Abstract
We have proposed a new model of rat intestinal sugar absorption in which high glucose concentrations promote rapid insertion of GLUT2 into the apical membrane, so that absorptive capacity is precisely regulated to match dietary intake. Construction and building work during expansion and refurbishment of our department permitted opportunistic experiments on the effects of building-induced stress on the GLUT2 component of absorption. In fed rats perfused with 75 mm glucose in vivo, stress rapidly inhibited glucose absorption 36.4 ± 3.0% compared with control rats. Selective inhibition of the GLUT2 component with phloretin demonstrated that stress inhibited the GLUT2 component by 42.8 ± 3.8%, which correlated with a corresponding diminution in apical GLUT2 levels: the SGLT1 component and its level were unaltered by stress. Effects of stress were reversed by the administration in drinking water of metyrapone, which inhibits 11-β-hydroxylase. Injection of dexamethasone into control rats 60 min before perfusion resulted in absorption and transporter properties indistinguishable from stressed rats. Our data are consistent with the view that stress activates the hypothalamus–pituitary–adrenal (HPA) axis, causing release of glucocorticoid. The ensuing inhibition of GLUT2 trafficking and absorption seems necessary to prevent enhanced intestinal delivery of glucose to the circulation from antagonizing the essential stress response of glucorticoid in mobilizing peripheral energy stores for emergency purposes.
We have proposed a new model for sugar absorption across the brush-border membrane of rat small intestine. When the intestine is challenged with high concentrations of glucose, the facilitative transporter, GLUT2, is rapidly activated and inserted into the brush-border membrane. Regulation of the GLUT2-facilitated component of absorption involves a PKC-dependent pathway, which is activated by glucose transport through the Na+–glucose cotransporter, SGLT1 (Affleck et al. 2003; Helliwell et al. 2000a; Kellett & Helliwell, 2000). Inhibition of SGLT1 with phloridzin diminishes the level of GLUT2 at the brush-border membrane and so inhibits the facilitated as well as the active component. SGLT1 therefore exerts an important control function in addition to its established functions as scavenger and transporter (Kellett, 2001). Regulation also involves phosphatidylinosital (PI) 3-kinase, extracellular signal-regulated kinase (ERK) and p38 signalling pathways (Helliwell et al. 2000b; Helliwell et al. 2003) and is altered in experimental diabetes (Corpe et al. 1996). The ability to detect regulation of the facilitated component depends crucially on the design of the perfusion experiment, for inhibition of the GLUT2 component is observed in low (physiological) but not in high mechanical stress perfusions (Helliwell & Kellett, 2002).
GLUT2 is a high capacity transporter, with a high Km, which displays a normal Michaelis-Menten type saturation response in basolateral membrane vesicles. However, the activation and rapid insertion of GLUT2 into the brush-border membrane results in a co-operative response by which absorptive capacity is matched precisely to dietary intake, so that GLUT2 affords the major route of absorption at high glucose concentrations (Kellett & Helliwell, 2000). In addition, and of equal importance, the intrinsic activity of GLUT2 is rapidly regulated over a 9-fold range in response to the same stimuli (Helliwell et al. 2000b). In this model, since GLUT2 transports not only glucose but also fructose (Cheeseman, 1993), it follows that fructose absorption across the brush-border membrane is mediated not only by GLUT5, which is highly specific for fructose, but also by GLUT2 (Helliwell et al. 2000a, 2000b; Au et al. 2002; Gouyon et al. 2003).
The GLUT2 component of intestinal glucose absorption has the potential to be regulated by hormones through the various intracellular signalling pathways. Until now, however, the only hormone identified as a regulator is glucagon-like peptide-2 (GLP-2; Au et al. 2002). In this paper, we now report that the GLUT2 component is regulated by glucocorticoids in response to stress stimuli. The stress response was caused by major building and renovation works in the expansion and modernization of the Department of Biology at York. Throughout the work there was no adverse effect on animal welfare and animals are now housed in a new, state-of-the-art animal facility. The first part of this paper therefore describes an opportunistic set of experiments in response to variable stress stimuli of ill-defined origin and character. The second part of the paper describes controlled experiments to support the conclusions drawn from the opportunistic experiments.
Methods
Animals
All procedures used conformed to the UK Animals (Scientific Procedures) Act 1986. Male Wistar rats (240–270 g) were fed ad libitum on standard Bantin and Kingman rat and mouse diet with free access to water or saline solution as required.
As soon as we observed inhibition of the GLUT2 component of glucose absorption during the first phase of building works (see Results section), we informed the Animal House Manager and the Department's Home Office Liaison Officer. The in-house veterinary surgeon, the Department's Ethics Committee and the Home Office Inspector were also duly informed. Throughout the period of construction and modernization work, regular monitoring of animals by these authorities found that there was no discernible effect on animal welfare. There was no effect on frequency of breeding, feeding or drinking habits, cage behaviour, illness or disease, or in visual animal condition, such as coat appearance. Regular screens for viral and bacterial infection throughout the work were negative.
