Abstract
Sarcomas are often characterised by significant histopathologic heterogeneity, both between and within tumours. This heterogeneity reflects physiologic, biochemical and genetic processes that are amenable to characterisation by functional imaging. Although anatomically based imaging modalities such as plain radiography, X-ray computed tomography (CT) and magnetic resonance imaging (MRI) remain the primary diagnostic modalities for staging sarcomas, nuclear medicine approaches including gamma camera scintigraphy and positron emission tomography (PET) are being used increasingly to provide complementary information in specific clinical situations. These include biopsy guidance within anatomically complex masses, staging, therapeutic response assessment and evaluation of residual mass lesions after treatment. This review aims to address the range of nuclear medicine techniques available for evaluation of bone and soft tissue sarcomas. A subsequent review discusses the clinical application of these techniques with a particular focus on PET.
Keywords: PET, FDG, Tl-201, bone scintigraphy, In-111 octreotide
Introduction
Anatomical imaging techniques including radiography, ultrasound, computer X-ray tomography (CT) and magnetic resonance imaging (MRI) currently play a dominant role in the evaluation of suspected and known sarcomas. These modalities allow definition of intra-lesional structural characteristics, and of the relationship between tumour boundaries and adjacent normal tissues including bone and neurovascular structures. For this purpose, MRI is now probably the major diagnostic tool [1]. Regional anatomical information is important to determine the need for and method of biopsy, and to guide subsequent loco-regional therapies, including radiotherapy and surgery. However, tissue heterogeneity, which is a common feature of sarcomas, can make selection of the most appropriate biopsy site problematic (Fig. 1). It can also make interpretation of anatomical imaging results difficult following therapy. This is an important potential limitation since the behaviour of sarcomas, their prognosis and determination of the most appropriate management are influenced by the highest histological grade of tumour that is present [2]. Furthermore, for staging purposes, sensitive and specific whole-body screening capability is required.
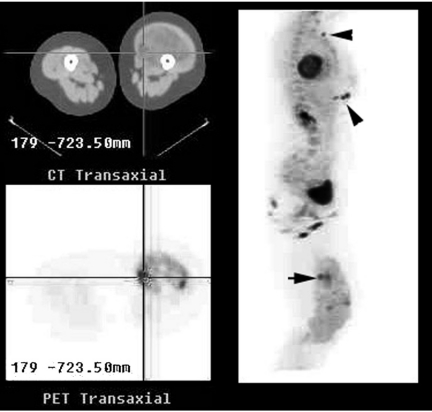
Figure 1.
Combined PET/CT imaging of a large soft tissue mass in the left thigh demonstrated marked heterogeneity of FDG uptake in the mass with small foci of very intense uptake superimposed on an overall pattern of only mildly to moderately increased activity. The reference transaxial CT and PET images correspond to the focus of highest FDG uptake seen on the right lateral maximum intensity projection images (horizontal arrow). Biopsy would normally have been directed at this site. However, multiple cutaneous metastases were identified (arrow heads). Note that the intensity of uptake at metastatic sites is similar to the intense primary tumoral uptake sites, consistent with the notion that the most metabolically active tumour sites are also the most aggressive and determine prognosis.
The cellular derangement that produces a sarcoma most likely begins with altered cell cycle regulation related to genetic damage. This, in turn, will often alter cellular function characteristics including enzymatic, cytosolic and cell-surface protein expression. These changes alter biochemical characteristics of the cancer cell and secondarily influence tissue physiology. It is only when the volume of abnormal cells becomes large enough to be detected by an imaging technique, direct visualisation or palpation, that a mass lesion becomes evident. Although this is a very simplistic account of tumorigenesis, it makes the point that anatomical imaging can only be expected to characterise the later consequences, and not the intrinsic drivers of tumour development.
Nuclear medicine techniques potentially offer unique information regarding tumour biology and thereby, provide complementary information to anatomical imaging, particularly within heterogeneous lesions. Based on the tracer kinetic model, developed by Georg von Hevesy early last century, nuclear medicine techniques involve administration of ‘radiotracers’ in minute amounts in order to mimic the fate of related, non-radioactive chemicals. Accordingly, nuclear medicine tests are extremely safe with an extremely low incidence of allergic reactions or other side effects [3].
The importance of functional imaging for sarcoma evaluation
Altered tissue physiology and biochemistry provide potential means for differentiating benign from malignant mesenchymal lesions and underpin functional imaging techniques [4]. However, it needs to be recognised that functional imaging is not the exclusive domain of nuclear medicine techniques. For example, dynamic-contrast CT [5] and MRI [6] exploit the hypervascularity and altered endothelial function of tumours, including sarcomas, and have emerged as important functional imaging techniques, eroding the traditional role of standard nuclear medicine techniques for assessing tumoral perfusion and ‘blood volume’. Alteration in tissue biochemistry is less easily evaluated by anatomical imaging techniques than by nuclear medicine. However, magnetic resonance spectroscopy (MRS) is now providing important information regarding the biochemical signature of sarcomas, albeit with a highly restricted sampling volume [7]. Characterisation of the whole-body distribution of biochemical processes is currently primarily the domain of nuclear medicine. This has led to the widespread use of the term ‘metabolic imaging’ in place of ‘nuclear medicine’ in recent times.
