC50 for zinc block of around 20 μm.
The findings in this study differ from the findings of Kim et al. (2000) who state that rat TASK-3 is insensitive to zinc (100 μm). This is not due to differences in either the expression system used or in the concentration of divalent ions present in the external solution, since experiments recording currents through TASK-3 channels transiently transfected into tsA cells, with 2 mm CaCl2 and 1 mm MgCl2 in the external solution, showed zinc to be at least as effective at blocking TASK-3 currents under these more physiological recording conditions (100 μm zinc reducing the current to 0.16 ± 0.1 of control, n = 3). Furthermore it is unlikely to be due to species differences, since hTASK-3 and rTASK-3 show high amino acid sequence identity around the pore regions (and complete identity in the putative regions of zinc interaction around the first pore region, below), although sequence identity is lower in the C-terminus. In this study, no significant difference in zinc sensitivity could be found between hTASK-1 and rTASK-1. These channels also differ in their C-terminus, but are identical around the first pore region.
H98 and E70 on TASK-3 are critical for zinc sensitivity
Histidine 98 is an essential component of the extracellular pH sensor of TASK-3 (see Results and Kim et al. 2000). Previous studies have shown histidine residues to be potential zinc interaction sites on other channels, such as P2X2 receptors (Clyne et al. 2002) and GABAA receptors (Hosie et al. 2003). In this study we show that the TASK-3 mutant H98A has a reduced sensitivity to block by zinc. This suggests that H98 is important for zinc binding. The fact that this mutation only led to a reduction in zinc sensitivity and not a complete loss, and also that TASK-1 has the equivalent histidine residue in the first pore region, but is relatively insensitive to zinc block, led us to consider other potential residues involved in zinc block.
E70, a residue that is an important determinant of sensitivity to extracellular divalent cations and ruthenium red in TASK-3 channels (Derst et al. 2002; Czirjak & Enyedi, 2003) is not present in TASK-1. Mutation of this glutamate to lysine (E70K), the equivalent residue in TASK-1, led to a reduction in the channel's sensitivity to zinc. Importantly, the reverse mutation in TASK-1 (K70E) induced zinc sensitivity in TASK-1 channels. Furthermore, in this study, the TASK-3–TASK-1 concatamer was relatively zinc insensitive. This concatamer will contain one glutamate and one lysine at position 70 compared to the usual two glutamates or two lysines for the respective TASK-3 and TASK-1 homodimers. It is of interest that TASK-1–TASK-3 heterodimers were also found to be resistant to block by ruthenium red (Czirjak & Enyedi, 2003).
It therefore seems that both H98 and E70 are important residues implicated in zinc binding in TASK-3 channels. Studies of the zinc ligands of zinc-binding enzymes have led to the recognition of several distinct types of zinc binding site. The most common amino acids that are donors for these sites are histidine, glutamate, aspartate and cysteine (Auld, 2001), often with either four or six amino acids co-ordinating the binding site. In addition, there are identified zinc binding sites at the interface of two distinct proteins or protein subunits (Auld, 2001). Glutamate and histidine residues have been established as interacting to form zinc binding sites for several proteins such as the binding sites for zinc on GABAA receptors (Hosie et al. 2003).
It is therefore possible that the binding site for zinc in TASK-3 is formed from H98 and E70 donors, with the formation of dimers completing a four co-ordinate zinc binding site between the two TASK-3 subunits. It is worth noting that E70 lies within the predicted M1–P1 loop of TASK-3, yet this hypothesis requires that this residue interacts with H98 (which lies within the mouth of the pore region) to bind zinc ions. Alternatively, E70 may have a role in allowing access of zinc to H98 in the pore, which is, itself, the binding site. In this scenario, K at position 70 in TASK-1 would occlude zinc access to the pore since TASK-1 is zinc insensitive.
TASK-2, which is also zinc insensitive, does not possess either an E at the equivalent to position 70 (position 75 for TASK-2) or a H at the equivalent to position 98 (position 103 for TASK-2) possessing instead a T (threonine) and N (asparagine), respectively, at these positions. Interestingly, although TASK-2 is pH sensitive, this occurs through a completely different region of the channel to TASK-1 and TASK-3, since changing the N at position 103 to H, makes TASK-2 channels less, rather than more, pH sensitive (Morton et al. 2003).
