Abstract
Many plants respond to herbivory by releasing a specific blend of volatiles that is attractive to natural enemies of the herbivores. In corn (Zea mays), this induced odor blend is mainly composed of terpenoids and indole. The induced signal varies with plant species and genotype, but little is known about the variation due to abiotic factors. Here, we tested the effect of soil humidity, air humidity, temperature, light, and fertilization rate on the emission of induced volatiles in young corn plants. Each factor was tested separately under constant conditions for the other factors. Plants released more when standing in dry soil than in wet soil, whereas for air humidity, the optimal release was found at around 60% relative humidity. Temperatures between 22°C and 27°C led to a higher emission than lower or higher temperatures. Light intensity had a dramatic effect. The emission of volatiles did not occur in the dark and increased steadily with an increase in the light intensity. An experiment with an unnatural light-dark cycle showed that the release was fully photophase dependent. Fertilization also had a strong positive effect; the emission of volatiles was minimal when plants were grown under low nutrition, even when results were corrected for plant biomass. Changes in all abiotic factors caused small but significant changes in the relative ratios among the different compounds (quality) in the induced odor blends, except for air humidity. Hence, climatic conditions and nutrient availability can be important factors in determining the intensity and variability in the release of induced plant volatiles.
Chemical changes in plants after insect damage have intrigued plant physiologists and ecologists ever since these changes have been suggested to function as a possible defense against herbivory (Green and Ryan, 1972). Herbivore-induced changes in volatile emissions may function as an indirect defense to attract natural enemies of the herbivores (Price et al., 1980; Dicke, 1994). The importance of the third trophic level as a part of the battery of plant defenses was first suggested by Price et al. (1980), and has since led to many examples in which plants produce odors after insect feeding that attract parasitoids and predators (Dicke et al., 1990; Turlings et al., 1990; Takabayashi et al., 1991; Agelopoulos and Keller, 1994; Mattiacci et al., 1994; McCall et al., 1994; Pallini et al., 1997; Röse et al., 1997). In Brussels sprouts (Brassica oleracea) and corn (Zea mays), it was shown that the production of volatiles is triggered by an elicitor in oral secretions of arthropod herbivores (Turlings et al., 1993; Mattiacci et al., 1995). A fatty acid derivative, N-[17-hydroxylinolenoyl]-l-Gln (volicitin), was isolated and identified from the regurgitant of Spodoptera exigua (Lepidoptera:Noctuidae; Alborn et al., 1997). This elicitor triggers the emission of the same odor blend in corn plants as does caterpillar feeding (Turlings et al., 2000).
Variation in induced plant odors has been studied to determine signal specificity, which could provide parasitoids and predators with information on the identity, stage, and suitability of herbivores attacking a plant. It was found, for instance, that induced odor can vary for different herbivore instars that attack a plant (Takabayashi et al., 1995). Also, different herbivore species trigger the release of different odor blends, which can be exploited by parasitoids and predators to locate plants that carry the most suitable host or prey species (Sabelis and van de Baan, 1983; Sabelis and Dicke, 1985; Takabayashi et al., 1991; De Moraes et al., 1998; Du et al., 1998; Guerrieri et al., 1999). The potential for specificity is complicated by a large variability in induced odor emission among plant genotypes (Takabayashi et al., 1991; Loughrin et al., 1995; Turlings et al., 1998; Krips, 2000; Gouinguené et al., 2001).
Variation in induced odor due to various biotic factors is well documented, but little information is available on how abiotic conditions affect the odor emission. Obviously, changes in environmental conditions affect the physiological state of plants. Growing plants under different light conditions will lead to different growth rates and similar effects can be easily demonstrated with different temperatures, levels of nutrition, and watering. Some studies provide information on how these environmental factors affect direct chemical defenses in plants. Green and Ryan (1973) showed that in young tomato (Lycopersicon esculentum) leaves, the accumulation of a proteinase inhibitor increases with light intensity and that optimal temperature for the highest accumulation level is about 36°C. The indole alkaloid content in Catharanthus roseus (Apocinacae) increases with water availability in soil (Frischknecht et al., 1987). Temperature and day length affect the induction of hydroxamic acid in wheat (Triticum aestivum) seedlings after cherry-oat aphid (Rhopalosiphum padi) infestation (Gianoli and Niemeyer, 1996). Studies on emission of fragrance in flowers provide information on the importance of light in the release of odor by plants. Altenburger and Matile (1990) found that the emission of flower volatiles can either be regulated by an internal clock (circadian rhythm), or only by a diurnal phenomenon that completely depends on the photoperiod of the environment.
Diurnal emissions have also been reported for induced volatiles. Loughrin et al. (1994) showed that the induced emission of volatiles in cotton (Gossypium hirsutum) plants (Malvaceae) was higher during the afternoon and significantly decreased at night. Similar results were reported by Takabayashi et al. (1994), who found that uninfested leaves of lima bean (Phaseolus lunatus; Fabaceae) placed under high light intensity are more attractive to predatory mites than when they are placed under low light conditions, which was due to different volatiles emission under the two light regimes. More recently, Maeda et al. (2000) reported the importance of light on the emission of induced volatiles in kidney beans (Phaseolus vulgaris) plants attacked by the spider mite (Tetranychus urticae). This corresponded nicely with the responsiveness of predatory mites; they were more active during light periods (Maeda et al., 2000). Water stress also affects the attractiveness of lima beans plants to predatory mites. Lima beans under water stress release higher amount of volatiles than non-stressed plants, which could explain their differential attractiveness to predatory mites (Takabayashi et al., 1990). Recently, Halitschke et al. (2000) reported that the emission of induced odor by tobacco (Nicotiana tabacum) plants was highly dependent on light, and that even when plants were induced to release volatiles, the emission began only with the onset of the next light period. In corn plants, information on the effects of environmental factors on the emission of induced volatiles is very limited. Only the effect of the photophase on the release of induced volatiles has been reported (Turlings et al., 1995; Tumlinson et al., 1999).