One of the most important characteristics of sarcomas is heightened proliferative activity. Increased mitochondrial numbers, increased protein synthesis, accelerated glycolysis, alteration in glucose-transporter protein expression are but a few of the biochemical correlates of this process. Most functional imaging techniques that are in current use for the evaluation of sarcoma rely on detection of the secondary metabolic consequences of heightened proliferation and some newer techniques directly trace cellular proliferation. The ability to directly and indirectly evaluate cellular proliferation over time provides an expanding opportunity for the use of metabolic imaging as a prognostic indicator and surrogate measure of therapeutic response.
More specific characterisation of malignant transformation may also be possible by identification of the key drivers of this process including: alteration in cell surface receptors and transport protein complexes; up-regulation or down-regulation of key cytosolic enzymes; and altered gene signalling.
Specific nuclear medicine investigations
Vascular phase imaging
Although contrast enhancement of anatomical imaging techniques provides excellent assessment of tumour vascularity, administration of any radiotracer in a sufficient activity and with suitable imaging characteristics can also allow assessment of regional perfusion. The blood flow phase of bone scanning is an example of this technique and provides additional diagnostic information to the subsequent metabolic phase of the investigation. For example, incorporation of information from the blood-flow and blood-pool phases substantially improves specificity and overall accuracy of bone scanning in the detection of bone tumours [8].
The best validated nuclear medicine technique for quantitatively evaluating the perfusion of sarcomas is, however, positron emission tomography (PET) with oxygen-15 (15O) water [9]. Osteosarcoma, chondrosarcoma, Ewing’s sarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma all tend to be highly vascular. Although many other sarcomas are also hypervascular, benign lesions can also have this characteristic. Osteoid osteomas, and inflammatory lesions such as osteomyelitis and recent fracture are examples. The pattern of perfusion abnormality can be helpful in differentiating benign from malignant processes with increased peripheral perfusion being more common in aggressive tumours than benign lesions. Tumour vascularity will generally decrease with effective therapy either returning to similar levels seen in adjacent bone or soft tissue or becoming relatively avascular if healing fibrosis occurs.
The technical and logistic advantages of dynamic-contrast CT and MRI (DC-MRI) will likely make these the preferred functional imaging techniques for characterisation of tumoral perfusion with 15O water studies reserved for research applications in specialised facilities with the expertise and resources required to perform such studies.
Bone scanning
Bone-forming tumours, particularly osteosarcoma, are characterised by malignant osteoid formation while other sarcomas can secondarily invade bone, invoking reactive osteoblastic activity that can be imaged on bone scanning. Evaluation of bone involvement remains an important indication for radionuclide bone scanning in known or suspected cases of sarcoma. The whole body screening capability of this scan provides cost-effective screening for metastatic spread or multifocal bone pathologies. Bone seeking radiotracers include 99mTc methylene diphosphonate (MDP) and 18F fluoride, which can be imaged by PET. High 99mTc MDP uptake in soft tissue sarcomas is uncommon except in osteosarcoma metastases. Bone scanning still has a role in the follow-up of patients with osteosarcoma (Fig. 2). With respect to other primary bone sarcomas, the intensity of osteoblastic activity with Ewing’s sarcoma is variable but tends to be less than with osteosarcoma. In our experience, high bone tracer uptake in relationship to chondroid-matrix lesions increases the likelihood of malignant transformation but is not specific for malignancy. Lack of abnormality on the bone uptake phase of the study favours a benign or mature bone process. Malignant fibrous histiocytoma in bone usually invokes moderately intense osteoblastic activity at the bone–tumour interface but the tumour, itself, does not usually concentrate bone-seeking radiopharmaceuticals.
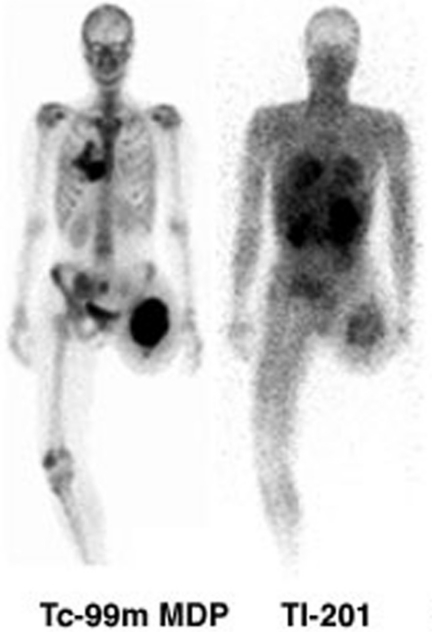
Figure 2.
