Abstract
Sinonasal disease is one of the most common clinical head and neck pathologies. The majority of sinonasal pathology is inflammatory with neoplasms comprising approximately 3% of all head and neck tumours. Although sinus tumours are rare, they portend a poor prognosis, often due to advanced disease at diagnosis. Like most neoplasms, early detection improves prognosis, therefore clinicians and radiologists should be aware of features separating tumours from inflammatory sinus disease. This article reviews the anatomy, clinical features, imaging findings, treatment and histopathology of selected sinonasal tumours. Benign neoplasms reviewed include osteoma, inverting papilloma, and juvenile nasal angiofibroma. Malignant neoplasms reviewed include squamous cell carcinoma, the minor salivary gland tumour, adenoid cystic carcinoma, adenocarcinoma, melanoma, lymphoma, and olfactory neuroblastoma (esthesioneuroblastoma).
Keywords: Sinuses, carcinoma, computed axial tomography (CT), magnetic resonance imaging (MRI)
Introduction
The sinonasal cavity extends from the nostrils to the posterior nasal septum ending posteriorly in the nasopharynx. The nasal cavity floor is the hard palate, also the roof of the mouth. Three turbinate bones project medially from the lateral walls. Four aerated paranasal sinuses: maxillary, ethmoid, frontal and sphenoid surround the nasal cavity. Ethmoidal sinuses create the superior, lateral and medial nasal cavity walls with bilateral maxillary antra forming the infero-lateral margins. The superior wall of the maxillary sinus forms the orbital floor, disrupted by the infraorbital groove containing the infraorbital nerve. The frontal sinuses anteriorly contribute to the orbital roofs and the sphenoid sinus posteriorly is the nasopharynx roof. Classically, benign neoplasms expand and remodel bone and aggressive malignancies destroy and invade adjacent tissues with ill-defined margins. These rules however, may be broken in sinonasal imaging. Computed tomography (CT) has superior bony definition while magnetic resonance imaging (MRI) better distinguishes tumour versus retained secretions. MRI gives superior soft tissue delineation in the adjacent infratemporal fossa, masticator space, and in evaluation of perineural, intraorbital and intracranial spread [1].
Staging for sinus cancer is via the T-system noted below:
T1: tumour confined to antral mucosa with no bone erosion or destruction
T2: tumour with erosion or destruction of infrastructure, hard palate, and/or middle nasal meatus
T3: tumour invasion into skin of cheek, posterior maxillary sinus wall, floor or medial wall of orbit, anterior ethmoid sinus
T4: massive tumour with invasion of cribiform plate, posterior ethmoids, sphenoid, nasopharynx, ptyergoid plates, base of skull or orbit.
Additional imaging considerations include determination of tumour margins for assessing resectability, surgical approach and radiation therapy fields. Imaging is vital in distinguishing tumour from infection, retained secretions, and granulation scar tissue. The majority of malignant sinonasal cavity tumours are epithelial in origin with approximately 80% squamous cell carcinomas [2]. Because these cellular tumours have little water content, they demonstrate low to intermediate signal intensity on MRI images. Ten percent of sinonasal tumours are lymphomas or olfactory neuroblastomas, sarcomas, and fibrous histiocytomas. These cellular carcinomas also display MRI characteristics similar to carcinomas found elsewhere. The final 10% of tumours arise from minor salivary glands, and reflect their diverse histology containing either serous or mucinous elements. The majority of tumours on MRI T2W sequences have intermediate signal intensity compared to inflammatory tissue which has increased signal intensity [3]. Approximately 5% of sinonasal tumours of minor salivary gland origin may have increased T2-signal, and a biopsy may be needed to determine if a tumour is present.
Benign tumours
Osteoma
Clinical features
The presentation of osteomas depends on their location, commonly originating in the frontal sinuses [4]. These benign tumours are usually incidental findings on imaging. They may block the fronto-ethmoidal recess giving rise to unilateral headaches or a mucocele, which may expand intracranially, causing pressure symptoms, personality changes and pneumatocoeles or intraorbitally, causing proptosis and diplopia [5, 6]. Ethmoidal osteomas invariably spread intraorbitally causing orbital symptoms and occasional nasal obstruction, epiphora and facial deformity [7]. Rarely, osteomas may present in the maxillary and sphenoid sinuses [8].
Imaging
Osteomas on CT scanning are hyperdense bony masses protruding into the sinus cavity. Because of the dense compact bone, they are poorly seen on MRI.
Treatment
Only osteomas causing symptoms are removed surgically. They are difficult removals due to the challenge of obtaining adequate exposure, spawning a variety of surgical approaches. The osteoplastic frontal sinus approach with coronal incision is preferred for best aesthetic results for frontal sinus osteomas [9]. Ethmoidal osteomas can be removed with a mid-facial degloving or a lateral rhinotomy combined with orbital exposure and medial maxillectomy. Endonasal approach with stereotactic localisation, is used increasingly for excision [10, 11]. Treatment is individualised and close follow-up is needed to rule out recurrence [12].
