Abstract
The common co-existence of fibromyalgia and chronic abdominal pain could be due to sensitization of spinal neurones (SNs), as a result of viscero-somatic convergence. The objective of this study is to explore the influence of acute nociceptive somatic stimulation in the form of acid injections, into the ipsilateral somatic receptive field of neurones responsive to colorectal distension (CRD), and the potential role of ionotropic glutamate receptors on sensitization. Action potentials of CRD-sensitive SNs were recorded extracellularly from the lumbar (L2–L5) spinal cord. Stimulus–response functions (SRFs) to graded CRD (10–80 mmHg, 30 s) were constructed before and 30 min after ipsilateral injection of low pH (4.0, 100 μl) saline into the somatic receptive fields. In some experiments, cervical (C1–C2) spinalization was performed to eliminate supraspinal influence. The selective NMDA receptor antagonist CGS 19755 and AMPA receptor antagonist NBQX were injected (25 μmol kg−1, i.v.) to examine their influence on sensitization. Three types of neurones were characterized as short-latency abrupt (SLA, n = 24), short latency sustained (SLS, n = 12), and long-latency (LL, n = 6) to CRD. Ipsilateral injection of low pH (4.0) in the somatic receptive field, but not the contralateral gastrocnemius (GN) or front leg muscles, sensitized responses of these neurones to CRD. Spinalization had no influence on the development of low pH-induced sensitization. Both CGS 19755 and NBQX significantly attenuated the sensitized response to CRD in intact and spinalized animals. Acute nociceptive somatic stimulus sensitizes CRD-sensitive SNs receiving viscero-somatic convergence. The sensitization occurs at the spinal level and is independent of supraspinal influence. Ionotropic glutamate receptors in the spinal cord are involved in sensitization.
Although significant advances have been made in understanding the neurophysiological basis of visceral sensation, visceral afferent processing in the spinal cord and the role of central influences remains poorly understood. The majority of spinal neurones (SNs) receiving synaptic input from visceral organs receive convergent input from somatic structures (Cervero & Connel, 1984; Cervero & Tattersall, 1987; Ness & Gebhart, 1991). It is known that noxious visceral stimulation induces expansion of the somatic convergent receptive field and sensitization of responses to mechanical stimuli (Cervero et al. 1992; Euchner-Wamser et al. 1993).
Fibromyalgia is a chronic, soft tissue disorder characterized by diffuse musculoskeletal pain with specific tender points. The common co-existence of fibromyalgia and chronic abdominal pain and/or irritable bowel syndrome (IBS) has been well documented (Yunus, 1981; Veale et al. 1991; Triadafilopoulos et al. 1991; Thompson et al. 1999). Although the underlying mechanism for this common co-existence is probably multifactorial, altered somatic afferent activity in patients with fibromyalgia could influence visceral sensation at the spinal level.
It has been shown that chronic musculoskeletal pain in the rat can be induced by two unilateral, low pH injections in the gastrocnemius (GN) muscle, which produce a nociceptive stimulation resulting in bilateral mechanical hyperalgesia (Sluka et al. 2001). Furthermore, we have recently reported a model of visceral hyperalgesia in the conscious rat which results from low pH injections in the GN muscle (Miranda et al. 2004). We suggest that somatic pain-induced visceral hyperalgesia is a phenomenon of spinal viscero-somatic convergence, since nociceptive somatic stimulus to distant somatic structures (e.g. front leg injection) did not result in visceral hyperalgesia (Pace et al. 2003).
The excitatory amino acid glutamate probably plays a major role in sensitization of SNs receiving synaptic input from the viscera (Kolhekar & Gebhart, 1996; Coutinho et al. 1998). Glutamate is an endogenous ligand for ionotropic (NMDA and AMPA/kainate) and metabotropic (mGlur I–III) glutamate receptors, which mediate excitatory synaptic transmission between primary afferent nociceptors and spinal dorsal horn neurones (Schneider & Perl, 1985, 1988; Yoshimura & Jessell, 1990) to develop and maintain the secondary mechanical hyperalgesia to tissue injury (Yoshimura & Jessell, 1990; Skyba et al. 2002). In our recent behavioural model of visceral hyperalgesia, we have shown that pre-emptive spinal administrations of NMDA and non-NMDA (AMPA/kainate) antagonists prevent the development of both somatic and visceral hyperalgesia induced by low pH injections in the GN muscle (Miranda et al. 2004). However, the effect of the intramuscular low pH injections on colorectal distention (CRD)-sensitive SN having viscero-somatic convergence has not been explored.
Sensitization of CRD-sensitive SNs due to nociceptive somatic stimuli may provide insight into the pathophysiology of visceral hyperalgesia often encountered in patients with co-existing fibromyalgia. The present study has three goals: (1) to characterize the behaviour of CRD-sensitive SNs after nociceptive somatic stimulation; (2) to evaluate supraspinal influences in the sensitization of SN; and (3) to study the effects of NMDA and AMPA receptor antagonists on sensitized SNs.