Drug treatments
Metyrapone
Rats were given 0.05% metyrapone (2-methyl-1,2-di-3-pyridyl-1-propane, Sigma Chemical Co.) in 0.9% NaCl as drinking water for a minimum of 72 h during stressful conditions prior to perfusion studies.
Dexamethasone
Control rats were given an intraperitoneal injection of 5.0 mg dexamethasone 21-sodium phosphate (Sigma Chemical Co.) per kilogram body weight in 0.9% NaCl approximately 60 min prior to perfusion studies. All perfusions using either metyrapone-treated rats or dexamethasone-injected rats were performed in conjunction with a control, i.e. a rat given 0.9% NaCl as a replacement for drinking water or as an i.p. injection was perfused simultaneously to confirm that either stressed or control conditions prevailed, where appropriate.
Perfusion of jejunal loops
Rats were anaesthetized by an i.p. injection of a mixture of 1.0 ml Hypnorm (Janssen Animal Health) and 0.5 ml Hypnovel (Roche Diagnostics) per kilogram body weight. Additional doses of 0.4 ml Hypnorm/0.2 ml Hypnovel per kilogram body weight were administered by an intramuscular route when required as determined by tail, foot and corneal reflexes, which were carefully monitored throughout the perfusion. Rats were humanely killed by exsanguination under anaesthetic at the end of the experiment. Jejunal loops were perfused luminally in vivo with 75 mmd-glucose in a modified Krebs-Henseleit buffer, pH 7.4, as previously described (Kellett & Helliwell, 2000). The perfusate also contained [3H]-inulin (Amersham International), a non-transportable marker, to permit the determination of water transport. Phloretin (1 mm; Sigma Chemical Co.), a specific inhibitor of GLUT2, was utilized to determine the relative contributions of SGLT1 and GLUT2 components in glucose absorption under different conditions. The perfusion consisted of a gas-segmented single pass system with perfusate and gas flow rates of 0.75 and 0.38 ml min−1, respectively, used to disrupt the unstirred layer. Luminal outflow was sampled every 5 min for a 1-min period, aliquots of which were analysed using a COBAS automatic analyser (Roche) for glucose concentration using a test kit (Trinder) obtained from Sigma Chemical Co. The concentration of glucose in the perfusate was calculated with correction for losses in perfusate volume caused by water transport. The rate of glucose transport, expressed as μmol min−1 (g dry weight)−1, was measured at the steady state period, achieved at t = 20 min.
Membrane vesicle preparation
Brush-border membrane vesicles were prepared as previously described (Corpe et al. 1996): every step of the procedure was performed at 0–4°C to prevent changes in trafficking after the intestine had been excised. Briefly, immediately following perfusion, the jejunum was flushed with ice-cold buffered mannitol (20 mm imidazole buffer, pH 7.5, containing 250 mm mannitol and 0.1 mm phenylmethane sulphonyl fluoride (PMSF)) in order to arrest potential trafficking of GLUT2. The jejunum was then placed on an ice-cold glass plate and slit longitudinally. Mucosal scrapings were taken with an ice-cold glass slide and homogenized immediately at 4°C in buffered mannitol using a Kinematica Polytron homogeniser (4 × 30 s bursts using the large probe at setting 7). The remainder of the preparation and its characterization for purity were as detailed by (Corpe et al. 1996).
SDS-PAGE analysis of membrane components
SDS-PAGE and Western blotting were performed as previously described using ECL (Enhanced Chemiluminescence) detection (Corpe et al. 1996; Helliwell et al. 2000a). All immmunoblotting was performed using rabbit polyclonal antibodies. For GLUT2, one was raised at York to 15 amino acids at the C-terminus (KATVQMEFLGSSETV), whereas the second was raised to a sequence contained in the large extracellular loop (SHYRHVLGVPLDDRRA) and was commissioned from Research Genetics, USA. An SGLT1 antibody, also from Research Genetics, was raised against the extracellular loop (RNSTEERIDLDA). For the detection of PKC βII, a C18 antibody to the last 18 amino acids was purchased from Santa Cruz, CA, USA. Neutralization of all antibodies with the corresponding peptide (antibody to peptide 1: 1 v/v, peptide 50 μg ml−1) prior to blotting abolished labelling, confirming the specificity of the antibodies. Quantification of Western blots was performed using a Flowgen AlphaImager 1200 analysis system (Alpha Innotech Corporation, CA, USA). Relative protein levels detected in brush-border membranes under various conditions, as indicated by band intensities, were expressed with respect to preparations from control (unstressed) rats or stressed rats as appropriate. The same loading of 20 μg protein was used for all samples.
Statistical analysis
Values are presented as means ± s.e.m and were tested for significance using paired or unpaired Student's t test where appropriate.
