Abstract
Motor nerve terminals possess multiple voltage-sensitive calcium channels operating acetylcholine (ACh) release. In this study, we investigated whether facilitation of neuromuscular transmission by adenosine generated during neuronal firing was operated by Ca2+ influx via ‘prevalent’ P-type or via the recruitment of ‘silent’ L-type channels. The release of [3H]ACh from rat phrenic nerve endings decreased upon increasing the stimulation frequency of the trains (750 pulses) from 5 Hz (83 ± 4 × 103 disintegrations per minute per gram (d.p.m. g−1); n = 11) to 50 Hz (30 ± 3 × 103 d.p.m. g−1; n = 5). The P-type Ca2+ channel blocker, ω-agatoxin IVA (100 nm) reduced (by 40 ± 10%; n = 6) the release of [3H]ACh evoked by 50-Hz trains, while nifedipine (1 μm, an L-type blocker) was inactive. Tetanic depression was overcome (88 ± 6 × 103 d.p.m. g−1; n = 12) by stimulating the phrenic nerve with 50-Hz bursts (five bursts of 150 pulses, 20 s interburst interval). In these conditions, ω-agatoxin IVA (100 nm) failed to affect transmitter release, but nifedipine (1 μm) decreased [3H]ACh release by 21 ± 7% (n = 4). Inactivation of endogenous adenosine with adenosine deaminase (ADA, 0.5 U ml−1) reduced (by 54 ± 8%, n = 5) the release of [3H]ACh evoked with 50-Hz bursts. This effect was opposite to the excitatory actions of adenosine (0.5 mm), S-(p-nitrobenzyl)-6-thioinosine (5 μm, an adenosine uptake blocker) and CGS 21680C (3 nm, a selective A2A receptor agonist); as the A1 receptor agonist R-N6-phenylisopropyl adenosine (R-PIA, 300 nm) failed to affect the release of [3H]ACh, the results indicate that adenosine generated during 50-Hz bursts exerts an A2A-receptor-mediated tonus. The effects of ADA (0.5 U ml−1) and CGS 21680C (3 nm) were prevented by nifedipine (1 μm). Blocking tonic A2A receptor activation, with ADA (0.5 U ml−1) or 3,7-dimethyl-1-propargyl xanthine (10 μm, an A2A antagonist), recovered ω-agatoxin IVA (100 nm) inhibition and caused the loss of function of nifedipine (1 μm). Data indicate that, in addition to the predominant P-type Ca2+ current triggering ACh release during brief tetanic trains, motoneurones possess L-type channels that may be recruited to facilitate transmitter release during high-frequency bursts. The fine-tuning control of Ca2+ influx through P- or L-type channels is likely to be mediated by endogenous adenosine. Therefore, tonic activation of presynaptic A2A receptors operating Ca2+ influx via L-type channels may contribute to overcome tetanic depression during neuronal firing.
Multiple subtypes of voltage-sensitive calcium channels (VSCC) are colocalized in nerve terminals, where they may act synergistically to cause transmitter release (Takahashi & Momiyama, 1993; Wheeler et al. 1994). It is known that brief depolarizations trigger Ca2+ currents that seem to be different from the ‘facilitatory’ currents, which are normally quiescent but can be activated by repetitive depolarizations (cf. Artalejo et al. 1992). At mature mammalian motor endplates, activation of synaptic vesicle exocytosis is primarily accomplished by P-type Ca2+ channels (e.g. Atchison, 1989; Protti & Uchitel, 1993; Wessler et al. 1995; Correia-de-Sá et al. 2000a), which are specifically targeted into presynaptic nerve terminals (Stanley, 1997). In contrast, L-type Ca2+ channels, presumably located away from the active zones, do not ordinarily participate in the release process but they might be involved in the integration of synaptic activity (Tsien et al. 1988; Robitaille et al. 1990). Recently, we showed that long-depolarizing stimuli unmask Ca2+ influx through high-capacity/slow-inactivating L-type channels contributing to the facilitation of acetylcholine (ACh) release from motor nerve terminals (Correia-de-Sá et al. 2000a; see also Urbano & Uchitel, 1999). The pathophysiological significance of L-type Ca2+ currents has been difficult to demonstrate. They may play a role in re-establishing neuromuscular transmission (1) during development and re-innervation of motor endplates, when P-type channels are functionally immature (Katz et al. 1996; Sugiura & Ko, 1997), (2) during functional recovery from botulinum toxin type-A poisoning (Santaféet al. 2000), and (3) in Lambert–Eaton myasthenic syndrome (LEMS), where spared L-type currents can be activated by repetitive nerve stimulation to compensate for down-regulation of P-type channels (Garcia & Beam, 1996).
Understanding the fine-tuning control of Ca2+ influx through neuronal P- or L-type channels at various stimulation patterns, as well as the sensitivity of different VSCCs to endogenous modulators, may be of clinical relevance to increase the safety margin of neuromuscular transmission. Adenosine build-up from the catabolism of released ATP is one of such mediators (Cunha et al. 1996; Magalhães-Cardoso et al. 2003) playing a key role in adjusting the modulatory pattern of neuromuscular transmission to the stimulation conditions (Correia-de-Sá et al. 1996). While adenosine acts predominantly as an inhibitory signal (via A1 receptors) under resting conditions, amplification of neuromuscular transmission due to A2A receptor activation becomes evident at high levels of synaptic adenosine, such as generated during high-frequency long-lasting stimuli. At most neuro-effector junctions, adenosine A2A receptors act via subtle modifications of the presynaptic inter-receptor dynamics (for review see Sebastião & Ribeiro, 2000) involving the generation of intracellular second messengers, such as cyclic AMP (Correia-de-Sá & Ribeiro, 1994) and Ca2+ (Correia-de-Sá et al. 2000b). The question then arises, of whether endogenous adenosine generated during neuronal firing might influence Ca2+ dynamics to adapt transmitter exocytosis to neuronal firing at the rat endplate.
Methods
Preparation and experimental conditions
Rats (Wistar, 150–200 g) of either sex (Charles River, Barcelona, Spain) were kept at a constant temperature (21°C) and a regular light (06.30–19.30 h)–dark (19.30–06.30 h) cycle, with food and water ad libitum. The animals were killed after stunning followed by exsanguination. Animal handling and experiments followed the guidelines of the International Council for Laboratory Animal Science (ICLAS). The experiments were performed on left phrenic nerve–hemidiaphragm preparations (4–6 mm width). Each muscle was superfused with gassed (95% O2–5% CO2) Tyrode solution (pH 7.4) containing (mm): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1, NaH2PO4 0.4, NaHCO3 11.9, glucose 11.2 and choline 0.001 at 37°C.
The procedures used for labelling the preparations and measuring evoked [3H]ACh release were as previously described (Correia-de-Sá et al. 1991), with minor modifications. Phrenic nerve–hemidiaphragm preparations were mounted in 3-ml capacity Perspex chambers heated to 37°C. Nerve terminals were labelled for 40 min with 1 μm[3H]choline (specific activity 2.5 μCi nmol−1) under electrical stimulation at a frequency of 1 Hz (0.04 ms duration, 8 mA). The phrenic nerve was stimulated with a glass–platinum suction electrode placed near the first division branch of the nerve trunk to avoid direct contact with muscle fibres. Washout of the preparations was performed for 60 min, by superfusion (15 ml min−1) with Tyrode solution supplemented with the choline uptake inhibitor, hemicholinium-3 (10 μm). Tritium outflow was evaluated by liquid scintillation spectrometry (percentage counting efficiency, 40 ± 2%) after appropriate background subtraction using 2-ml bath samples collected automatically every 3 min. After the loading and washout periods, the preparation contained 5542 ± 248 × 103 disintegrations per minute per gram wet weight of tissue (d.p.m. g−1) and the resting release was 132 ± 12 × 103 d.p.m. g−1 (n = 8). The fractional release was calculated to be 2.38 ± 0.14% of the radioactivity present in the tissue at the first collected sample (see e.g. Fig. 1).