The current study was conducted to obtain a more complete picture of the relative importance of various abiotic factors for induced odor emissions in corn plants. The effects of variations in soil humidity, air humidity, temperature, light intensity and phases, and fertilization rate were tested under highly controlled conditions.
RESULTS
Soil Humidity
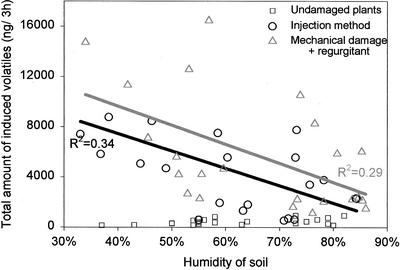
Caterpillar (Spodoptera littoralis) regurgitant was either injected into the stem or applied to the scratched leaves of 10-d-old plants. For both methods used to induce the release of volatiles, the soil humidity had an effect on the emissions, but no effect of soil humidity was found for the emission by untreated plants (Fig. 1). The emissions were highly variable, but overall the relation between the humidity of the soil and the amount of induced odor emitted was negative: The higher the humidity, the lower the release of induced volatiles (Fig. 1). On average, more odor was emitted from corn plants induced by mechanically damage and regurgitant on the wound than by injecting the regurgitant directly in the stem (Fig. 1).
Figure 1.
Total amount (ng/3 h) of induced volatiles emitted by corn plants submitted to different levels of soil humidity. Triangles represent the amount of volatiles released after induction by mechanical damage + regurgitant on the wounded site, circles represent the amount of volatiles emitted after injection of regurgitant directly into the stem, and squares represent the odor emitted by healthy undamaged plants. Light-gray line represents the linear regression for mechanical damage + regurgitant-treated plants (F = 8.577 and P = 0.008) and dark line represents the linear regression for injection method-treated plants (F = 8.667 and P = 0.0091).
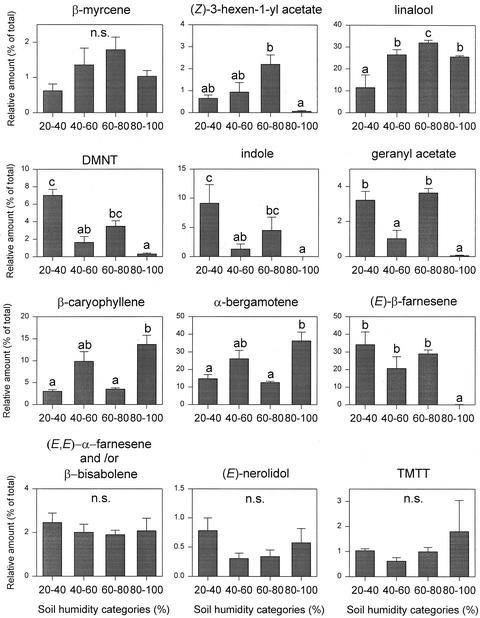
In the experiment using the mechanical damage plus regurgitant method, the soil humidity also had a significant effect on the quality of the odor blend (F = 1.925, P = 0.033, and degrees of freedom [df] = 39). The effect was not the same for the 12 dominant compounds plotted in Figure 2. Ratios among compound differed for different humidity classes. The compounds linalool, β-caryophyllene, and (E)-α-bergamotene showed a tendency to increase with humidity, whereas the release of (Z)-3-hexenyl acetate, (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), indole, geranyl acetate, and (E)-β-farnesene were very low at high humidity (Fig. 2). When the regurgitant was injected directly in the stem, no significant effect of the humidity of soil on the quality of the induced odor blend was found (F = 1.897, P = 0.091, and df = 39).
Figure 2.
Relative amount (mean of % of total + se) of the 12 dominant compounds in the odor blend induced after mechanical damage and regurgitant application at different soil humidities. Humidity was divided in four categories of 20% intervals. Letters above bars indicate significant differences among soil humidity categories after Student-Newman-Keuls post hoc test (α = 0.05).
Air Humidity
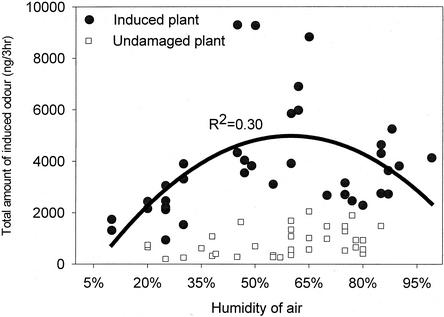
The air humidity inside the collection chamber also had an effect on the release of induced volatiles (Fig. 3). The highest release of induced odor occurred between 45% and 65% relative humidity; total amounts of volatiles released dropped at lower or higher air humidities (Fig. 3). The quality of the odor was not different over the range of air humidity regimes that the plants were subjected to (data not shown, F = 1.169, P = 0.259, and df = 52).
Figure 3.
Total amount (ng/3 h) of induced volatiles emitted by corn plants under different air humidities. Circles represent the amount released by induced corn plants and squares represent the odor released by undamaged plants. Black curve represents the relation between the amount emitted by induced plants and the air humidity (F = 7.09 and P = 0.0027).
Temperature
For the range of temperatures tested, we found significant differences in the total amount of induced volatiles released (F = 3.514, P = 0.02, and df = 3). Emissions were highest at 22°C and 27°C (Fig. 4). The total amount released by undamaged corn plants was significantly lower than the amount emitted by induced plants (F = 35.148, P < 0.001, and df = 1) and it was not affected by the different temperatures tested (F = 1.793 and P = 0.189).
Figure 4.