Zinc sensitivity of the native current IKSO, in CGNs
Our results show that IKSO is partially sensitive to block by zinc with a maximally effective concentration inducing inhibition of around 42%. Since zinc maximally blocks TASK-3 by around 70%, in our hands, this suggests that around 60% of the native IKSO is made up of TASK-3 homodimers (since TASK-1 and TASK-1–TASK-3 heterodimers are relatively zinc insensitive). Han et al. (2002) have suggested that four separate channels potentially contribute to IKSO, but that TASK-3 accounts for around half of the channels at the age of animals used in this study (see Methods). Kang et al. (2004) have extended these observations to suggest that in cells from 1- to 2-day-old cultures (when functional TASK-3 channels are at a low density) heterodimers of TASK-3 and TASK-1 may form. From their data, it seems that, at this age of culture, around 56% of the channels formed are homodimers of TASK-3 while 44% are TASK-1–TASK-3 heterodimers. These single-channel data suggest therefore that anything between 30 and 50% of the channels in the membrane are TASK-3 homodimers.
It is not clear, however, how these numbers alter when the proportion of functional TASK-3 channels (compared to TASK-1 channels) increases greatly with time in culture (Han et al. 2002). Furthermore, the number of channels present is not, of course, necessarily proportional to the contribution of these channels to the whole-cell current due to differences in open probablility and single-channel conductance between channels. Indeed, Lauritzen et al. (2003), on the basis of ruthenium red sensitivity of the whole-cell IKSO current in cerebellar granule cells, suggest that the vast majority of the whole-cell current observed is through TASK-3 homodimer channels (with around 70% inhibition of the whole-cell current by 1 μm ruthenium red). TREK channels, which, potentially, may also underlie a component of IKSO (Han et al. 2002), are relatively insensitive to zinc block with an IC50 of around 650 μm for TREK-1 (M. Gruss unpublished observations). Our data would therefore be consistent with the suggestion that TASK-3 homodimers are a major component of IKSO. In further support of this hypothesis, IKSO is enhanced in Ca2+-free solution, a property shared with TASK-3 channels but not with TASK-1 channels (Derst et al. 2002).
Free zinc is sequestered into vesicles in the terminals of a number of different neuronal types throughout the mammalian brain (Li et al. 2003). This free zinc is then secreted as a neurotransmitter and acts as an essential modulator and mediator of cell signalling (Fredrickson, 2003). An increase of zinc in the extracellular solution alters the activity of several membrane receptor-channels such as GABAA receptors and NMDA receptors (e.g. Harrison & Gibbons, 1994; Smart et al. 1994; Huang, 1997). The concentration of zinc in the synaptic cleft has been suggested to reach levels in the tens of micromolar range (Li et al. 2001) and may even reach peak levels as high as 300 μm in synaptic cleft microdomains (Minami et al. 2002).
It has recently been suggested that zinc may be released onto cerebellar granule cells from zinc enriched inhibitory Golgi cell synaptic terminals (Wang et al. 2002). If TASK-3 channels were located close to the synapse, zinc would act to increase granule cell excitability both by depolarizing the neurone and increasing neuronal input resistance.
This may have important consequences for cerebellar excitability, particularly since excessive synaptic zinc release occurs during certain acute conditions, including epilepsy and transient global ischaemia (Weiss et al. 2000).
Takayasu et al. (2003) have shown that the action of ACh (released from cholinergic mossy fibres) can be attributed to block of IKSO in granule cells, which strongly suggests that IKSO currents (and therefore TASK-3 channels) are present and of functional importance at cerebellar glomeruli, where mossy fibre and Golgi cell termini converge with granule cells dendrites.
In preliminary experiments, we found that bath application of the zinc-chelating agent N,N,N′,N′ tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; 1 μm) had no effect on the amplitude of IKSO in adult mouse cerebellar slices (S. Brickley, unpublished observation). This suggests that there is no tonic inhibition of IKSO in mouse cerebellar granule cells by zinc. IKSO in mouse cerebellar slices is a comparatively small current, however, which varies in composition and size with development. In order to clearly observe it (and its regulation) in isolation, we have to use recording conditions where synaptic transmission is considerably reduced (with room temperature recordings, AMPA and GABAA receptor blockade and low Ca2+/high Mg2+ recording solutions). In future experiments we aim to develop our recording technique further, to allow us to stimulate release of zinc from Golgi cells and test whether IKSO is affected by synaptically released zinc. However, the situation is further complicated by the suggestion that synaptic release of zinc is considerably depressed in brain slice preparations (Suh et al. 2000).