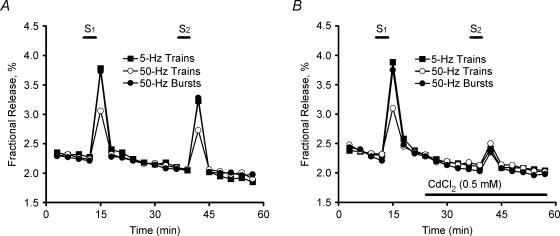
Figure 1. Time course of tritium outflow from electrically stimulated phrenic nerve terminals.
Time course of tritium outflow in control conditions (A) and in the presence of 0.5 mm CdCl2 (B). Tritium outflow (ordinate) is expressed as a percentage of the total radioactivity present in the tissue at the beginning of the collection period. The abscissa indicates the times at which samples were collected. [3H]ACh release was elicited by stimulating the phrenic nerve trunk with 750 electrical pulses delivered with frequencies of 5 Hz (▪) and 50 Hz; either one train (15 s) (○) or a series of five bursts (3 s, 150 pulses, 20-s interburst interval) (•) were delivered when 50-Hz stimulation was applied. Each period of stimulation was applied twice, starting at the 12th (S1) and 39th (S2) minutes after the end of washout (zero time). In (B), CdCl2 (0.5 mm) was added to the incubation media 15 min before S2 (horizontal bar). Note that spontaneous tritium outflow was not changed in the presence of CdCl2.
[3H]ACh release was evoked by two periods of electrical stimulation of the phrenic nerve, starting at the 12th (S1) and 39th (S2) minutes after the end of washout (zero time). Supramaximal intensity pulses were delivered at 5 Hz or 50 Hz. Either one train (750 pulses) or a series of five bursts of 150 pulses applied with a 20-s interburst interval were used when the stimulation frequency was 50 Hz (tetanus). Electrical stimulation increased only the release of [3H]ACh in a Ca2+- and tetrodotoxin-sensitive manner (Correia-de-Sá et al. 2000a), while the output of [3H]choline remained unchanged (Wessler & Kilbinger, 1986). Therefore, evoked [3H]ACh release was calculated by subtracting the basal tritium outflow from the total tritium outflow during the stimulation period (cf. Correia-de-Sá et al. 1991).
Drug effects and pharmacological interactions
Test drugs were added 15 min before S2 and were present up to the end of the experiments (see e.g. Fig. 1). The percentage change in the ratio between the evoked [3H]ACh release during the two stimulation periods (S2/S1) relative to that observed in control situations (in the absence of test drugs) was taken as a measure of the effect of the tested drugs. Positive and negative values represent facilitation and inhibition of evoked [3H]ACh release, respectively. When we evaluated changes in the effect of tested drugs induced by a modifier, the modifier was applied 15 min before starting sample collection and hence was present during S1 and S2. When the same drug was present in S1 and S2, the S2/S1 ratios were not significantly (P > 0.05) different from controls, that is, without addition of drugs; the S2/S1 values were 0.79 ± 0.03 (n = 11), 0.81 ± 0.05 (n = 5) and 0.88 ± 0.03 (n = 12) for 5-Hz trains, 50-Hz trains and 50-Hz bursts, respectively (Fig. 1). None of the drugs changed basal tritium outflow significantly (P > 0.05).
Materials and solutions
Adenosine, adenosine deaminase (ADA, type VI, 1803 U ml−1, EC 3.5.4.4), adenosine 5′-monophosphate (AMP), CdCl2, choline chloride, ω-conotoxin GVIA (ω-CgTx GVIA), ω-conotoxin MVIIC (ω-CmTx MVIIC), ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA), hemicholinium-3, nifedipine, R-N6-phenylisopropyl adenosine (R-PIA) and S-(p-nitrobenzyl)-6-thioinosine (NBTI) were from Sigma, USA. ω-Agatoxin IVA (ω-AgaTx IVA) was from Peptide Institute Inc., Japan. 2-[4-(2-p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamido adenosine (CGS 21680C) and 3,7-dimethyl-1-propargylxanthine (DMPX) were from Research Biochemicals Inc., USA. [Methyl-3H]-choline chloride (ethanol solution, 80 Ci mmol−1) was from Amersham, UK. All reagents were of the highest purity available. Nifedipine was made up in a 10 mm stock solution in ethanol, and was kept protected from the light to prevent photodecomposition. NBTI and R-PIA were made up as 10 mm and 50 mm stock solutions in dimethylsulphoxide, respectively. All stock solutions were stored as frozen aliquots at −20°C. Dilutions of these stock solutions were made daily and appropriate solvent controls were performed. The pH of the superfusion solution did not change following addition of the drugs at the maximum concentrations applied to the preparations.
Statistics
The data are expressed as mean ± s.e.m. from n experiments. Statistical analysis of data was carried out using paired or unpaired Student's t test or one-way analysis of variance (ANOVA) followed by Dunnett's modified t test. Values of P < 0.05 were considered to represent significant differences.
Results
Influence of stimulation conditions on [3H]ACh release from motor nerve endings: differential role of P- and L-type VSCCs
Figure 1 shows the time course of tritium outflow from phrenic nerve–hemidiaphragm preparations stimulated electrically. Stimulation of the phrenic nerve with 5-Hz trains (750 pulses) increased tritium outflow by an average of 83 ± 4 × 103 d.p.m. g−1 (n = 11) above the basal tritium outflow. The average evoked [3H]ACh release decreased to 30 ± 3 × 103 d.p.m. g−1(n = 10) upon increasing the frequency of the stimulation train to 50 Hz, keeping the number of pulses constant (Fig. 1A, tetanic depression). The amount of [3H]ACh release recovered to 88 ± 6 × 103 d.p.m. g−1(n = 11) when the phrenic nerve was stimulated with 50-Hz frequency bursts (5 × 150 pulses, 20 s interburst interval). In all these conditions, [3H]ACh release was largely attenuated by CdCl2 (500 μm) indicating that transmitter exocytosis is dependent on the Ca2+ influx through voltage-sensitive channels (VSCCs) (Fig. 1B).
