Abstract
For every course of radiotherapy treatment, the potential benefit has to be weighed against the risk of normal tissue damage. Radiation-induced proctitis during and after radical radiotherapy for prostate cancer can be decreased by reducing both the size of the target volume and the margins required around this volume. In the future, target volumes could be reduced by both CT/MRI co-registration and dose painting using MR spectroscopy of choline and citrate in the prostate. Improved immobilisation and image-guided radiotherapy should allow reduced margins without compromising the effectiveness of treatment. Similarly, in breast radiotherapy treatment, lung and cardiac complications can be reduced by better patient positioning and ensuring that doses to the heart and lung are minimised during radiotherapy treatment planning. Cosmesis can be improved by using 3D breast planning techniques rather than the conventional 2D approach. These ongoing improvements and developments in radiotherapy treatment planning are leading to treatments which offer both better tumour volume coverage, and are minimising the risk of treatment-related complications. In time, these changes should allow the escalation in dose delivered to the tumour volume with the potential for increased cure rates.
Keywords: Radiotherapy, therapeutic index, breast, prostate
Introduction
Every dose of radiation delivered to a patient, with the aim of cure of a tumour, is limited by the possibility of serious damage to normal tissues. This risk is of course inherent in all forms of medical therapy, including drug therapy and surgery, and is not peculiar to radiotherapy. The balance between the probability of tumour control (TCP) and the risk of normal tissue complications (NTCP) is a measure of the therapeutic ratio of the treatment. Normal tissue damage cannot be completely avoided because the doses necessary to achieve tumour control usually overlap with those that can cause complications. For some tumours, such as carcinoma of the prostate, there is evidence of a dose–response curve with dose escalation leading to increased tumour control rates. As shown in Fig. 1, there is also a dose–response curve for normal tissues and dose escalation may lead to increased normal tissue damage. The damage to normal organs depends upon the volume of tissue irradiated, the dose delivered and the inherent radiosensitivity of the organ. Whilst the radiosensitivity of the organ cannot be easily altered, the volume of tissue irradiated and the dose delivered may be minimised through careful design of the radiotherapy treatment technique. This paper takes the examples of radical radiotherapy to the prostate and breast to demonstrate how improved tumour imaging and treatment delivery techniques can minimise normal tissue damage and lead to an improvement of the therapeutic ratio.
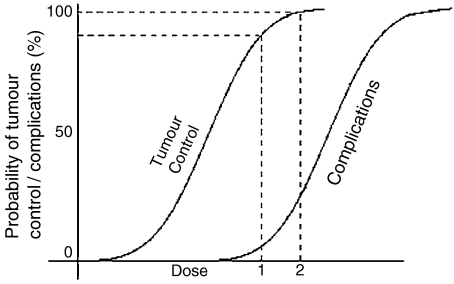
Figure 1.
Idealised dose–response curve. For increase in dose from level 1 to 2 there is a small increase in tumour control but a much larger increase in treatment complication probability.
Prostate cancer
Predicting rectal morbidity
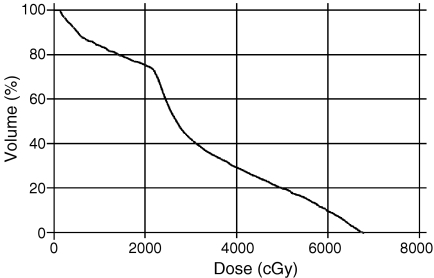
Due to both the close proximity of the anterior rectal wall and the relative radiosensitivity of the rectum, proctitis is the major morbidity associated with radical radiotherapy to the prostate. In order to effectively monitor improvements in radiotherapy delivery, it is essential to have a method of accurately measuring the radiation dose delivered to the rectum. In practice, the dose delivered is not measured during treatment, but is predicted by computer systems used to plan the radiotherapy treatment. Along with any other organs at risk (OAR), such as the bladder, the rectum is outlined in two dimensions on each axial slice of the planning CT scan to create a three-dimensional (3D) representation of the organ. A graph of the volume of the organ plotted against the predicted dose may then be drawn as a dose volume histogram (DVH) (Fig. 2). Some validation of the predicative value of the rectal DVH has been provided by Fiorino et al. [1]. They showed that if the volume of the rectum receiving over 50 Gy on the rectal DVH was above 60%–65% then this was associated with an increased risk of late rectal bleeding, a symptom of radiation-induced rectal damage.
Figure 2.
Dose volume histogram (DVH) for rectum in radical prostate radiotherapy showing that 20% of the rectum receives over 50 Gy for this patient.
The predictive value of a rectal DVH is, however, directly dependent on the rectal volume which is delineated. Although the rectum is a hollow organ, DVHs are often generated using outlines of the outer rectal wall and so consider the total rectal volume (including the rectal contents) as the OAR. It is likely, however, that a DVH of the rectal wall itself may be more clinically relevant than a DVH of the whole rectum. This DVH can be calculated by carefully outlining both the outer and inner rectal wall, so excluding the rectal contents. This is a more time-consuming practice, and may be difficult due to the close proximity of the inner and outer rectal wall on the CT scans. An alternative method is described by Meijer et al. [2]. They show that mathematical modelling may allow a good estimation of rectal wall volume based on outlining the outer rectal wall only. This offers the potential for a more accurate measurement of the true rectal dose in this hollow organ without adding significantly to planning time.
Whilst the techniques described above may accurately predict the dose that would have been received by the patient on the day of their planning CT scan, they will not necessarily accurately model the dose received on each subsequent treatment fraction (over 7 weeks of daily treatment). Numerous studies [3, 4] have shown that both the position and the volume of the rectum may change over the course of treatment, so reducing the validity of the rectal DVH as a predictor of rectal toxicity. In the next section, methods for reducing this variability in rectal size and position are examined.
Reducing the toxicity of prostate irradiation
One of the most promising ways of reducing the toxicity of radiotherapy to the prostate is by reducing the size of the target volume. This may be achieved in two main ways: by improving imaging used in defining the prostate volume, and by reducing the margins used during radiotherapy treatment planning.
The first step in treatment planning is to define the 3D extent of the volume to be irradiated. In prostate radiotherapy this is usually the clinical target volume (CTV). The CTV is defined as the demonstrable disease, plus a margin for sub-clinical spread. In this case, the CTV therefore includes the whole prostate gland. CT is the imaging modality of choice in radiotherapy treatment planning, due to the useful electron density information, availability of scanners and lack of spatial distortion. CT is not, however, the best modality for visualisation of the prostate. Magnetic resonance imaging (MRI) has become the most accurate method for evaluating tumours of the prostate gland, using phased array pelvic coils or dedicated endorectal coils. Comparison of CT/MRI delineation of the prostate shows that the average CT volume is 1.4 times greater than an axial MRI volume [5]. The differences are at the apex, where CT adds around 6 mm inferiorly due to difficulty in defining the relation of the apex to the base of the penis [5], and the base of the prostate adjacent to the seminal vesicles. Co-registration of CT planning images with MR images will provide more accurate and smaller prostate target volumes resulting in less rectal morbidity. In addition to resulting in smaller volumes encompassing the prostate (and therefore reducing the volume of rectum included), the use of MRI also increases the accuracy of target definition. The MRC RT-01 Trial target volume definition quality assurance programme evaluated 15 radiotherapists outlining three patients [6]. The most variable margins were prostatic apex, superior prostate projecting into bladder and seminal vesicle outlining, a conclusion confirmed by Fiorino et al. [7]. Parker [8] showed improved target volume localisation using intraprostatic fiducial markers and MRI co-registered with CT images. The size of the CTV may be further reduced by careful evaluation of the need to include the seminal vesicles within the treatment volume. Partin’s tables [9] are used to determine if the seminal vesicles are likely to be involved and therefore need to be included. Excluding seminal vesicles from the CTV reduces the volume of irradiated rectum by 40%–50% [10] and hence affects the risk of long-term complications.
In addition to better defining the whole prostate organ, new imaging modalities may be used to highlight areas within the prostate that require a higher dosage of radiation. MR spectroscopy (MRS) using spectra from protons in choline and citrate can be used to define the gross tumour volume (GTV) because prostate secretions contain high concentrations of citrate. In prostate tumours, there is a reduction in citrate production and hence citrate levels fall and higher choline levels are produced due to increased epithelium proliferation turnover of cell membrane lipids. Combined MRS and T2 weighted axial MR helps to improve the localisation of GTV and prediction of extracapsular extension. This may permit dose painting with higher doses aimed at these detailed maps of tumour cells using intensity modulated radiotherapy treatment (IMRT). In addition, choline levels have been shown to correlate with Gleason grade [11] so predicting tumour behaviour and giving the potential for biological conformality. In this way, high dose areas may be limited to within the prostate gland itself, further reducing dose delivered to the rectum and the risk of radiation induced proctitis.
The other main way in which toxicity of radiotherapy to the prostate may be reduced is through a greater conformance of the treated volume of tissue to the actual tumour volume. This conformance of the high dose volume to the tumour volume is termed conformal radiotherapy, and naturally reduces the overall volume of tissue irradiated. This should result in less normal tissue damage but it is important to show that this translates into a clinically relevant decrease in morbidity. Dearnaley et al. [12] carried out a trial of patients with carcinoma of the prostate randomised to conventional or conformal radiotherapy treatment. After a median of 3.6 years follow-up significantly fewer patients developed radiation-induced proctitis and bleeding in the conformal arm (34%) compared with after conventional treatment (51%). Newer developments such as IMRT should allow further reductions in high dose volumes.
It is very important to note that the volume of tissue which is targeted by the radiation treatment is not confined to the identifiable disease (or diseased organ). After the CTV has been defined (i.e. the prostate), a further margin needs to be added to ensure that the treatment delivered does actually result in the CTV being consistently treated. The planning target volume (PTV) is the CTV with a 3D margin around it, acting as a ‘safety margin’ for all of the uncertainties inherent in the radiotherapy process. If this safety margin can be reduced (by reducing the uncertainties in the treatment process), so the volume of tissue treated and the associated treatment morbidity can be reduced. The uncertainties which are accounted for by the PTV margin are numerous, and include mechanical variations of the treatment machine, positional uncertainties of the patient on the couch, patient internal organ motion and clinician variability in tumour volume delineation. Of these, uncertainties due to patient positioning and internal organ motion are most susceptible to monitoring and reduction. A large amount of effort has been expended to ensure that the patient undergoing radiotherapy to the prostate lies on the couch in a consistent manner from initial planning CT scan to subsequent daily treatments. Immobilisation devices have been developed, and their efficacy assessed using portal images taken during radiotherapy treatment. The gold standard of immobilisation is called indexed immobilisation. Devices are relocated (indexed) onto the couch top, ensuring that the patient lies on the same part of the couch on a daily basis. This ensures that machine variations due to daily movement of the patient are minimised. Ideally, the patient would be relocated on to the treatment couch with their head, pelvis and legs immobilised. This three point immobilisation ensures that the patient is consistently straight, and un-rotated in all three planes.
Whilst the positional uncertainties due to patient positioning may be improved through immobilisation devices, it is more difficult to reduce the uncertainties due to internal organ motion. It has been shown that the position of the prostate varies both between treatment fractions [13] and during the treatments themselves [14]. The inter fraction variability is largely due to variations in rectal filling, and to a lesser extent bladder filling [15]. As the rectum empties, so the prostate gland moves posteriorly. This movement may be reduced through dietary advice (starting a low fibre diet prior to radiotherapy) or more actively managed through endo-rectal balloons inserted for each radiotherapy treatment [16].
A newer approach is to acknowledge the variation in prostate position during radiotherapy, and to measure and compensate for this. This approach is collectively known as image-guided radiotherapy. An example is to implant fiducial markers (e.g. gold grain seeds) into the prostate prior to treatment planning and to use the visualisation of these markers as an indication of the prostate position during treatment. Images may be generated through the megavoltage X-ray treatment beams that show the position of these markers and allow the treatment to be adapted on a daily basis to ensure target coverage [17].
It can be seen that the whole process of radiotherapy from initial definition of the tumour volume through to the actual delivery of the treatment may be improved though improved imaging techniques. These techniques allow a smaller tumour volume to be defined (CTV) and a smaller safety margin used to cover this volume (PTV). These improvements, in conjunction with new conformal radiotherapy techniques give the potential for significantly reducing rectal toxicity for patients undergoing radical radiotherapy for prostate cancer.