Total amount (mean + se) of odor emitted by corn plants under different temperatures (°C). Black bars represent induced plants and white bars represent undamaged plants. Stars indicate significant differences between induced plants and undamaged plants (F = 35.148 and P < 0.001) and letters above black bars indicate significant differences among the different temperature tested for induced plants by Student-Newman-Keuls post hoc test (α = 0.05).
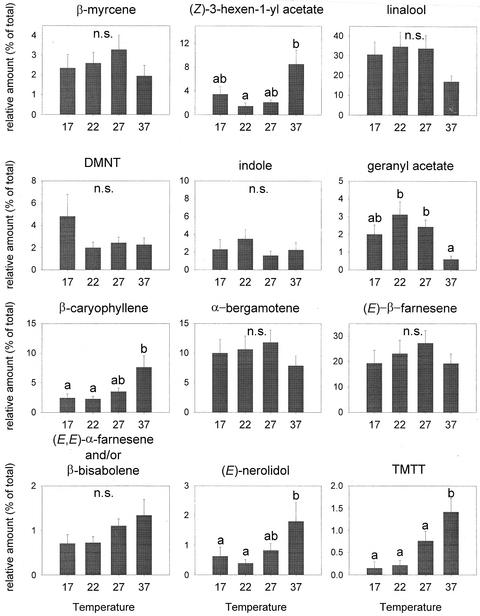
Temperature had also an effect on the quality of the odor (F = 2.630, P < 0.0001, and df = 42; Fig. 5). For example, the proportion of β-caryophyllene was highest at the highest temperature (37°C; Fig. 5). The same was observed for (Z)-3-hexenyl acetate, (E)-nerolidol, and (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (TMTT; Fig. 5). β-Bisabolene followed the same trend, but the differences were not significant. In contrast, the proportion of geranyl acetate was higher at 22°C and that of DMNT was higher at lower temperatures (Fig. 5). The other compounds followed the same trend as for the total amount; their relative amount was highest at 22°C and 27°C (Fig. 5).
Figure 5.
Relative amount (mean of % of total + se) of the 12 dominant compounds in the induced odor blend at different temperatures. Letters above bars indicate significant differences among temperatures after Student-Newman-Keuls post hoc test (α = 0.05).
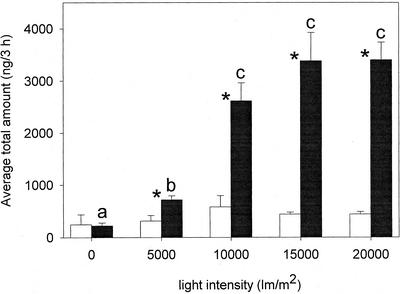
Light Intensity
Light intensity had a dramatic effect (F = 19.174, P < 0.001, and df = 4), with an increase in release of volatiles as light intensity increased (Fig. 6). Induced plants in the dark emitted very little odor and their releases were not different from the odor of undamaged plants. No significant effect of the light intensity was found for the releases by undamaged plants (F = 0.755, P = 0.577, and df = 4).
Figure 6.
Total amount (mean + se) of volatiles emitted by corn plants under different light intensities. Black bars represent induced corn plants, and white bars represent undamaged plants. Stars indicate significant differences between the total amount of odor released by induced and undamaged plants (α = 0.05), and letters indicate significant differences after Student-Newman-Keuls post hoc test among light intensity for induced plants (α = 0.05).
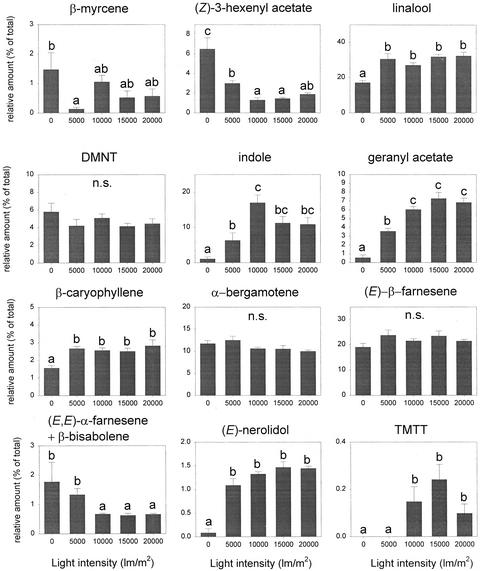
Light intensity also affected the quality of the induced odor blend (F = 3.792, P < 0.001, and df = 52). The relative amount of β-myrcene, (Z)-3-hexenyl acetate, and β-bisabolene + (E,E)-α-farnesene significantly decreased with increases in light intensity, whereas most of the other compounds showed a significant increase in their relative proportion when the light was on (Fig. 7). Only DMNT, α-bergamotene, and (E)-β-farnesene did not show a significant change in their proportion among the different light intensity tested even though the release of these compounds significantly increased with light intensity.
Figure 7.
Relative amount (mean of % of total + se) of the 12 dominant compounds in the induced odor blend at different light intensities. Letters above bars indicate significant differences among light intensities after Student-Newman-Keuls post hoc test (α = 0.05).
Induced Odor Emission in Relation to Light Cycle
As with the previous results plants in the dark released no induced volatiles, but when the light was on (8 h after damage) the emission of volatiles dramatically increased (Fig. 8). Interestingly, the emission of odor ceased again when the light was switched off (Fig. 8). Obviously, significant differences in the amount of induced odor released were found among the different light phases (F = 38.405, P = 0.001, and df = 5). The odor signal was “switched” on or off with the presence or absence of light, independently of a possible “circadian” rhythm, as is the case for floral odors in certain plants (Altenburger and Matile, 1990). The total amount of induced volatiles released slowly decreased with time after damage, as was previously shown by Turlings et al. (1998).