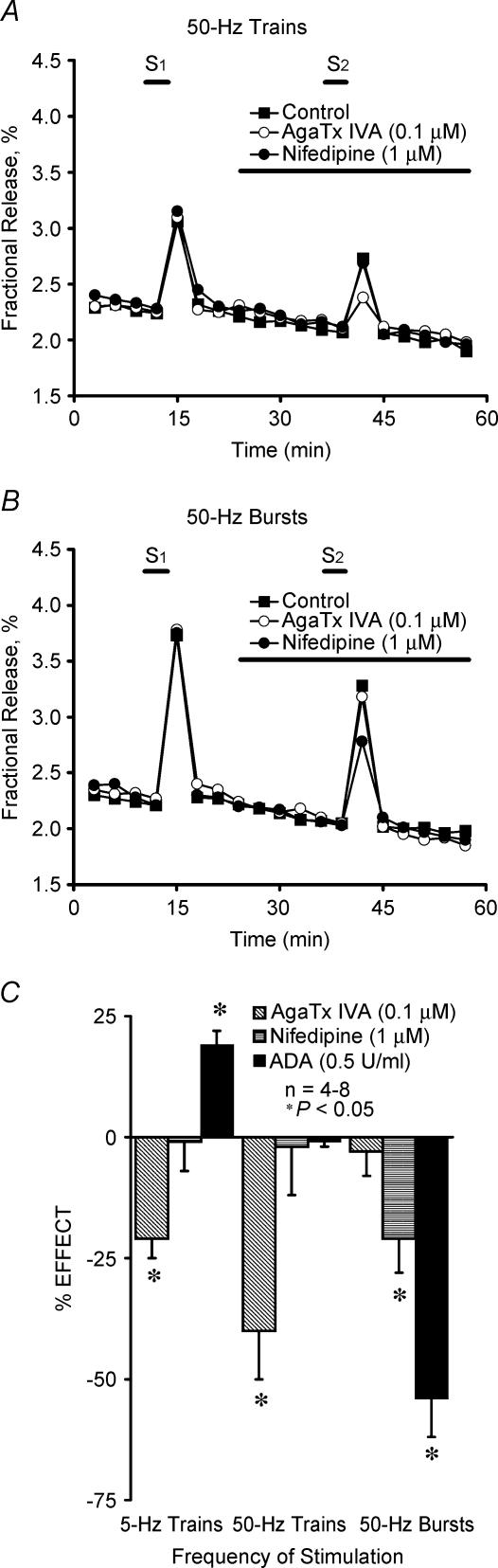
In order to investigate whether the influx of Ca2+ through different VSCCs could alternatively contribute to ACh exocytosis from electrically stimulated motor nerve terminals, we studied the effects of several type-specific blockers of VSCCs. The drugs used were: ω-agatoxin IVA (ω-AgaTx IVA), which blocks P-type channels in the nanomolar concentration range (Zhang et al. 1993); ω-conotoxin GVIA (ω-CgTx GVIA), which selectively blocks the N-type channels (Olivera et al. 1994; Dunlap et al. 1995); ω-conotoxin MVIIC (ω-CmTx MVIIC), a blocker of P-, Q- and N-type channels (Wheeler et al. 1994); and nifedipine, a blocker of L-type Ca2+ channels (Olivera et al. 1994; Dunlap et al. 1995). Due to the slow binding kinetics of the peptides, we performed experiments where the pre-incubation time was prolonged from 15 to 45 min and the results were not statistically different (P > 0.05) (see e.g. Correia-de-Sá et al. 2000b). Evoked [3H]ACh release was unaffected by ω-CgTx GVIA (1 μm) or ω-CmTx MVIIC (150 nm) (data not shown), thus indicating that neither N- nor Q-type VSCCs are involved in the release process. ω-AgaTx IVA, applied at a concentration (100 nm) that selectively blocked P-type VSCCs (Dunlap et al. 1995), inhibited evoked [3H]ACh release by 21 ± 4% (n = 4) and 40 ± 10% (n = 6) when the phrenic nerve was stimulated with 5- and 50-Hz trains, respectively (Fig. 2A and C). However, ω-AgaTx IVA (100 nm) failed to affect evoked [3H]ACh release during 50-Hz bursts (Fig. 2B and C). In contrast, nifedipine (1 μm) inhibited (21 ± 7%, n = 4) the release of [3H]ACh only when the phrenic nerve was stimulated with 50-Hz bursts, having no significant effect when 5- or 50-Hz trains were delivered (Fig. 2). These experiments demonstrate that ACh exocytosis from motor nerve terminals results mainly from the influx of Ca2+ through P-type channels during low frequency stimulation or high frequency trains, but L-type currents can be alternatively recruited to facilitate transmitter release during high frequency bursts. A putative role of ‘resistant’ R-type channels mediating transmitter release from motor nerve terminals in the presence of VSCC blockers cannot be excluded (see e.g. Lang et al. 2003).
Figure 2. Influence of the stimulation parameters on the effects of P- and L-type Ca2+ channel blockers and endogenous adenosine on evoked [3H]ACh release from motor nerve terminals.
A and B, show the time course of tritium outflow from electrically stimulated phrenic nerve terminals taken from typical experiments in the absence (Control, ▪) and in the presence of ω-Agatoxin IVA (ω-AgaTx IVA, 0.1 μm, ○) and nifedipine (1 μm, •). Tritium outflow (ordinate) is expressed as a percentage of the total radioactivity present in the tissue at the beginning of the collection period. The abscissa indicates the times at which samples were collected. [3H]ACh release was elicited twice (S1 and S2) by stimulating the phrenic nerve trunk with 750 electrical pulses delivered with frequencies of 5 Hz and 50 Hz; either one train (15 s) (A) or a series of five bursts (3 s, 150 pulses, 20-s interburst interval) (B) were delivered when 50-Hz stimulation was applied. ω-AgaTx IVA (0.1 μm), nifedipine (1 μm) and adenosine deaminase (ADA, 0.5 U ml−1) were applied 15 min before the end of S2, as represented by the horizontal bars. Note that spontaneous tritium outflow was not significantly modified in the presence of the drugs. C, the ordinates are percentage change in S2/S1 ratio in the presence of test drugs as compared with the S2/S1 ratio in control experiments using a similar stimulation protocol. Zero percent represents identity between the two ratios. Each column represents pooled data from n = 4–8 individual experiments. The vertical bars represent s.e.m. *P < 0.05 (one-way ANOVA followed by Dunnett's modified t test) when compared with controls obtained in the absence of test drugs.
Endogenous adenosine activating A2A receptors shifts neuronal Ca2+ influx from constitutive P-type channels to facilitatory L-type channels during high frequency bursts
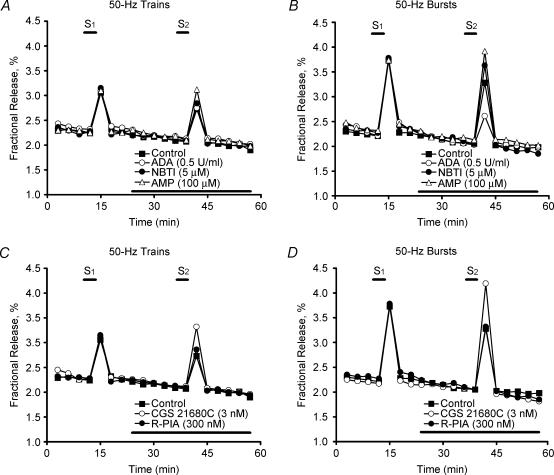
Previously, we showed that tonic A1/A2A receptor activation balance depends on the concentration of adenosine at the rat neuromuscular junction, which seems to be tightly regulated by the nerve stimulation conditions (Correia-de-Sá et al. 1996). Figure 2C shows that removal of endogenous adenosine tonus using adenosine deaminase (ADA, 0.5 U ml−1), disinhibited (facilitated, 19 ± 3%, n = 5) the release of [3H]ACh induced by 5-Hz trains, but it significantly (P < 0.05) decreased (54 ± 8%, n = 5) transmitter release during 50-Hz bursts. The adenosine uptake blocker, S-(p-nitrobenzyl)-6-thioinosine (NBTI, 5 μm) (Paterson et al. 1977), which promotes extracellular adenosine accumulation, increased evoked [3H]ACh release by 24 ± 8%(n = 5) when the bursting protocol was used (Fig. 3B). This contrasts with (1) the failure of NBTI (5 μm) to change [3H]ACh release when only one high-frequency (50-Hz) train was delivered (Fig. 3A) and with (2) the situation where NBTI (5 μm) inhibited (by 18 ± 3%, n = 5) the release of [3H]ACh induced by 5-Hz trains (Correia-de-Sá & Ribeiro, 1996). These results are in keeping with the predominance of a facilitatory A2A-receptor-mediated tonus during high frequency bursts (see Correia-de-Sá et al. 1996). Because high amounts of ATP are released by high frequency (50-Hz) trains (Magalhães-Cardoso et al. 2003), adenosine formation is blocked during the time needed for ATP and ADP levels to fall below the threshold for feedforward inhibition of ecto-5′ nucleotidase (Cunha et al. 1996; Magalhães-Cardoso et al. 2003). This might explain the absence of effects of ADA (0.5 U ml−1) and NBTI (5 μm) when [3H]ACh release was induced by brief 50-Hz trains (Fig. 3A). To investigate this hypothesis further, we performed experiments in which the adenosine precursor that is inactive on ecto-5′-nucleotidase activity, AMP (100 μm), was tested in both high-frequency stimulation protocols. When only one 50-Hz train was applied,
Figure 3. Comparison of adenosine modulation of [3H]ACh release induced by high-frequency (50 Hz) nerve stimulation delivered as continuous trains or intermittent bursts.