Methods
General surgery
Experiments were performed on 46 male Sprague–Dawley rats (Harlan Indianapolis, IN, USA) weighing 400–500 g. Rats were deprived of food, but not water, 16–18 h before the experiment for complete evacuation of fecal material from the descending colon. All rats were anaesthetized with pentobarbital sodium (50 mg kg−1 i.p.) and maintained with constant intravenous infusion of pentobarbital (5–10 mg kg−1 h−1). The right femoral vein was cannulated for constant infusion of anaesthetic and the left carotid artery was cannulated for monitoring the blood pressure. The trachea was intubated for ventilation. The rats were paralysed with an initial dose of gallamine triethiodide (10 mg kg−1 i.v., Flaxedil) and mechanically ventilated with room air (∼60 strokes min−1). Paralysis was maintained throughout the experiment with supplemental doses of gallamine triethiodide (5 mg kg−1 h−1). The level of anaesthesia following paralysis was maintained by observing the blood pressure and heart rate fluctuations. When a change in blood pressure and heart rate was observed to a painful stimulation (pinch to the plantar surface of the hind leg) an additional dose of pentobarbital sodium (10 mg kg−1 i.v.) was injected. The body temperature was kept within physiological range (36–37°C) with an overhead lamp. After completion of the experiments, rats were killed by a lethal injection of Beuthanasia-D (390 mg pentobarbital, 50 mg phenytoin sodium, 2% benzyl alcohol; Schering-Plough Animal Health) at a dose of 2 ml kg−1. All experimental protocols were approved by the Medical College of Wisconsin Animal Use and Care Committee.
Spinal preparation
After the initial surgery, rats were placed in a stereotaxic head holder. The lumbar (L2–L5) spinal cord was exposed by laminectomy. The dura membrane was carefully removed and the exposed spinal cord was then covered with a 1–2 cm long saline-soaked gelatin sponge (Gelfoam, PharmaciaUpjohn Company, MI, USA). The vertebral column was rigidly clamped by using spinal clamps. The skin was reflected laterally to make a pool for an agar solution (1.7% in saline), which was allowed to harden. A block of agar was cut to expose the spinal cord, and the gelatin sponge was removed. The dorsal surface of the exposed spinal cord was then covered with warm mineral oil (37°C).
Visceral stimulation
CRD was employed as the visceral stimulus. A 6–7 cm long, 2–3 cm diameter, flaccid latex balloon (made from a condom) was inserted into the descending colon. The balloon was secured in place by taping the balloon catheter to the base of the tail. The balloon catheter was connected to a barostatic distension device for isobaric distension.
Somatic mapping
Somatic mapping was performed by light touch, brushing and retraction of the skin. In addition, strong pinch of the GN muscle, thigh, rump area, perianal region and testicles was performed with a pair of non-serrated forceps. The receptive fields in the knee and ankle joint, if any, were identified by extension–flexion of the joint. The tail was rotated clockwise and counter-clockwise to test for propiospinal neurones (Ness & Gebhart, 1987a). The neurones that responded to CRD and tail rotation were excluded from the study.
Nerve recording
Stainless steel microelectrodes (3–4 MΩ, FHC, Bowdoinham, ME, USA) were used for extracellular single-unit recording from the lumbar spinal cord (L2–L5). The placement of the electrode was 0–0.3 mm lateral to midline and 0.5–1.5 mm ventral from the dorsal surface. The nerve action potentials were continuously monitored and displayed on an oscilloscope after amplification through an AC differential amplifier (model 3000; A-M Systems). A dual window discriminator (model DDIS-1; BAK Electronics, Mount Airy, MD, USA) was used to discriminate the action potentials and to convert them to rectangular TTL pulses. The frequency of TTL pulses was counted online by using the Spike2/CED 1401 data acquisition system (Cambridge Electronic Design, UK). The nerve action potentials, intracolonic pressure and blood pressure were recorded online. Following the completion of the experiment, recordings were run through the Wave Mark analysis using the Spike 4 software (Cambridge Electronic Design). The Wave Mark builds templates of all action potentials in the recording and discriminates them by the shape of the action potential. Wave Mark analysis ensured that the same neurone was captured by the window discriminator throughout the experiment. Data were also recorded on a digital audiotape (DAT) for off-line analysis.
Spinalization
In a set of experiments (n = 7), responses of SNs to CRD were recorded before and after cervical (C1–C2) spinal transection. A laminectomy was performed to expose the cervical (C1–C2) spinal cord. The dura membrane was gently removed and 10–15 μl of 4% xylocaine was applied to the surface of the exposed spinal cord. After 10 min, the spinal cord was completely transected by using a scalpel blade. The transected area was covered with warm mineral oil. Animals exhibited a vasodepressor response immediately after spinal transection, but recovered in 15–30 min. Responses of the neurones to CRD were recorded only after complete recovery of the blood pressure. The spinalization was verified post mortem.
Experimental protocol
CRD (60 mmHg, 10–15 s) was used as a search stimulus to identify a CRD-sensitive SN. The neurones that responded positively to CRD, but not to tail rotation, were included in the study. The somatic receptive fields of all CRD-sensitive neurones were mapped by applying brush, pinch of the muscle and skin retraction. Only those that exhibited a response to somatic stimulation in the GN, thigh or rump area were included in the study for the convenience of low pH injection. Responses of these neurones were then tested to graded CRD (10, 20, 30, 40, 60 and 80 mmHg, 30 s duration and 3 min interstimulus time interval) to construct a stimulus–response function (SRF). In a group of five rats, SRF was repeated three times to test whether repeated distensions produce progressive sensitization of the neurone.