Results
Over the period October 2000 to April 2003, the Biology Department of the University of York undertook a major programme of expansion and modernization. The bottom left hand corner of Fig. 1 shows the old research department before work began, comprising wing D, wing E containing the old animal house on the first floor and wing F. In the first phase of works, a new building was constructed (right hand side of Fig. 1), which is situated adjacent to wing E of the original building. Pile driving and steel construction were undertaken from December 2000 until the end of August 2001. There followed a 10 month interval in which the new building was equipped and no major construction works took place. In the second phase, wings F and E were renovated and modernised in sequence and a new state-of-the-art animal facility provided in wing F.
Figure 1. Construction of the new building at the Department of Biology, University of York.
The original research Department is shown on the left; wing D, wing E containing the old animal house and wing F.
During the initial phase of construction and modernization work, we observed that the GLUT2 component of intestinal glucose absorption was partially or totally inhibited (see below). The influence of external stimuli arising from building work on the GLUT2 component of absorption was relatively rapid. A weekend without building work was sufficient to ensure that on Monday mornings the GLUT2 component was fully restored, but in the second phase, during periods of intense renovation and modernization work, the GLUT2 component was inhibited again by Tuesday following commencement of work on Monday. In what follows, we therefore characterize rats in which the GLUT2 component was inhibited as ‘stressed’ and rats having the full rate of absorption after several days as ‘control’.
The stress stimuli were ill-defined. Some were obvious to humans, such as intermittent audible noise. On other occasions vibrations were apparent as a direct result of work being carried out near the animal house. On many occasions when the system was not working, however, nothing could be readily identified as responsible for inhibition of the GLUT2 component of absorption. We were left to surmise that the rats were sensitive to noise frequencies and vibration levels not detectable by humans. Because of the nature of the work in different phases, the fact that it was a major building project and the relative inability to monitor the stress stimuli, all the experiments on stressed rats were opportunistic.
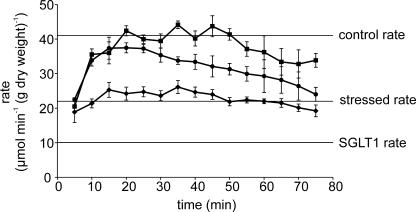
In control perfusions in unstressed rats in vivo with 75 mm glucose (Fig. 2, squares), the rate of glucose absorption reached a steady state rate at 15–20 min of 40.65 ± 2.9 μmol min−1 (g dry weight)−1 (mean ± s.e.m.). This was sustained for 75–90 min, although occasionally, as in Fig. 2, there was a slight tailing off towards the end. Work on the new building commenced with pile driving and steel construction. During this initial phase, we observed for the first time that the rate of glucose absorption was not adequately sustained throughout the full period of the perfusion (Fig. 2, circles). With 75 mm glucose perfused through the lumen, rates peaked at 15 min at a value not significantly different from that of control perfusions. However, the rate of absorption after 25 min began to fall and continued to do so. When heavy construction work ceased, perfusions returned rapidly to control properties and a large, strongly regulated GLUT2 component of absorption operated perfectly until the renovation and modernization of the old building began.
Figure 2. The effect of different types of building work on the time-course of glucose perfusions.
Rat jejunum was perfused in vivo with 75 mm glucose as described in the Methods section. Data are shown for: (▪) control rats under stress-free conditions, no building work (n = 4); (•) rats during construction of the new building (n = 11); (♦) rats during a period of intense renovation of wing F in the old building (n = 8).
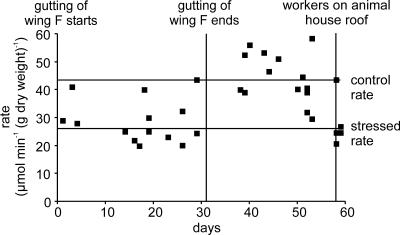
Almost immediately following the start of renovation, we observed that the rate of glucose absorption (Fig. 2, diamonds) was strongly inhibited even at the start of the perfusion and remained inhibited throughout. It became clear the rats were showing a stress response. The rate of absorption in stressed rats was inhibited by 36.4 ± 3.0% (25.87 ± 0.81 compared with 40.65 ± 2.9 μmol min−1 (g dry weight)−1 for control rats, P < 0.0001). The adverse relationship between renovation works and glucose absorption is shown in Fig. 3. At the start of renovation, wing F was gutted over a period of 31 days. Of the 14 perfusions over this period, 11 displayed strong inhibition with an absorption rate of 25.87 ± 0.81 μmol min−1 (g dry weight)−1. Three displayed control rates, reflecting the fact that about one in five rats appear to be resistant to stress (Vogel & Jensh, 1988). Gutting of wing F finished after 32 days and the first perfusion afterwards on day 37 showed a control rate. The rates remained high until our last perfusion of that series on day 53: during this period, there was no work and the average control rate of absorption was 43.4 ± 1.8 μmol min−1 (g dry weight)−1. Figure 2 also provides a very clear demonstration that inhibition of absorption caused by stress stimuli occurs very quickly. On day 58, two workers carried out some work on the roof of wing E directly over the old animal house. Two perfusions on that day show rates for stressed rats, but one, presumably a stress-resistant rat, showed the control rate. The workers finished the job next day, when two more perfusions showed the stressed rate.