The time course of tritium outflow is shown from electrically stimulated phrenic nerve terminals taken from typical experiments. Tritium outflow (ordinate) is expressed as a percentage of the total radioactivity present in the tissue at the beginning of the collection period. The abscissa indicates the times at which samples were collected. [3H]ACh release was elicited twice (S1 and S2) by stimulating the phrenic nerve trunk with 750 electrical pulses delivered at a frequency of 50 Hz; either one train (15 s) (A and C) or a series of five bursts (3 s, 150 pulses, 20-s interburst interval) (B and D) were applied. Adenosine deaminase (ADA, 0.5 U ml−1), S-(p-nitrobenzyl)-6-thioinosine (NBTI, 5 μm), adenosine 5′-monophosphate (AMP, 100 μm), CGS 21680C (3 nm), and R-N6-phenylisopropyl adenosine (R-PIA, 300 nm) were added to the incubation media 15 min before S2 (horizontal bar). Note that spontaneous tritium outflow was not changed in the presence of the drugs.
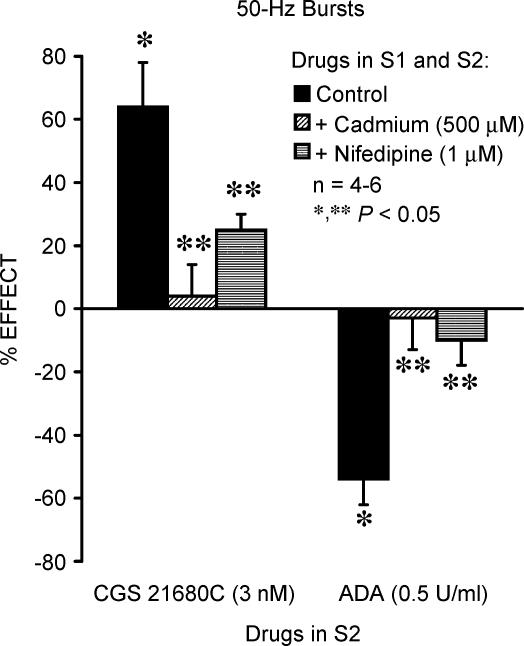
AMP (100 μm) facilitated [3H]ACh release by 26 ± 1%(n = 5) (Fig. 3A). The facilitatory effect of AMP (100 μm) was significantly (P < 0.05) enhanced (44 ± 6%, n = 5) when the release of [3H]ACh was induced by five 50-Hz bursts (Fig. 3B). The exogenously applied adenosine (100–500 μm) significantly (P < 0.05) increased the release of [3H]ACh in a concentration-dependent manner, independent of the protocol used to stimulate the phrenic nerve; for instance, adenosine (500 μm) facilitated [3H]ACh release, respectively, by 44 ± 3%(n = 4) and by 49 ± 16%(n = 5) when nerve stimulation was delivered as 50-Hz trains and 50-Hz bursts. Likewise, the selective adenosine A2A receptor agonist, CGS 21680C (3 nm), consistently facilitated (∼60%) the release of [3H]ACh induced by high-frequency stimuli (Fig. 3C and D, see also Correia-de-Sá et al. 1996). It is worth noting that R-N6-phenylisopropyl adenosine (R-PIA, 300 nm), an adenosine A1 receptor agonist, failed to modify transmitter release (Fig. 3C and D), indicating that during high-frequency nerve stimulation inhibition of [3H]ACh release operated by adenosine A1 receptors becomes less effective, as previously shown for other inhibitory presynaptic receptors (Duckles & Budai, 1990). Consistent with this hypothesis is the finding that the enzymatically stable adenosine analogue, R-PIA (300 nm), was still unable to modify [3H]ACh release evoked by high-frequency stimuli in the presence of ADA (0.5 U ml−1) (data not shown). Using 50-Hz bursts, a situation where ACh exocytosis depends mainly on Ca2+ influx through L-type channels (see above), both CdCl2 (500 μm) and nifedipine (1 μm) significantly (P < 0.05) reduced CGS 21680C-induced (3 nm) excitation (64 ± 14%, n = 4) (Fig. 4). These results suggest that facilitation of [3H]ACh release by A2A receptor activation depends on neuronal L-type Ca2+ influx. Likewise, blockade of L-type channels with CdCl2 (500 μm) or nifedipine (1 μm) abolished the inhibitory effect of ADA (0.5 U ml−1) (Fig. 4), further indicating that Ca2+ influx through L-type VSCCs is required to facilitate transmitter release by endogenous adenosine.
Figure 4. Tonic adenosine A2A-receptor-mediated facilitation of evoked [3H]ACh release from motor nerve terminals depends on Ca2+ influx through L-type channels.
[3H]ACh release was elicited twice (S1 and S2) by stimulating the phrenic nerve trunk with 50-Hz bursts (five bursts of 150 pulses each, delivered with a 20-s interburst interval). CGS 21680C (3 nm) and adenosine deaminase (ADA, 0.5 U ml−1) were applied 15 min before the end of S2; CdCl2 (500 μm) and nifedipine (1 μm) were added to the incubation solution at the beginning of the release period (time zero), i.e. they were present during the whole assay including S1 and S2. The ordinates are percentage change in S2/S1 ratio in the presence of CGS 21680C (3 nm) or ADA (0.5 U ml−1) as compared with the S2/S1 ratio in control conditions, either in the absence or in the presence of CdCl2 (500 μm) or nifedipine (1 μm). Zero percent represents identity between the two ratios. Each column represents pooled data from n = 4–6 individual experiments. The vertical bars represent s.e.m. *P < 0.05 (one-way ANOVA followed by Dunnett's modified t test) represent significant differences from the control; **P < 0.05 (one-way ANOVA followed by Dunnett's modified t test) represent significant differences when compared with the effects of CGS 21680C (3 nm) or ADA (0.5 U ml−1) alone.