Evidence for synaptic release of zinc is much better established in other regions of the brain such as at hippocampal mossy fibre synapses (Ruiz et al. 2004) and in the thalamocortical system (Gibbs et al. 2000). It is of interest that both these regions have high levels of TASK-3 expression (Talley et al. 2001; Meuth et al. 2003). Furthermore, there is good evidence in thalamocortical relay neurones that TASK-3 channels are inhibited by released neurotransmitters from thalamic terminals of the ascending brainstem system, thereby altering the activity mode of thalamocortical networks (Meuth et al. 2003).
TASK-3 channels have recently been implicated in neuronal apoptosis (Lauritzen et al. 2003) and the amplification and overexpression of TASK-3 have been suggested to play a direct role in the proliferation of a number of human tumours including breast cancer (Mu et al. 2003; Pei et al. 2003). Therefore, the development of specific modulators of TASK-3 channels, perhaps by compounds acting through residue E70, such as zinc and ruthenium red, may provide a useful pharmacological strategy for the treatment of neurodegenerative and proliferative diseases.
Acknowledgments
This work was supported by the MRC and BBSRC. CEC was funded by a BBSRC CASE studentship with GlaxoSmithKline. Thanks to Jane Saffell and Michael Delves for cultured CGNs for some experiments and to Marco Gruss and Stephen Brickley for helpful discussions and advice.
References
- Auld DS. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- Barbuti A, Ishii S, Shimizu T, Robinson R, Feinmark SJ. Block of the background K+ channel TASK-1 contributes to arrythmogenic effects of platelet-activating factor. Am J Physiol Heart Circ Physiol. 2002;282:H2024–H2030. doi: 10.1152/ajpheart.00956.2001. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Buckler K, Williams B, Honore E. An oxygen-, acid-, and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CG, Meadows HJ, Godden R, Campbell DA, Duckworth M, Kelsell RE, Murdock PR, Randall AD, Rennie GI, Gloger IS. Cloning, localisation and functional expression of a novel human cerebellum specific, two pore domain potassium channel. Mol Brain Res. 2000;82:74–83. doi: 10.1016/s0169-328x(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Green PJ, Veale EL, Meadows HJ, Mathie A. The involvement of residues H98 and E70 in the block of the human two pore domain potassium channel, TASK-3, by zinc. J Physiol. 2003;547.P:C46. doi: 10.1113/jphysiol.2004.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, LaPointe LD, Hume RI. The role of histidine residues in modulation of the rat P2X2 purinoceptor by zinc and pH. J Physiol. 2002;539:347–359. doi: 10.1113/jphysiol.2001.013244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirják G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Czirják G, Enyedi P. Ruthenium red inhibits TASK-3 potassium channel by interconnecting glutamate 70 of the two subunits. Mol Pharmacol. 2003;63:646–652. doi: 10.1124/mol.63.3.646. [DOI] [PubMed] [Google Scholar]
- Czirják G, Fischer T, Spät A, Lesage F, Enyedi P. TASK (TWIK-Related Acid-Sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- Derst C, Liu GX, Musset B, Rajan S, Preisig-Müller R, Daut J. Molecular analysis of divalent cation sensitivity of TASK channels. Pflugers Arch. 2002;443(S2):P44–10. S340. [Google Scholar]
- Frederickson CJ, Moncrieff DW. Zinc-containing neurons. Biol Signals. 1994;3:127–139. doi: 10.1159/000109536. [DOI] [PubMed] [Google Scholar]
- Fredrickson C. Imaging zinc: Old and new tools. Science STKE. 2003;182:pe18. doi: 10.1126/stke.2003.182.pe18. [DOI] [PubMed] [Google Scholar]
- Gallo V, Kingsbury A, Balazs R, Jorgensen OS. The role of depolarisation in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JW, Zhang Y-F, Shumate MD, Coulter DA. Regionally selective blockade of GABAergic inhibition by zinc in the thalamocortical system: functional significance. J Neurophysiol. 2000;83:1510–1521. doi: 10.1152/jn.2000.83.3.1510. [DOI] [PubMed] [Google Scholar]
- Goldstein SAN, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nature Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacol. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Hartness ME, Lewis A, Searle GJ, O'Kelly I, Peers C, Kemp PJ. Combined antisense and pharmacological approaches implicate hTASK as an airway O2 sensing K+ channel. J Biol Chem. 2001;276:26499–26508. doi: 10.1074/jbc.M010357200. [DOI] [PubMed] [Google Scholar]