Pathology
Tumour origin is ascribed to embryologic tissue maldevelopment, trauma, or infection. The tumours are hard and lobulated with an ivory-like appearance, often mixed with a coarse granular component. The bone is compact or cancellous, with vascular or connective tissue components [4].
Inverted papilloma
Clinical features
Inverted papilloma is a benign tumour classically arising in the lateral nasal wall near the middle turbinate [13]. The tumour is predominant in males, and is staged according to disease extent and surgical planning [14, 15]. Tumours are often unilateral and have a pernicious ability to recur after partial resection. They are unfortunately associated with squamous cell carcinomas, with reports varying from 2% to 15% [20, 21].
Imaging
By location, tumours arise from the nasal cavity lateral walls and septum. It is virtually impossible to distinguish benign versus malignant papilloma on imaging as both have intermediate signal on T2W MRI and solid enhancement. Associated bone loss is often a sign of aggressiveness either neoplastic or in some cases due to infection or inflammation (Fig. 1).
Figure 1.
Coronal CT scan, bone windows, soft tissue mass obstructing the right osteomeatal unit and complete opacification of the right maxillary antrum. Bony erosion noted along the right maxillary medial wall.
Treatment
Traditional techniques such as Caldwell-Luc and conservative trans-nasal ethmoidectomy or spheno-ethmoidectomy have given way to endoscopic surgery as an effective treatment for inverted papillomas [16]. Proper patient selection, meticulous use of sub-periosteal dissection, and regular follow-up are key for success [17–19]. Radiation therapy may be considered in incompletely resectable lesions, recurrent tumours, and tumours associated with malignancy [20].
Pathology
Inverted papillomas have marked patchy squamous metaplasia in ductal and surface epithelium and numerous microcysts containing macrophages in the epithelia. Low-grade squamous carcinoma is difficult to distinguish from inverted papilloma on imaging and biopsy with histopathology is the best option [21].
Juvenile nasopharyngeal angiofibroma
Clinical features
Juvenile nasopharyngeal angiofibroma (JNA) is a histologically benign, locally invasive tumour found primarily in the pubescent male [22]. Patients present with recurrent epistaxis and nasal obstruction [23]. Two constant features include: (1) mass in posterior nasal cavity and pterygopalatine fossa; (2) erosion of bone behind the sphenopalatine foramen extending to the upper medial pterygoid plate [24]. Chandler’s, Fisch’s and Radkowski’s staging are based on tumour extent and spread and demarcate surgical approaches and prognosis. Grade 1 is confined to nasopharynx, Grade 2 is spread into pterygopalatine fossa or masticator space, Grade 3 is spread intraorbitally or intracranially.
Imaging
Characteristics on either CT or MRI include bowing of the posterior wall of maxillary antrum anteriorly, enlargement of pterygopalatine fossa with bone erosion posterior to the sphenopalatine foramen extending towards the medial pterygoid plate [24] and on post-contrast studies dense enhancement with the mass predominately fed by the ipsilateral internal maxillary artery and small ascending pharyngeal artery branches (Figs 2–4).
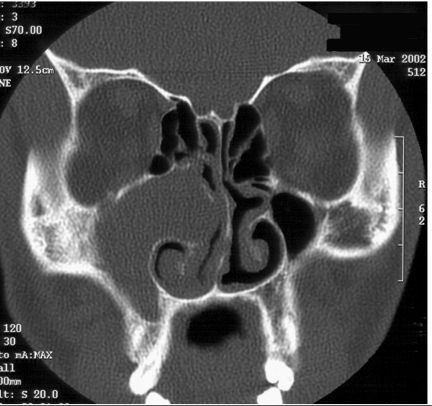
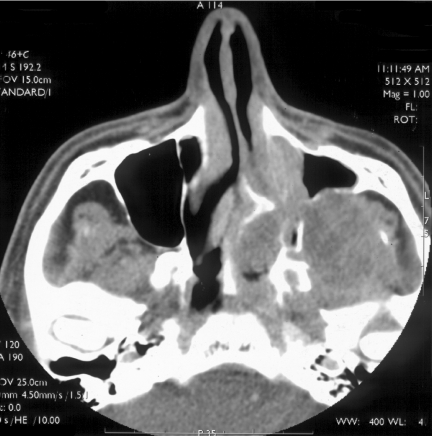
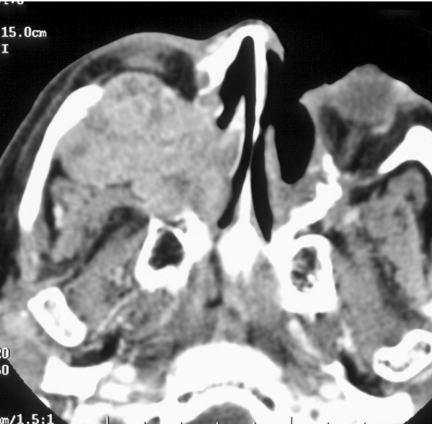
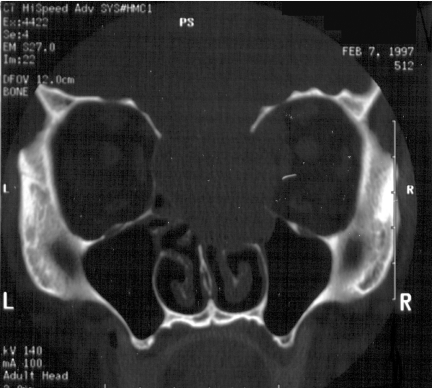
Figure 2.
Axial CT bone windows of a juvenile nasal angiofibroma (JNA) completely opacifying the left nasal cavity, enlarging the left pterygopalatine fossa with extension to the left foramen rotundum and vidian canals.
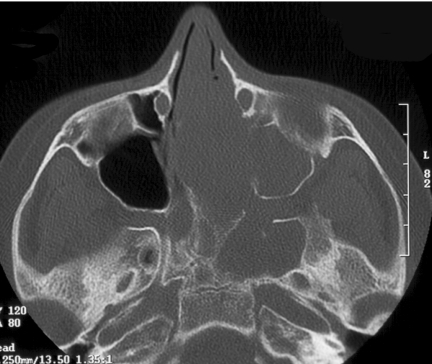
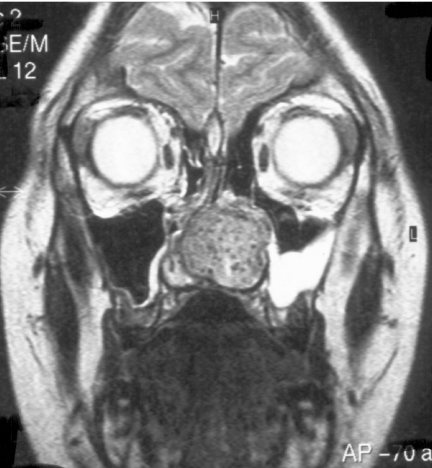
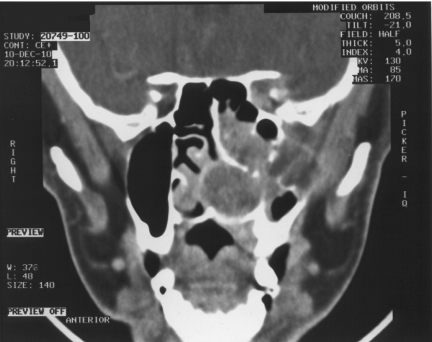
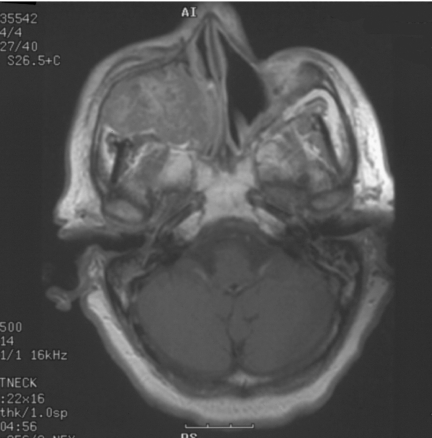
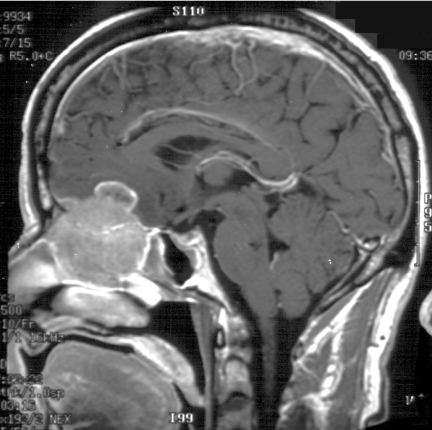
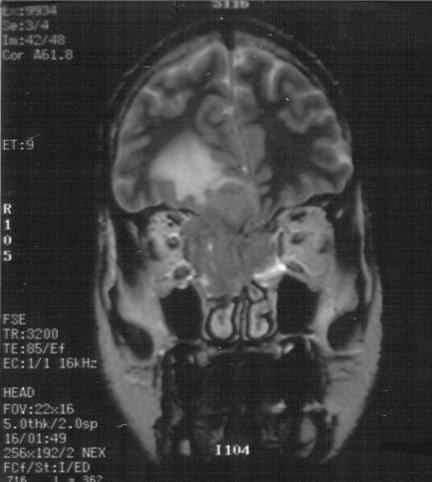
Figure 3.
Coronal T2W, MRI of same patient as in Fig. 2, JNA demonstrating multiple small flow voids in the vascular tumour and the utility of MRI in distinguishing tumour from retained secretions in the left maxillary antrum.
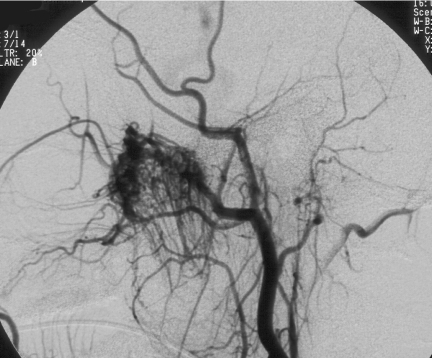
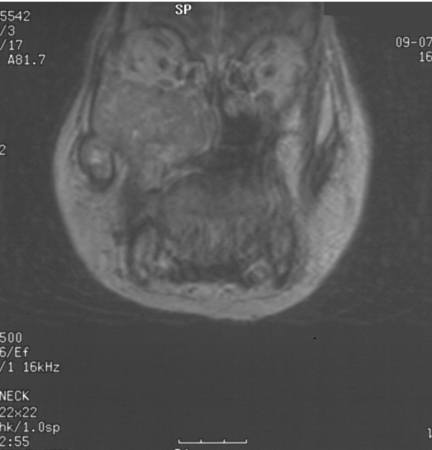
Figure 4.
Same patient as in Figs 2 and 3. Cerebral angiogram demonstrating marked vascularity of the JNA with feeding vessels from the left internal maxillary artery and branches of the left ascending pharyngeal artery.
Treatment
Surgical resection is the preferred treatment. Pre-operative embolisation may significantly reduce operative blood loss and the need for transfusions [25]. Adjunctive therapy includes oestrogens, cryotherapy and arterial ligation. Radiotherapy is contraindicated except in select cases [26]. Lesions limited to nasal and nasopharyngeal cavities with sphenoid and ethmoid invasions can be removed endoscopically [27–29]. Larger tumours—Radkowski’s stage III and above—require external approaches, including trans-palatal, mid-facial degloving, lateral rhinotomy, trans-zygomatic and facial lowering by Le Fort I. Intracranial extension requires a combined intra- and extracranial approach. Additional radiotherapy is reserved for incomplete resections [22, 30, 31].
Pathology
The tumours show a characteristic zonal organisation of a sub-epithelial myxoid-fibrous zone and a proliferative capillary fibroblastic cambium layer made of capillary/vascular channels and fibrous components in varying amounts. The latter exhibits a changing cellularity and fibre content. Large areas of hyalinisation predominate centrally, with fibrous tissue prevailing in older lesions [32].
Malignant tumours
Squamous cell carcinoma
Clinical features
Squamous cell carcinoma (SCCA) is the commonest malignant tumour of the sinonasal cavity [33]. They are more frequent in males [34]. Nickel workers are particularly susceptible to SCCA of the sinonasal cavity [42]. Like other nasal malignancies, they present as a nasal mass with symptoms of obstruction, discharge and bleeding and facial swelling [36]. An SCCA may present as a non-healing ulcer inside the nose. Most series identify the maxillary sinus or nasal cavity as the most common site of origin [33–36]. Because of late presentation, the exact site of origin is often unidentifiable. Delayed presentation is often due to initial non-specific symptoms, with more than half being T3 or T4 [34, 36].
Imaging
Most SCCAs are in the maxillary sinuses. On MRI, most SCCAs are hypointense on T2W images and heterogeneous with solid enhancement, as opposed to the uniform homogenous appearance of secretions which have peripheral rim enhancement of the sinus mucosa. Other tumours including benign inverting papillomas or neoplastic lymphomas may have similar imaging characteristics. An important radiographic finding for malignancies is bone destruction, best seen on CT and noted on initial exams in approximately 80% of sinonasal SCCAs. Staging follows TNM classification, with Ohngren’s line extending from the orbital medial canthus to the mandibular angle, dividing the inferior anterior margins from the superior posterior margins.
Treatment
In resectable tumours, the optimal treatment is combined surgery and radiotherapy [35–38]. Adjunctive chemotherapy is used for larger tumours. Histology, localisation and nodal involvement are significant prognostic factors for locoregional control and survival [33]. Orbital and neural invasion significantly affect local control [36]. Local failure remains the dominant cause for poor outcome [36]. Cervical metastases developing subsequent to initial therapy is associated with poor survival rates [39, 40, 42]. The 5-year corrected survival for sinonasal SCCA is 35% [41].
Pathology
Squamous cell carcinoma arises from atypical epidermal cells resulting in a hypertrophic nodule or a non-healing ulcer. Breach of the basal membrane converts it from an in situ to an invasive carcinoma.
Adenoid cystic carcinoma
Clinical features
Sinonasal adenoid cystic carcinoma (ACC) arises from minor salivary glands as an aggressive neoplasm with a high incidence of local recurrence and distant metastasis, regardless of treatment modality [43]. It presents in females twice as much as males [45, 46], while cervical lymphadenopathy is rare [47]. Local spread in ACC may occur by lysis of adjacent bone and/or by peri-neural and peri-vascular spread [48]. Local recurrence is more common in incomplete excision or with perineural spread [47]. Metastatic development is independent of local recurrence. Bony metastases are more rapidly aggressive than pulmonary metastases, which may remain asymptomatic [47].
Imaging
Because of the varied histology related to cell density, cysts, tubular or cribiform patterns, the signal intensity on MRI sequences can vary widely. Adenoid cystic carcinoma has a propensity for perineural spread. A careful imaging assessment of trigeminal nerve branches, especially within the ptergyopalatine fossa, is essential to evaluate for intracranial extension. Key signposts include a mass with loss of adjacent fat, and perineural enhancement suggestive of perineural spread along routes within foramen rotundum and the infraorbital fissure (V2), and foramen ovale (V3). It is important to remember that nearly all neoplasms, not just adenoid cystic can also spread perineurally (Figs 5–8).
Figure 5.
Axial CT post-contrast soft tissue windows in a patient with adenoid cystic carcinoma (ACC) extending throughout the left nasal cavity, left maxillary sinus and left pterygoid fossa with enlargement and loss of fat in the left pterygopalatine fossa and left infratemporal fossa. Perineural tumour extension is expected along the V2 branches in pterygopalatine fossa, infraorbital nerve and foramen rotundum.
Figure 6.
Coronal CT post-contrast same patient as Fig. 5 with ACC. Demonstrating tumour throughout the left nasal cavity with bony destruction of the left maxillary sinus, loss of fat in the left infratemporal fossa and extension in the left orbital apex along the V2 division.
Figure 7.
Same patient as in Figs 5 and 6. Coronal T1 MRI without contrast of ACC filling the left nasal cavity and left maxillary sinus with extension and loss of fat in the left infratemporal fossa. Perineural tumour spread again noted along V2 in the infraorbital foramen.
Figure 8.
Same patient as Figs 5–7. Axial T2W MRI in patient with ACC demonstrating increased signal. Adenoid cystic tumours violate the rule of decreased signal on T2W MRI seen with most other cellular tumours, because of their varied histology.
Treatment
Though the 5-year survival may be better than other sinonasal cancers, most cases are ultimately fatal, with long disease-free intervals being observed. A combination of surgery and post-operative radiotherapy offers the best chance for disease control compared with the either treatment modality alone [43, 45, 48].
Pathology
Tumours of ‘massive’ or solid or adenoid cystic histological type carry a poorer prognosis than the cribiform or mucoepidermoid type [44, 45, 48].
Adenocarcinoma
Clinical features
Sinonasal adenocarcinomas are unusual tumours with variable clinical courses. Nasal obstruction is the most common presentation. Age, tumour grade and intracranial extension are associated with overall survival and death from disease [49]. The relationship between ethmoidal adenocarcinomas and ‘hard wood’ dust exposure is well established. Duration and average level contribute independently to the overall elevated risk. In addition formaldehyde exposure in the leather industry increases the risks for developing sinonasal adenocarcinoma [50].
Imaging
Adenocarcinomas tend to occur in the ethmoid sinus. On MRI, the tumour usually has a slightly hypointense signal on T2W images. Occasional masses may show increased signal intensity as seen in adenoid cystic tumours (Figs 9–11).
Figure 9.
Axial CT post contrast soft tissue windows in patient with adenocarcinoma extending throughout the right nasal cavity, maxillary sinus, and right infratemporal fossa with right intraorbital invasion causing proptosis.
Figure 10.
Same patient as in Fig. 9. MRI T1W post-gadolinium of adenocarcinoma showing heterogeneous enhancement of tumour throughout the right sinonasal cavity and maxillary sinus.
Figure 11.
Same patient as Figs 9 and 10. Coronal T1W post-gadolinium of adenocarcinoma throughout the right nasal cavity and maxillary sinus with extension into the right infratemporal fossa.
Treatment
Treatment for nasal adenocarcinoma varies widely depending on tumour stage and metastasis. Low-grade variants of nasal adenocarcinomas are associated with a favourable prognosis and can be treated with less aggressive therapy [49]. Surgical excision is the standard treatment. Either a trans-facial approach (lateral nasal and degloving) or a cranio-facial approach may be used, depending on the site and tumour extent [51]. Post-operative radiotherapy is used adjunctively for better tumour free survival [52]. Some authors claim a combination of surgical debulking and repeated topical chemotherapy provide equally good results [53]. Extensive tumours are palliated with low-dose radiotherapy [52].
Pathology
Adenocarcinomas are subdivided into well, moderately, and poorly differentiated adenocarcinomas, and mucinous adenocarcinomas. Patients with mucinous and poorly differentiated adenocarcinomas have significantly shorter disease-free intervals and survival rates than those with well differentiated and moderately differentiated adenocarcinomas [54].
Lymphoma
Clinical features
Sinonasal lymphomas are relatively uncommon, representing less than 1% of all head and neck malignancies. They are predominantly non-Hodgkin’s lymphomas (NHL) [55]. Currently, two distinct subgroups are recognised, characterised by phenotype, location, prognosis, and treatment. Lymphomas of the B-cell phenotype are the most frequent sinonasal tumours. They are less aggressive with a better prognosis. The rarer T/NK-cell lymphomas are mostly found in the nasal cavity; though in South America and Asia, a T-cell phenotype predominates [56]. These are aggressive with a worse prognosis [55]. Burkitt’s lymphoma (BL) is a high-grade B-cell non-Hodgkin’s lymphoma, associated with Epstein Barr virus usually involving the maxilla and facial bones in the endemic African variant; head and neck lesions in non-endemic BL are rare [57]. The disease occurs in a predominantly male elderly population, except BL which mainly affects children [56, 58].
Low grade lymphomas present with a sinonasal mass associated with obstructive symptoms. High grade lymphomas are more likely to present with aggressive symptoms, including non-healing ulcer, cranial nerve manifestations, facial swelling, epistaxis, or pain. High grade B-cell lymphomas tend to present with soft tissue or osseous destruction, particularly of the orbit with associated proptosis. T-cell lymphomas are associated with nasal septal perforation and/or destruction [56].
Imaging
In the sinonasal cavity, the majority of lymphomas are non-Hodgkin’s histiocytic lymphoma. The tumours are bulky masses with intermediate signal intensity on MRI images with moderate enhancement. The lesions remodel and may erode adjacent bone. Tumours are usually within the nasal fossa and maxillary sinus, and less commonly in the ethmoids and very rarely in the sphenoid and frontal sinuses (Figs 12–15).
Figure 12.
Axial CT bone windows in patient with olfactory neuroblastoma with tumour extension through the right lamina papyrecea causing lateral bowing of the right medial rectus muscle.
Figure 13.
Same patient as in Fig. 12; note the right and left sides are reversed on this coronal image. The olfactory neuroblastoma again extends intraorbitally through the lamina papyrecea as well as through the cribiform plate.
Figure 14.
Saggital T1W post-gadolinium contrast MRI; same patient as Figs 12 and 13. The olfactory neuroblastoma extends cephalad through the cribiform plate into the anterior cranial fossa and is shown with a cystic component superiorly.
Figure 15.
Same patient as Figs 13 and 14. Coronal T2W MRI demonstrating the slightly hypointense olfactory neuroblastoma extending both intraorbitally and intracranially with extensive edema in the right frontal lobe.
Treatment
Patients with localised sinonasal lymphoma have a favourable prognosis when treated with a combined modality treatment (CMT) of radiotherapy and chemotherapy [59, 60]. Patients with primary non-Hodgkin’s lymphoma of the nasal cavity, Ann Arbor stage IE limited to one nasal cavity, have better survival rates than those with the same stage but with tumour extension beyond the nasal cavity. Patients with stages IIE, IIIE and IV have a poor prognosis unaffected by conventional chemotherapy [59].
Pathology
The nasal type of extranodal NK/T-cell lymphoma has a characteristic histologic pattern, which is angio-centric, angio-invasive and angio-destructive and a thorough immuno-histological study should always be conducted in these cases [61].
Melanoma
Clinical features
Melanomas in the sinonasal cavity can be similar in appearance to other malignant tumours when they are amelanotic [62]. Patients present with epistaxis and nasal obstruction [63]. A significant proportion arises from the septum [64].
Imaging
The nasal septum requires close inspection, as this is where melanoma most commonly occurs; the next most common site is the turbinates. Melanomas containing melanin have a paramagnetic effect shortening the T1 and T2 relaxation times, creating increased signal on T1W images and decreased signal on T2W scans. Amelanotic tumours may demonstrate the reverse, with increased signal on T1 and high signal on T2W images. Tumours may have hemorrhagic components also altering the signal intensities.
Treatment
Wide surgical excision is the mainstay of treatment with adjunctive radiotherapy and/or chemotherapy [62, 63]. Treatment success depends on the patient’s immune status. Biologic response modifiers like interferon α2b, interleukin 2, interferon γ, monoclonal antibodies, and gene therapy are used more frequently as adjuncts or primary treatment [64].
Pathology
Histologically, tumours are composed of a variety of cell types, epithelioid, spindled, undifferentiated, frequently arranged in a peritheliomatous distribution [63]. Immuno-histochemical studies are necessary if amelanotic type is suspected and aid in diagnosis of carcinoma, lymphoma, sarcoma, and olfactory neuroblastoma [59]. Positive reactions for S-100 protein, tyrosinase, HMB-45, melan A and microphthalmia transcription factor indicate melanoma [63].
Olfactory neuroblastoma
Clinical features
Olfactory neuroblastomas (ENB) originate from olfactory epithelium in the upper part of the nasal cavity. The common presenting symptoms include hyposmia, anosmia, nasal congestion, facial pain and epistaxis, headache and personality changes if the frontal lobe is affected. Rarely, ectopic tumour hormone secretion gives rise to SIADH or Cushing’s Syndrome [65–68]. Orbit extension may cause exophthalmos, ophthalmoplegia and or visual loss [68]. Staging predicts prognosis; in group A, tumour is limited to the nasal cavity; in group B, the tumour is localised to the nasal cavity and para-nasal sinuses; and in group C, the tumour extends beyond the nasal cavity and para-nasal sinuses [69].
Imaging
On CT and MRI, the tumour is often centred at the cribiform plate. The mass may be homogenous or have areas of inhomogeneity and moderate enhancement. On T1W images the tumour is decreased in signal compared to brain parenchyma and may be isointense or increased in signal relative to brain on T2W scans. Important associated imaging characteristics include cysts along the superior tumour margins especially within the anterior cranial fossa intracranially.
Treatment
Multi-modality treatment involving surgery, chemotherapy and radiotherapy appears highly effective in preventing relapse in advanced ENB [70, 71]. The preferred surgical approach is a cranio-facial resection [72]. Large tumours are considered for pre-operative chemotherapy and post-operative radiotherapy [73].
Pathology
Histologically, the tumour contains epithelial nests of small round cells and small short spindle cells surrounded by a net of fibrous connective tissue. Immuno-histochemistry is essential for diagnosis [73]. Neuron-specific enolase (NSE) is uniformly distributed throughout tumour cell clusters within tumour nodules while S-100 protein is distributed at the periphery of tumour cell nests. Anti-synaptophysin, microtubule-associated protein-2, and class III beta-tubulin isotype are present in most ENBs.
Conclusion
Although rare, sinonasal tumours often have a poor prognosis due to a delay in diagnosis. This article reviews the salient clinical features, imaging, treatment and pathology of selected benign and malignant neoplasms within the nasal cavity, in order to help clinicians and radiologists better distinguish between sinonasal inflammatory disease and neoplasms.
References
- 1.Som P. Tumors and tumorlike conditions of the sinonasal cavity. In: Som P, Bergeron RT, editors. Head and Neck Imaging. 2nd ed. St. Louis: Mosby-Year Book; 1990. pp. 169–227. [Google Scholar]
- 2.Boring CC, Squires TS, Tong T. Cancer statistics. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Som PM, Dillon WP, Sze G, et al. Benign and malignant sinonasal lesions with intracranial extension:differentiation with MR imaging. Radiology. 1989;172:763–6. doi: 10.1148/radiology.172.3.2772185. [DOI] [PubMed] [Google Scholar]
- 4.Namdar I, Edelstein DR, Huo J, Lazar A, Kimmelman CP, Soletic R. Management of osteomas of the paranasal sinuses. Am J Rhinol. 1998;12:393–8. doi: 10.2500/105065898780707955. [DOI] [PubMed] [Google Scholar]
- 5.Rappaport JM, Attia EL. Pneumocephalus in frontal sinus osteoma: a case report. J Otolaryngol. 1994;23:430–6. [PubMed] [Google Scholar]
- 6.Hehar SS, Jones NS. Fronto-ethmoid osteoma: the place of surgery. J Laryngol Otol. 1997;111:372–5. doi: 10.1017/s0022215100137351. [DOI] [PubMed] [Google Scholar]
- 7.Osma U, Yaldiz M, Tekin M, Topcu I. Giant ethmoid osteoma with orbital extension presenting with epiphora. Rhinology. 2003;41:122–4. [PubMed] [Google Scholar]
- 8.Boysen M. Osteomas of the paranasal sinuses. J Otolaryngol. 1978;7:366–70. [PubMed] [Google Scholar]
- 9.Schick B, Steigerwald C, el Rahman el Tahan A, Draf W. The role of endonasal surgery in the management of frontoethmoidal osteomas. Rhinology. 2001;39:66–70. [PubMed] [Google Scholar]
- 10.Selva D, Chen C, Wormald PJ. Frontoethmoidal osteoma: a stereotactic-assisted sino-orbital approach. Ophthal Plast Reconstr Surg. 2003;19:237–8. doi: 10.1097/01.iop.0000062847.27980.ce. [DOI] [PubMed] [Google Scholar]
- 11.Brodish BN, Morgan CE, Sillers MJ. Endoscopic resection of fibro-osseous lesions of the paranasal sinuses. Am J Rhinol. 1999;13:111–6. doi: 10.2500/105065899782106779. [DOI] [PubMed] [Google Scholar]
- 12.London SD, Schlosser RJ, Gross CW. Endoscopic management of benign sinonasal tumors: a decade of experience. Am J Rhinol. 2002;16:221–7. [PubMed] [Google Scholar]
- 13.Savy L, Lloyd G, Lund VJ, Howard D. Optimum imaging for inverted papilloma. J Laryngol Otol. 2000;114:891–3. doi: 10.1258/0022215001904284. [DOI] [PubMed] [Google Scholar]
- 14.Outzen KE, Grontved A, Jorgensen K, Clausen PP. Inverted papilloma of the nose and paranasal sinuses: a study of 67 patients. Clin Otolaryngol Allied Sci. 1991;16:309–12. doi: 10.1111/j.1365-2273.1991.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 15.Krouse JH. Development of a staging system for inverted papilloma. Laryngoscope. 2000;110:965–8. doi: 10.1097/00005537-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Pasquini E, Sciarretta V, Farneti G, Modugno GC, Ceroni AR. Inverted papilloma: report of 89 cases. Am J Otolaryngol. 2004;25:178–85. doi: 10.1016/j.amjoto.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Stankiewicz JA, Girgis SJ. Endoscopic surgical treatment of nasal and paranasal sinus inverted papilloma. Otolaryngol Head Neck Surg. 1993;109:988–95. doi: 10.1177/019459989310900603. [DOI] [PubMed] [Google Scholar]
- 18.Tomenzoli D, Castelnuovo P, Pagella F, et al. Different endoscopic surgical strategies in the management of inverted papilloma of the sinonasal tract: experience with 47 patients. Laryngoscope. 2004;114:193–200. doi: 10.1097/00005537-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lee TJ, Huang SF, Lee LA, Huang CC. Endoscopic surgery for recurrent inverted papilloma. Laryngoscope. 2004;114:106–12. doi: 10.1097/00005537-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Gomez JA, Mendenhall WM, Tannehill SP, Stringer SP, Cassisi NJ. Radiation therapy in inverted papillomas of the nasal cavity and paranasal sinuses. Am J Otolaryngol. 2000;21:174–8. doi: 10.1016/s0196-0709(00)85020-6. [DOI] [PubMed] [Google Scholar]
- 21.Michaels L. Benign mucosal tumors of the nose and paranasal sinuses. Semin Diagn Pathol. 1996;13:113–7. [PubMed] [Google Scholar]
- 22.Jafek BW, Krekorian EA, Kirsch WM, Wood RP. Juvenile nasopharyngeal angiofibroma: management of intracranial extension. Head Neck Surg. 1979;2:119–28. doi: 10.1002/hed.2890020207. [DOI] [PubMed] [Google Scholar]
- 23.Witt TR, Shah JP, Sternberg SS. Juvenile nasopharyngeal angiofibroma. A 30 year clinical review. Am J Surg. 1983;146:521–5. doi: 10.1016/0002-9610(83)90245-3. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd G, Howard D, Lund VJ, Savy L. Imaging for juvenile angiofibroma. J Laryngol Otol. 2000;114:727–30. doi: 10.1258/0022215001906642. [DOI] [PubMed] [Google Scholar]
- 25.Economou TS, Abemayor E, Ward PH. Juvenile nasopharyngeal angiofibroma: an update of the UCLA experience, 1960–1985. Laryngoscope. 1988;98:170–5. doi: 10.1288/00005537-198802000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Biller HF. Juvenile nasopharyngeal angiofibroma. Ann Otol Rhinol Laryngol. 1978;87(5 Pt 1):630–2. doi: 10.1177/000348947808700505. [DOI] [PubMed] [Google Scholar]
- 27.Han D, Chen X, Wang J. Endoscopic nasal surgery in treatment of nasopharyngeal angiofibroma. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1998;33:358–60. [PubMed] [Google Scholar]
- 28.Roger G, Tran Ba Huy P, Froehlich P, et al. Exclusively endoscopic removal of juvenile nasopharyngeal angiofibroma: trends and limits. Arch Otolaryngol Head Neck Surg. 2002;128:928–35. doi: 10.1001/archotol.128.8.928. [DOI] [PubMed] [Google Scholar]
- 29.Enepekides DJ. Recent advances in the treatment of juvenile angiofibroma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:495–9. doi: 10.1097/01.moo.0000143970.19992.64. [DOI] [PubMed] [Google Scholar]
- 30.Waldman SR, Levine HL, Astor F, Wood BG, Weinstein M, Tucker HM. Surgical experience with nasopharyngeal angiofibroma. Arch Otolaryngol. 1981;107:677–82. doi: 10.1001/archotol.1981.00790470025007. [DOI] [PubMed] [Google Scholar]
- 31.Antonelli AR, Cappiello J, Di Lorenzo D, Donajo CA, Nicolai P, Orlandini A. Diagnosis, staging, and treatment of juvenile nasopharyngeal angiofibroma (JNA) Laryngoscope. 1987;97:1319–25. doi: 10.1288/00005537-198711000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Stiller D, Kuttner K. Growth patterns of juvenile nasopharyngeal fibromas. A histological analysis on the basis of 40 cases. Zentralbl Allg Pathol. 1988;134:409–22. [PubMed] [Google Scholar]
- 33.Grau C, Jakobsen MH, Harbo G, et al. Sino-nasal cancer in Denmark 1982–1991—a nationwide survey. Acta Oncol. 2001;40:19–23. doi: 10.1080/028418601750070993. [DOI] [PubMed] [Google Scholar]
- 34.Hone SW, O’Leary TG, Maguire A, Burns H, Timon CI. Malignant sinonasal tumours: the Dublin Eye and Ear Hospital experience. Ir J Med Sci. 1995;164:139–41. doi: 10.1007/BF02973281. [DOI] [PubMed] [Google Scholar]
- 35.Spiro JD, Soo KC, Spiro RH. Squamous carcinoma of the nasal cavity and paranasal sinuses. Am J Surg. 1989;158:328–32. doi: 10.1016/0002-9610(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 36.Porceddu S, Martin J, Shanker G, et al. Paranasal sinus tumors: Peter MacCallum Cancer Institute experience. Head Neck. 2004;26:322–30. doi: 10.1002/hed.10388. [DOI] [PubMed] [Google Scholar]
- 37.Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821–9. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 38.Anniko M, Franzen L, Lofroth PO. Long-term survival of patients with paranasal sinus carcinoma. ORL J Otorhinolaryngol Relat Spec. 1990;52:187–93. doi: 10.1159/000276132. [DOI] [PubMed] [Google Scholar]
- 39.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012–29. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 40.St-Pierre S, Baker SR. Squamous cell carcinoma of the maxillary sinus: analysis of 66 cases. