Abstract
Hepatocellular carcinoma (HCC) is the commonest malignancy of the liver and is usually due to cirrhosis. Early detection of HCC and the premalignant dysplastic nodules has implications on the management options of tumor ablation, liver resection and transplantation. Magnetic resonance imaging is useful for the detection and characterization of lesions, in the identification of dysplastic nodules and their malignant transformation into HCC.
Keywords: Cirrhosis, liver tumors, hepatocellular carcinoma, imaging, magnetic resonance imaging, computed tomography, ultrasonography, regenerative nodule, dysplastic nodule
Introduction
HCC is the commonest primary malignancy of the liver accounting for one million deaths annually worldwide. The incidence of HCC in North America has almost doubled during the past 20 years, more significantly among younger people of 40–60 years [1]. More than 80% of patients with HCC have underlying cirrhosis of varied etiology.
Cirrhosis of the liver is a progressive diffuse fibrosis with architectural distortion and nodular regeneration. Chronic viral hepatitis due to the Hepatitis B and Hepatitis C virus is the most important etiologic factor in North America followed by alcoholic liver disease. Other uncommon causes include hemochromatosis, hemosiderosis, Wilson’s Disease, alpha1 antitrypsin deficiency, Budd–Chiari Syndrome, primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, toxins like aflatoxin and cryptogenic etiology. All types of cirrhosis predispose to HCC.
Pathology of nodules in cirrhosis
Regenerative nodules represent focal proliferation of hepatocytes in response to various injurious stimuli. They can be classified as micronodular (<5 mm) or macronodular (>5 mm). Their blood supply is from the portal vein and hence they show an enhancement pattern similar to the normal liver parenchyma. Some nodules may contain iron (siderotic nodules) and these have a greater propensity towards dysplasia.
Dysplastic nodules are premalignant and contain atypical hepatocytes without definite features of malignancy on histology. According to the severity of the cellular atypia, they can be low grade or high grade and can undergo malignant transformation in a short duration of 4 months [2]. They receive their blood supply from the portal vein and are hypovascular. However, occasionally they can have increased arterial flow [3].
HCC may be solitary, multifocal or diffusely infiltrative. Small HCC (<3 cm) are usually well differentiated whereas larger and diffuse HCC are poorly differentiated. They receive their blood supply mostly from the hepatic artery although rarely portal venous supply may be noted. Fibrous capsule and fat may be noted in well differentiated HCC thus differentiating them from dysplastic nodules. Fibrous septa, necrosis, hemorrhage and invasion of veins and bile ducts can also be present.
Importance of early detection
Early detection and staging of HCC are necessary for the more effective triage of patients and in planning management strategies like resection, transplantation, tumor ablation (using radiofrequency, cryotherapy or percutaneous ethanol injection) and chemo-embolization. Although long term survival is poor, it is significantly increased following early diagnosis and treatment. The survival of cirrhotic patients undergoing transplantation with a solitary HCC of less than 2 cm is similar to that of patients transplanted for non-malignant disease [4] whereas patients transplanted with up to three discrete lesions of less than 3 cm or a solitary 2–5 cm lesion have a 75% 4-year survival [5]. The commonly used criteria for liver transplantation include a single tumor of less than 5 cm, fewer than three tumors (largest no greater than 3–4 cm), no invasion of major blood vessels and no lymph node or extra-hepatic site involvement. When patients fall outside these criteria, the survival rate is reduced. There is an advantage of liver transplantation over liver resection, even in potentially resectable HCC. In patients with cirrhosis and HCC, living donor liver transplantation yields superior results when the waiting time for a cadaveric organ exceeded 7 months [6].
Diagnosis of HCC
Measurement of serum alpha fetoprotein (AFP) is used for the screening of patients with cirrhosis and rising serum AFP levels are diagnostic of HCC. However, it is neither sensitive nor specific if used alone. Moreover, with dysplastic nodules and small HCC, the level of AFP is usually in the normal range and imaging plays an important role in the early detection of dysplastic nodules and HCC.