Figure 8.
Total amount (mean + se) of volatiles emitted by corn plants under dark-light phases. Black bars represent induced corn plants, and white bars represent undamaged plants. The horizontal bar represents the respective dark and light phases.
Fertilization Rate
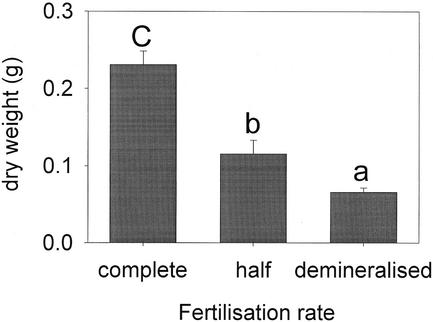
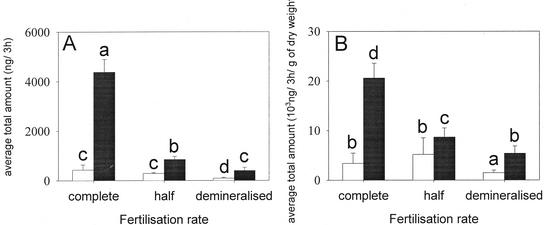
The fertilization had a significant effect on plant size, which was expressed in dry weight at the end of an experiment (Fig. 9). Plants watered with only demineralized water were 3.5 times smaller than plants watered with the complete nutritive solution. Fertilization rate had also a significant effect on the emissions (F = 36.160, P < 0.001, and df = 5), even when the total amount was corrected for biomass of plants (F = 33.490, P < 0.001, and df = 5). Plants that received little fertilization released significantly lower amounts of volatiles (Fig. 10, A and B). Interestingly, unfertilized undamaged plants also released less than fertilized undamaged plants.
Figure 9.
Dry weight (mean + se) of corn plants grown under three different fertilization rates (F = 40.707 and P < 0.001). Letters above bars indicate significant differences among the different fertilization treatments after Student-Newman-Keuls post hoc test (α = 0.05).
Figure 10.
Total amount (mean + se) of volatiles emitted by corn plants under three different fertilization rates (see text for details). The graph in A represents the amount without correction for biomass and the graph in B represents the amount corrected for biomass. Black bars represent induced plants, and white bars represent undamaged plants. Letters above bars indicate significant difference among fertilization rates after Student-Newman-Keuls post hoc test (α = 0.05). The fertilization rate also had an effect on the overall odor blend composed of the 12 dominant compounds (F = 2.689 and P = 0.001).
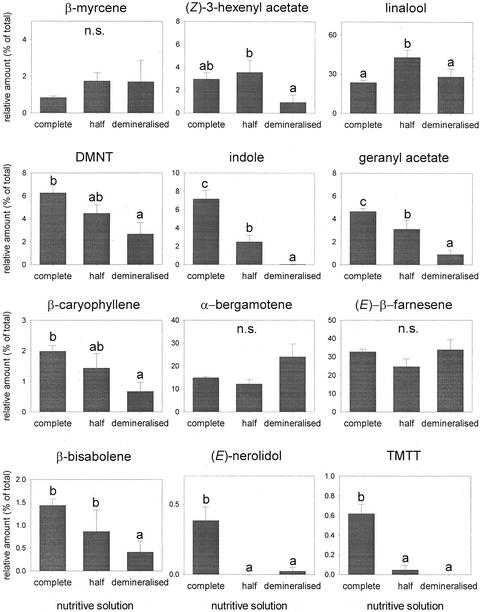
Only the proportions of β-myrcene, α-bergamotene, and (E)-β-farnesene were not significantly affected by the rate of fertilization (Fig. 11). Most of the other compounds tested were released in a lower proportion when the plants were grown under low fertilization. Only the proportion of linalool was higher for the half-fertilization treatment than for the two other fertilization treatments. A surprising result is that the relative amount of linalool did not differ for plants watered with the complete nutritive solution and the ones watered only with demineralized water.
Figure 11.
Relative amount (mean of % of total + se) of the 12 dominant compounds in the induced odor blend for plants at different fertilization levels. Letters above bars indicate significant differences among fertilization rates after Student-Newman-Keuls post hoc test (α = 0.05).
DISCUSSION
All abiotic factors that were tested had a significant effect on the quantity of induced volatiles emitted by corn plants. The magnitude and direction of this effect was different for each factor considered. Higher emission of induced volatiles occurred when the soil was relatively dry, the relative air humidity was between 45% and 65%, the temperature between 22°C and 27°C, with high light intensity, and with continuous fertilization of the soil. In many cases, the different abiotic factors also affected the quality of the odor blend, which is the relative ratio among the substances within a blend. This composition of the blend seems important in the context of the signal that insects may perceive. It is likely that differences in ratios may result in distinguishable differences in the odors perceived, whereas mere differences in the total amount will only affect the detectability of a signal. Soil humidity affected the ratio among the 12 dominant compounds only when plants were mechanically damaged and regurgitant of Spodoptera littoralis was applied to the wounded site and not when the regurgitant was directly injected in the stem. We found no evidence that air humidity affects the quality of the odor blend, but temperature, light intensity, and fertilization rate all had an effect on the composition of the odor blend. Some compounds seem less sensitive to changes in environmental factors than others. For example, the proportion in which (E)-β-farnesene was released was very stable, whereas (E)-nerolidol and TMTT were released in very variable proportions. Fertilization rate had the greatest effect on the proportion of these compounds.