99mTc MDP bone (left) and 201Tl (right) scans of a patient with recurrence of osteosarcoma in the left thigh and metastases to lung and bone are demonstrated. Although 201Tl demonstrates the peripheral and lung lesions quite well, the contrast is less than that observed on bone scanning, potentially limiting its sensitivity for other metastatic sites. The high uptake in abdominal and pelvic structures obscure the iliac and sacral metastases that are clearly seen on bone scan.
While a decrease in bone turnover throughout a lesion following therapy strongly suggests therapeutic response, the converse is not as predictive of lack of response. Increased osteoblastic activity may accompany bone healing following successful treatment: this is often termed a ‘flare’ response.
Metabolic imaging with single-photon agents
The term metabolic imaging is pertinent to radiotracers that have active uptake into tumours based on basal metabolic rates or increased proliferative activity.
Thallium-201 (201Tl), a mono-cationic analogue of potassium, is concentrated in tumour cells by the sodium–potassium ATPase pump [10]. Enhanced metabolic activity often increases activity of this pump and therefore malignant tumours frequently concentrate this tracer more intensely than normal soft tissues or bone. Increased perfusion probably also plays a role in the increased uptake of 201Tl in tumours. 201Tl was first reported as a tumour-imaging radiotracer following serendipitous discovery of uptake in a lung cancer during myocardial perfusion scanning [11] and since then has been evaluated in a range of malignancies including glioma, lymphoma, and carcinoma of the lung, breast and thyroid. High affinity for 201Tl has also been observed in various bone (Fig. 2) and soft tissue sarcomas (Fig. 3). An early study involving 38 patients with surgically proven bone and soft tissue sarcomas found increased uptake in all cases [12]. A subsequent Japanese study demonstrated abnormal 201Tl uptake in all 30 patients with newly diagnosed osteosarcoma [13]. The ability of 201Tl to detect osteosarcoma involving pagetic bone has also been documented [14]. Soft tissue tumours, including leiomyosarcoma and malignant hemangioendothelioma, have also been identified on 201Tl imaging. Increased 201Tl uptake in a lesion is not, however, unequivocal evidence of a malignant aetiology. Various granulomatous diseases can have increased uptake early, possibly reflecting third space trapping, but retention is usually quite low, and therefore either both early and delayed, or only delayed imaging, is recommended for assessing the likelihood of malignancy in lesions with equivocal anatomical features. It is also important to recognise that sarcomas containing a large amount of acellular matrix material, such as myxoid liposarcomas or chondroid matrix tumours, can be false negative on 201Tl imaging. Recognition of the likelihood of these entities based on anatomical imaging appearances can allow for either omission of 201Tl scintigraphy or complementary use of additional isotopic studies more suited to the detection of these acellular matrix components (see below).
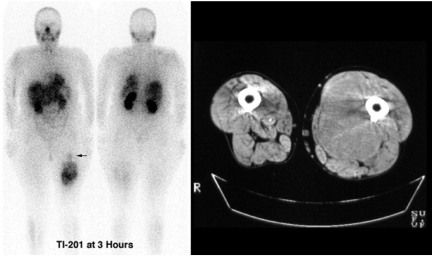
Figure 3.
This patient presented with recent enlargement of a long-standing mass in the medial aspect of the left thigh, thought previously to represent a lipoma. The upper part of the mass had characteristics of fat on CT and MRI while the lower part had features suggesting sarcomatous degeneration. The upper part of the tumour (arrow) demonstrated no 201Tl uptake whereas the lower part had heterogeneous uptake, most marked in the inferior aspect. The latter site was chosen for biopsy and demonstrated high-grade liposarcoma. Following neoadjuvant radiotherapy only low-grade tumour elements remained in the mid-part of the tumour.
99mTc sestamibi (MIBI) and 99mTc tetrofosmin are other myocardial blood-flow imaging agents that have been shown to actively concentrate in tumour cells. The higher photon energy and shorter half-life of 99mTc offer theoretical advantages compared to 201Tl but efflux of these radiopharmaceuticals mediated by p-glycoprotein, a membrane-associated channel involved in multi-drug resistance (MDR), may limit their clinical utility [15]. Enhanced uptake of 99mTc MIBI in malignant tumours is thought to reflect increased numbers of mitochondria in cancer cells. A decrease in 99mTc MIBI uptake may reflect favourable therapeutic response [16]. Unlike 99mTc MIBI, 99mTc tetrofosmin does not accumulate significantly in mitochondria [17] and therefore may provide different information regarding cell viability following therapy. However, for tumour visualisation both agents appear to perform comparably [18]. Similarly a comparison of 99mTc MIBI and 201Tl also suggests that the diagnostic performance of these tracers is comparable [19]. Despite concerns regarding the influence of MDR on the negative predictive value of 99mTc MIBI scans, a series of 84 patients with musculoskeletal tumours (31 malignant and 53 benign) demonstrated a negative predictive value of 88% [20]. A comparison of 99mTc MIBI and FDG PET imaging in 48 patients with suspected recurrent or residual musculoskeletal sarcoma demonstrated that PET had a significantly higher sensitivity (98% vs. 82%) and a non-significantly higher specificity (90% vs. 80%) than MIBI [21]. For these reasons, we currently use either 201Tl or FDG PET for appendicular sarcomas and FDG PET for sarcomas of the abdomen and pelvis (due to the high background uptake in normal organs by the available SPECT tracers).