Following the SRF to graded CRD, rats received an injection of 0.1 ml of pH 4.0 saline (adjusted using 0.1 n HCl) to the ipsilateral somatic receptive field. The injection was given intramuscularly to the main body of the GN, thigh or rump muscles. A second SRF was constructed 30 min after low pH (4.0) injection. In addition, responses to somatic stimuli (brush, pinch and skin retraction) were tested after low pH injection. To investigate the convergence-specific sensitization, two groups of rats received either a low pH saline injection to the contralateral GN muscle (n = 3) or the ipsilateral antebrachial region of the front leg (n = 3). SRFs to graded CRD were constructed before and after low pH injections in these two areas.
Pharmacology
To test the involvement of glutamate receptors (NMDA and non-NMDA) in the sensitization of CRD-sensitive SNs after low pH injection, a selective NMDA receptor antagonist CGS 19755 (25 μmol kg−1 i.v.) or non-NMDA (AMPA/kainate) receptor antagonist NBQX (25 μmol kg−1 i.v.) was systemically administered. A SRF to graded CRD was constructed before and after low pH injection. A third SRF was constructed 10–15 min following the application of the glutamate receptor antagonist.
Drugs
CGS 19755 and NBQX (Tocris Cookson Inc. Ellisville, MO, USA) were dissolved freshly in saline on the day of the experiment.
Data analysis and statistics
The neuronal response to CRD was calculated by subtracting the mean firing frequency of the ongoing spontaneous activity (30 s) prior to distension, from the mean firing frequency during distension (30 s). Responses were normalized as a percentage of maximum baseline response (80 mmHg CRD) prior to somatic stimulation. Data are expressed as means ± s.e.m. and were analysed at each distention pressure using one-way repeated measures ANOVA. A P value < 0.05 was considered as significant.
Results
Sample
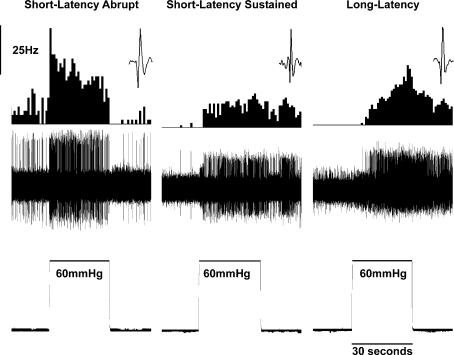
Forty-two neurones from the lumbar (L2–L5) spinal cord responded to CRD and were characterized as short latency sustained (SLS, n = 12), short latency abrupt (SLA, n = 28) and long latency (LL, n = 6). No differences in responses were noted across different segments of the spinal cord. The sustained neurones (SLS) were characterized by the presence of a sustained poststimulus enhanced firing following CRD. The abrupt neurones (SLA) on the other hand had an abrupt cessation (i.e. time-locked response) of activity immediately following the end distension. The long latency neurones (LL) were excited by CRD with a latency of 5–10 s following the onset of distention. Typical examples of these three types of neurones are shown in Fig. 1.
Figure 1. Three types of CRD-sensitive SNs recorded in the lumbar spinal cord.
In each panel, the top trace is the frequency histogram (1 s bin width) of the firing of the neurone, the middle trace is the nerve action potentials and the lower trace is the distending pressure (60 mmHg for 30 s). The depths of recordings of these neurones were 1142 μm for SLA, 1224 μm for SLS and 1434.5 μm for LL ventral from the dorsal surface.
The mean spontaneous firing frequencies of SLA, SLS and LL neurones were 6.7 ± 0.9, 1.8 ± 0.6 and 2.8 ± 1.1 Hz, respectively. The spontaneous firing rate of SLA group neurones was significantly different from SLS neurones (P < 0.05).
Somatic receptive fields
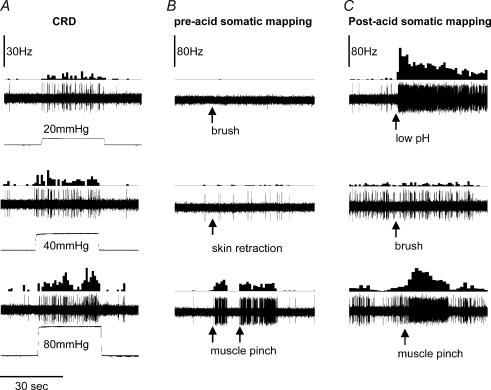
All CRD-sensitive neurone's convergent somatic receptive areas were checked by applying brush, pinch and skin retraction. To check the receptive field deep in the muscle, pressure was applied to the belly of the GN, thigh and rump area. Figure 2 shows an example of the response of an SLA-CRD sensitive neurone to somatic stimuli before and after low pH injection. Of 42 neurones, 15/24 SLA, 5/12 SLS and 1/6 LL exhibited a wide dynamic range with a cutaneous receptive field that included the rump and ipsilateral thigh. The rest of the neurones exhibited a response only to pinch at the ipsilateral GN muscle.
Figure 2. Response of a CRD-sensitive neurone with a convergent somatic receptive field in the thigh muscle.
The recording depth of the neurone was 718.5 μm. Each panel in the columns shows frequency histogram of firing of the neurone and action potentials. A shows the intensity-dependent response of the neurone to graded (20, 40 and 80 mmHg) CRD. B shows responses of the neurone to brush, skin retraction with a forceps and muscle pinch, respectively. C shows the response of the neurone to low pH injection into the thigh muscle, brush and muscle pinch after low pH injection, respectively. The baseline ongoing firing of the neurone increased after low pH injection. The neurone exhibited greater response to muscle pinch, but not to brush. Arrows indicate the point of application of mechanical or chemical stimulus.