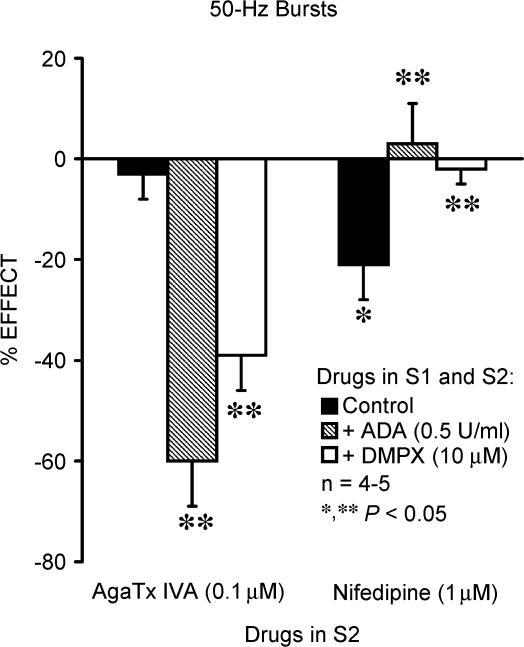
In view of the putative interactions between synaptic adenosine generation and Ca2+ influx through distinct VSCCs, we tested whether Ca2+ recruitment through P- or L-type channels could be modified during neuronal firing upon blocking tonic activation of A2A receptors with either ADA or 3,7-dipropyl-1-propargylxanthine (DMPX, an adenosine A2A receptor antagonist). As shown in Fig. 5, both ADA (0.5 U ml−1) and DMPX (10 μm) switched the pharmacology of Ca2+ channels coupled to [3H]ACh release during 50-Hz bursts; i.e. the action of nifedipine (1 μm) was completely abolished, while ω-AgaTx IVA (100 nm)-induced inhibition recovered to levels observed in conditions where adenosine had no tonic effect (e.g. 50-Hz trains). Therefore, the fine-tuning Ca2+ influx through either P- or L-type VSCCs can be modulated by synaptic accumulation of endogenous adenosine.
Figure 5. Activation of excitatory A2A receptors by endogenous adenosine switch Ca2+ influx triggering [3H]ACh release from prevalent P-type to silent L-type neuronal Ca2+ channels.
[3H]ACh release was elicited twice (S1 and S2) by stimulating the phrenic nerve trunk with 50-Hz bursts (five bursts of 150 pulses each, delivered with a 20-s interburst interval). ω-AgaTx IVA (0.1 μm) and nifedipine (1 μm) were applied 15 min before the end of S2. Adenosine deaminase (ADA, 0.5 U ml−1) and the adenosine A2 receptor antagonist, 3,7-dipropyl-1-propargylxanthine (DMPX, 10 μm), were added to the incubation solution at the beginning of the release period (time zero), i.e. they were present during the whole assay including S1 and S2. The ordinates are percentage change in S2/S1 ratio in the presence of ω-AgaTx IVA (0.1 μm) and nifedipine (1 μm) as compared with the S2/S1 ratio in control conditions, either in the absence or in the presence of ADA (0.5 U ml−1) or DMPX (10 μm). Zero percent represents identity between the two ratios. Each column represents pooled data from n = 4–5 individual experiments. The vertical bars represent s.e.m. *P < 0.05(one-way ANOVA followed by Dunnett's modified t test) represent significant differences from the control; **P < 0.05 (one-way ANOVA followed by Dunnett's modified t test) represent significant differences when compared with the effects of ω-AgaTx IVA (0.1 μm) or nifedipine (1 μm) alone.
Discussion
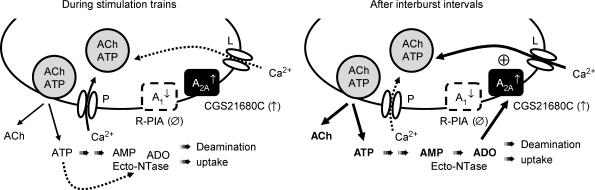
The present results support the hypothesis that VSCCs other than prevalent P-type become involved in ACh exocytosis during high frequency bursts; in particular, our results suggest that motor nerve terminals possess L-type Ca2+ channels that may be recruited to facilitate transmitter release under this condition. The fine-tuning control of Ca2+ influx through either P- or L-type channels is likely to be mediated by endogenous adenosine activating presynaptic A2A receptors. Data also indicate that tetanic depression of ACh release may be overcome during intermittent neuronal firing due to tonic activation of A2A receptors operating additional Ca2+ recruitment via L-type channels (see Fig. 6).
Figure 6. Fine-tuning control of Ca2+ recruitment through P- and L-type VSCCs by endogenous adenosine generated during motoneuronal firing.
During short stimulation trains, P-type Ca2+ channels clustered at active zones control nerve-evoked ACh release from adult mammalian motor nerve terminals. The function of P-type channels declines rapidly as consequence of a Ca2+-dependent inactivation system, which might contribute to neurotransmission tetanic failure. During intermittent high-frequency bursts, decline of transmission within a train may completely recover by the onset of the next train probably due to the recruitment of Ca2+ via quiescent high-capacity/slow-inactivating L-type channels located away from the active zones. The fine-tuning control of Ca2+ currents through P- or L-type channels is likely to be mediated by endogenous adenosine. The predominance of inhibitory A1/excitatory A2A tonus is balanced by the stimulation pattern (trains versus bursts), which also tightly regulates the amount of extracellular adenosine formed from released ATP. As ATP is released synchronously with ACh in a frequency-dependent manner, it can reach high enough levels during 50-Hz trains to be able to inhibit ecto-5′-nucleotidase (ecto-NTase), the rate-limiting enzyme responsible for adenosine formation from adenine nucleotides. Interburst intervals favour recovery from enzymatic inhibition, leading to a burst-like formation of adenosine and channelling to the activation of facilitatory A2A receptors. In parallel, there is a co-ordinated shift in Ca2+ channel dynamics operating ACh exocytosis, from the prevalent P-type to the ‘facilitatory’ L-type channel, in a way completely reversed by blocking A2A receptor activation. This mechanism represents a novel form of synaptic plasticity mediated by adenosine at the rat motor endplate and may function to overcome tetanic depression during intermittent neuronal firing. The transducing pathway mediating the crosstalk between adenosine and VSCCs at the motor endplate remains to be elucidated.
Neurotransmission failure may result from three processes: failure of action potential conduction along the axon; diminished release of ACh from the presynaptic terminal; and reduced excitability of the endplate due to ACh receptor desensitization. Neuromuscular junction endplate potentials decrease greatly and quickly during continuous 5- to 100-Hz stimulation without a significant change in the size of miniature endplate potentials (MEPPs) (Moyer & Van Lunteren, 1999). Our results measuring [3H]ACh release fully agree with the interpretation that rundown of endplate potentials during high frequency nerve stimulation is due to rapid changes in the magnitude of neurotransmitter release rather than to axonal block or postsynaptic receptor desensitization. Previous estimates of the susceptibility of neurotransmission failure are based almost entirely on protocols in which motor nerves are stimulated continuously without interruption (e.g. Wilson, 1979; Hong & Chang, 1991; Fournier et al. 1991; Bazzy, 1994). For some muscles, especially those used for postural tasks, this is a physiologically relevant activation pattern. However, many muscles (in particular thoracic muscles used for breathing) are activated intermittently, with a duty cycle as short as ∼0.25–0.35 (see Kong & Berger, 1986). This pattern of activation potentially allows decrements in neurotransmission during repetitive stimulation to recover, thereby substantially preserving synaptic transmission during intermittent compared with continuous stimulation. Recovery from tetanic depression of [3H]ACh release in the course of 50-Hz bursts is in keeping with previous findings showing a complete mitigation of endplate potential drop-out during intermittent stimulation (Moyer & Van Lunteren, 1999). Although the interburst interval (20 s) used for the intermittent 50-Hz stimulation was longer than would normally occur during rhythmic activities, data suggest that recovery between trains can be sufficient to substantially improve neuromuscular junction function.