Figure 3. The adverse relationship between renovation work and the rate of glucose absorption.
Gutting starts: wing F, adjacent to wing E containing the old animal house, was refurbished by gutting over a period of 31 days. Gutting ends: after the gutting ended, all work then stopped until two workers carried out a job in wing E on the roof of the animal house on days 58 and 59 (roof workers). For explanation, see text.
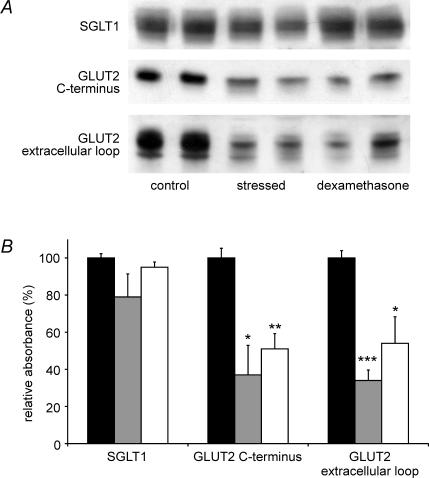
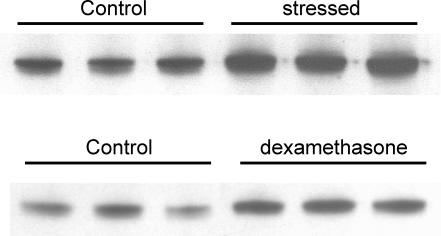
We have previously reported that intestinal glucose absorption comprises SGLT1- and GLUT2-mediated components, which can be resolved in whole intestine by selective inhibition of the latter by phloretin (Kellett & Helliwell, 2000). Perfusion of rats under control and stress conditions with phloretin revealed that stress inhibited the GLUT2 component by 42.8 ± 3.8% (P < 0.01); the SGLT1 component was unaffected (Table 1). Western blots of brush-border membrane vesicles prepared from control and stressed rats, initially perfused with 75 mm glucose to confirm the appropriate rates of perfusion, were probed with SGLT1 and GLUT2 antibodies (Fig. 4). Antigen-specific bands were identified by elimination on pre-incubation of anti-serum with excess antigenic peptide (data not shown). All bands obtained with the SGLT1 anti-serum and the C-terminal GLUT2 anti-serum were eliminated, but with the GLUT2 extracellular loop antibody, only the upper band was eliminated. C-terminal and extracellular loop antibodies to GLUT2 revealed a reduction in GLUT2 level in stressed rats (as judged by the intensity of specific bands) of 66.0 ± 15.9 and 66.3 ± 5.6%, respectively (P < 0.05 and P < 0.001), which correlates with the observed inhibition of absorption within experimental error. The fact that both C-terminal and extracellular loop antibodies yielded the same result provides strong evidence that protein really is GLUT2 and not some other unidentified GLUT with which a cross-reaction occurs. There was no significant change in the level of SGLT1 (−21.3 ± 12.4%, P = 0.17 in stressed compared with control rats). Interestingly, the level of PKC βII was increased in stressed rats by 72.8 ± 7.9% (P < 0.001) and in control rats injected with dexamethasone by 58.5 ± 4.3% (P < 0.05), yet apical GLUT2 levels were diminished, not increased (Fig. 5). The purity of brush-border membrane vesicle preparations was unaffected by stress or glucocorticoid treatment, as judged by the enrichment of apical marker alkaline phosphatase (Quaroni et al. 1999).
Table 1.
The effect of stress, metyrapone and dexamethasone on the rates of glucose and water
| Control rats | Stressed rats | Stressed rats + metyrapone | Control rats + dexamethasone | |
|---|---|---|---|---|
| Glucose absorption | ||||
| no phloretin | 40.65 ± 2.9(9) | 25.87 ± 0.81****(10) | 38.26 ± 1.79§§§§(12) | 23.97 ± 1.43****(11) |
| Glucose absorption | ||||
| plus phloretin | 11.32 ± 0.62††(4) | 9.73 ± 1.58†††(5) | 11.55 ± 3.59††(5) | 16.66 ± 2.83††(6) |
| Water absorption | 0.17 ± 0.02(9) | 0.14 ± 0.02(10) | 0.18 ± 0.01(12) | 0.07 ± 0.01***(11)§§§ |
Rats were maintained on 0.05% metyrapone in 0.9% NaCl as drinking water for a minimum of 72 h at the end of which paired perfusions of untreated rats maintained on 0.9% NaCl showed that a stress response was present as monitored by inhibition of glucose absorption. Control rats (under stress-free conditions) were given an intraperitoneal injection of dexamethasone 21-sodium phosphate (5.0 mg (kg body weight)−1 in 0.9% NaCl) approximately 60 min prior to perfusion studies. Paired perfusions of saline-injected rats showed that during this series of experiments a stress response was not present. Glucose absorption (μmol min−1 (g dry weight)−1) was measured in the presence and absence of phloretin (1 mm). Water transport is expressed in ml min−1 (g dry weight)−1. Values are given as mean ± s.e.m. with the number of experiments in parentheses.