Acid-induced sensitization of SNs
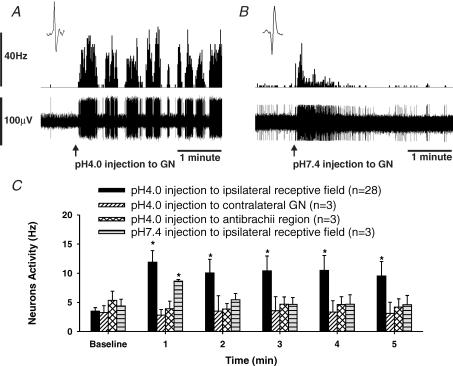
Responses of 29 CRD-sensitive SNs were recorded before and after the injection of 0.1 ml of low pH (4.0)-buffered saline to the somatic receptive field. Figure 3A shows the response of a neurone, with a somatic receptive field in the GN muscle to ipsilateral low pH (4.0) injection. Typically, the firing frequency of these neurones increased following the onset of injection and became sensitized with an increase of 388 ± 22% in firing frequency over a 5 min period (n = 29, P < 0.01 versus pre-injection firing frequency). Three neurones with convergent receptive fields in the GN muscle were tested with ipsilateral injection of 0.1 ml of pH 7.4-buffered saline. Figure 3B shows the response of a neurone with a similar receptive field to the neurone in Fig. 3A that received pH 7.4-buffered saline injection to the ipsilateral GN muscle. The initial burst of firing observed in neurones that received the pH 7.4 injection was due to mechanical stimulation produced by the penetrating needle (Fig. 3B). However, these neurones did not show sustained increase in firing. In six experiments, rats received pH 4.0 injection to either the contralateral GN muscle (n = 3), or to the muscle of the ipsilateral antebrachial region of the front leg (n = 3). In either case low pH had no effect on the neurones. Figure 3C summarizes the data for all low pH (4.0) and pH 7.4 injections.
Figure 3. Response of CRD-sensitive neurons to saline injection into the somatic receptive field.
A, response of a CRD-sensitive SN to an injection of 0.1 ml of pH 4.0-buffered saline to its somatic receptive field in the ipsilateral GN muscle. The neurone was recorded 1142 μm from the dorsal surface of the spinal cord. The top trace is the activity of the nerve represented as frequency histogram (1 s bin width), and the lower trace is the nerve action potentials. The neurone had low ongoing firing, which markedly increased following low pH injection. The enhanced firing of the neurone lasted for the 2 h tested. B, response of a CRD-sensitive SN to an injection of 0.1 ml of pH 7.4-buffered saline to its somatic receptive field in the ipsilateral GN muscle. The neurone was recorded 1027 μm from the dorsal surface of the spinal cord. A transient increase in firing occurred at the time of injection. However, the increased firing progressively declined. The initial burst of firing was due to mechanical stimulus occurred during needle prick. C, summary data of neuronal response to injection to the somatic receptive field, contralateral GN muscle and anti-brachial region of the forelimb. Only low pH injections given to the ipsilateral somatic receptive field produced sustained increase in firing on the CRD-sensitive SNs. (*P < 0.001 versus baseline).
Sensitization to CRD induced by pH 4.0 injections to the ipsilateral somatic receptive field
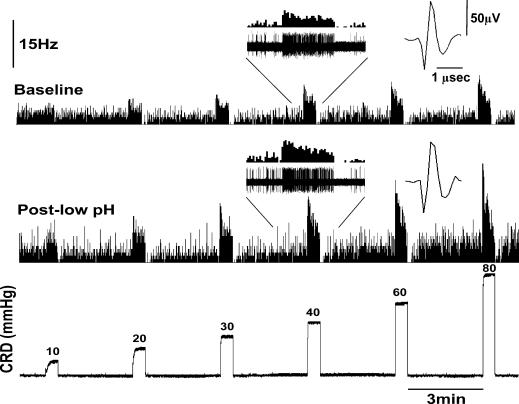
The responses of 29 SNs (15 SLA, 9 SLS and 5 LL) were tested by constructing a SRF to graded CRD prior to, and 30 min following, the pH 4.0 saline injection to the ipsilateral somatic receptive field. Figure 4 shows the effect of the low pH injection on the response of a CRD-sensitive SLA neurone.
Figure 4. Response of a CRD-sensitive SLA neurone to graded CRD before and after low pH (4.0) injection to its somatic receptive field (GN muscle).
The electrode position was 1224 μm from the dorsal surface of the spinal cord. Both baseline spontaneous firing and response to distensions significantly increased following the low pH 4.0 injection. Post-low pH recording was made 30 min post low pH injection.
