Abstract
Gastrointestinal stromal tumours (GISTs) comprise a group of smooth muscle mesenchymal alimentary tract tumours of variable malignancy. Recently, the pathophysiology and radiology of these tumours has generated enormous interest following the discovery of a specific, highly effective, chemotherapeutic agent in the form of ST-571 (Imatinib; Glivec, Novartis, Frimley UK). At the time of this review, 106 patients with malignant gastrointestinal stromal tumours seen at the Royal Marsden Hospital have been entered into trials examining the efficacy of varying doses of Imatinib. Burkill et al., also from the Royal Marsden Hospital, have previously reported the distribution, imaging features and pattern of metastatic spread of these tumours (Burkill GJ, Badran M, Al-Muderis O et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 2003; 226: 527–32). This new review re-examines the radiological features of GISTs at presentation and well as their changed imaging features following treatment with Imatinib.
Keywords: Gastrointestinal stromal tumour (GIST), ST-571, Imatinib, radiological features
Introduction
A heterogeneous group of gastrointestinal smooth muscle tumours has been recognised for many years. These tumours are now grouped under the umbrella title of gastrointestinal stromal tumours (GISTs), of which leiomyomata are the benign form and generally form 70%–80%. Some malignant lesions, composed primarily of spindle shaped cells, comprise leiomyosarcomas, whereas those comprised primarily of epithelioid cells are designated leiomyoblastomas. The cellular features in these tumours are now recognised to be closely related to the interstitial cells of Cajal which form a structure within the GI tract involved in physiological control of smooth muscle activity. They therefore act as pacemakers of intestinal peristaltic activity [1, 2].
The crucial development in the understanding of these tumours has been, however, the demonstration that some highly malignant forms exhibit a growth factor receptor with tyrosine kinase activity named KIT. Activation of mutated tyrosine kinase leads to abnormal cellular proliferation. C-kit is the proto-oncogene responsible for the production of KIT and immunohistochemistry of these cells will display positivity for CD117 and CD34 [3]. Ninety percent of malignant GISTs harbour a mutation in KIT which is specifically inhibited by Imatinib. This agent acts by competing for the ATP binding site on the target kinase, inhibiting the tyrosine kinase thus reducing cellular proliferation.
Epidemiology and presentation
In the UK, the true incidence of GIST is not known precisely, but it is estimated to be as high as 16–20 /million population, and these tumours are thought to constitute approximately 5% all sarcomas. Between 5000 and 10000 people annually are estimated to develop this tumour worldwide. There is no reported significant sex difference [2] although the original Royal Marsden study identified 76 males and 40 females in their cohort. The median age at presentation is around 60 years. Grading of malignancy is a continuum based on assessment of tumour size and mitotic index [4, 5].
Small lesions of less than 2 cm are rarely symptomatic and are usually benign, often having been detected incidentally during the investigation of non-specific symptoms. Our series did, however, include one patient with a 3 cm duodenal tumour who developed obstructive jaundice. When tumours become larger, however, they may stimulate bleeding, abdominal pain, anaemia, abdominal distension or abdominal mass. In their report Burkill et al. identified a mean tumour diameter of 13 cm in a population of 116 patients. Because of their submucosal position and absence of local invasive characteristics, GISTs frequently reach considerable size without producing pain or signs of bowel obstruction. Tumours which arise in the stomach tend to have a less aggressive histology when compared with those elsewhere in the GI tract but 50% of GISTs will have metastasised by the time of presentation and on long term follow-up studies up to 85% of all patients develop local recurrence or metastasis [6]. In a study reported by Samiian et al. recurrence rate in malignant GIST after surgical excision was 53% with a mean time to recurrence of 16 months. Eighty percent of recurrences occurred within 2 years of excision [7]. The best hope for cure can only be achieved by complete and wide excision of the tumour and Langer et al. looked at long term survival in 39 patients following surgery in whom complete en bloc excision was achieved in 35. In this group only five died from recurrent disease, whereas in the four who had involved margins, three succumbed to local recurrence [8].
The median survival with metastatic GIST has been calculated as 20 months but in those patients with locally recurrent tumour, 9–12 months [2]. Hitherto, conventional doxorubicin based chemotherapy has been reported as effecting a response in less than 5% of cases [9]. Most recurrent GISTs are diagnosed on routine follow-up computed tomography (CT) studies and a single follow-up CT scan would seem appropriate for those patients with completely resected low risk tumours, whilst those with medium and high risk disease might usefully have a CT scan at 6 months followed by annually thereafter for 5 years. No evidence based follow-up protocols exist, as yet, however.