P < 0.001
P < 0.0001 when compared with control
P < 0.001
P < 0.0001 versus stressed by unpaired Students t test.
P < 0.01
P < 0.001 in paired Student's t test of glucose transport rate ± phloretin.
Figure 4. Effect of stress and dexamethasone on the levels of SGLT1 and GLUT2 in the brush-border membrane.
Vesicles were prepared from the jejunum of control (filled bars), stressed (grey bars) and control/dexamethasone-treated (open bars) rats, following perfusion with 75 mm d-glucose for 30 min. A, Vesicle protein (20 μg) was separated on 10% SDS-PAGE gels, transblotted onto PVDF membrane and Western blotted with SGLT1 antibody and with GLUT2 C-terminal and extracellular loop antibodies. B, Expression of transporter protein relative to controls (100%) determined from three separate preparations using two rats each; *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired Student's t test.
Figure 5. Effect of stress and dexamethasone on the levels of PKC βII in the brush-border membrane.
Vesicles were prepared as described in the legend to Fig. 4. Western blots were probed with C18 antibody to the last 18 amino acids of PKC βII.
Two of the major stress responses in animals are the release of adrenaline and cortisol. Perfusion of stressed rats with the PKA inhibitor, H89 (2 μm), failed to restore the rate of absorption in stressed rats to its control value, effectively ruling out adrenaline (data not shown). Stressed rats were treated with metyrapone in saline (0.9% NaCl as drinking water) for a minimum of 3 days during a period of renovation when the rate of absorption of untreated rats was strongly inhibited. Metyrapone inhibits 11-β-hydroxylase, which catalyses the last step in the pathway leading to cortisol synthesis and so blocks stress-induced cortisol and corticosterone synthesis without affecting basal levels (Calvo et al. 1998). Perfusions performed on metyrapone-treated rats under stress conditions demonstrated that the drug was able to block the effects of stress and hence restore the rate of glucose absorption to a value of 38.26 ± 1.79 μmol min−1 (g dry weight)−1 (P < 0.0001 and P = 0.47 compared with perfusions for stressed and control rats, respectively, Table 1). Parallel perfusions on untreated rats (administered 0.9% NaCl only) confirmed that a stress response was present throughout the period of these experiments (24.37 ± 1.09 μmol min−1 (g dry weight)−1, n = (4). In addition, there was no effect of metyrapone when administered to unstressed control rats (36.53 ± 1.09 μmol min−1 (g dry weight)−1 (n = 5), P = 0.49 compared with control rats not given metyrapone).
Since metyrapone inhibits 11-β-hydroxylase, we investigated the effect of a poorly metabolized analogue of cortisol, the synthetic glucocorticoid, dexamethasone 21-sodium phosphate. Dexamethasone was injected intraperitoneally into control rats; these were subsequently demonstrated to be initially stress-free by simultaneous injection of a rat with saline only. These corresponding controls show a rate of 37.17 ± 1.64 μmol min−1 (g dry weight)−1 (n = 5) confirming that no stress response to building work was present throughout these experiments. Table 1 shows that injection of dexamethasone 60 min prior to perfusion resulted in a reduction in the GLUT2 component of absorption of 71.4 ± 3.9% (P < 0.0001 and P = 0.27 for comparison with control and stressed rats, respectively). There was no effect of dexamethasone on glucose absorption in stressed rats, i.e. the effects were not additive (28.73 ± 1.84 μmol min−1 (g dry weight)−1 (n = 4), P = 0.1 compared with stressed rats not injected with dexamethasone). Again, simultaneous perfusions in rats injected with saline only confirmed that stress conditions prevailed (23.69 ± 1.15 μmol min−1 (g dry weight)−1 (n = 3)). The reduction in absorption observed in dexamethasone-injected control rats was accompanied by a 49.2 ± 8.2% and 46.0 ± 14.3% reduction in the level of GLUT2, detected by C-terminus and extracellular loop antibodies (P < 0.01 and P < 0.05, respectively; Fig. 4). SGLT1 was not affected (−5.4 ± 2.8%, P = 0.21 compared with control rats).
In a parallel project during renovation of wing F, rats deprived of food overnight (18 h-starved) were also perfused on days when fed rats were stressed and glucose absorption was strongly inhibited. Of particular interest, the absorption of glucose in 18 h-starved rats was unaffected by stress: the control rate of absorption for starved rats was 48.61 ± 1.1 μmol min−1 (g dry weight)−1 (n = 4) and the rate for starved rats under proven stress conditions (as indicated by the rate of absorption in fed rats) was 39.72 ± 0.8 μmol min−1 (g dry weight)−1 (n = 4) (P = 0.14).
Table 1 shows the effects of stress stimuli and dexamethasone injection on the absorption of water by the jejunum during the perfusions, as measured by the non-transportable marker [3H]-inulin. A 17.6 ± 2.6% reduction in the mean value of water absorption in perfusions with stressed rats was not statistically significant compared with control perfusions (P = 0.34). However, dexamethasone injection into control rats caused a severe 58.8 ± 2.4% inhibition in water absorption (P < 0.001). Metyrapone administration caused a 28.6 ± 1.8% increase in water absorption in stressed rats (P < 0.05), although there was no difference when compared with control values (P = 0.53).
Discussion
Experiments on the effects of stress caused by construction and modernization work were of necessity opportunistic. The effects of stress were intermittent over quite long periods, depending as they did on the intensity of the work; moreover, onset and reversal of effects were relatively rapid. Nevertheless, we were able over time to gather sufficient data to propose a possible mechanism for the inhibition of glucose absorption caused by stress, although we were not able to collect all the data we would have wished.
During renovation work, when the effects were more intense, glucose absorption was inhibited 36.4 ± 3.0%; moreover, absorption was inhibited as soon as the steady state was achieved. Use of phloretin to inhibit the GLUT2 component selectively in whole intestine in stressed and control rats revealed that stress caused a 42.8 ± 3.8% inhibition of the GLUT2 component (Table 1): there was a concomitant diminution of 66.0 ± 15.9 and 66.3 ± 5.6% in the level of GLUT2, when determined with the C-terminal and the extracellular loop antibody, respectively (Fig. 4). There was no significant effect of stress on the SGLT1 component of absorption or on the level of SGLT1 protein (Table 1, Fig. 4). The effect of stress was relatively rapid, as shown on days 58 and 59 in Fig. 2, when work on the animal house roof was undertaken.
Three observations implicate the glucocorticoid cortisol as the most likely hormone involved in the inhibition of glucose absorption in stressed rats. First, the administration of metyrapone in the drinking water reversed the effects of stress; metyrapone inhibits 11-β-hydroxylase to maintain plasma cortisol at basal levels. Metyrapone has also been shown to cause an increase in noradrenaline and dopamine production (Bratt et al. 2001; Laborie et al. 2003); however, the fact that metyrapone has no effect on glucose absorption in control animals excludes a role of these hormones and adrenaline in the stress response, a conclusion supported by our observation that H89 fails to reverse the effects of stress, thus ruling out the major alternative stress signals to cortisol. Second, injection of the poorly metabolized synthetic analogue of cortisol, dexamethasone-21-phosphate, into control rats 60 min before perfusion strongly inhibited the GLUT2 component of glucose absorption (Table 1). The GLUT2 component is inhibited to a slightly greater extent by dexamethasone than that observed in the stress response from building works. The differences in the two situations may simply be due to the fact that dexamethasone is 30 times more potent than cortisol (Quaroni et al. 1999). Third, dexamethasone treatment of control rats diminished the level of GLUT2 but increased the level of PKC βII by approximately 48 and 58.5%, respectively, a response very similar to that observed in stressed rats (Figs 4 and 5). This observation was unexpected, since increased apical GLUT2 insertion correlated with increased PKC activation in all previous investigations. Ideally, we would like to have measured changes in circulating levels of cortisol in stressed rats; however, this opportunity was lost because of the opportunistic nature of our experiments on the stress response.
Inhibition of glucose absorption by stress and dexamethasone therefore results from inhibition of GLUT2 trafficking. Trafficking is a rapid process with a half-time of the order of 3 min (Kellett & Helliwell, 2000). Cortisol has a half-life of 30–60 min in vivo and stress causes an increase in its synthesis coupled with an increase in its half-life in the circulation (Herman et al. 1992; Calvo & Volosin, 2001). The effects of stress were rapid and dexamethasone exerted full inhibition of absorption within 60 min. The rapidity of the glucocorticoid response most probably represents a non-genomic, direct effect on the trafficking mechanism. The GLUT2 system shows significant similarities to the inhibition of insulin-induced 2-deoxyglucose uptake through GLUT4 by treatment of adipocytes or soleus muscle for 60 min with dexamethasone: in both adipocytes and soleus muscle, dexamethasone causes translocation of PKC to the plasma membrane and in adipocytes the isoform involved is PKC β (Ishizuka et al. 1995; Kajita et al. 2001). Moreover, chronic administration of dexamethasone inhibits rapid trafficking of GLUT4 to the membrane of rat soleus muscle without altering the cellular content of GLUT4, thereby inducing insulin resistance (Dimitriadis et al. 1997). The fact that PKC βII is activated but apical GLUT2 levels are diminished in response to stress or dexamethasone suggests that glucocorticoid inhibits GLUT2 insertion by a parallel pathway acting downstream of PKC βII activation. The possibility of such a pathway deserves detailed investigation. Prolonged administration of dexamethasone had no effect on glucose uptake as measured by in vitro methods, in which transport was mediated exclusively by SGLT1 at the brush-border membrane (Thiesen et al. 2003a, b). In addition, protein and mRNA levels of SGLT1 remain unchanged. These data agree with the present short-term regulation studies implicating inhibition of GLUT2 trafficking as the mechanism of inhibition of glucose absorption by glucocorticoids.