Breast cancer
Given postoperatively, radiotherapy for early breast cancer reduces the local recurrence rate from around 35% to 10% [18]. Due to the position of the breast overlying the chest wall, the irradiated volume may include parts of the lung and, in left-sided disease, the heart. Morbidity must be kept to negligible levels, especially for patients with ductal carcinoma in situ (DCIS) and good prognosis tumours with cure rates of 80%–95%.
Cardiac morbidity
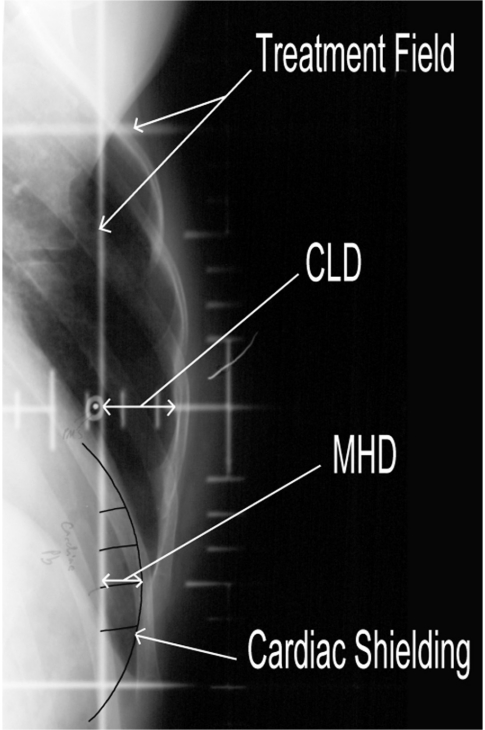
Overview analyses for breast radiotherapy given in the 1960s and 1970s show an increase in non-breast cancer deaths amongst patients given breast radiotherapy [19]. This mortality from treatment was largely due to cardiovascular deaths associated with early techniques, which often included myocardium and large vessels in the target volume. Traditionally, radiotherapy to the breast is localised using plain X-ray film. The films taken represent the final treatment field borders, which are ‘glancing’ across the breast and chest wall. On these two-dimensional images, the extent of the heart within the field may be measured from the heart shadow, termed the maximum heart distance (MHD) (Fig. 3). Correlation has been shown between the MHD and the percentage risk of cardiac normal tissue complication probability [20]. Part of the left anterior descending artery lies within the high dose area for some patients with left-sided breast cancer, and may be the end organ at risk [21].
Figure 3.
Radiotherapy treatment planning film for breast radiotherapy showing cardiac shielding. Maximum heart distance (MHD)=1 cm, central lung distance (CLD)=2 cm.
Pneumonitis
Irradiation of lung tissue during breast radiotherapy may be associated with acute pneumonitis and/or late lung fibrosis depending on the volume of lung irradiated and other coincident lung pathology. Lung symptoms after breast radiotherapy do not correlate with chest radiograph findings [22] and assessment of lung reactions can best be made by asking the patient. In an individual patient, the risk of pneumonitis must be weighed against the risk of regional node recurrence. When the risk of recurrence is high, supraclavicular nodes are treated with an anterior field which includes the apex of the lung, resulting in an increased percentage of lung volume irradiated than during breast radiotherapy alone.
Women with good prognosis breast tumours and negative lymph nodes have the breast only irradiated and the rates of moderate pneumonitis are less than 1% [23]. The chance of recurrent breast tumour in these women is very low, and so it is critical to minimise the volume of lung irradiated. Consequently, there has been considerable interest in trying to quantify and limit the volume of lung irradiated.
It has been shown that the perpendicular distance from the posterior tangential field edge to the posterior part of the anterior chest wall at the centre of the field, termed the central lung distance (CLD), is a good predictor of the percentage of ipsilateral lung volume treated [24] and symptomatic pneumonitis [25]. If the CLD is kept below 2 cm then the amount of lung volume treated is likely to be low (100–125 cm 3) and hence the incidence of pneumonitis is minimal.