- Herrup K, Kuemerle B. The compartmentalisation of the cerebellum. Ann Rev Neurosci. 1997;20:61–90. doi: 10.1146/annurev.neuro.20.1.61. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAA receptors: discrete binding sites underlie subtype specificity. Nature Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Huang EP. Metal ions and synaptic transmission: think zinc. Proc Natl Acad Sci U S A. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston E, Cullen G, Sweeney MI, Pearson H, Fazeli MS, Dolphin AC. Pertussis toxin treatment increases glutamate release and dihydropyridine binding-sites in cultured rat cerebellar granule neurons. Neurosci. 1993;52:787–798. doi: 10.1016/0306-4522(93)90529-o. [DOI] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;544:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik K-H, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3 and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honore E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis: role of TASK leak K+ channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacol. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Li YV, Hough CJ, Fredrickson CJ, Sarvey JM. Induction of mossy fiber to CA3 long-term potentiation requires translocation of synaptically released Zn2+ J Neurosci. 2001;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YV, Hough CJ, Sarvey JM. Do we need zinc to think? Science STKE. 2003;182:pe19. doi: 10.1126/stke.2003.182.pe19. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Zilberg N, Goldstein SAN. Block of KCNK-3 by protons: Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem. 2001;276:24449–24452. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Clarke CE, Ranatunga KM, Veale EL. What are the roles of the many different types of potassium channel expressed in cerebellar granule cells? Cerebellum. 2003;2:11–25. doi: 10.1080/14734220310015593. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Randall AD. Functional characterization of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacol. 2001;40:551–559. doi: 10.1016/s0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape H-C. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe REW, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel, TASK-1, in cerebellar granule neurons. Proc Natl Acad Sci U S A. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A, Takeda A, Yamaide R, Oku N. Relationship between zinc and neurotransmitters released into the amygdalar extracellular space. Brain Res. 2002;936:91–94. doi: 10.1016/s0006-8993(02)02499-x. [DOI] [PubMed] [Google Scholar]
- Morton MJ, O'Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K channels TASK-1 and -2. Pflugers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- Mu D, Chen L, Zhang X, See L-H, Koch CM, Yen C, Tong JJ, Spiegel L, Nguyen KCQ, Servoss A, Peng Y, Pei L, Marks JR, Lowe S, Hoey T, Jan LY, McCombie WR, Wigler MH, Powers S. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. 2003;3:297–302. doi: 10.1016/s1535-6108(03)00054-0. [DOI] [PubMed] [Google Scholar]
- O'Connell AD, Morton MJ, Hunter M. Two-pore domain K+ channels – molecular sensors. Biochem Biophys Acta Biomemb. 2002;1566:152–161. doi: 10.1016/s0005-2736(02)00597-7. [DOI] [PubMed] [Google Scholar]
- Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci U S A. 2003;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Liu GX, Preisig-Muller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel: an extracellular histidine as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. Endogenous zinc inhibits GABAA receptors in a hippocampal pathway. J Neurophysiol. 2004;91:1091–1096. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localised in the spinal cord. J Biol Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Suh SW, Danscher G, Jensen MS, Thompson R, Motamedi M, Frederickson CJ. Release of synaptic zinc is substantially depressed by conventional brain slice preparations. Brain Res. 2000;879:7–12. doi: 10.1016/s0006-8993(00)02675-5. [DOI] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Furuya N, Ozawa S. Muscarine induced increase in frequency of spontaneous EPSCs in Purkinje cells in the vestibulo-cerebellum of the rat. J Neurosci. 2003;23:6200–6208. doi: 10.1523/JNEUROSCI.23-15-06200.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Rev. 2000;34:137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Danscher G, Kim YK, Dahlstrom A, Mook Jo S. Inhibitory zinc-enriched terminals in the mouse cerebellum: double-immunohistochemistry for zinc transporter 3 and glutamate decarboxylase. Neurosci Lett. 2002;321:37–40. doi: 10.1016/s0304-3940(01)02560-5. [DOI] [PubMed] [Google Scholar]
- Watkins CS, Mathie A. A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. J Physiol. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JH, Sensi SL, Koh JY. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]