An inhibitory effect of dexamethasone on jejunal water absorption was evident from the perfusions. Thus a 71.4 ± 3.9% inhibition of the GLUT2 component of glucose absorption was accompanied by a 58.8 ± 2.4% inhibition of water absorption (Table 1). However, in stressed animals, there was no significant inhibition of water absorption, despite a similar inhibition of the GLUT2 component. This may also be attributed to the potency of dexamethasone. Nevertheless, there is a clear dissociation between water and glucose absorption. Such dissociation is not consistent with the proposal that glucose absorption at concentrations above those required to saturate SGLT1 is the result of paracellular flow (Pappenheimer & Reiss, 1987). Gruzdkov and colleagues have recently provided a definitive demonstration that water transport and glucose absorption can be dissociated. Accordingly, by manipulating the osmolarity of the perfusion medium at high glucose concentrations, Gromova et al. (2001) have shown that very high rates of glucose absorption can occur not only in the presence of water absorption, but also in the face of water secretion. Moreover, we have previously reported that simultaneous inhibition of the GLUT2 and SGLT1 components can account for within experimental error for all glucose absorption (Helliwell & Kellett, 2002).
Stress and gastrointestinal function
Stress has been frequently associated with gastrointestinal manifestations, for example, changes in gastric emptying, increased colonic and small intestinal motility, and intestinal barrier function (Bijlsma et al. 2001). A variety of clinical evidence demonstrates a relationship between stress and specific gastrointestinal functions, thought to be mediated by neural pathways linking the CNS and the gut (summarized in Saunders et al. 1994).
An elevated plasma cortisol concentration is recognized as a classic response to stress (Saunders et al. 1994; Calvo et al. 1998). The initial response of individuals or animals to stress is activation of the sympathetic nervous system followed by rapid secretion of adrenaline by the adrenal medulla. The resulting fright, fight or flight response includes enhanced mobilization of glucose and fat and redistribution of blood flow from the skin and gut to the brain and muscle. However, the adrenal medulla releases only minute amounts of material and so response to prolonged stress is taken over by sustained secretion of cortisol in response to activation of the HPA axis. Thus cortisol secretion from the adrenal cortex is stimulated by rapid secretion of adrenocorticotrophic hormone (ACTH) from the anterior pituitary, which in turn is caused by the secretion of corticotrophin releasing hormone (CRF) from the hypothalamus (DeRijk et al. 2002). Access to dietary glucose is a pre-requisite for HPA stress responses. Thus, in humans fasted for 8–11 h before being subjected to a controlled stress test, there was a doubling in plasma cortisol levels within 15 min, provided that the subjects received a 100 g glucose load 1 h before the test: if, instead of glucose, they received either tap water or a protein or a fat load, there was no increase in plasma cortisol levels above basal (Kirschbaum et al. 1997; Gonzalez-Bono et al. 2002). Consistent with these findings, we observed during the renovation of wing F that stress had no effect on the rate of glucose absorption in rats from which food was withheld overnight.
Animal welfare studies on the transport of piglets have revealed that stress induced by vibration causes an increase in plasma cortisol levels (Perremans et al. 2001). At times there was undoubtedly detectable vibration of the old animal house in wing E as a result of the renovation work in wing F and the effects of stress were blocked by metyrapone. Vibration therefore seems likely to have been a significant factor in the stress response of rats; indeed, it has long been known that rats are very much more sensitive to vibration than humans.
Why should cortisol inhibit the GLUT2 component of intestinal glucose absorption? As noted earlier, cortisol antagonizes the actions of insulin. At physiological concentrations, cortisol mobilizes energy stores in the periphery, stimulating glycogenolysis and lipolysis. Cortisol also inhibits protein synthesis, resulting in release of amino acids and loss of skeletal mass. The degradation products, lactate, glycerol and amino acids, are recovered by enhanced gluconeogenesis, assisted by stimulation of amino acid uptake in liver. In the periphery, cortisol inhibits both amino acid and glucose uptake, preventing potential inhibition of the mobilization process. After a meal high in free sugars or complex carbohydrate of high glycaemic index, the major route of transintestinal delivery of glucose into the circulation is mediated by GLUT2 in the brush-border membrane. The resulting increase in blood glucose levels would stimulate peripheral glucose uptake and restrict gluconeogenesis. The ability of cortisol to inhibit GLUT2 trafficking and absorption therefore seems necessary to prevent enhanced intestinal delivery of glucose from antagonizing the ability of cortisol to mobilize peripheral energy stores in response to prolonged stress. Our opportunistic experiments have afforded what is perhaps a unique demonstration that the possibility of such a mechanism is not just a deduction from a laboratory experiment; it operates in a real-life physiological situation.
Acknowledgments
This work was supported by The Wellcome Trust. O.J.M., E.L.M. and N.P. are recipients of BBSRC studentships.