Takabayashi et al. (1994) reported that lima beans under water stress were more attractive to spider mites. With chemical analyses, they confirmed that lima bean plants under water stress produce more of the attractive volatiles than non-stressed plants. Their study was done with undamaged plants and they did not report on any effect of water stress on the emission of induced volatiles (Takabayashi et al., 1994). In the case of corn, undamaged plants were not affected by soil humidity, but the emission of induced volatiles was considerably higher when plants were kept in dry soil, even if the plants did not show any symptoms of desiccation. This can perhaps be explained by the fact that plants in dry soil have a faster take-up and transportation of the regurgitant than when they are grown in wet soil (Taiz and Zeiger, 1998). This must certainly be true for the injection method. It has also been suggested that plant water status might have a considerable influence on the way stomata aperture is controlled (Jones, 1998; Tardieu and Simonneau, 1998). Corn is considered an isohydric plant species, which means that it adjusts its stomata such that leaf water potential is kept constant under varying soil water conditions (Tardieu and Simonneau, 1998). Hence, stomata tend to close under water stress conditions. The physiological response and actual mechanism that leads to the observed odor emission is not yet understood, but the fact that we find high emissions from plants in dry soil may suggest that the volatiles are not only released through the stomata. This hypothesis should be tested in a follow-up experiment by correlating stomatal conductance with the release of induced volatiles. The emission of terpenoids by plants has been suggested to be dependent on their vapor pressure in the leaves (Tingey et al., 1980). Perhaps in dry soil, corn plants attacked by herbivores invest more in the synthesis of induced defense compounds. This could be a trade-off between growth and defense. When water is available, plants can invest more in the growth and thus compensate for herbivory, whereas with less water available, growth is reduced and protection of the vegetative part becomes more important. In fact, corn under water stress does show higher concentrations of typical defense signals (Garcia et al., 1987; Xin et al., 1997). This contrasts with the suggestion by Masters et al. (1993) that water stress may hamper photosynthesis and thus reduce carbon levels and increase the level of nitrogen, which would improve food quality.
The effect of air humidity on the emission of induced volatiles has not been studied before. In corn, this emission was highest at about 60% relative humidity. Loreto et al. (1996) found that in Quercus ilex leaves, the release of constitutive monoterpenes is not affected by air humidity, but there was a slight tendency of a decrease in the emission when the leaves were exposed to dry conditions. In this case, our results favor the notion that odor release may be correlated with the aperture of stomata. In corn, stomata opening increases with the dryness of the air, but only up to a certain limit, after which the stomata close (Taiz and Zeiger, 1998). If at least a part of the emission of induced volatiles occurs through the stomata, it could explain the higher emission at intermediate humidity (Fig. 3).
Temperature has been shown to have an effect on the induced chemical defense in wheat after aphid infestation (Gianoli and Niemeyer, 1996). In their study, lower temperature led to higher production of hydroxamic acids, an induced compound accumulated after aphid infestation. However, this effect was mainly due to the growth rate of the plants; they grew faster under higher temperatures, and the concentration of hydroxamic acid was negatively correlated with growth rate. In tomato, Green and Ryan (1973) found that both light and temperature have an effect on the induced production of proteinase inhibitors and that temperatures below 22°C led to a severe reduction of this defense compound. Here, we show that in corn, the temperature under light-controlled conditions affects the release of induced volatiles. The emission was high at 22°C and 27°C, but decreased at lower or higher temperature. Again, it could have something to do with the aperture of stomata increase, which can be affected by temperature (Taiz and Zeiger, 1998). Variation in temperature may have affected the air humidity inside the collection glass, but such changes cannot explain the differences in the release of induced volatiles observed in this experiment.
The effect of light on the production of induced defense compounds is better documented. The production of a proteinase inhibitor factor was shown to be temperature, but also light dependent. In darkness, only a very low accumulation of this defense factor occurs after induction (Green and Ryan, 1973). In lima bean, spider mites are more attracted to healthy plants placed under high intensity light than plants under low light intensity (Takabayashi et al., 1994). This differential attractiveness was correlated with a differential release of volatiles (Takabayashi et al., 1994). Similarly, levels of volatiles released by kidney bean plants in response to spider mite infestation is higher in the presence of light (Maeda et al., 2000). Loughrin et al. (1994) showed that herbivore-injured cotton plants in a greenhouse emit more induced volatiles during the day than at night. Similar results were obtained in greenhouse experiments with corn (Turlings et al., 1995). However, the results of cotton and corn could also be explained by considerable increases in temperature during the day. Here, we show that in corn the emission of induced volatiles increases with light intensity even under constant temperature. This indicates that the production of induced volatiles is correlated with the photosynthesis activity in plants, which is corroborated by the fact that emissions occurred only during the photophase, independently of the internal rhythm of the plants. The study by Paré and Tumlinson (1997) that demonstrates the de novo production of induced volatiles in cotton also tightly couples this production to photosynthesis.
The level of nutrients available in soil greatly influences the growth rate of plants (Taiz and Zeiger, 1998). The carbon-nutrient balance theory predicts that any lack in nutrients will affect the production of secondary metabolites (Gershenzon and Croteau, 1991). When nutrient availability is limited growth rate is reduced, but photosynthesis remains constant due to carbon availability. Concentrations of nitrogen-based compounds will decline, but accumulation of carbohydrate will lead to the synthesis of terpenoids (Gershenzon, 1994). In corn, however, the release of induced terpenoids was lower when nutrient availability was low. No fertilization and intermediate fertilization reduced the plants' growth rate, but even after correction for biomass, these plants were found to emit far less of the induced volatiles than fully fertilized plants. A lack in constitutive elements and nutrients that are needed for the de novo synthesis of induced compounds may explain this observed reduction, but this is contradictory to what the carbon-nutrient theory predicts. These results confirm the notion by Hamilton et al. (2001) that the carbon-nutrient balance hypothesis frequently fails its predictions.
All of the environmental factors tested here had an effect on the total production of induced odor and most of them also on the quality of the odor blend. Further experiments on the interactions between these different factors should reveal more on their relative importance for the production of induced volatiles. Cross effects among the different factors cannot be predicted as long as the mechanisms are poorly understood. It also remains to be studied how the observed differences in emission affect the behavior of parasitoid and other natural enemies of herbivores. We do know that parasitoids are usually diurnal and are most active at higher temperatures, under which conditions the releases of induced volatiles are particularly high. This could be coincidence, but perhaps also the result of adaptations by insect and/or plant to each other's activities.
MATERIALS AND METHODS
Plant Material
Corn (Zea mays var Delprim) plants were used in all experiments. Seeds were planted individually in plastic pots (360 mL, 10-cm diameter, 7 cm high) filled with fertilized soil (Coop, Basel). The seedlings were grown in a climate chamber (Type 10′US/+5 DU-PI, Weiss Umwelttechnik GmbH, Kilchberg, Switzerland) at 23°C, 60% relative humidity, and 16-h-light:8-h-dark light regime (45,000 lm.m−2 during photophase). The plants were watered daily in the morning. Ten- to 11-d-old plants were used for each experiment; at this age, seedlings carry three to four leaves. For the experiment on the effect of fertilization on volatile emissions, the method for growing plants is described below.
Induction of Odor
Oral secretion was collected from third and fourth instar Spodoptera littoralis, which were provided by Novartis (Syngenta) Insect Control (Basel). They were fed with corn (Zea mays) leaves for at least 1 d before their regurgitant was collected as described by Turlings et al. (1993). Corn seedlings were induced to emit volatile synomones by scratching 2 cm2 of the leaf surface with a razor blade and applying 10 μL of S. littoralis regurgitant (which is equivalent to what we collect on average per caterpillar) to the damaged site. The second and third leaves were treated this way. An additional induction method was used in the experiments on the effects of soil humidity. For this method, we injected the caterpillar oral secretions directly into the stem of corn plants (T. Degen, unpublished data). Just above the soil level, we injected two times 10 μL into the center of the stem. All treatments took place during the dark period, 7 h before lights turned on. In these experiments, the injection method was used because it could provide some information on the importance of take-up and circulation of the regurgitant in the plant, which should vary for different soil humidity levels. However, the scratching method is closer to the natural damage done to plants by caterpillars. Using these standardized methods instead of having caterpillars feeding on the plants avoided undesirable variation micro in the amount of damage on plant.
Collection of Induced Odor
The volatile collection system has been described in detail by Turlings et al. (1998). It basically consists of six vertically placed cylindrical glasses (9.5-cm i.d., 54 cm high). A split Teflon plate with a hole in the center at the base of a cylinder closed loosely around the stem of a plant, allowing the separation of the aerial part of a plant, which was placed in a glass cylinder, from the pot, which remained outside (Turlings et al., 1998). Purified and humidified air was pushed into each cylinder at a rate of 1 L.min−1 and passed down over the plant. Around the base of each cylinder, just above the Teflon disc, eight openings served as ports that could hold the collection traps. Only one port was used during an experiment. For collections, air was pulled (0.8 L.min−1) through a Super-Q adsorbent trap (Heath and Manukian, 1994), whereas the rest of the air vented out through the hole in the bottom, thus preventing outside, impure air from entering. The automated part of the collection system (Analytical Research System, Gainesville, FL) controlled the flow through the trap. The climate chamber (CMP4030, CONVIRON, Winnipeg, Canada) in which the collection cylinders were housed was kept at 17.5°C; due to the irradiation heat, the temperature inside the cylinders was 23°C ± 3°C. During the light cycle, light intensity was about 20,000 lm.m−2.
Collections started 3 h after lights went on, 10 h after treatment. Each collection lasted 3 h. After each collection, traps were extracted with 150 μL of methylene chloride (Lichrosolv., Merck, Whitehouse Station, NJ) and 200 ng of n-octane and nonyl acetate (Sigma, Switzerland) in 10 μL of methylene chloride were added to the samples as internal standards.
Analyses were done with an HP 6890 series gas chromatograph equipped with an automated on-column injection system (HP G1513 A, Hewlett-Packard, Palo Alto, CA) and a flame ionization detector. Of each sample, a 3-μL aliquot was injected onto an apolar EC-1 capillary column (30 m, 0.25-mm i.d., 0.25-μm film thickness, Alltech, Deerfield, IL) preceded by a deactivated retention gap (5 m, 0.25-mm i.d., Connex, Agilent, Palo Alto, CA) and a deactivated precolumn (30 cm, 0.530-mm i.d., Connex). Helium (24 cm.s−1) was used as carrier gas. After injection, the column temperature was maintained at 50°C for 3 min, increased to 230°C at 8°C min−1, and held at 230°C for 9.5 min. The detector signal was processed with HP GC Chemstation software.
Abiotic Factors Tested
Soil Humidity
Three plants were each placed in an individual pot that was watered before the treatment with regurgitant, whereas the three other potted plants were not watered for 18 h. The three plants in dry or moist soil were either left unharmed, scratched and treated with regurgitant, or injected with regurgitant. Actual soil humidity was measured after odor collection. This was done by weighting the soil in each pot without corn roots just after an experiment and again after the soil was dried in an oven at 70°C for 3 d. The percentage of water in the soil was then calculated. Twenty-three plants were treated with the scratching method, 21 plants with the injection method, and 22 undamaged plants were tested as control.
Air Humidity
To manipulate air humidity, air was passed through a 1-L bubbler just before it entered a glass collection chamber. For two chambers, the bubblers were filled with 300 mL of silica gel to create a dry atmosphere in each chamber, whereas the other two bubblers were left empty and the last two were filled with 300 mL of demineralized water to create high humidity. For each replication and each air treatment, one corn seedling was induced to emit odor while another one was left unharmed as control. Hygrometers were placed in the collection chamber to measure the relative humidity inside the glass chamber. Twelve replications were performed.