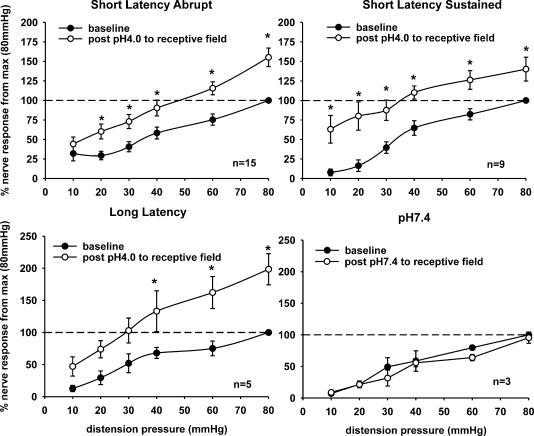
Following the injection of low pH saline, the ongoing firing of the neurone markedly increased, and the neurone exhibited increased response to CRD. To test whether the increased firing of the neurones after the injection was due to the somatic convergence and not due to the repeated distension, in five experiments neurones underwent three repeated SRF to CRD (10–80 mmHg, 30 s). No sensitization was observed due to the repeated distensions. On the other hand, an increase in ongoing firing after low pH injection lasted up to 2 h in the majority of neurones. Responses of SLA neurones significantly increased to a distending pressure range of 20–80 mmHg. SLS neurones showed significant increases in firing to all distending pressures (10–80 mmHg). LL neurones only showed a significant increase in response at distending pressure > 40 mmHg. Three neurones (one of each subtype) were tested with pH 7.4 injections to the ipsilateral somatic receptive field, but did not exhibit sensitization of response to graded CRD. Figure 5 shows the summary data for responses to all the CRD-sensitive neurones before and after low pH injection.
Figure 5. Summary data of the mean SRFs of three subtypes of SNs before and 30 min following low pH 4.0 injection to the somatic receptive field.
SLA (n = 15), SLS (n = 9) and LL (n = 5) neurones showed sensitization to CRD following the low pH injection. Buffered saline injection (pH 7.4) to the somatic receptive field of three CRD-sensitive neurones did not produce change in response to graded CRD (*P < 0.05 versus baseline).
Attenuation of sensitization to CRD by NMDA and AMPA receptor antagonists
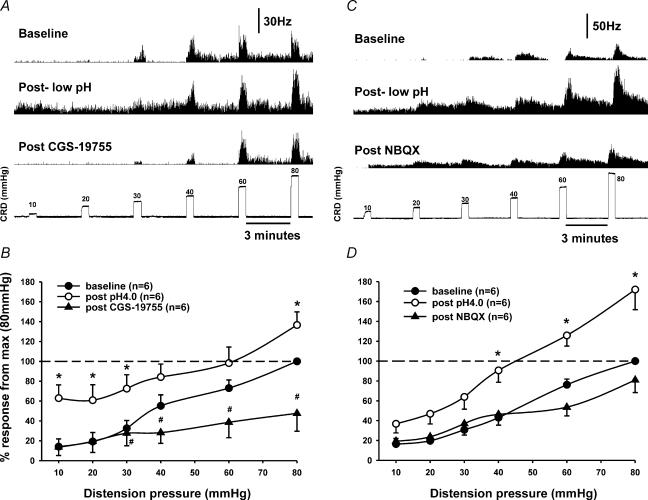
The NMDA receptor antagonist CGS 19755 (25 μmol kg−1 i.v.) attenuated the responses of the low pH-induced sensitization of neurones (n = 5) to CRD (Fig. 6A). The enhanced firing and sensitized response to CRD were significantly reduced. Responses to distending pressure of 40 mmHg decreased below the pre-acid baseline responses after CGS 19755 injection (Fig. 6B). The AMPA receptor antagonist NBQX (25 μmol kg−1 i.v.) also attenuated responses of low pH-induced sensitization of neurones (n = 4, Fig. 6C). Responses to distending pressures of 60 mmHg were reduced below the pre-acid baseline responses (Fig. 6D).
Figure 6. Effect of NMDA and AMPA receptor antagonists on sensitized responses of CRD-sensitive SNs.
A, responses of a SLA neurone (depth: 1035 μm) to graded CRD before and after low pH (4.0) injection and followed by intravenous injection of NMDA antagonist CGS 19755 (25 μmol kg−1). CGS 19755 attenuated the post-acid-enhanced spontaneous firing and sensitized response to CRD. B, summary data of the mean SRFs of six neurones tested with CGS 19755. C, responses of a LL neurone (depth: 1133 μm) to graded CRD before and after low pH (4.0) and followed by intravenous injection of the AMPA receptor antagonist NBQX (25 μmol kg−1). NBQX attenuated the post-acid-enhanced spontaneous firing and sensitized response to CRD. D, summary data of the mean SRFs of six neurones tested with NBQX. (*post pH 4.0 versus baseline, #post pH 4.0 versus post drug in B and D).
Spinalization
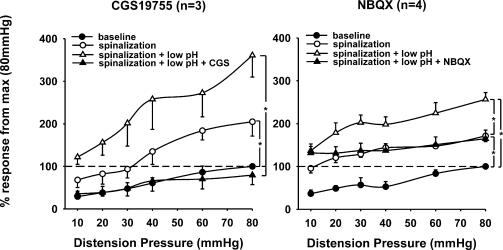
Seven rats underwent spinalization (C1–C2) after constructing a SRF to graded CRD. After spinal transection basal neuronal activity increased significantly by 294 ± 54% (n = 7, P < 0.01) due to the removal of supraspinal descending inhibitory influences. The mean slopes of the SRFs before and after spinal transaction was 13.1 ± 1.4 and 23.3 ± 4.2, respectively (P = 0.032, n = 7). The low pH injection to the somatic receptive field following spinalization produced further sensitization to CRD (Fig. 7). The mean slope of the SRFs following the low pH injection was 31.6 ± 6.2 and was significantly larger than in intact animals, yet was no different from post-spinalization (P = 0.013 versus intact, n = 7). CGS 19755 (25 μmol kg−1) inhibited the response of the neurones to the pre-spinalized (i.e. spinal intact) condition. NBQX (25 μmol kg−1) attenuated the responses of sensitized neurones to post-spinalization level. Results indicate that both antagonists attenuate SNs sensitization at the spinal level, and that CGS 19755 is more effective in blocking the sensitization of SNs in this model.