Ultrasonography
Sonography has variable sensitivity in the detection of HCC in the cirrhotic liver ranging from 33 to 96% with a high sensitivity of 80% if HCC is suspected clinically. However, there are no specific features to distinguish dysplastic nodules from HCC and it has low sensitivity for the detection of dysplastic nodules (0–1.6%) and also for small HCC less than 1 cm [7]. Harmonic imaging and sonographic contrast agents may improve detection of the lesions [8–10].
Computed tomography
Multiphasic dynamic helical computed tomography (CT) is useful in the evaluation of nodular lesions in the cirrhotic liver. Arterial phase imaging is most useful for the detection of HCC as its predominant blood supply is from the hepatic artery. However, it is less sensitive for the detection of small HCC and for dysplastic nodules which appear isodense to the liver parenchyma due to their predominant blood supply from the portal vein [11, 12]. CT arterio-portography and CT hepatic arteriography are more sensitive for the detection of HCC but the false positive rate is high due to benign hypervascular lesions like arterioportal shunts [13, 14]. Multidetector CT has a higher sensitivity in the detection of HCC in patients with cirrhosis due to increased speed and improved spatial and temporal resolution. Double arterial phase imaging is useful for the evaluation of hepatic arterial anatomy which is essential in patients who are likely to undergo surgery as well as in improved detection of HCC.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is extremely useful in the detection and characterization of regenerating and dysplastic nodules and HCC (Table 1). Several studies have demonstrated the superiority of MRI in both lesion detection and characterization for focal hepatic lesions when compared to CT [15, 16].
Table 1.
MR features of focal liver lesions in cirrhosis
| Lesion | T1 W image | T2 W image | Contrast enhancement pattern | SPIO uptake | Other features |
|---|---|---|---|---|---|
| Regenerative nodule | Variable | Hypointense | Enhances during portal venous phase | Present | Siderosis |
| Dysplastic nodule | Hyperintense | Hypointense | Enhances during portal venous phase | Present | Siderosis, nodule-in-nodule |
| HCC (small) | Hypointense | Hyperintense | Enhances during arterial phase | Absent | Nodule-in-nodule |
| HCC (large) | Heterogeneous | Hyperintense | Enhances during arterial phase | Absent | Fibrous capsule, satellite |
| nodules, invasion, fat | |||||
| Pseudolesion | Variable | Hypointense | Enhances during arterial phase | Absent | |
Techniques
There are various new techniques that can be employed in the evaluation of HCC and nodular lesions of the liver. Non-contrast sequences include fast spin echo sequence with short and long echo times (T1 and T2 W images), in-phase and opposed phase T1 W gradient echo images and fat suppression with T2 W fast spin echo sequences.
The choice of sequence is generally dictated by field strength, gradient factors and patient cooperation. In general T1 weighted imaging is performed with the breath hold spoiled gradient echo (SGE) technique. The advantages of this sequence include fast data acquisition, avoidance of breathing artifacts and complete coverage of the liver in a single breath hold. Optimal parameters for this sequence include relatively long TR (100–150 ms) and flip angle of approximately 60–90 degrees. This sequence generates 14–22 sections in a 20-s breath hold period. The drawback of SGE is that it requires the patient to suspend respiration. In patients who cannot cooperate, spin echo techniques are effective if the breathing is regular, otherwise breathing independent techniques such as turbo FLASH are necessary. T2 weighted imaging is most frequently performed as echo train spin echo (FSE or TSE). Short tau inversion recovery (STIR) may also be used as a T2 weighted sequence and this can be modified to turbo STIR to save time. In-phase and opposed phase T1 W gradient echo images are useful for the evaluation of fatty infiltration of lesions.
Multiphasic dynamic gadolinium contrast enhanced T1 W images in hepatic arterial, portal venous and delayed phases improve the detection and characterization of lesions, particularly small HCC, and they are superior to multiphasic helical CT [17–19]. It is performed with a T1 weighted GRE sequence with the shortest possible TE and with a breath-hold technique. 3-D sequences are recommended as they provide both a higher signal to noise ratio and thinner effective slice thickness. Fat suppression is desirable because the conspicuity of contrast enhancement is improved. However, small HCC may not always be detected.
Superparamagnetic iron oxide (SPIO) or Ferumoxide particles are taken up by Kuppfer cells and result in decreased signal intensity on T2 W images. Uptake of SPIO occurs in benign hepatocellular lesions but not in HCC, thus improving the detection of small HCC and also helping in the differentiation of HCC from regenerative and dysplastic nodules, both of which show SPIO uptake. Hypovascular HCC not seen on a dynamic Gd-contrast study may be detected on SPIO images. Double contrast MR imaging using gadolinium and SPIO is found to be highly sensitive (92%) in the diagnosis of HCC larger than 1 cm and better than either of them alone. However, the sensitivity for the detection of subcentimeter lesions (38%) is still poor [20–22].
Tissue or liver specific MR contrast agents like Mangafodipir (MnDPDP), Gadobenate (Gd-BOPTA) and Gadoxetic acid (Gd-EOB-DTPA) may be useful in the detection of hepatocellular tumors. However, they are not superior to gadolinium and are not useful in the differentiation of well-differentiated HCC from benign nodules [23–25].
Imaging characteristics
Regenerative nodules
These have variable intensity on T1 W images, are hypointense on T2 W images with an enhancement pattern similar to normal liver parenchyma and without abnormal enhancement during the arterial phase. They also take up SPIO [26–28]. Siderotic nodules appear hypointense on T1 and T2 W gradient recalled echo images. However, siderosis can also be noted in dysplastic nodules.
Dysplastic nodules
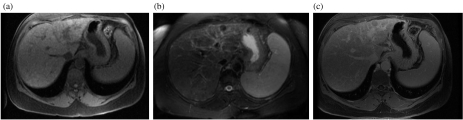
Due to the gradual change of a regenerative nodule to a dysplastic nodule and then to HCC, it may not always be possible to differentiate one from the other. Dysplastic nodules appear hyperintense on T1 W and hypointense on T2 W images (Fig. 1). They enhance in the portal venous phase and appear iso/hyperintense to liver parenchyma. Uptake of SPIO and the presence of siderotic foci may also be noted. Malignant change appears as nodule-within-a-nodule with hyperintense foci within a hypointense nodule on T2 W images. However, the nodule-within-nodule appearance can also occur in small HCC. The absence of a fibrous capsule, fat, arterial phase enhancement and hyperintensity on T2 W images help in differentiating dysplastic nodules from HCC. However, occasionally, dysplastic nodules can enhance during the hepatic arterial phase [29, 30].
Figure 1.
MRI of the dysplastic nodule. (a) T1 weighted image showing a hyperintense dysplastic nodule in the left lobe of the liver. (b) Nodule is characteristically hypointense on T2 weighted image. (c) Non enhancing after IV gadolinium administration.
HCC
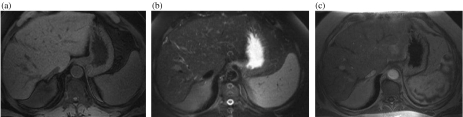
Most HCC are characteristically hypointense on T1 W and hyperintense on T2 W images with intense enhancement during the hepatic arterial phase of a dynamic gadolinium contrast study (Fig. 2). However, the intensity on T1 W images can be variable due to hemorrhage and the presence of copper, protein, lipid and glycogen. HCC show no uptake of SPIO.
Figure 2.
Small HCC in segment 8 of the liver. (a) T1 weighted image showing a small hypointense nodule adjacent to the right hepatic vein. (b) Nodule is characteristically hyperintense on T2 weighted image. (c) Enhancement during arterial phase after administration of IV gadolinium.
Small HCC, in addition may have nodule-within-a-nodule appearance. Large HCC may have certain characteristic features including: (a) a fibrous capsule that appears hypointense on T1 W and T2 W images with enhancement during the portal venous or delayed phases of a dynamic contrast study, depending on the extent of vascularity [31, 32]; (b) a mosaic appearance due to areas of necrosis and hemorrhage; (c) extra capsular extension into adjacent parenchyma and vessels; (d) satellite nodules; (e) lymph nodal and distant metastases.
Diffuse type HCC is an extensive, ill-defined, infiltrative, heterogeneous tumor with variable intensity on T1 W and heterogeneous hyperintensity on T2 W images and variable enhancement on the early phase of dynamic contrast study [33].
There are certain benign lesions like focal fibrosis, hemangioma, arterio-portal shunts and other pseudolesions of unknown etiology which can be mistaken for HCC on imaging. However, the different techniques of MR are useful in differentiating them from HCC. A multi-phasic dynamic contrast study helps in differentiating HCC from focal fibrosis which enhances only during the portal venous and delayed phases. Hemangiomas show peripheral enhancement during the early arterial phase with complete intense enhancement during the delayed phase, unlike HCC which enhances completely during the early arterial phase [34, 35]. MRI with blood pool contrast agent Code 7227 has also been found to be useful in differentiating HCC from hemangiomas [36]. Diffusion weighted MRI, though not routinely performed, may also be useful in the differentiation of HCC from other focal lesions like metastases and hemangiomas, as their mean ADC values are different [37]. Other lesions that enhance during the early arterial phase, like arterio-portal shunts and other pseudolesions of unknown etiology, can be differentiated from HCC by the signal intensity on T2 W images; pseudolesions are of variable signal intensity on T1 W images and hypointense on T2 W images, whereas HCC is hyperintense [38, 39].
Dynamic MRI is also useful for the evaluation of the effect of chemoembolization on HCC as it shows enhancement on the hepatic arterial phase but only in the viable tissue [40–42].
Conclusion
The various techniques of MRI are useful and more sensitive than other modalities in the early detection and characterization of HCC in the cirrhotic liver and in differentiating it from dysplastic nodules and pseudolesions.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Takayama T, Makuuchi M, Hirohashi S, et al. Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet. 1990;336:1150–3. doi: 10.1016/0140-6736(90)92768-d. [DOI] [PubMed] [Google Scholar]
- 3.Matsui O, Kadoya M, Kameyama T, et al. Benign and malignant nodules in cirrhotic livers: distinction on blood supply. Radiology. 1991;178:493–7. doi: 10.1148/radiology.178.2.1846240. [DOI] [PubMed] [Google Scholar]
- 4.Hemming AW, Cattral MS, Reed AI, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652–9. doi: 10.1097/00000658-200105000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Patt CH, Thuluvath PJ. Role of liver transplantation in the management of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S205–10. doi: 10.1016/s1051-0443(07)61788-6. [DOI] [PubMed] [Google Scholar]
- 7.Bennett GL, Krinsky GA, Abitbol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis. Correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR. 2002;179:75–80. doi: 10.2214/ajr.179.1.1790075. [DOI] [PubMed] [Google Scholar]
- 8.Hann LE, Bach AM, Cramer LD, et al. Hepatic sonography: comparison of tissue harmonic and standard sonography techniques. AJR. 1999;173:201–6. doi: 10.2214/ajr.173.1.10397127. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SR, Burns PN, Muradali D, et al. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153–61. doi: 10.1148/radiology.215.1.r00ap08153. [DOI] [PubMed] [Google Scholar]
- 10.Harvey CJ, Blomley MJ, Eckersley RJ, et al. Hepatic malignancies: improved detection with pulse-inversion US in late phase of enhancement with SH U 508A – early experience. Radiology. 2000;216:903–8. doi: 10.1148/radiology.216.3.r00se22903. [DOI] [PubMed] [Google Scholar]
- 11.Lim JH, Kim CK, Lee WJ, Park CK, Koh KC, Paik SW, Joh JW. Detection of hepatocellular carcinoma and dysplastic nodules in cirrhotic livers. Accuracy of helical CT in transplant patients. AJR. 2000;175:693–8. doi: 10.2214/ajr.175.3.1750693. [DOI] [PubMed] [Google Scholar]
- 12.Peterson MS, Baron RL, Marsh JW Jr, et al. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology. 2000;217:743–9. doi: 10.1148/radiology.217.3.r00dc28743. [DOI] [PubMed] [Google Scholar]
- 13.Jang HJ, Lim JH, Lee SJ, et al. Hepatocellular carcinoma: are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology. 2000;215:373–80. doi: 10.1148/radiology.215.2.r00ma30373. [DOI] [PubMed] [Google Scholar]
- 14.Kim TK, Choi BI, Han JK, et al. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998;208:597–603. doi: 10.1148/radiology.208.3.9722834. [DOI] [PubMed] [Google Scholar]
- 15.Semelka RC, Martin DR, Balci C, Lance T. Focal hepatic lesions: comparison of dual phase CT and multisequence multiplanar MR imaging including dynamic gadolinium enhancement. J Magn Reson Imaging. 2001;13:397. doi: 10.1002/jmri.1057. [DOI] [PubMed] [Google Scholar]
- 16.Yamshita Y, Mitsuzaki K, Yi T, et al. Small hepatocellular carcinoma in patient with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology. 1996;200:79–84. doi: 10.1148/radiology.200.1.8657948. [DOI] [PubMed] [Google Scholar]
- 17.Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology. 1996;201:337–45. doi: 10.1148/radiology.201.2.8888220. [DOI] [PubMed] [Google Scholar]
- 18.Oi H, Murakami T, Kim T, et al. Dynamic MR imaging and early phase helical CT for detecting small intrahepatic metastases of hepatocellular carcinoma. AJR. 1996;166:369–74. doi: 10.2214/ajr.166.2.8553950. [DOI] [PubMed] [Google Scholar]
- 19.Rode A, Bancel B, Douek P, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT and MR and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327–36. doi: 10.1097/00004728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto H, Yamashita Y, Yoshimatsu S, et al. Hepatocellular carcinoma in cirrhotic livers: detection with unenhanced and iron oxide-enhanced MR imaging. Radiology. 1995;195:106–12. doi: 10.1148/radiology.195.1.7892448. [DOI] [PubMed] [Google Scholar]
- 21.Ward J, Guthrie JA, Scott DJ, et al. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology. 2000;216:154–62. doi: 10.1148/radiology.216.1.r00jl24154. [DOI] [PubMed] [Google Scholar]
- 22.Bhartia B, Ward J, Guthrie A, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR. 2003;180:577–84. doi: 10.2214/ajr.180.3.1800577. [DOI] [PubMed] [Google Scholar]
- 23.Sahani DV, O’Malley ME, Bhat S, et al. Contrast-enhanced MRI of the liver with mangafodipir trisodium: imaging technique and results. J Comput Assist Tomogr. 2002;26:216–22. doi: 10.1097/00004728-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Murakami T, Baron RL, Peterson MS, et al. Hepatocellular carcinoma: MR imaging with mangafodipir trisodium (Mn-DPDP) Radiology. 1996;200:69–77. doi: 10.1148/radiology.200.1.8657947. [DOI] [PubMed] [Google Scholar]
- 25.Rofsky NM, Weinreb JC, Bernardino ME, et al. Hepatocellular tumors: characterization with Mn-DPDP-enhanced MR imaging. Radiology. 1993;188:53–9. doi: 10.1148/radiology.188.1.8390072. [DOI] [PubMed] [Google Scholar]
- 26.Earls JP, Theise ND, Weinreb JC, et al. Dysplastic nodules and hepatocellular carcinoma: thin-section MR imaging of explanted cirrhotic livers with pathologic correlation. Radiology. 1996;201:207–14. doi: 10.1148/radiology.201.1.8816545. [DOI] [PubMed] [Google Scholar]
- 27.Matsui O, Kadoya M, Kameyama T, et al. Adenomatous hyperplastic nodules in the cirrhotic liver: differentiation from hepatocellular carcinoma with MR imaging. Radiology. 1989;173:123–6. doi: 10.1148/radiology.173.1.2550995. [DOI] [PubMed] [Google Scholar]
- 28.Muramatsu Y, Nawano S, Takayasu K, et al. Early hepatocellular carcinoma: MR imaging. Radiology. 1991;181:209–13. doi: 10.1148/radiology.181.1.1653443. [DOI] [PubMed] [Google Scholar]
- 29.Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–54. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 30.Krinsky GA, Theise ND, Rofsky NM, et al. Dysplastic nodules in cirrhotic liver: arterial phase enhancement at CT and MR imaging – a case report. Radiology. 1998;209:461–4. doi: 10.1148/radiology.209.2.9807574. [DOI] [PubMed] [Google Scholar]
- 31.Kadoya M, Matsui O, Takashima T, et al. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992;183:819–25. doi: 10.1148/radiology.183.3.1316622. [DOI] [PubMed] [Google Scholar]
- 32.Grazioli L, Olivetti L, Fugazzola C, et al. The pseudocapsule in hepatocellular carcinoma: correlation between dynamic MR imaging and pathology. Eur Radiol. 1999;9:62–7. doi: 10.1007/s003300050629. [DOI] [PubMed] [Google Scholar]
- 33.Kanematsu M, Semelka RC, Leonardou P, et al. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging. 2003;18:189–95. doi: 10.1002/jmri.10336. [DOI] [PubMed] [Google Scholar]
- 34.Murakami T, Mitani T, Nakamura H, et al. Differentiation between hepatoma and hemangioma with inversion-recovery snapshot FLASH MRI and Gd-DTPA. J Comput Assist Tomogr. 1992;16:198–205. doi: 10.1097/00004728-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Itai Y, Ohtomo K, et al. Small hepatocellular carcinoma and cavernous hemangioma: differentiation with dynamic FLASH MR imaging with Gd-DTPA. Radiology. 1989;171:339–42. doi: 10.1148/radiology.171.2.2539606. [DOI] [PubMed] [Google Scholar]
- 36.Harisinghani MG, Saini S, Weissleder R, et al. Differentiation of liver hemangiomas from metastases and hepatocellular carcinoma at MR imaging enhanced with blood-pool contrast agent Code-7227. Radiology. 1997;202:687–91. doi: 10.1148/radiology.202.3.9051017. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa T, Haradome H, Hachiya J, et al. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR. 1998;170:397–402. doi: 10.2214/ajr.170.2.9456953. [DOI] [PubMed] [Google Scholar]
- 38.Kamura T, Kimura M, Sakai K, et al. Small hypervascular hepatocellular carcinoma versus hypervascular pseudolesions: differential diagnosis on MRI. Abdom Imaging. 2002;27:315–24. doi: 10.1007/s00261-001-0074-z. [DOI] [PubMed] [Google Scholar]
- 39.Ueda K, Matsui O, Kadoya M, et al. Pseudolesion in segment IV of the liver on MRI: prevalence and morphology in 250 cirrhotic livers compared with 250 normal livers. J Comput Assist Tomogr. 1999;23:63–8. doi: 10.1097/00004728-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 40.De Cobelli F, Castrucci M, Sironi S, et al. Role of magnetic resonance in the follow-up of hepatocarcinoma treated with percutaneous ethanol injection (PEI) or transarterial chemoembolization (TACE) Radiol Med (Torino) 1994;88:806–17. [PubMed] [Google Scholar]
- 41.Murakami T, Nakamura H, Hori S, et al. Detection of viable tumor cells in hepatocellular carcinoma following transcatheter arterial chemoembolization with iodized oil. Pathologic correlation with dynamic turbo-FLASH MR imaging with Gd-DTPA. Acta Radiol. 1993;34:399–403. [PubMed] [Google Scholar]
- 42.Sironi S, Livraghi T, Angeli E, et al. Small hepatocellular carcinoma: MR follow-up of treatment with percutaneous ethanol injection. Radiology. 1993;187:119–23. doi: 10.1148/radiology.187.1.8383864. [DOI] [PubMed] [Google Scholar]