References
- Affleck JA, Helliwell PA, Kellett GL. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J Histochem Cytochem. 2003;51:1567–1574. doi: 10.1177/002215540305101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma PB, van Raaij MT, Dobbe CJ, Timmerman A, Kiliaan AJ, Taminiau JA, Groot JA. Subchronic mild noise stress increases HRP permeability in rat small intestine in vitro. Physiol Behav. 2001;73:43–49. doi: 10.1016/s0031-9384(01)00424-3. [DOI] [PubMed] [Google Scholar]
- Bratt AM, Kelley SP, Knowles JP, Barrett J, Davis K, Davis M, Mittleman G. Long term modulation of the HPA axis by the hippocampus. Behavioral, biochemical and immunological endpoints in rats exposed to chronic mild stress. Psychoneuroendocrinology. 2001;26:121–145. doi: 10.1016/s0306-4530(00)00033-0. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res. 1998;800:227–235. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73:261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology. 1993;105:1050–1056. doi: 10.1016/0016-5085(93)90948-c. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Basaleh MM, Affleck J, Gould G, Jess TJ, Kellett GL. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996;432:192–201. doi: 10.1007/s004240050124. [DOI] [PubMed] [Google Scholar]
- DeRijk RH, Schaaf M, de Kloet ER. Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol. 2002;81:103–122. doi: 10.1016/s0960-0760(02)00062-6. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G, Leighton B, ParryBillings M, Sasson S, Young M, Krause U, Bevan S, Piva T, Wegener G, Newsholme EA. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J. 1997;321:707–712. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Horm Behav. 2002;41:328–333. doi: 10.1006/hbeh.2002.1766. [DOI] [PubMed] [Google Scholar]
- Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552:823–832. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromova L, Al A, Gruzdkov Gruzdkov AA. Mechanisms of glucose absorption at a high carbohydrate level in the rat small intestine in vivo. [Russian] Russian J Physiol. 2001;87:973–981. [PubMed] [Google Scholar]
- Helliwell PA, Kellett GL. The active and passive components of glucose absorption in rat jejunum under low and high perfusion stress. J Physiol. 2002;544:579–589. doi: 10.1113/jphysiol.2002.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000a;350:149–154. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000b;350:163–169. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C betaII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem. 2003;278:28644–28650. doi: 10.1074/jbc.M301479200. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Yamamoto M, Nagashima T, Kajita K, Taniguchi O, Yasuda K, Miura K. Effect of dexamethasone and prednisolone on insulin-induced activation of protein kinase C in rat adipocytes and soleus muscles. Metabolism. 1995;44:298–306. doi: 10.1016/0026-0495(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Kajita K, Ishizuka T, Miura A, Kanoh Y, Ishizawa M, Kimura M, Muto N, Yasuda K. Glucocorticoid-induced insulin resistance associates with activation of protein kinase C isoforms. Cell Signal. 2001;13:169–175. doi: 10.1016/s0898-6568(01)00143-7. [DOI] [PubMed] [Google Scholar]
- Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Bono EG, Rohleder N, Gessner C, Pirke M, Salvador A, Hellhammer DH. Effects of fasting and glucose load on free cortisol responses to stress and nicotine. J Clin Endocrin Met. 1997;82:1101–1105. doi: 10.1210/jcem.82.4.3882. [DOI] [PubMed] [Google Scholar]
- Laborie C, Van Camp G, Bernet F, Montel V, Dupouy JP. Metyrapone-induced glucocorticoid depletion modulates tyrosine hydroxylase and phenylethanolamine N-methyltransferase gene expression in the rat adrenal gland by a noncholinergic transsynaptic activation. J Neuroendocrinol. 2003;15:15–23. doi: 10.1046/j.1365-2826.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- Perremans S, Randall JM, Rombouts G, Decuypere E, Geers R. Effect of whole-body vibration in the vertical axis on cortisol and adrenocorticotropic hormone levels in piglets. J Anim Sci. 2001;79:975–981. doi: 10.2527/2001.794975x. [DOI] [PubMed] [Google Scholar]
- Quaroni A, Tian JQ, Goke M, Podolsky DK. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol. 1999;277:G1027–G1040. doi: 10.1152/ajpgi.1999.277.5.G1027. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994;267:G794–G799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- Thiesen A, Wild GE, Keelan M, Clandinin MT, Thomson AB. Locally and systemically active glucocorticosteroids modify intestinal absorption of sugars in rats. J Appl Physiol. 2003a;94:583–590. doi: 10.1152/japplphysiol.00134.2002. [DOI] [PubMed] [Google Scholar]
- Thiesen A, Wild GE, Tappenden KA, Drozdowski L, Keelan M, Thomson BK, McBurney MI, Clandinin MT, Thomson AB. The locally acting glucocorticosteroid budesonide enhances intestinal sugar uptake following intestinal resection in rats. Gut. 2003b;52:252–259. doi: 10.1136/gut.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel WH, Jensh R. Chronic stress and plasma catecholamine and corticosterone levels in male rats. Neurosci Lett. 1988;87:183–188. doi: 10.1016/0304-3940(88)90167-x. [DOI] [PubMed] [Google Scholar]