Metabolic imaging with PET
The use of metabolic tracers imaged with PET is playing an increasing role in oncology [22]. The most widely used PET tracer is fluorine-18 fluorodeoxyglucose ([18F]FDG), which is an analogue of glucose that is transported into cells, phosphorylated and trapped within cancer cells more avidly than by normal cells. Up-regulation of glucose transporters is probably a major factor driving this process [23]. The intensity of FDG-uptake has been shown to correlate with histologic grade and proliferative activity in a range of cancer types [24].
[18F]FDG has been shown to concentrate in a wide range of sarcomas. A recent meta-analysis of the use of FDG PET for the detection of sarcoma pooled results from 29 studies that met predefined inclusion criteria gave a sensitivity, specificity and accuracy of 91%, 85% and 88%, respectively [25]. However, the ability of FDG PET to differentiate between benign and malignant lesions has been variable in individual studies. Using quantitative measurement of glucose metabolic rate, a small study in 19 malignant and 7 benign bone tumours was unable to reliably characterise lesions [26]. The dynamic acquisition protocol used for this study only evaluated the uptake characteristics over the first 50 min after injection of radiotracer. There is now evidence that the peak intensity of FDG uptake in benign lesions is reached by around 30 min after injection, whereas the peak uptake of FDG is delayed to around 4 h in malignant soft tissue lesions [27]. Accordingly, characterisation of the initial phase of FDG uptake, even if quantitative, may not have as great discriminatory power as delayed imaging. This raises the possibility that ‘dual-phase’ FDG PET scanning may be a helpful technique for helping to differentiate between malignant and inflammatory lesions. With this technique, a standard whole-body screening study would be performed at around 1 h after injection; this is a practical time-point in a clinical PET facility. If there is an isolated focus of FDG uptake that could reflect an inflammatory or malignant condition, a delayed single bed position acquisition could be acquired at 3–4 h post-injection. An increase in the measured FDG uptake would increase the likelihood of malignancy, whereas washout would favour an inflammatory basis. The most common method of measurement of FDG uptake from static images is the so-called ‘standardised uptake value’ (SUV). This methodology calibrates uptake in any given tissue against a known sample and corrects for the mass of the patient, the administered dose of activity and radioactive decay. Assuming a uniform distribution of FDG throughout the body, a SUV of 1.0 would be obtained. However, tissues that actively concentrate FDG have SUVs of significantly higher than this, and generally in excess of 2.5. Arguing against a semi-quantitative approach as opposed to formal quantitative analysis of glucose metabolism, a previous study comparing dynamic and delayed static PET imaging demonstrated better correlation between the quantitative measure and histopathological malignancy grade than with SUV measurement [28] and another that demonstrated that a fully quantitative approach provided better discrimination of grades I and III than did SUV analysis [29]. However, for practical reasons, we favour a delayed whole-body imaging protocol in clinical practice and are encouraged by a methodological study performed by investigators at the University of Washington who demonstrated an excellent correlation co-efficient of 0.94 between an SUV obtained by summing the last 30 min of a dynamic imaging series with a full dynamic acquisition obtained over 60 min [30], even though this study may also suffer from the limitations of progressively rising SUV over time in malignant lesions but not in benign ones.
Relative hypoxia in tumours appears to be an important stimulus for up-regulation of the primary glucose transporter in cancer cells, GLUT 1, and of expression of the rate-limiting enzyme of glycolysis, hexokinase [31]. Hypoxia may therefore be an important reason for the high FDG in many sarcomas. However, hypoxia can also be more directly evaluated by PET using [18F]fluoromisonidazole (FMISO), an imidazole compound that is specifically trapped within hypoxic cells. Since hypoxia decreases the sensitivity of tissues to radiation and thus lessens the efficacy of radiotherapy, PET scanning with FMISO may provide useful information in selecting the most appropriate form of adjuvant therapy for bone and soft tissue tumours [32].
Increased protein synthesis in tumours can be assessed using a range of radiopharmaceuticals based on naturally occurring amino acids. These can be labelled with single photon agents or with positron emitting radionuclides. The most intensively evaluated agent is carbon-11 methionine, a PET agent [33]. Incorporation of this agent in tumour tissues is correlated with proliferative activity. A decrease in protein synthesis may precede and be more predictive of therapeutic response than changes in [18F]FDG uptake and retention. [11C]Tyrosine has been compared to FDG in 55 patients with soft tissue sarcoma, 28 of whom had follow-up imaging after treatment [34]. In this study, FDG correlated better with tumour grade but [11C]tyrosine uptake seemed to provide better prediction of therapeutic response. [18F]Fluoroethyltyrosine represents a new radiolabelled amino acid suitable for imaging using PET [35]. Its role in sarcoma imaging is currently unclear.