Imaging features
Although abdominal ultrasound is often the initial imaging test employed in the investigation of a patient with abdominal pain or mass, the tumour discovered is frequently so large as to render the organ of origin unidentifiable. The sonogram report frequently indicates the presence of a huge mass, often filling the abdomen, of heterogeneous reflectivity and frequent necrosis.
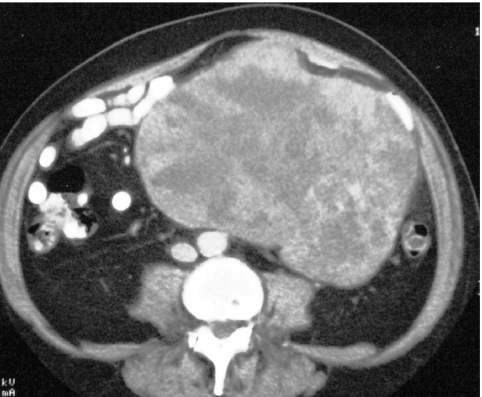
CT therefore provides the basis for diagnosis and staging in most patients. Tumours are usually of varying density, and show patchy enhancement after intra-venous contrast (Fig. 1). Varying degrees of necrosis may be frequently demonstrated within the mass.
Figure 1.
Axial enhanced CT showing a large, well-defined soft tissue mass with heterogenous enhancement in a bulky primary small bowel GIST.
The CT study will usually provide rapid and reproducible assessment of the size of the primary tumour, as well as its relationship to other structures. Metastatic disease may well be demonstrated at the outset [1]. Small primary tumours are frequently well marginated and, on unenhanced CT, are of low soft tissue density. On enhanced CT these tumours, when small, typically show homogeneous enhancement (Fig. 2) but, due to their rarity and absence of characteristic features, the diagnosis of GIST is seldom considered initially. They may mimic more common tumours such as pancreatic (Fig. 3), or oesophageal cancer (Fig. 4) and not infrequently small bowel GISTs mimic bowel lymphoma (Fig. 5). Occasionally, the demonstration of a well marginated rounded exophytic mass in the stomach may prompt a suggested diagnosis of GIST (Fig. 6).
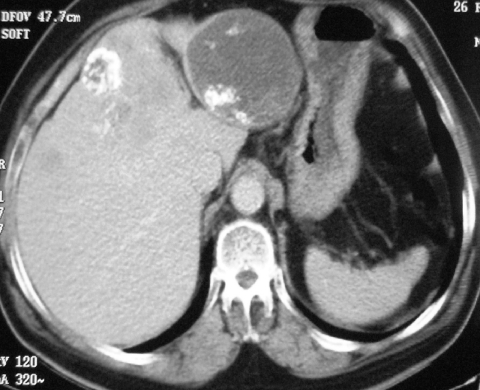
Figure 2.
Axial CT showing a small, well-defined soft tissue tumour exhibiting homogeneous enhancement arising from the proximal third part of the duodenum.
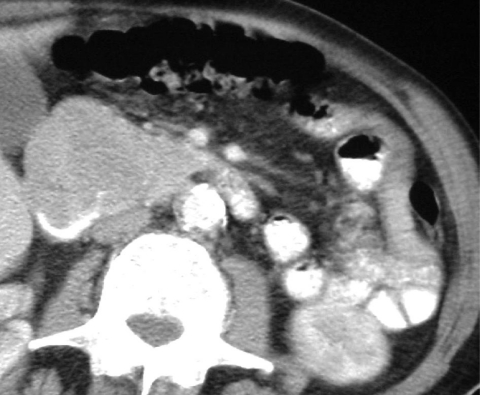
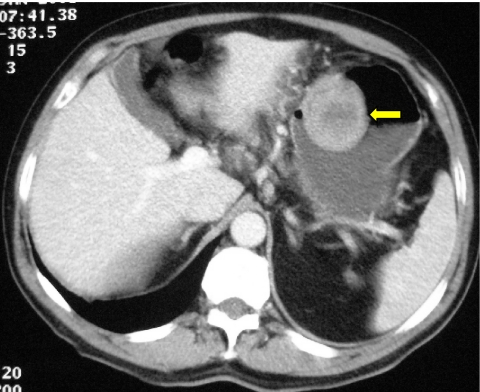
Figure 3.
Low attenuation GIST involving pancreatic head (arrowed) mimicking primary pancreatic adeno-carcinoma. Note lack of dilatation of biliary tree.
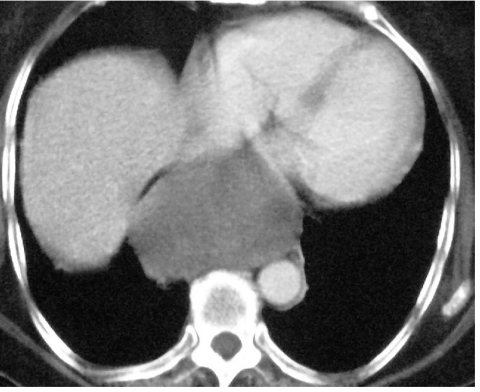
Figure 4.
Well-marginated low density mass in the position of the distal oesophagus. Biopsy proven GIST.
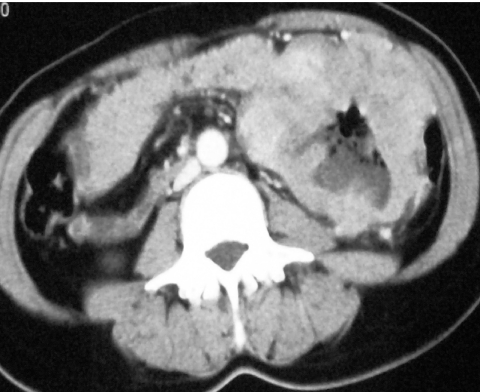
Figure 5.
Axial CT through the mid abdomen demonstrating a large, cavitating fistulating small bowel GIST.
Figure 6.
Well-defined exophytic gastric stromal tumour, showing homogeneous enhancement intruding into the body of the stomach (arrow).
Plain radiography has no role in the diagnosis of GIST but the frequency of gastric involvement may result in diagnosis at barium meal (Fig. 7(a)) with subsequent confirmation on CT (Fig. 7(b)). Increasingly frequently, however, gastric GISTs are the unsuspected finding at endoscopy performed in the investigation of vague abdominal symptoms.
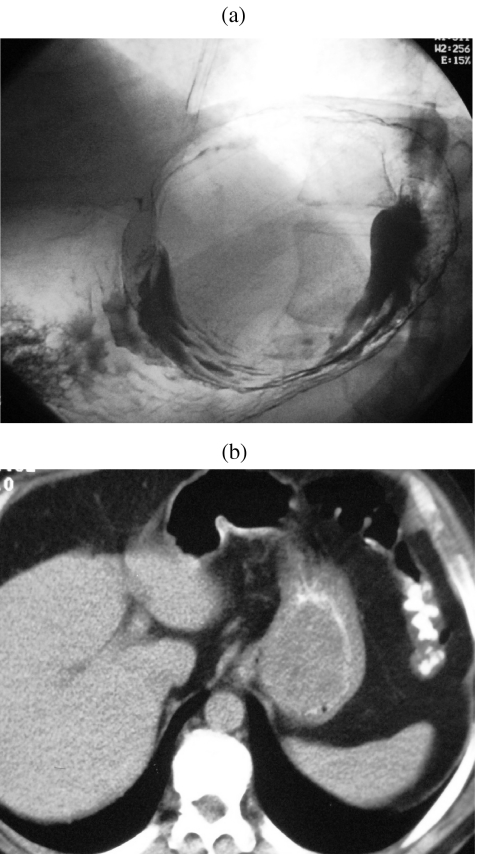
Figure 7.
(a) Smooth rounded filling defect resulting from sub-mucosal situation of GIST discovered on barium meal. (b) Axial CT showing gastric soft tissue mass with displaced intraluminal oral contrast.
Many GISTs achieve enormous size before diagnosis and demonstrate considerable cystic change usually associated with a surrounding rim of viable enhancing tumour (Fig. 8). Necrosis may lead to enteric fistulation and calcification within the tumour is occasionally recognised in association with this tumour necrosis (Fig. 9) [11].
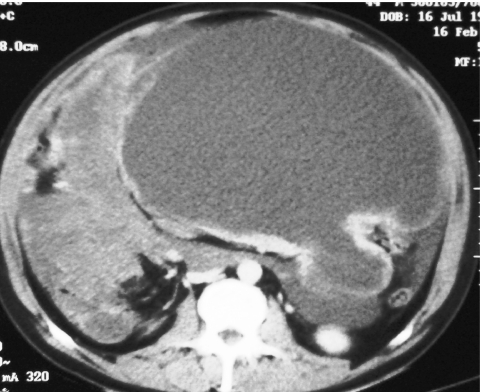
Figure 8.
Enhance axial CT demonstrating thin walled ‘cystic’ small bowel GIST.
Figure 9.
Punctate foci of calcific density on CT of a thin walled GIST.
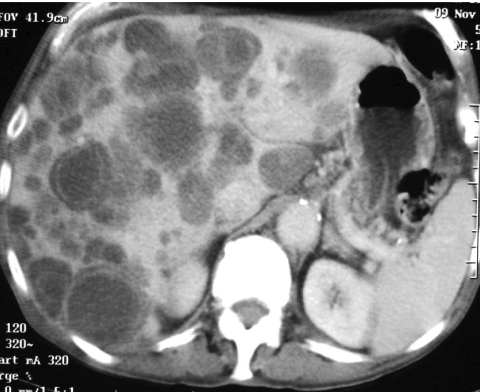
In their paper based on the cohort of 116 patients at the Royal Marsden, Burkill and colleagues demonstrated that 49 tumours arose in the small bowel, 43 in the stomach and 13 in colon and rectum [1]. In the 38 patients whose CT scans they were able to review, 23 showed metastases at presentation of which 13 involved the liver and eight were peritoneal. Both liver and peritoneal deposits are typically of large volume (Figs 10 and 11).
Figure 10.
Enhanced axial CT demonstrating extensive intrahepatic metastases of low and cystic density.
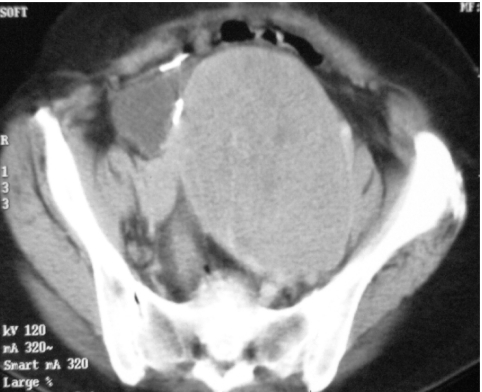
Figure 11.
CT showing very large, well-defined soft tissue tumour arising in the pelvic peritoneum.
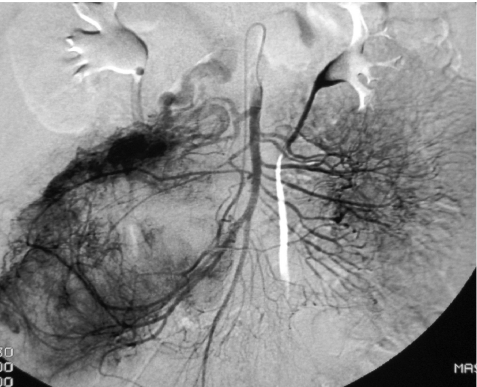
GI bleeding may complicate the management of patients with GISTs and angiography may be performed as part of the investigation into its cause. Frequently, prominent increased arterial and venous branching patterns will be displayed, together with, occasionally, evidence of extravasation. The demonstration of vascular anatomy may also aid in route mapping prior to surgery (Fig. 12).
Figure 12.
Hypervascular GIST shown on superior mesenteric angiogram.
The role of imaging in the management of GISTs
The principal treatment for GIST is surgery, involving wide local excision with a margin of 1–2 cm being ideal. The intention should be to achieve en bloc removal of the whole tumour (R0 resection). When achieved successfully R0 resection has been shown to be a good prognostic indicator for the development of metastatic disease, whereas those patients with involved margins have a high incidence of metastases [8]. Complete surgical resection has been reported, in a study of 200 patients, to be is accompanied by a 5 year survival of 54% [6]. Small gastric tumours may be managed without total gastrectomy. Small and large bowel tumours will usually require resection of adjacent mesentery and regional nodes although lymph node involvement in GIST is not common and formal lymphadenectomy is therefore not indicated [6, 10]. Whilst it has been recommended that pre-operative biopsies should be avoided due to risk of peritoneal seeding or tumour dehiscence of necrotic debris [12], GISTs are not infrequently diagnosed after image guided biopsy performed during the investigation of a patient with an abdominal mass of unknown origin.
Prior to the development of Imatinib, conventional chemotherapy was based upon standard sarcoma regimens employing doxorubicin most of which were consistently shown to have no impact on survival. In one study the response rate to doxorubicin was less than 5% [9]. Those patients with oesophageal tumours tend to have more favourable outcomes, whereas those with small intestinal primaries fare the worst [13]. Imatinib has now been shown in many studies to be a uniquely effective therapeutic agent by virtue of its selective inhibition of KIT.
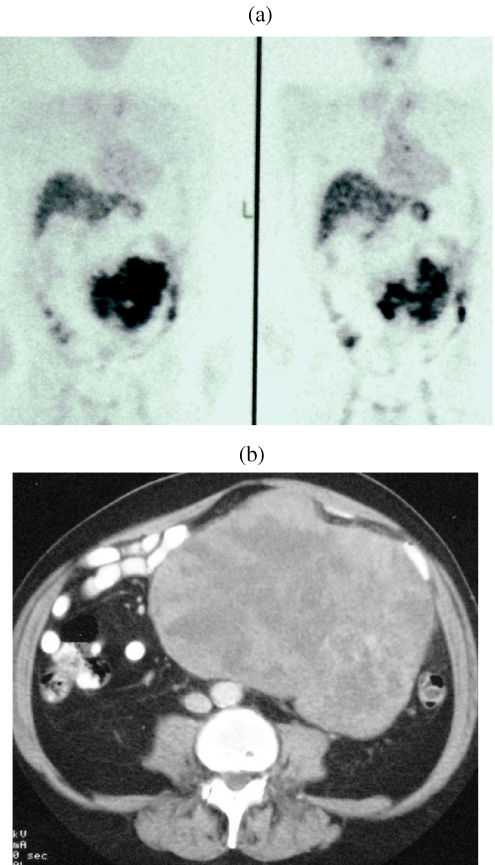
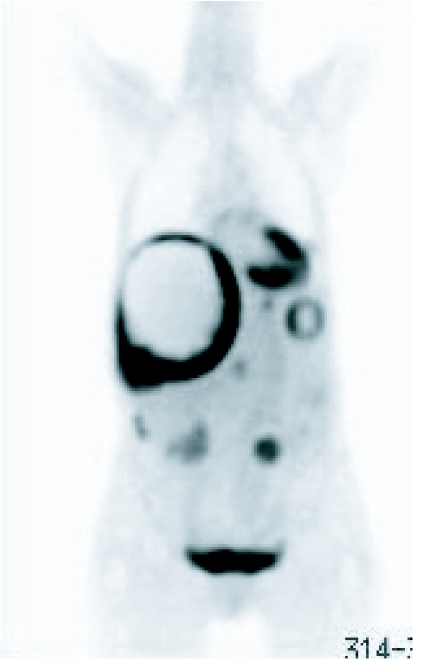
Most trials into the efficacy of Imatinib have employed serial CT examinations as a dominator of response but more recently the addition of positron emission tomography (PET) in the imaging armamentarium has added significantly to the specificity of imaging by virtue of its functional characteristics (Fig. 13(a), (b)). In their study of the value of PET, CT and PET/CT in monitoring response to treatment, Antoch et al. observed that PET/CT displayed more metastases from GISTs than CT and PET alone [14]. In 20 patients with GIST, PET/CT demonstrated 282 lesions, whereas 249 were detected on CT alone and 135 by PET alone. In characterising response to Imatinib the same authors demonstrated similar improved accuracy by the application of PET/CT. Indeed they demonstrated that in evaluating disease activity at 3 and 6 months, all methods using PET correctly assessed response in 100% patients, whereas CT viewed on its own was correct in 60% patients at 3 months and in 57% at 6 months [14]. They also observed that the functional imaging properties of PET provided much earlier evidence of response than the CT morpholgical imaging. A small number of false positive observations were, however, observed. Goerres et al. have reported the results of a study evaluating the prognostic power of PET, contrast enhanced CT and PET/CT in evaluating therapeutic impact of treatment by Imatinib in 34 patients with GIST. In a group of 28 patients they showed that patients without FDG activity after Imatinib had a better prognosis than those with residual activity. They also showed that although CT demonstrated more lesions, it was inferior in predicting prognosis [15].
Figure 13.
((a), (b)) PET image showing localized increased activity in association with large peritoneal GIST.