Reducing the toxicity of breast irradiation
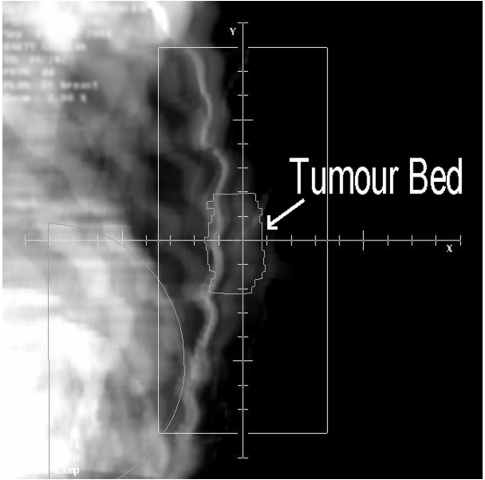
In breast radiotherapy utilising traditional tangential glancing fields, it is the posterior aspect of the treatment fields that can irradiate both the heart and lung. A consequence of this is that techniques that minimise heart dose also tend to minimise lung dose. Where radiotherapy to the breast is localised using plain X-ray film, the basic precaution is to ensure that the MHD and CLD are kept within acceptable limits. A MHD less than 1 cm and a CLD less than 2 cm are the criteria used for planning tangential breast fields to minimise heart and lung morbidity. It is sometimes the case that in order to adequately irradiate the entire breast CTV, the MHD cannot be minimised sufficiently. In this case it may be necessary to use cardiac shielding, where the treatment field is shaped to specifically exclude the heart from the treatment fields. In a series of 17 left-sided breast cancer patients, Hurkmans et al. [26] found that by using a conformal technique which shaped the radiation fields to the shape of the breast, the NTCP for late cardiac mortality was reduced from 5.9% (for rectangular fields) to 4.0%. Due to the glancing nature of the treatment fields, the addition of shielding may have consequences for coverage of the breast tissue lying medially and laterally to the heart. In order to ensure adequate CTV coverage, the use of full CT data in radiotherapy planning is highly recommended. Through the use of CT data, it may be possible to identify the post lumpectomy cavity, which is at highest risk of recurrence, and to ensure that coverage is not compromised by the shielding. Fig. 4 shows a digitally reconstructed radiograph (DRR) of the same patient imaged in Fig. 3. The tumour bed has been outlined on each axial slice and it can be clearly seen that the cardiac shielding is not compromising coverage of this volume of higher risk breast tissue.
Figure 4.
CT generated digitally reconstructed radiograph (DRR) of breast treatment field showing that cardiac shielding is not compromising dose to outlined tumour bed in this patient.
Attempts have been made to reduce cardiac dose delivered during breast radiotherapy through positional manoeuvres. An effective and simple method is to immobilise the patient for treatment with their arms raised above their heads, instead of with their arms at right angles as is more traditionally used. Canney et al. [27], found that in this position the lateral chest wall tissues were raised superiorly and anteriorly, allowing a reduced cardiac dose for the same breast tissue coverage. It is fortuitous that this position is also most suitable for CT scanning.
A similar positional advantage may be gained through respiratory manoeuvres. A number of centres have shown that in deep inspiration the volume of heart within the tangential breast treatment fields is reduced. In order to consistently reproduce the deep inspiration achieved during treatment planning, either respiratory monitoring [28] or mechanical suspension of breathing [29], known as active breathing control (ABC) need to be used.
Cosmesis
In patients with large breasts, studies have indicated a tendency for a greater level of dose inhomogeneity when conventional 2D (non-CT) planning methods are used [30]. In 2D planning the distribution of radiation is optimised on a single axial slice through the centre of the breast, and the distribution superiorly and inferiorly is not calculated. If a plan generated is this way is analysed in 3D (using CT data) it is often seen that areas of high dose occur in the superior and inferior portions of the breast. A consequence of this is that if 50 Gy is the prescribed dose to 100% on the central slice, parts of the breast off-axis may receive up to 110% per fraction. The breast at these points receives the biological equivalent of 58 Gy. Yarnold et al. [31] reported a randomised study of standard 2D breast radiotherapy vs. 3D intensity modulated radiotherapy in patients receiving radiotherapy for early breast cancer. They demonstrated that the dosimetric improvements of the IMRT treatment resulted in a significant improvement in cosmetic results when compared with the conventional planning method.
Conclusion
The reduction of treatment-related morbidity is at the forefront of developments in radiotherapy planning and treatment. This improvement is a necessary first step in the move towards dose escalation, with the potential for improved cure rates.
Technology that is enabling the reduction in treatment-related morbidity is varied, but improvements in imaging underpin the advances taking place. In treatment sites where the use of CT data is well established (such as radical prostate radiotherapy), the addition of other imaging modalities (such as MRI) is improving the accuracy of tumour volume and organ at risk definition. This is allowing a greater conformance of the treatment to the tumour volume and reducing the margin of normal tissue needed. The delivery of treatment is also improving with a move towards real time (on-line) correction of field placement, again reducing the size of treatment margins needed. In treatment sites where the use of CT is not yet widely established (such as radical breast radiotherapy), the evidence is mounting for a need to move from a 2D approach to a 3D approach to treatment planning.
For both of these sites more complex treatment delivery techniques such as IMRT, which allow intricate shaping of the treatment to the tumour volume, are becoming more commonly used as evidence for their efficacy is established.
References
- 1.Fiorino C, Cozzarini C, Vavassori V, et al. Relationships between DVHs and late rectal bleeding after radiotherapy for prostate cancer: analysis of a large group of patients pooled from three institutions. Radiother Oncol. 2002;64:1–12. doi: 10.1016/s0167-8140(02)00147-0. [DOI] [PubMed] [Google Scholar]
- 2.Meijer GJ, van den Brink M, Hoogeman MS, Meinders J, Lebesque JV. Dose-wall histograms and normalized dose-surface histograms for the rectum: a new method to analyze the dose distribution over the rectum in conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:1073–80. doi: 10.1016/s0360-3016(99)00270-9. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 4.Zellars RC, Roberson PL, Strawderman M, et al. Prostate position late in the course of external beam therapy: patterns and predictors. Int J Radiat Oncol Biol Phys. 2000;47:655–60. doi: 10.1016/s0360-3016(00)00469-7. [DOI] [PubMed] [Google Scholar]
- 5.Rasch C, Barillot I, Remeijer P, Touw A, van Herk M, Lebesque JV. Definition of the prostate in CT and MRI: a multi observer study. Int J Radiat Oncol Biol Phys. 1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 6.Seddon B, Bidmead M, Wilson J, Khoo V, Dearnaley D. Target volume definition in conformal radiotherapy for prostate cancer: quality assurance in the MRC RT-01 trial. Radiother Oncol. 2000;56:73–83. doi: 10.1016/s0167-8140(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 7.Fiorino C, Reni M, Bolognesi A, Bonini A, Cattaneo GM, Calandrino R. Intra and inter observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol. 1998;49:133–41. doi: 10.1016/s0167-8140(98)00021-8. [DOI] [PubMed] [Google Scholar]
- 8.Parker CC, Damyanovich A, Haycocks T, Haider M, Bayley A, Catton CN. Magnetic resonance imaging in the radiation treatment planning of localised prostate cancer using intra-prostatic fiducial markers for CT coregistration. Radiother Oncol. 2003;66:217–24. doi: 10.1016/s0167-8140(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 9.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score in men with localised prostate cancer. J Urol. 1993;150:110–4. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 10.Katcher J, Kupelian PA, Zippe C, Klein EA, Sohn JW. Indications for excluding the seminal vesicles when treating clinically localised prostatic adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1997;37:871–6. doi: 10.1016/s0360-3016(96)00617-7. [DOI] [PubMed] [Google Scholar]
- 11.Kurhanewicz J, Vigneron DB, Males RG, et al. The prostate: MRI imaging and spectroscopy: present and future. Radiol Clin North Am. 2000;38:115–38. doi: 10.1016/s0033-8389(05)70152-4. [DOI] [PubMed] [Google Scholar]
- 12.Dearnaley D, Khoo V, Norman A, et al. Comparison of radiation side effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet. 1999;352:267–71. doi: 10.1016/S0140-6736(98)05180-0. [DOI] [PubMed] [Google Scholar]
- 13.Antolak JA, Rosen II, Childress CH, Zagars GK, Pollack A. Prostate target volume variations during a course of radiotherapy. Int J Radiat Oncol Biol Phys. 1998;42:661–72. doi: 10.1016/s0360-3016(98)00248-x. [DOI] [PubMed] [Google Scholar]
- 14.Padhani AR, Khoo VS, Suckling J, Husband JE, Leach MO, Dearnaley DP. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999;44:525–33. doi: 10.1016/s0360-3016(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 15.van Herk M, Bruce A, Kroes AP, Shouman T, Touw A, Lebesque JV. Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int J Radiat Oncol Biol Phys. 1995;33:1311–20. doi: 10.1016/0360-3016(95)00116-6. [DOI] [PubMed] [Google Scholar]
- 16.Ciernik IF, Baumert BG, Egli P, Glanzmann C, Lütolf UM. On-line correction of beam portals in the treatment of prostate cancer using an endorectal balloon device. Radiother Oncol. 2002;65:39–45. doi: 10.1016/s0167-8140(02)00187-1. [DOI] [PubMed] [Google Scholar]
- 17.Chung PW, Haycocks T, Brown T, et al. On-line aSi portal imaging of implanted fiducial markers for the reduction of interfraction error during conformal radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 2004;60:329–34. doi: 10.1016/j.ijrobp.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow up in a randomised clinical trial comparing total mastectomy with laparotomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;22:1456–61. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists’ Collaboration Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of randomised trials. Lancet. 2000;355:1757–70. [PubMed] [Google Scholar]
- 20.Hurkmans CW, Borger JH, Bos LJ, et al. Cardiac and lung complication probabilities after breast cancer irradiation. Radiother Oncol. 2000;55:145–51. doi: 10.1016/s0167-8140(00)00152-3. [DOI] [PubMed] [Google Scholar]
- 21.Fuller SA, Haybittle JL, Smith RE, Dobbs HJ. Cardiac doses in post-operative breast irradiation. Radiother Oncol. 1992;25:19–24. doi: 10.1016/0167-8140(92)90190-6. [DOI] [PubMed] [Google Scholar]
- 22.Holli K, Pitkänen M, Järvenpää R, et al. Early skin and lung reactions in breast cancer patients after radiotherapy: prospective study. Radiother Oncol. 2002;64:163–9. doi: 10.1016/s0167-8140(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 23.Lind PA, Wennberg B, Gagliardi G, Fornander T. Pulmonary complications following different radiotherapy techniques for breast cancer, and association to the irradiated lung volume and dose. Breast Cancer Res Treat. 2001;68:199–210. doi: 10.1023/a:1012292019599. [DOI] [PubMed] [Google Scholar]
- 24.Neal AJ, Yarnold JR. Estimating the volume of lung irradiated during tangential breast irradiation using the central lung distance. Br J Radiol. 1995;68:1004–8. doi: 10.1259/0007-1285-68-813-1004. [DOI] [PubMed] [Google Scholar]
- 25.Bornstein BA, Cheng CW, Rhodes LM, et al. Can simulation measurements be used to predict the irradiated lung volume in the tangential fields in patients treated for breast cancer? Int J Radiat Oncol Biol Phys. 1990;18:181–7. doi: 10.1016/0360-3016(90)90282-o. [DOI] [PubMed] [Google Scholar]
- 26.Hurkmans CW, Cho BC, Damen E, Zijp L, Mijnheer BJ. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol. 2002;62:163–71. doi: 10.1016/s0167-8140(01)00473-x. [DOI] [PubMed] [Google Scholar]
- 27.Canney PA, Deehan C, Glegg M, Dickson J. Reducing cardiac dose in post-operative irradiation of breast cancer patients: the relative importance of patient positioning and CT scan planning. Br J Radiol. 1999;72:986–93. doi: 10.1259/bjr.72.862.10673950. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen AN, Korreman S, Nyström H, Specht L. Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol. 2004;72:53–60. doi: 10.1016/j.radonc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 30.Neal AJ, Torr M, Helyer S, Yarnold JR. Correlation of breast dose heterogeneity with breast size using 3D CT planning and dose-volume histograms. Radiother Oncol. 1995;34:210–8. doi: 10.1016/0167-8140(95)01521-h. [DOI] [PubMed] [Google Scholar]
- 31.Yarnold JR, Donovan E, Bleackley N, et al. on behalf of Breast Technology Group. Randomised trial of standardised 2D radiotherapy vs. 3D intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2002;64:5–15. [Google Scholar]