Similarly, new tracers for evaluating cellular proliferation have recently been described. Currently, the best characterised of these is [18F]fluorothymidine (FLT) [36]. There is relatively little experience with this agent in sarcomas but preliminary studies suggest that it may have a role in tumour grading [37]. Our own experience also suggests that it may be helpful for assessing therapeutic response (Fig. 4).
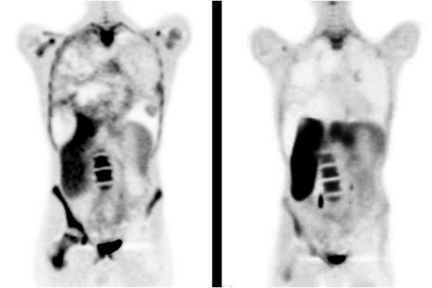
Figure 4.
Baseline (left) and follow-up (right) 18F FLT PET scans demonstrate a decrease in uptake in the actively proliferating components of a large, heterogenous mass completely filling the right hemithorax in an adolescent with previous radiotherapy for a synovial sarcoma of the left hip. The loss of bone marrow uptake in the left femur (arrows) is consistent with ablation of haemopoietic cells by radiotherapy.
Indirect imaging of acellular matrix components
As discussed above, false negative metabolic imaging studies can occur with some high-grade sarcomas if the metabolic signal from the malignant cell lineage is significantly diluted by the presence of abundant acellular matrix material within the tumour mass. In many series chondroid matrix tumours account for a significant proportion of the relatively few false negative 201Tl, 99mTc MIBI and FDG PET scans. The large amount of chondroid matrix that can be present in these tumours and the relatively scant malignant cells, even when the cells are poorly differentiated, makes this observation easy to comprehend. Nevertheless, since the classification of chondroid matrix tumours as being benign or malignant has significant prognostic and management implications, a non-invasive technique that could make this distinction accurately would be of benefit. The demonstration that [99mTc(V)]DMSA is actively concentrated in almost all chondrosarcomas and not by the majority of benign chondrogenic tumours [38] suggests that this may be a useful technique for this dichotomisation. Our own experience with combined 201Tl and [99mTc(V)]DMSA suggests that high-grade chondrosarcomas generally have high uptake of both tracers, whereas intermediate- and low-grade chondrosarcomas are generally only positive on the [99mTc(V)]DMSA scan (Fig. 5). Most benign lesions are negative on both. The exception is that a positive [99mTc(V)]DMSA scan can occur in adolescents and young adults (up to around the age of 30), in whom there is presumably ongoing active growth of the benign lesion. Although the exact mechanism of uptake of [99mTc(V)]DMSA scan in chondrosarcomas is not clear, the intensity of uptake suggests that it must be in the chondroid matrix but reflect biochemical difference in the nature of the matrix in benign and malignant lesions. Given the uptake of [99mTc(V)]DMSA scan in amyloid [39], this may relate to the valency of the protein component. Immature osteoid is a possible target and would explain the false-positive results in actively growing osteochondromas and enchondromas in adolescents and young adults. However, uptake of this agent has also been described in other sarcomas without a significant acellular matrix component [40] and therefore the possibility that it might reflect intense direct uptake in the malignant chondrocytes cannot be excluded. We routinely perform [99mTc(V)]DMSA scanning in patients with chondroid matrix tumours that are negative on 201Tl or FDG PET imaging.
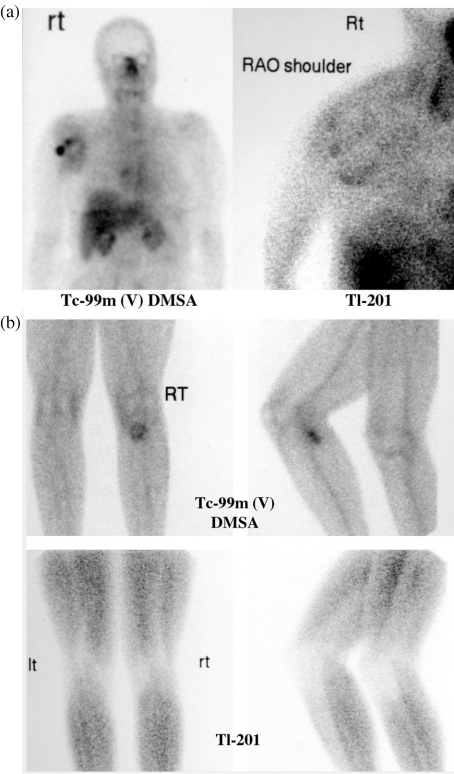
Figure 5.
(a) Plain X-ray and MRI suggested a chondroid matrix tumour in the right shoulder in a middle-aged female. Foci of high [99mTc(V)]DMSA (right) and 201Tl (left) uptake suggested a probable chondrosarcoma. Subsequent histopathology indicated a high-grade chondrosarcoma. (b) An exophytic chondroid lesion in proximal left tibia had recently caused pain in a male in his twenties. High [99mTc(V)]DMSA (right) was apparent over the cap of the lesion but the 201Tl scan (left) was negative. While high DMSA uptake generally indicates malignant degeneration in adult patients, benign exostoses usually have uptake as long as skeletal growth is present and for a few years after normal epiphyseal fusion. The resected specimen confirmed a low-grade chondrosarcoma.
Myxoid tumours are also recognised to lead to false-negative metabolic imaging studies with 201Tl, 67Ga and FDG PET. Avid uptake of [99mTc]pertechnetate has been described in myxoid sarcomas [41]. The pertechnetate uptake by myxoid tumours is a delayed rather than an acute phenomenon and therefore more likely reflects binding of tracer to the myxomatous elements rather than passive third-space trapping. Our imaging protocol is to do dynamic blood flow and early blood pool images followed by 2–3 h delayed scanning. We routinely perform [99mTc]pertechnetate scans for soft tissue sarcomas that are negative from 201Tl or FDG PET imaging.
Immunological and receptor imaging
Advances in the understanding of the molecular biology of tumour cells coupled with improved methods for radiolabelling of biological compounds will increasingly allow development of specific radiotracers for targeting sarcomas. Monoclonal antibodies to cell surface antigens are an example of this type of approach. The anti-3F8 monoclonal antibody directly against gangliosides has affinity for a range of sarcomas including malignant fibrous histiocytoma [42]. However, the relatively large molecular size of monoclonal antibodies can limit tissue penetration and, therefore, contrast between blood and tumour.
Peptides that act as receptor ligands and cell messenger proteins offer attractive prospects for tumour imaging. For example, [111In]pentreotide binds particularly to subclass 2 somatostatin receptors and has been shown to be taken up in osteosarcoma [43]. This interesting paper demonstrated a higher sensitivity for primary tumours than for metastatic sites and tumours with high uptake had a better response to chemotherapy than lesions without uptake, suggesting that somatostatin receptor expression may provide useful biological behaviour characterisation.
As the growth factors, autocrine and paracrine substances that regulate tumour growth of bone and soft tissue sarcomas become better understood, it may be possible to radiolabel these to allow metabolic imaging.
Genomic imaging
Oligonucleotides including anti-sense codons that form triplexes within the groove of double-stranded DNA potentially allow detection of specific genetic sequences within cells. Where an aberrant gene is implicated in tumorigenesis, a radiolabelled anti-sense-oligonucleotide could be used to target tumour cells. This approach is at a very early stage of development. Its potential has recently been demonstrated using optical imaging in a rat model [44]. Although it is feasible to label anti-sense oligonucleotides with radioisotopes suitable for SPECT or PET imaging, this approach is considered likely to be more effective for therapeutic intervention than for diagnostic imaging due to the very low concentration of potential target genes within tumour cells. A type of ionising radiation called an Auger electron is ideally suited to therapeutic application of this type. The Auger electrons emitted from a range of radionuclides such as iodine-125 (125I) travel only a few micrometres from where they arise. Oligonucleotides labelled with these agents are capable of inducing lethal double-stranded DNA breaks at specific sites in the chromosome. The sensitivity of PET may make it feasible to image such genomic targeting using 124I.
Conclusion
The wide range of nuclear medicine techniques available for evaluation of sarcomas reflects the wide diversity of biological features that characterise sarcomas. The choice of investigation should be guided by the clinical question that needs to be answered and the results of other investigations that are more routinely performed. Nevertheless, nuclear medicine techniques in general, and PET in particular, are an important component of the diagnostic armamentarium used for evaluation of known or suspected sarcoma.
References
- 1.Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39:701–20. doi: 10.1016/s0033-8389(05)70306-7. [DOI] [PubMed] [Google Scholar]
- 2.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 3.Keeling DH. Adverse reactions and untoward events associated with the use of radiopharmaceuticals. In: Sampson CB, editor. Textbook of radiopharmacy: theory and practice. 2nd edn. Switzerland: Gordon and Breach Scientific Publishers; 1994. pp. 285–98. [Google Scholar]
- 4.Hicks RJ. Nuclear medicine techniques provide unique physiologic characterization of suspected and known soft tissue and bone sarcomas. Acta Orthop Scand. 1997;273:25–36. doi: 10.1080/17453674.1997.11744699. [DOI] [PubMed] [Google Scholar]
- 5.Miles KA. Measurement of tissue perfusion by dynamic computed tomography. Br J Radiol. 1991;64:409–12. doi: 10.1259/0007-1285-64-761-409. [DOI] [PubMed] [Google Scholar]
- 6.Tacikowska M. Dynamic magnetic resonance imaging in soft tissue tumors—assessment of the diagnostic value of tumor enhancement rate indices. Med Sci Monit. 2002;8:MT53–7. [PubMed] [Google Scholar]
- 7.Negendank WG. MR spectroscopy of musculoskeletal soft-tissue tumors. Magn Reson Imaging Clin N Am. 1995;3:713–25. [PubMed] [Google Scholar]
- 8.Caluser CI, Abdel-Dayem HM, Macapinlac HA, et al. The value of thallium and three-phase bone scans in evaluation of bone and soft tissue sarcomas. Eur J Nucl Med. 1994;21:1198–205. doi: 10.1007/BF00182353. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzbach M, Willeke F, Dimitrakopoulou-Strauss A, et al. Functional imaging and detection of local recurrence in soft tissue sarcomas by positron emission tomography. Anticancer Res. 1999;19:1343–9. [PubMed] [Google Scholar]
- 10.Sehweil AM, McKillop JH, Milroy R, Wilson R, Abdel-Dayem HM, Omar YT. Mechanism of 201Tl uptake in tumours. Eur J Nucl Med. 1989;15:376–9. doi: 10.1007/BF00449228. [DOI] [PubMed] [Google Scholar]
- 11.Cox PH, Belfer AJ, van der Pompe WB. Thallium-201 chloride uptake in tumors, a possible implication in heart scintigraphy. Br J Radiol. 1976;49:767–8. doi: 10.1259/0007-1285-49-585-767. [DOI] [PubMed] [Google Scholar]
- 12.Ramanna L, Waxman A, Binney G, Waxman S, Mirra J, Rosen G. Thallium-201 scintigraphy in bone sarcoma: comparison with gallium-67 and technetium-MDP in the evaluation of chemotherapeutic response. J Nucl Med. 1990;31:567–72. [PubMed] [Google Scholar]
- 13.Ohtomo K, Terui S, Yokoyama R, et al. Thallium-201 scintigraphy to assess effect of chemotherapy in osteosarcoma. J Nucl Med. 1996;37:1444–8. [PubMed] [Google Scholar]
- 14.Colarinha P, Fonseca AT, Salgado L, Vierira MR. Diagnosis of malignant change in Paget’s disease by Tl-201. Clin Nucl Med. 1996;21:299–301. doi: 10.1097/00003072-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ballinger JR, Bannerman J, Boxen I, Firby P, Hartman NG, Moore MJ. Technetium- 99m-tetrofosmin as a substrate for p-glycoprotein: in vitro studies in multidrug-resistant breast tumor cells. J Nucl Med. 1996;37:1578–82. [PubMed] [Google Scholar]
- 16.Caner B, Kitapçl M, Unlü M, et al. Technetium- 99m-MIBI uptake in benign and malignant bone lesions: a comparative study. J Nucl Med. 1992;33:319–24. [PubMed] [Google Scholar]
- 17.Arbab AS, Koizumi K, Toyama K, Araki T. Uptake of technetium- 99m-tetrofosmin, technetium- 99m-MIBI and thallium-201 in tumor cell lines. J Nucl Med. 1996;37:1551–6. [PubMed] [Google Scholar]
- 18.Söderlund V, Larsson SA, Bauer HCF, Brosjö O, Larsson O, Jacobsson H. Use of 99mTc-MIBI scintigraphy in the evaluation of the response of osteosarcoma to chemotherapy. Eur J Nucl Med. 1997;24:511–5. doi: 10.1007/BF01267682. [DOI] [PubMed] [Google Scholar]
- 19.Taki J, Sumiya H, Tsuchiya H, Tomita K, Nonomura A, Tonami N. Evaluating benign and malignant bone and soft-tissue lesions with technetium- 99m-MIBI scintigraphy. J Nucl Med. 1997;38:501–6. [PubMed] [Google Scholar]
- 20.Pinkas L, Robinson D, Halperin N, et al. 99mTc-MIBI scintigraphy in musculoskeletal tumors. J Nucl Med. 2001;42:33–7. [PubMed] [Google Scholar]
- 21.Garcia R, Kim EE, Wong FC, et al. Comparison of fluorine-18-FDG PET and technetium- 99m-MIBI SPECT in evaluation of musculoskeletal sarcomas. J Nucl Med. 1996;37:1476–9. [PubMed] [Google Scholar]