Figure 7. Summary data of the mean SRFs of seven neurones tested before and after cervical (C1–C2) spinal transection.
All SNs exhibited greater responses to graded CRD after spinal transection. Low pH (4.0) produced further increase in response of these neurones under spinalized condition. Both NMDA receptor antagonist CGS 19755 (25 μmol kg−1) and AMPA receptor antagonist NBQX (25 μmol kg−1) significantly attenuated the enhanced spontaneous firing and the sensitized response to CRD (*P < 0.05).
Discussion
The present study describes the sensitization of several subpopulations of CRD-sensitive neurones in the lumbar (L2–L5) spinal cord in response to nociceptive somatic stimulation. The observed sensitization also occurred in the spinalized rats suggesting a spinally mediated process independent of supraspinal influence. In addition, the present study demonstrates the importance of ionotropic glutamate receptors in the mechanisms of spinal sensitization, which also appears to be independent of supra-spinal influences.
Viscero-somatic convergence
Convergence of visceral and somatic sensory afferent neurones on SNs mediating nociceptive transmission has been well documented in previous electrophysiological studies (Pomeranz et al. 1968; Foreman et al. 1981; Cervero & Tattersall, 1986; Ness & Gebhart, 1991). Visceral primary afferents from the distal colon in the rat travel via the splanchnic, hypogastric and pelvic nerve to project on thoraco-lumbar and lumbo-sacral spinal segments, respectively. These afferents project superficially to lamina I, medially to intermediate and deep laminae including the dorsal grey commissure and lamina X (Cervero & Tattersall, 1987; Janig, 1996). The deep laminae are of particular importance in this study since lamina X has been shown to receive primary visceral afferent projections as well as descending projections from the brainstem (Martin et al. 1982; Light, 1983).
In the context of visceral nociception and viscero-somatic convergence, it has been well established that repetitive noxious distension of the oesophagus, gallbladder or colon in rats and cats produces an increase in the size of the convergent cutaneous receptive fields of the corresponding neurones (Ness & Gebhart, 1991; Cervero et al. 1992; Euchner-Wamser et al. 1993). The size of the convergent cutaneous receptive field in rats can also be increased after colonic irritation with turpentine (Ness & Gebhart, 2000). Clinically, it has been shown that repeated colonic distension in humans similarly leads to an increase in the area of referred sensation in the abdomen, a phenomenon which is best explained by the convergence of visceral and somatic afferents onto second-order spinal neurones (Ness et al. 1990). Our previous behavioural study has documented the development of visceral hyperalgesia in the rat induced by two unilateral low pH (4.0) injections into the GN muscle, 2 days apart (Miranda et al. 2004). The present electrophysiological study attempts to elucidate the mechanism responsible for the visceral hyperalgesia observed in our behavioural study (Miranda et al. 2004).
The current study is in accordance with previous studies showing that spinal neurones receiving visceral input can be distinguished from each other on the basis of their responses to phasic isobaric distension (Ness & Gebhart, 1988). These neurones can be classified as SLA, SLS and LL as previously described. Both SLA and SLS neurones are excited by nociceptive cutaneous stimuli to the same segmental receptive field (Ness & Gebhart, 1988). It has been suggested that SLS neurones may have a predominant role in signalling visceral nociception. These neurones exhibit an increase in spontaneous firing and response to CRD after intracolonic irritation with turpentine, whereas SLA neurones are unaffected (Ness & Gebhart, 2000). Other studies have also suggested that neuronal subtypes respond differently to visceral and somatic stimulation after central administration of the AMPA/kainate receptor antagonist CNQX (Ji & Traub, 2002). In addition, the μ-opioid receptor agonist fentanyl also differentially inhibits the response of SLS neurones to CRD, having no effect on SLA neurones (Kozlowski et al. 2000). Taking previous studies into consideration, it can be speculated that SLS neurones are relatively more involved than SLA neurones in processing visceral nociceptive transmission. However, in the present study all three subtypes of neurones (SLA, SLS and LL) were sensitized with a higher spontaneous activity and increased magnitude of response to CRD after a single, unilateral low pH injection into the somatic field. The mode of sensitization (low pH injection) of CRD-sensitive SN could account for the undifferentiated effects on all three subtypes, unlike previous studies.
Spinal sensitization
It is likely that visceral hyperalgesia developed by low pH injection in the GN muscle is dependent on viscero-somatic convergence. This has been supported by our recent observation that low pH injections, at a site distant from the convergent area (i.e. antebrachial region of forelimb) had no influence on visceral sensation (Miranda et al. 2004). Similarly, the present study shows that a nociceptive somatic stimulus away from the convergent area (i.e. antebrachial muscle of the forelimb) does not acutely alter spontaneous firing or response to CRD of lumbar SNs. In addition, contralateral GN muscle injection of low pH failed to sensitize the ipsilateral CRD-sensitive SN. Our recent chronic behavioural study showed that ipsilateral low pH injection produced contralateral somatic hyperalgesia (Miranda et al. 2004). A possible explanation is the chronic, more prolonged nociceptive primary afferent input (two injections 2 days apart), which might be necessary to see contralateral expansion versus the acute nature of the response tested in the electrophysiology study. The widespread changes may involve late-onset transcription-dependent central sensitization (Dubner & Ruda, 1992; Ji & Rupp, 1997; Ji & Woolf, 2001). Neuroplasticity in the dorsal horn involves activation of transcription factors, which can take hours to become manifest and last for prolonged periods (Dubner & Ruda, 1992; Ji & Rupp, 1997; Ji & Woolf, 2001; Samad et al. 2001). This may not be manifested in the acute study. In addition, buffered saline (pH 7.4) not only failed to alter the response of SN in our current study, but also did not alter the visceral sensation in our previous behavioural model (Miranda et al. 2004).
This observed spinal sensitization may partially explain the clinically observed overlap of chronic abdominal pain and fibromyalgia. Although fibromyalgia is predominant in females, we have used male rats because the pain perception in female rats is very much dependent on the oestrous cycle. In order to avoid the complexity, male rats were selected to eliminate the influence of the oestrous cycle. The male rat model did not change the main focus of our study (i.e. to investigate the influence of viscero-somatic convergence).
Supraspinal influence
The effects of supraspinal descending influences on spinal sensitization have been well documented (Gebhart & Randich, 1990). Neurones in the medial thoraco-lumbar spinal cord encoding visceral nociception also involved in the processing of somatic afferents are under tonic descending inhibitory and facilitatory influences (Giesler & Liebeskind, 1976; Fields et al. 1977; Cervero & Tattersall, 1986; Tattersall et al. 1986; Ness & Gebhart, 1987b; Zhuo & Gebhart, 1992; Zhuo & Gebhart, 2002; Zhuo et al. 2002). A decrease in inhibitory processes is also involved in central sensitization and hyperalgesia (Sivilotti & Woolf, 1994; Lin et al. 1996). In order to investigate the supraspinal influence on observed sensitization of SNs, cervical spinalization was performed. The increased spontaneous firing and response to CRD following spinalization supports previous studies emphasizing the presence of continuous tonic descending inhibitory influence on SNs (Fields et al. 1977; Ness & Gebhart, 1987b; Zhuo & Gebhart, 1992). However, the increased response to CRD of the SNs to subsequent low pH injection in the spinalized condition suggests that sensitization of SNs is independent of descending facilitatory pathways in the acute setting (Fig. 7).
Pharmacology
There are several target neurotransmitters that could potentially be involved in the central sensitization including glutamate, substance P and serotonin. Glutamate has been shown to mediate excitatory synaptic transmission (Schneider & Perl, 1985). Fast excitatory synaptic transmission between primary afferents and SNs in response to nociceptive stimulation is thought to be mediated by non-NMDA ionotropic glutamate receptors (AMPA/kainate) (Skyba et al. 2002). It is widely accepted that NMDA and non-NMDA glutamate receptors contribute to colonic inflammation-evoked hyperalgesia and dorsal horn neurone hyperexcitability (Coutinho et al. 1996; Kolhekar & Gebhart, 1996; Ide et al. 1997). In addition, administration of NMDA has been shown to expand the convergent cutaneous receptive fields of viscerosomatic convergent neurones (Kolhekar & Gebhart, 1996). We have previously shown in our behavioural study that intrathecal administration of either CGS 19755 or NBQX prior to the low pH injections in the GN muscle prevents the development of somatic and visceral hyperalgesia (Miranda et al. 2004). In the present study, attenuation of the response of acutely sensitized SNs was evident after systemic administration of NMDA or AMPA/kainate receptor antagonists CGS 19755 and NBQX, respectively (Fig. 6). Contrary to other studies, we found no differential effects of either antagonist on different subtypes (SLS, SLA and LL) of neurones (Ji & Traub, 2002). In addition, cervical spinal transection does not alter the effects of these antagonists suggesting that the attenuation occurs at the spinal level (Fig. 7).
To our knowledge this is the first study documenting the sensitization of CRD-sensitive neurones by a nociceptive stimulus to the somatic receptive field. It is possible that nociceptive stimuli involving different somatic regions may cause sensitization at different convergent spinal segments. The spinal sensitization may give insights to the pathophysiology of overlapping chronic abdominal pain and fibromyalgia.
Acknowledgments
The work was supported by New Faculty Grant, Digestive Disease Center (DDC) Grant and International Foundation for Gastrointestinal Disorders (IFFGD) Senior Scientist Award awarded to Jyoti N. Sengupta.