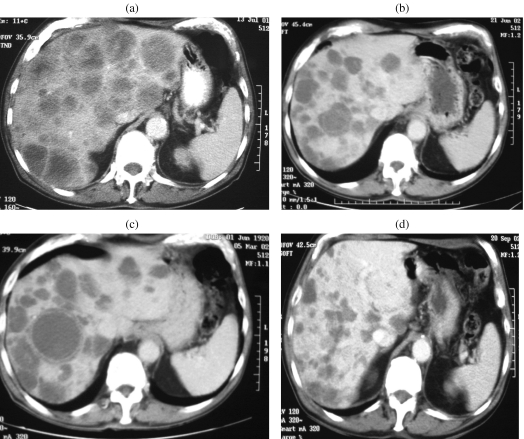
Cystic degeneration of metastases was seen in many of our patients (Fig. 14(a)–(d)) during treatment and this concurs with the observations of Chen et al. who described four patients in whom the hepatic metastases became cystic after treatment with Imatinaib [16]. In this area PET clearly has an advantage in being able to demonstrate tumour activity on the margins of the necrosis (Fig. 15).
Figure 14.
((a)–(d)) Imaging impact of Imatinib. Serial axial enhanced CT scans showing initial cystic change in hepatic metastases followed by significant involution of tumour deposits.
Figure 15.
Frontal PET image showing residual tumour activity in viable intra-hepatic mass surrounding large inactive area of cystic degeneration.
Conclusion
The management of malignant GISTs has been revolutionised by the development of Imatinib which is, uniquely, a therapeutic agent that targets a specific abnormal intracellular signalling molecule. The effective management of patients with these tumours requires regular imaging assessment for which CT has conventionally been the method of choice. Whilst it remains most valuable in the initial diagnosis and staging of GISTs, it is now clear that PET imaging, preferably combined with CT, will become the gold standard method for assessment of response by virtue of its unique dynamic functional characteristic which, when combined with CT, provides a more accurate assessment and prediction of the quality of response.
References
- 1.Burkill GJ, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003;226:527–32. doi: 10.1148/radiol.2262011880. [DOI] [PubMed] [Google Scholar]
- 2.Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC. The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol. 2001;54:96–102. doi: 10.1136/jcp.54.2.96. (review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarlamo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–34. [PubMed] [Google Scholar]
- 4.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. (review). [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–18. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 6.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samiian L, Weaver M, Velanovich V. Evaluation of gastrointestinal stromal tumors for recurrence rates and patterns of long-term follow-up. Am Surg. 2004;70:187–91. (discussion 191–2). [PubMed] [Google Scholar]
- 8.Langer C, Gunawan B, Schuler P, Huber W, Fuzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003;90:332–9. doi: 10.1002/bjs.4046. [DOI] [PubMed] [Google Scholar]
- 9.Goss GS, Merriam P, Manola J, Singer F, Fletcher CD, Demitri GD. Clinical and pathological characteristics of gastrointestinal stromal tumors. Prog Proc Am Soc Clin Oncol. 2000;19:A599. (abstract). [Google Scholar]
- 10.Lev D, Kariv Y, Issakov J, et al. Gastrointestinal stromal sarcomas. Br J Surg. 1999;86:545–9. doi: 10.1046/j.1365-2168.1999.01075.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304, 456. doi: 10.1148/rg.232025146. (quiz 532; review). [DOI] [PubMed] [Google Scholar]
- 12.De Matteo RP. The GIST of targeted cancer therapy: a tumour (gastrointestinal stromal tumour), a mutated gene (c-kit) and a molecular inhibitor (STI1571) Ann Surg Oncol. 2002;9:831–9. doi: 10.1007/BF02557518. [DOI] [PubMed] [Google Scholar]
- 13.Emory TS, Sobin LH, Lukes L, Lee DH, O’Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82–7. doi: 10.1097/00000478-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–65. [PubMed] [Google Scholar]
- 15.Goerres GW, Stupp R, Barghouth G, et al. The value of PET, CT and in-line PET/CT in patients with gastrointestinal tumours: long-term outcome of treatment with Imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32:153–62. doi: 10.1007/s00259-004-1633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MY, Bechtold RE, Savage PD. Cystic changes in hepatic metastases from gastrointestinal stromal tumors (GISTs) treated with Gleevec (imatinib mesylate) Am J Roentgenol. 2002;179:1059–62. doi: 10.2214/ajr.179.4.1791059. [DOI] [PubMed] [Google Scholar]