- 22.Leskinen-Kallio S. Positron emission tomography in oncology. Clin Physiol. 1994;14:329–5. doi: 10.1111/j.1475-097x.1994.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Seino Y, Fukumoto H, et al. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–30. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- 24.Okada J, Yoshikawa K, Itami M, et al. Positron emission tomography using fluorine-18-fluorodeoxyglucose in malignant lymphoma: a comparison with proliferative activity. J Nucl Med. 1992;33:325–9. [PubMed] [Google Scholar]
- 25.Bastiaannet E, Groen H, Jager PL, et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev. 2004;30:83–101. doi: 10.1016/j.ctrv.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Kole AC, Nieweg OE, Hoekstra HJ, van Horn JR, Koops HS, Vaalburg W. Fluorine-18-fluorodeoxyglucose assessment of glucose metabolism in bone tumors. J Nucl Med. 1998;39:810–5. [PubMed] [Google Scholar]
- 27.Lodge MA, Lucas JD, Marsden PK, Cronin BF, O’Doherty MJ, Smith MA. A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med. 1999;26:22–30. doi: 10.1007/s002590050355. [DOI] [PubMed] [Google Scholar]
- 28.Nieweg OE, Pruim J, van Ginkel RJ, et al. Fluorine-18-fluorodeoxyglucose PET imaging of soft-tissue sarcoma. J Nucl Med. 1996;37:257–61. [PubMed] [Google Scholar]
- 29.Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M, et al. Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J Nucl Med. 2001;42:713–20. [PubMed] [Google Scholar]
- 30.Eary JF, Mankoff DA. Tumor metabolic rates in sarcoma using FDG PET. J Nucl Med. 1998;39:250–4. [PubMed] [Google Scholar]
- 31.Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Active and passive mechanisms of [fluorine-18] fluorodeoxyglucose uptake by proliferating and prenecrotic cancer cells in vivo: a microautoradiographic study. J Nucl Med. 1994;35:1067–75. [PubMed] [Google Scholar]
- 32.Koh WJ, Rasay JS, Evans ML, et al. Imaging tumor hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys. 1992;22:199–212. doi: 10.1016/0360-3016(92)91001-4. [DOI] [PubMed] [Google Scholar]
- 33.Kubota K, Yamada S, Ishiwata K, et al. Evaluation of the treatment response of lung cancer with positron emission tomography and L-[methyl- 11C]methionine: a preliminary study. Eur J Nucl Med. 1993;20:495–501. doi: 10.1007/BF00175162. [DOI] [PubMed] [Google Scholar]
- 34.Kole AC, Plaat BE, Hoekstra HJ, Vaalburg W, Molenaar WM. FDG and L-[1-11C]-tyrosine imaging of soft-tissue tumors before and after therapy. J Nucl Med. 1999;40:381–6. [PubMed] [Google Scholar]
- 35.Wester HJ, Herz M, Weber W, Heiss P, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40:205–12. [PubMed] [Google Scholar]
- 36.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 37.Cobben DC, Elsinga PH, Suurmeijer AJ, et al. Detection and grading of soft tissue sarcomas of the extremities with 18F-3 ′-fluoro-3 ′-deoxy-L-thymidine. Clin Cancer Res. 2004;10:1685–90. doi: 10.1158/1078-0432.ccr-03-0040. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi H, Kotoura Y, Hosono M, et al. Diagnostic value of Tc- 99m (V) DMSA for chondrogenic tumours with positive Tc- 99m HMDP uptake on bone scintigraphy. Clin Nucl Med. 1995;20:361–4. doi: 10.1097/00003072-199504000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi H, Sakahara H, Itoh T, et al. Technetium- 99m(V)dimercaptosuccinic acid uptake in intra-abdominal massive deposit of amyloid protein. J Nucl Med. 1993;34:815–7. [PubMed] [Google Scholar]
- 40.Kobayashi H, Sakahara H, Hosono M, et al. Soft-tissue tumors: diagnosis with Tc- 99m (V) dimercaptosuccinic acid scintigraphy. Radiology. 1994;190:277–80. doi: 10.1148/radiology.190.1.8259419. [DOI] [PubMed] [Google Scholar]
- 41.Abe H, Terui S, Terauchi T, et al. Comparison of Tc- 99m pertechnetate with Tl-201 and Ga-67 scintigraphy of malignant soft-tissue tumors. Clin Nucl Med. 1997;22:38–41. doi: 10.1097/00003072-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Cheung NK, Canete A, Cheung IY, Ye JN, Liu C. Disialoganglioside GD2 anti-idiotypic monoclonal antibodies. Int J Cancer. 1993;54:499–505. doi: 10.1002/ijc.2910540324. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari S, Dondi M, Fanti S, et al. Somatostatin receptor (SSTR) scintigraphy in patients with osteosarcoma. Cancer Biother Radiopharm. 2003;18:847–51. doi: 10.1089/108497803770418391. [DOI] [PubMed] [Google Scholar]
- 44.Bhaumik S, Walls Z, Puttaraju M, Mitchell LG, Gambhir SS. Molecular imaging of gene expression in living subjects by spliceosome-mediated RNA trans-splicing. Proc Natl Acad Sci USA. 2004;101:8693–8. doi: 10.1073/pnas.0402772101. [DOI] [PMC free article] [PubMed] [Google Scholar]