References
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;230:88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM, Pozo MA. Selective changes of receptive field properties of spinal nociceptive neurons induced by noxious visceral stimulation in the cat. Pain. 1992;51:335–342. doi: 10.1016/0304-3959(92)90219-2. [DOI] [PubMed] [Google Scholar]
- Cervero F, Tattersall JE. Somatic and visceral sensory integration in the thoracic spinal cord. Prog Brain Res. 1986;67:189–205. doi: 10.1016/s0079-6123(08)62763-6. [DOI] [PubMed] [Google Scholar]
- Cervero F, Tattersall JE. Somatic and visceral inputs to the thoracic spinal cord of the cat: marginal zone (lamina I) of the dorsal horn. J Physiol. 1987;388:383–395. doi: 10.1113/jphysiol.1987.sp016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Euchner-Wamser I, Sengupta JN, Gebhart GF, Meller ST. Characterization of responses of T2–T4 spinal cord neurons to esophageal distension in the rat. J Neurophysiol. 1993;69:868–883. doi: 10.1152/jn.1993.69.3.868. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Clanton CH, Anderson SD. Nucleus raphe magnus inhibition of spinal cord dorsal horn neurons. Brain Res. 1977;126:441–453. doi: 10.1016/0006-8993(77)90596-0. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Hancock MB, Willis WD. Responses of spinothalamic tract cells in the thoracic spinal cord of the monkey to cutaneous and visceral inputs. Pain. 1981;11:149–162. doi: 10.1016/0304-3959(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Randich A. Brainstem modulation of nociception. In: Klemm WR, Vertes RP, editors. Brainstem Mechanism of Behavior. New York: Wiley; 1990. pp. 315–352. [Google Scholar]
- Giesler GJ, Jr, Liebeskind JC. Inhibition of visceral pain by electrical stimulation of the periaqueductal gray matter. Pain. 1976;2:43–48. doi: 10.1016/0304-3959(76)90045-2. [DOI] [PubMed] [Google Scholar]
- Ide Y, Maehara Y, Tsukahara S, Kitahata LM, Collins JG. The effects of an intrathecal NMDA antagonist (AP5) on the behavioral changes induced by colorectal inflammation with turpentine in rats. Life Sci. 1997;60:1359–1363. doi: 10.1016/s0024-3205(97)00081-7. [DOI] [PubMed] [Google Scholar]
- Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Ji Y, Traub RJ. Differential effects of spinal CNQX on two populations of dorsal horn neurons responding to colorectal distension in the rat. Pain. 2002;99:217–222. doi: 10.1016/s0304-3959(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Kolhekar R, Gebhart GF. Modulation of spinal visceral nociceptive transmission by NMDA receptor activation in the rat. J Neurophysiol. 1996;75:2344–2353. doi: 10.1152/jn.1996.75.6.2344. [DOI] [PubMed] [Google Scholar]
- Kozlowski CM, Bountra C, Grundy D. The effect of fentanyl, DNQX and MK-801 on dorsal horn neurones responsive to colorectal distension in the anaesthetized rat. Neurogastroenterol Motil. 2000;12:239–247. doi: 10.1046/j.1365-2982.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Light AR. Spinal projections of physiologically identified axons from the rostral medulla of cats. In: Bonica JJ, Lindblom U, Iggo A, editors. Advances in Pain Research and Therapy. New York: Raven; 1983. pp. 373–379. 5. [Google Scholar]
- Lin Q, Peng YB, Willis WD. Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is reduced during central sensitization. J Neurophysiol. 1996;76:1005–1014. doi: 10.1152/jn.1996.76.2.1005. [DOI] [PubMed] [Google Scholar]
- Martin GF, Cabana T, Ditirro FJ, Ho RH, Humbertson AO., Jr Raphespinal projections in the North American opossum: evidence for connectional heterogeneity. J Comp Neurol. 1982;208:67–84. doi: 10.1002/cne.902080106. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987a;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Quantitative comparison of inhibition of visceral and cutaneous spinal nociceptive transmission from the midbrain and medulla in the rat. J Neurophysiol. 1987b;58:850–865. doi: 10.1152/jn.1987.58.4.850. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13–L2 spinal cord of the rat. J Neurophysiol. 1988;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991;66:20–28. doi: 10.1152/jn.1991.66.1.20. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Acute inflammation differentially alters the activity of two classes of rat spinal visceral nociceptive neurons. Neurosci Lett. 2000;281:131–134. doi: 10.1016/s0304-3940(00)00832-6. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- Pace F, Molteni P, Bollani S, Sarzi-Puttini P, Stockbrugger R, Porro GB, Drossman DA. Inflammatory bowel disease versus irritable bowel syndrome: a hospital-based, case-control study of disease impact on quality of life. Scand J Gastroenterol. 2003;38:1031–1038. doi: 10.1080/00365520310004524. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Wall PD, Weber WV. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968;199:511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Perl ER. Selective excitation of neurons in the mammalian spinal dorsal horn by aspartate and glutamate in vitro: correlation with location and excitation input. Brain Res. 1985;360:339–343. doi: 10.1016/0006-8993(85)91251-x. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Perl ER. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J Neuroscience. 1988;8:2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Tattersall JE, Cervero F, Lumb BM. Effects of reversible spinalization on the visceral input to viscerosomatic neurons in the lower thoracic spinal cord of the cat. J Neurophysiol. 1986;56:785–796. doi: 10.1152/jn.1986.56.3.785. [DOI] [PubMed] [Google Scholar]
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl. 2):43–47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci. 1991;36:59–64. doi: 10.1007/BF01300088. [DOI] [PubMed] [Google Scholar]
- Veale D, Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the irritable bowel syndrome: Different expressions of a common pathogenic process. Br J Rheumatol. 1991;30:220–222. doi: 10.1093/rheumatology/30.3.220. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus MB. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arth Rheum. 1981;11:151–171. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. J Neurophysiol. 1992;67:1599–1614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87:2225–2236. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]