Temperature
Temperature inside the climate chamber with the odor collection system was set at either 10°C, 15°C, 20°C, or 30°C, which corresponded to 17°C, 22°C, 27°C, or 37°C inside the collection glasses. Light intensity was kept constant during all collections (n = 12 per temperature).
Light Intensity
Corn var Delprim plants were grown as described earlier, at a 45,000 lm.m−2, 16-h-light regime. Five light intensities were used during odor collections ranging from 0 to 20,000 lm.m−2, differing in 5,000 lm.m−2 increments. The light in the climate chamber was provided by bulb (Sylvania Satin, 100W) and fluorescence tubes (Sylvania Cool White, F72T12 V.H.O., 160W). The temperature in the climate chamber was decreased when the light intensity increased such that the temperature inside the collection vessel remained constant (23°C ± 3°C) for all the light intensity tested. Collection lasted for 3 h, with 12 replicates for each light intensity.
Induced Odor Emission in Relation to Light Rhythm
Induced corn var Delprim plants were submitted to an alternating dark-light regime of 3 h each. Odor collection was performed during the last 2 h of each period. A total of six collections were done per plant, starting 5 h after treatment while plants were in the dark. Light intensity was 20,000 lm.m−2 during the lights-on periods. A total of 12 plants were tested over four replicates.
Fertilization Rate
Seeds of the var Delprim were washed carefully with demineralized water and 2% (v/v) chlorine solution, then the seeds were placed in demineralized water and aerated during one night. Seeds were rolled in wet paper and the roll was put in a beaker covered with a plastic film. The beaker was placed in a dark cabinet at lab conditions. Seeds were left to germinated for 3 d. Then, the root and embryo were excised from the seed with a razor blade and planted in pots (360 mL, 10-cm diameter, 7 cm high) filled with a mixture of vermiculite and non fertilized regular soil (Coop). This way, the nutrient reserves of the seeds were removed to avoid any use of these reserves for odor production. Pots were kept in a climate chamber (Type 10′US/+5 DU-PI, Weiss Umwelttechnik GmbH) under the following conditions: 23°C ± 4°C, 60% ± 10% relative humidity, and 40,000 lm.m−2 with a photoperiod of 16 h of light and 8 h of darkness. The plants were watered daily either with a complete nutritive solution for the fertilized plants or with demineralized water for the unfertilized plants. A third (intermediate) treatment was obtained by watering certain plants with the complete nutritive solution for 7 d immediately after planting and then, for the next 8 d, with demineralized water only. The plants were used 15 d after planting when they carried four developed leaves. Each treatment was replicated 12 times.
Statistical Analyses
Regression analyses were performed for experiments on soil humidity and air humidity. For the other abiotic factors tested, an univariate analysis of variance was used to compare the total amount of induced odor blend. For comparison of odor blend quality (the relative importance of the different compounds in the composition of the odor blend), the ratios among the 12 dominant compounds were calculated by dividing the amount for each compound by the total amount of all compounds in each blend. For the experiments testing the effect of soil and air humidity, data were grouped into five humidity categories of 20% intervals. Student-Newman-Keuls was done as a post hoc test. Repeated measures ANOVA was used to test for differences in induced odor emission between treatments in the experiment on the effect of light cycle.
ACKNOWLEDGMENTS
We thank Thomas Degen for his useful suggestions on the experiments on soil humidity and Céline Schwaar for assistance with the fertilization experiment. We are grateful to Martine Rahier for her advice and continuous support.
Footnotes
This work was supported by the Swiss National Science Foundation (grant nos. 31–46237.95 and 31–58865.99).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001941.
LITERATURE CITED
- Agelopoulos NG, Keller MA. Plant-natural enemy association in tritrophic system, Cotesia rubecula-Pieris rapae-Brassicaceae (Cruciferae): III. Collection and identification of plant and grass volatiles. J Chem Ecol. 1994;20:1955–1967. doi: 10.1007/BF02066236. [DOI] [PubMed] [Google Scholar]
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- Altenburger R, Matile P. Further observation on rhythmic emission of fragrance in flowers. Planta. 1990;180:194–197. doi: 10.1007/BF00193995. [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- Dicke M. Why do plants “ talk” ? Chemoecology. 1994;5/6,3/4:159–165. [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol. 1990;16:3091–3118. doi: 10.1007/BF00979614. [DOI] [PubMed] [Google Scholar]
- Du Y, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol. 1998;24:1355–1368. [Google Scholar]
- Frischknecht PM, Bättig M, Baumann TW. Effect of drought and wounding stress on indole alkaloid formation in Catharanthus roseus. Phytochemistry. 1987;26:707–710. [Google Scholar]
- Garcia AL, Torrecillas AL, Leon A, Ruiz Sanchez MC. Biochemical indicators of the water stress in corn seedlings. Biol Plant. 1987;29:45–48. [Google Scholar]
- Gershenzon J. Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol. 1994;20:1281–1321. doi: 10.1007/BF02059810. [DOI] [PubMed] [Google Scholar]
- Gershenzon J, Croteau R. Terpenoids. In: Rosenthal GA, Berembaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. Boca Raton, FL: CRC Press; 1991. pp. 165–219. [Google Scholar]
- Gianoli E, Niemeyer HM. Environmental effects on the induction of wheat chemical defences by aphid infestation. Oecologia. 1996;107:549–552. doi: 10.1007/BF00333947. [DOI] [PubMed] [Google Scholar]
- Gouinguené S, Degen T, Turlings TCJ. Variability in herbivore-induced odour emissions among corn cultivars and their wild ancestors (teosinte) Chemoecology. 2001;11:9–16. [Google Scholar]
- Green TR, Ryan CA. Wound induced proteinase inhibitor in plant leaves: a possible defence mechanism against insects. Science. 1972;175:776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Green TR, Ryan CA. Wound-induced proteinase inhibition in tomato leaves: some effects of light and temperature on the wound response. Plant Physiol. 1973;51:19–21. doi: 10.1104/pp.51.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri E, Poppy GM, Powell W, Tremblay E, Pennachio F. Induction and systemic release of herbivore-induced plant volatiles mediating in-flight orientation of Aphidius ervi. J Chem Ecol. 1999;25:1247–1261. [Google Scholar]
- Halitschke R, Kebler A, Kahl J, Lorenz A, Baldwin IT. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia. 2000;124:408–417. doi: 10.1007/s004420000389. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett. 2001;4:86–95. [Google Scholar]
- Heath B, Manukian A. An automated system for use in collecting volatile chemicals released from plants. J Chem Ecol. 1994;20:593–608. doi: 10.1007/BF02059600. [DOI] [PubMed] [Google Scholar]
- Jones HG. Stomatal control of photosynthesis and transpiration. J Exp Bot. 1998;49:387–398. [Google Scholar]
- Krips OE. Plant Effects on Biological Control of Spider Mites in the Ornamental Crop Gerbera. Laboratory of Entomology. Dissertation. The Netherlands: Wageningen University; 2000. [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Tricoli D. Influence of environmental factors and air composition on the emission of α-pinene from Quercus ilex leaves. Plant Physiol. 1996;110:267–275. doi: 10.1104/pp.110.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Tumlinson JH. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc Natl Acad Sci USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall PJ, Turlings TCJ, Loughrin J, Proveaux AT, Tumlinson JH. Herbivore-induced volatiles emissions from cotton (Gossypium hirsutum L.) seedlings. J Chem Ecol. 1994;20:3039–3050. doi: 10.1007/BF02033709. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takabayashi J, Yano S, Takafuji A. Effects of light on the tritrophic interaction between kidney bean plants, two-spotted spider mites and predatory mites, Amblyseius womersleyi (Acari:Phytoseiidae) Exp Appl Acar. 2000;24:415–425. doi: 10.1023/a:1006449108245. [DOI] [PubMed] [Google Scholar]
- Masters GJ, Brown VK, Gange AC. Plant mediated interaction between above- and below-ground insect herbivores. Oikos. 1993;66:148–151. [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. Induction of parasitoid attracting synomone in brussel sprouts plants by feeding of Pieris brassicae larvae: role of mechanical damage and herbivore elicitor. J Chem Ecol. 1994;20:2229–2247. doi: 10.1007/BF02033199. [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA. β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallini A, Janssen A, Sabelis MW. Odour-mediated responses of phytophagous mites to conspecific and heterospecific competitors. Oecologia. 1997;110:179–185. doi: 10.1007/s004420050147. [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weiss AE. Interaction among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Ann Rev Ecol Syst. 1980;11:41–65. [Google Scholar]
- Röse USR, Alborn HT, Makranczy G, Lewis WJ, Tumlinson JH. Host recognition by the specialist endoparasitoid Microplitis Croceipes (Hymenoptera, Braconidae): role of host- and plant-related volatiles. J Insect Behav. 1997;10:313–330. [Google Scholar]
- Sabelis MW, Dicke M. Long-range dispersal and searching behaviour. In: Helle W, Sabelis MW, editors. Spider Mites. Their Biology, Natural Enemies and Control. World Crop Pests. Amsterdam: Elsevier Science Publishers; 1985. pp. 141–160. [Google Scholar]
- Sabelis MW, van de Baan HE. Location of distant spider mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tretranichus urticae and Panonychus ulmi. Entomol Exp App. 1983;33:303–314. [Google Scholar]
- Taiz L, Zeiger E. Plant Physiology. Sunderland, MA: Sinauer Associates, Inc.; 1998. [Google Scholar]
- Takabayashi J, Dicke M, Kemerink J, Veldhuizen T. Environmental effects on production of a plant synomones that attracts predatory mites. Symp Biol Hung. 1990;39:541–542. [Google Scholar]
- Takabayashi J, Dicke M, Posthumus M. Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology. 1991;2:1–6. [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA. Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol. 1994;20:1329–1354. doi: 10.1007/BF02059811. [DOI] [PubMed] [Google Scholar]
- Takabayashi J, Takahashi S, Dicke M, Posthumus MA. Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol. 1995;21:273–287. doi: 10.1007/BF02036717. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot. 1998;49:419–432. [Google Scholar]
- Tingey DT, Manning M, Grothaus LC, Burns WF. Influence of light and temperature on monoterpene emission rates from slash pine. Plant Physiol. 1980;65:797–801. doi: 10.1104/pp.65.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlinson JH, Paré PW, Lewis WJ. Plant production of volatile semiochemicals in response to insect-derived elicitors. In: Chadwick DJ, Goode J, editors. Insect-Plant Interactions and Induced Plant Defence. Novartis Foundation. London: John Wiley & Sons; 1999. pp. 95–109. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH. Volicitin, an elicitor of corn volatiles in oral secretion of Spodopters exigua: isolation and bioactivity. J Chem Ecol. 2000;26:189–202. [Google Scholar]
- Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D. Timing of induced volatile emissions in corn seedlings. Planta. 1998;207:146–152. [Google Scholar]
- Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol. 1993;19:411–425. doi: 10.1007/BF00994314. [DOI] [PubMed] [Google Scholar]
- Turlings TJC, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Xin Z-Y, Zhou X, Pilet P-E. Level changes of jasmonic, abscisic, and indole-3-yl-acetic acids in corn under desiccation stress. J Plant Physiol. 1997;151:120–124. [Google Scholar]