The extent to which modulation of spontaneous (asynchronous) quantal exocytosis influences neuromuscular transmission is unknown. Continuous nerve stimulation (20–50 Hz) causes slow transient increases in MEPP frequency (MEPP-hump) and intracellular free Ca2+ in motor nerve terminals (Narita et al. 1998). Such a mechanism might not contribute to facilitation of [3H]ACh release during 50-Hz bursts, because short interruptions of tetanus (5–9 s) during MEPP-hump quickly reduced MEPP frequency to the basal level (Narita et al. 1998). Furthermore, these authors also demonstrated that resuming tetanus swiftly raised MEPP frequency to the previous or a higher level in a manner inversely dependent on pause duration (< 1 min). This situation was opposite to our findings showing that enhanced amounts of [3H]ACh are released upon increasing the interburst interval (0–20 s), which may be required to generate enough endogenous adenosine capable of activating facilitatory A2A receptors (Correia-de-Sá et al. 1996). Although it has been shown that exogenous adenosine (via A1 receptors) may reduce spontaneous ACh release by modulating Ca2+ influx via L-type VSCCs in mouse motoneurones (De Lorenzo et al. 2004), our findings showed that adenosine A1 receptor activation with R-PIA (300 nm) was without effect on [3H]ACh release induced by high-frequency nerve stimulation. Failure of R-PIA (300 nm) to inhibit tetanus-induced [3H]ACh release may not be due to occupation of A1 receptors by endogenously generated adenosine, because (1) the Kd of R-PIA for A1 receptors is several orders of magnitude lower (low nanomolar range) than that of adenosine (high micromolar range) (e.g. Jacobson et al. 1992), and (2) the effect of R-PIA (300 nm) was not significantly (P > 0.05) modified in the presence of ADA (0.5 U ml−1) (see also Correia-de-Sá et al. 1996). Moreover, both adenosine (500 μm) and the selective A2A receptor agonist, CGS 21680C (3 nm), consistently facilitated evoked [3H]ACh release independently of the protocol of stimulation, without significantly changing the resting tritium outflow.
Ca2+ entry through presynaptic VSCCs links membrane depolarization and exocytosis of synaptic vesicles in the nerve terminal. The amount of transmitter released is steeply dependent on presynaptic Ca2+ concentrations (Dodge & Rahamimoff, 1967; Mintz et al. 1995) such that increases or decreases in Ca2+ influx can powerfully alter neurotransmission. Normally, P/Q-type Ca2+ channels control nerve-stimulated ACh release from adult mammalian motor nerve terminals (e.g. Atchison, 1989; Protti & Uchitel, 1993; Wessler et al. 1995; Correia-de-Sá et al. 2000a). In the present study, we showed that nifedipine had no effect on [3H]ACh release induced by 5- and 50-Hz trains, but it caused a significant inhibitory action during intermittent high frequency stimulation bursts, a situation where blockade of the P-type VSCC with ω-AgaTx IVA was ineffective. Transmitter release was not modified either by the N-type channel blocker, ω-CgTx GVIA (1 μm) (Olivera et al. 1994; Dunlap et al. 1995), or by ω-CmTx MVIIC (150 nm), which blocks P-, Q- and N-type channels (Wheeler et al. 1994). These findings are consistent with the observation that induction of quiescent L-type Ca2+ currents may represent an adaptive response to a function loss of prevalent P-type Ca2+ channels during periods of high intensity long-lasting nerve stimulation (Correia-de-Sá et al. 2000a; see also Urbano & Uchitel, 1999). Inactivation of P-type channels may be enhanced by additional Ca2+ influx via nearby located VSCCs (for review see e.g. Tareilus & Breer, 1995), whereas Ca2+ recruitment through L-type channels requires strong depolarizations to become functionally apparent (Miller, 1987). This might happen because L-type channels are distributed away from the active zones (Tsien et al. 1988; Robitaille et al. 1990), are more resistant to inactivation in neurones, and possess a high conductance capacity (∼25 pS) able to saturate intraterminal buffers which limit Ca2+ diffusion to the release apparatus (for review see e.g. Tareilus & Breer, 1995).
Adenosine present at the mammalian neuromuscular junction results mainly from the hydrolysis of ATP released synchronously with ACh (Smith, 1991; Magalhães-Cardoso et al. 2003). The activity of the ecto-nucleotidase pathway appears critical to define the pattern of formation of ATP-derived adenosine to allow the activation of either inhibitory A1 or facilitatory A2A receptors. As ATP is released from motor nerve terminals in a frequency-dependent manner (Silinsky, 1975; Cunha & Sebastião, 1993; Magalhães-Cardoso et al. 2003), it can reach levels high enough to inhibit ecto-5′-nucleotidase, the enzyme that forms adenosine from released adenine nucleotides (discussed by Cunha, 2001). This prediction was confirmed as the facilitatory effect of AMP (100 μm), the adenosine precursor that does not inhibit the enzyme, was almost doubled when 50-Hz bursts were used (see Fig. 3A and B), reaching a magnitude comparable to that observed with adenosine (500 μm) and CGS 21680C (3 nm) under similar stimulation conditions. During intermittent high-frequency stimulation, there is a delayed burst-like formation of adenosine leading to high synaptic concentrations of the nucleoside similar to those required to activate facilitatory A2A receptors (Correia-de-Sá et al. 1996; Cunha et al. 1996). This might explain the absence of adenosine tonus when [3H]ACh release was induced by brief 50-Hz trains (see also Malinowski et al. 1997). To bypass feedforward inhibition of ecto-5′-nucleotidase, we used high frequency (50 Hz) intermittent (five bursts, 150 pulses, 20 s interburst interval) nerve stimulations (cf. Correia-de-Sá et al. 1996). Under these conditions, inactivation of endogenous adenosine with ADA (0.5 U ml−1) significantly reduced (∼50%), whereas inhibition of adenosine uptake with NBTI (5 μm) facilitated the evoked [3H]ACh release, indicating that adenosine exerts a predominant facilitatory tonus. Therefore, the predominance of A1versus A2A tonus is balanced by the stimulation pattern. Thus, if the release of ATP is small, A1 inhibition prevails, whereas when ATP release is high, adenosine formed in a burst-like manner preferentially activates A2A receptors. In addition, the tonic facilitatory action emerging with increased stimulation frequency may also depend on the attenuation of A1-mediated inhibition of evoked [3H]ACh release (see above). Taken together these results indicate that during tetanic stimulation both time-dependent endogenous adenosine formation from released adenine nucleotides and attenuation of the inhibitory action mediated by A1 receptors contribute to the strong adenosine facilitatory tonus operated by A2A receptors on evoked [3H]ACh release (see Fig. 6).
Additionally, our data showed a co-ordinated shift on Ca2+ channel subtypes operating ACh exocytosis from stimulated motor nerve terminals. The P-type channels are operative when the amount of endogenous adenosine is small and, hence, the nucleoside activates preferentially inhibitory A1 receptors. During high frequency bursts, activation of A2A receptors by high extracellular adenosine facilitates Ca2+ recruitment through quiescent L-type channels and a correspondent decrease in the proportion of P-type currents. Blocking the activation of A2A receptors with ADA or DMPX could relieve down-regulation of P-type channel function. Moreover, facilitation of [3H]ACh release operated by A2A receptors seems to be highly dependent on L-type Ca2+ currents, as nifedipine largely, but not completely, eliminated the excitatory effect of CGS 21680C. This might be partially explained because activation of adenosine A2A receptors may alternatively mobilize Ca2+ from thapsigargin-sensitive endoplasmic stores in the presence of nifedipine (Correia-de-Sá et al. 2000b). It is well established that the activity of presynaptic Ca2+ channels is modulated by a membrane-delimiting pathway involving G proteins, as well as by activation of cytoplasmic messenger molecules. For instance, high levels of intracellular cyclic AMP enhance the open probability, reduce the rundown, and recruit newly functioning L-type Ca2+ channels (reviewed by Carbone et al. 2001). Likewise, protein kinase C phosphorylation increases quantal ACh release by opening quiescent L-type Ca2+ channels in frog motor nerves at resting potential, without needing for terminal depolarization (Arenson & Evans, 2001). In contrast, N-type and P/Q-type Ca2+ channels are inhibited by modulators acting through G protein-coupled receptors through a direct involvement of Gβγ binding to pore-forming subunits (Sandoz et al. 2004; for review see e.g. Jarvis & Zamponi, 2001). This supports the hypothesis that, besides the negative shift in P-type channel function, adenosine A2A receptor stimulation of the adenylate cyclase/cyclic AMP transducing system (Correia-de-Sá & Ribeiro, 1994) would activate L-type Ca2+ currents via protein kinase A-mediated phosphorylation (see e.g. Miller, 1987; Artalejo et al. 1992). So, much Ca2+ entering through this pathway will be sensed by the release apparatus facilitating evoked [3H]ACh release and, thereby, contribute to overcome neuronal tetanic depression. Thus, endogenous formation of adenosine during neuronal firing appears to operate a novel form of synaptic plasticity at the rat motor endplate involving Ca2+ recruitment through different Ca2+ channel subtypes.
The physiological significance of the expression of multiple VSCC subtypes in nerve terminals is still not completely clear. Ca2+ channel subtypes differ from each other in their distinct biophysical properties, differential modulation by mediators released during cell activity and variable coupling to intracellular processes (for review see Miller, 1987). The existence of different Ca2+ channel subtypes at motor nerve terminals would allow a more flexible and versatile regulation of ACh release, especially under non-physiological conditions. Different VSCC subtypes may play important and selective roles during development and regeneration of neuromuscular junctions (Gray et al. 1992; Fu & Huang, 1994; Santaféet al. 2001) and in pathological conditions when transmitter release is compromised. For instance, LEMS is an autoimmune disorder associated with the loss of function of motoneuronal P-type VSCCs due to high titres of anti-P/Q-type antibodies, which is characterized by a decreased release of vesicles in response to single nerve impulses (quantal content), facilitation following high frequency stimulation and normal postsynaptic responses (Lambert & Elmqvist, 1971). Following treatment with LEMS sera, down-regulation of P/Q-type channels is accompanied by a concomitant rise or unmasking of dihydropyridine-sensitive L-type, as well as in ‘resistant’ R-type, Ca2+ currents (for review see Lang et al. 2003). Similarly, compensatory increases in the proportion of facilitatory L-type Ca2+ currents become apparent following amyotrophic lateral sclerosis (ALS) IgG-treatment of murine neuromuscular junctions (Fratantoni et al. 2000). As under normal conditions the Ca2+ influx through P/Q-type VSCCs makes up > 95% of the current evoked at the mammalian neuromuscular junction, this dynamic regulation may explain why these patients are not more severely affected than in fact they are. Our results show for the first time that, under physiological conditions, endogenous adenosine generated during intermittent neuronal firing may facilitate extracellular Ca2+ recruitment through silent L-type channels. Specifically, the tonic A2A-receptor-mediated switch of Ca2+ influx into motor nerve terminals, from a predominant fast desensitizing P-type component to a high-capacity/slow-inactivating L-type current, allowed for a near complete recovery of neurotransmitter release from the decline that had occurred during a previous train. On the basis of these findings, manipulation of adenosine A2A receptor activation might be of clinical interest to recruit spared VSCCs in order to preserve neuromuscular transmission in compromised (low safety factor) motor endplates, particularly those involved in respiratory activity.
Acknowledgments
This research was partially supported by Fundacão para a Ciencia e a Tecnologia (FCT) projects (POCTI/FCB/36545/2000, POCTI/CVT/43368/2001 and UMIB-215/94). We also thank Mrs M. Helena Costa e Silva, Suzete Liça and Belmira Silva for their technical assistance.
References
- Arenson MS, Evans SC. Activation of protein kinase C increases acetylcholine release from frog motor nerves by a direct action ob L-type Ca2+ channels and apparaently not by depolarisation of the terminal. Neuroscience. 2001;104:1157–1164. doi: 10.1016/s0306-4522(01)00114-2. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Rossie S, Perlman RL, Fox AP. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992;358:63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Dihydropyridine-sensitive and -insensitive components of acetylcholine release from rat motor nerve terminals. J Pharmacol Exp Ther. 1989;251:672–678. [PubMed] [Google Scholar]
- Bazzy AR. Developmental changes in rat diaphragm endplate response to repetitive stimulation. Dev Brain Res. 1994;81:314–317. doi: 10.1016/0165-3806(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Carbone E, Carabelli V, Cesetti T, Baldelli P, Hernández-Guijo JM, Giusta L. G-Protein- and cAMP-dependent L-channel gating modulation: a manifold system to control calcium entry in neurosecretory cells. Pflugers Arch. 2001;442:801–813. doi: 10.1007/s004240100607. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Ribeiro JA. Evidence that the presynaptic A2A-adenosine receptor of the rat motor nerve endings is positively coupled to adenylate cyclase. Naunyn-Schmiedeberg's Arch Pharmacol. 1994;350:514–522. doi: 10.1007/BF00173021. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Ribeiro JA. Adenosine uptake and deamination regulate tonic A2A- receptor facilitation of evoked [3H]acetylcholine release from the rat motor nerve terminals. Neuroscience. 1996;73:85–92. doi: 10.1016/0306-4522(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Sebastião AM, Ribeiro JA. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve endings of the rat. Br J Pharmacol. 1991;103:1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-de-Sá P, Timóteo MA, Ribeiro JA. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J Neurophysiol. 1996;76:3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Timóteo MA, Ribeiro JA. Influence of stimulation on Ca2+ recruitment triggering [3H]acetylcholine release from the rat motor-nerve endings. Eur J Pharmacol. 2000a;406:355–363. doi: 10.1016/s0014-2999(00)00686-5. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P, Timóteo MA, Ribeiro JA. A2A adenosine receptor facilitation of neuromuscular transmission: influence of stimulus paradigm on calcium mobilization. J Neurochem. 2000b;74:2462–2469. doi: 10.1046/j.1471-4159.2000.0742462.x. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Correia-de-Sá P, Sebastião AM, Ribeiro JA. Preferential activation of excitatory adenosine receptors at the rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br J Pharmacol. 1996;119:253–260. doi: 10.1111/j.1476-5381.1996.tb15979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Sebastião AM. Adenosine and adenine nucleotides are independently released from both the nerve terminals and the muscle fibres upon electrical stimulation of the innervated skeletal muscle of the frog. Pflugers Arch. 1993;424:503–510. doi: 10.1007/BF00374914. [DOI] [PubMed] [Google Scholar]
- De Lorenzo S, Veggetti M, Muchnik S, Losavio A. Presynaptic inhibition of spontaneous acetylcholine release induced by adenosine at the mouse neuromuscular junction. Br J Pharmacol. 2004;142:113–124. doi: 10.1038/sj.bjp.0705656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles SP, Budai D. Stimulation intensity as critical determinant of presynaptic receptor effectiveness. Trends Pharmacol Sci. 1990;11:440–443. doi: 10.1016/0165-6147(90)90124-q. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central nervous system. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett. 1991;125:34–36. doi: 10.1016/0304-3940(91)90124-c. [DOI] [PubMed] [Google Scholar]
- Fratantoni SA, Weisz G, Pardal AM, Reisin RC, Uchitel OD. Amyotrophic lateral sclerosis IgG-treated neuromuscular junctions develop sensitivity to L-type calcium channel blocker. Muscle Nerve. 2000;23:543–550. doi: 10.1002/(sici)1097-4598(200004)23:4<543::aid-mus13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fu WM, Huang FL. L-type Ca2+ channel is involved in the regulation of spontaneous transmitter release at developing neuromuscular synapses. Neuroscience. 1994;58:131–140. doi: 10.1016/0306-4522(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Garcia KD, Beam KG. Reduction of Ca2+ currents by Lambert–Eaton syndrome sera: motoneurons are preferentially affected, and L-type currents are spared. J Neurosci. 1996;16:4903–4913. doi: 10.1523/JNEUROSCI.16-16-04903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DB, Bruses JL, Pilar GR. Developmental switch in the pharmacology of Ca2+ channels coupled to acetylcholine release. Neuron. 1992;8:715–724. doi: 10.1016/0896-6273(92)90092-r. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Chang CC. Run-down of neuromuscular transmission during repetitive nerve activity by nicotinic antagonists is not due to desensitization of the postsynaptic receptor. Br J Pharmacol. 1991;102:817–822. doi: 10.1111/j.1476-5381.1991.tb12258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, van Galen PJM, Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem. 1992;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Interactions between presynaptic Ca2+ channels, cytoplasmic messengers and proteins of the synaptic vesicle release complex. Trends Pharmacol Sci. 2001;22:519–525. doi: 10.1016/s0165-6147(00)01800-9. [DOI] [PubMed] [Google Scholar]
- Katz E, Ferro PA, Weisz G, Uchitel OD. Ca2+ channels involved in synaptic transmission at the mature and regenerating mouse neuromuscular junction. J Physiol. 1996;497:687–697. doi: 10.1113/jphysiol.1996.sp021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl hysiol. 1986;61:1999–2004. doi: 10.1152/jappl.1986.61.6.1999. [DOI] [PubMed] [Google Scholar]
- Lambert EH, Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci. 1971;183:183–199. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- Lang B, Pinto A, Giovannini F, Newsom-Davis J, Vincent A. pathogenic autoantibodies in the Lambert–Eaton myasthenic syndrome. In: Agius MA, Richman DP, Fairclough RT, Maselli RA, editors. Myasthenia Gravis & Related Disorders- Biochemical Basis for Disease of the Neuromuscular Junction. New York: The New York Academy of Sciences; 2003. pp. 187–195. [DOI] [PubMed] [Google Scholar]
- Magalhães-Cardoso MT, Pereira MF, Oliveira L, Ribeiro JA, Cunha RA, Correia-de-Sá P. Ecto-AMP deaminase blunts the ATP-derived adenosine A2A receptor facilitation of acetylcholine release at rat motor nerve terminals. J Physiol. 2003;549:399–408. doi: 10.1113/jphysiol.2003.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski MN, Cannady SB, Schmit KV, Barr PM, Schrock JW, Wilson DF. Adenosine depresses transmitter release but is not the basis for ‘tetanic fade’ at the neuromuscular junction of the rat. Neurosci Lett. 1997;230:81–84. doi: 10.1016/s0304-3940(97)00480-1. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Multiple calcium channels and neuronal function. Science. 1987;235:46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Moyer M, Van Lunteren E. Effect of phasic activation on endplate potential in rat diaphragm. J Neurophysiol. 1999;82:3030–3040. doi: 10.1152/jn.1999.82.6.3030. [DOI] [PubMed] [Google Scholar]
- Narita K, Akita T, Osanai M, Shirasaki T, Kijima H, Kuba K. A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals. J Gen Physiol. 1998;112:593–609. doi: 10.1085/jgp.112.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: the ω-conotoxins and ω-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Paterson AR, Babb LR, Paran JH, Cass CE. Inhibition by nitrobenzylthionosine of adenosine uptake by asynchrous HeLa cells. Mol Pharmacol. 1997;13:1147–1158. [PubMed] [Google Scholar]
- Protti DA, Uchitel OD. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin ω-Aga-IVA. Neuroreport. 1993;5:333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular junctions. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Sandoz G, Lopez-Gonzalez I, Grunwald D, Bichet D, Altafaj X, Weiss N, Ronjat M, Dupuis A, De Waard M. Cavβ-subunit displacement is a key step to induce the reluctant state of P/Q calcium channels by direct G protein regulation. Proc Natl Acad Sci U S A. 2004;101:6267–6272. doi: 10.1073/pnas.0306804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santafé MM, Garcia N, Lanuza MA, Uchitel OD, Tomás J. Calcium channels coupled to neurotransmitter release at dually innervated neuromuscular junctions in the newborn rat. Neuroscience. 2001;102:697–708. doi: 10.1016/s0306-4522(00)00507-8. [DOI] [PubMed] [Google Scholar]
- Santafé MM, Urbano FJ, Lanuza MA, Uchitel OD. Multiple types of calcium channels mediate transmitter release during functional recovery of botulinum toxin type A-poisoned mouse motor nerve terminals. Neuroscience. 2000;95:227–234. doi: 10.1016/s0306-4522(99)00382-6. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Fine-tuning neuromodulation by adenosine. Trends Pharmacol Sci. 2000;21:341–346. doi: 10.1016/s0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975;247:145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DO. Sources of adenosine released during neuromuscular transmission in the rat. J Physiol. 1991;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Ko CP. Novel modulatory effect of L-type calcium channels at newly formed neuromuscular junctions. J Neurosci. 1997;17:1101–1111. doi: 10.1523/JNEUROSCI.17-03-01101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tareilus E, Breer H. Presynaptic calcium channels: pharmacology and regulation. Neurochem Int. 1995;26:539–558. doi: 10.1016/0197-0186(94)00149-o. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Uchitel OD. L-type calcium channels unmasked by cell-permeant Ca2+ buffer at mouse motor nerve terminals. Pflugers Arch. 1999;437:523–528. doi: 10.1007/s004240050813. [DOI] [PubMed] [Google Scholar]
- Wessler I, Dooley DJ, Lohr B. P-type Ca2+ channels trigger stimulus-evoked [3H]-acetylcholine release from mammalian motor endplates. Eur J Pharmacol. 1995;278:83–86. doi: 10.1016/0014-2999(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kilbinger H. Release of [3H]-acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn-Schmiedeberg's Arch Pharmacol. 1986;334:357–364. doi: 10.1007/BF00569370. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Tsien RW, Randall A. Identification of calcium channels that control neurosecretion. Science. 1994;266:830–831. [Google Scholar]
- Wilson DF. Depression, facilitation, and mobilizations of transmitter at the rat diaphragm neuromuscular junction. Am J Physiol. 1979;237:C31–C37. doi: 10.1152/ajpcell.1979.237.1.C31. [DOI] [PubMed] [Google Scholar]
- Zhang J-F, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwartz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]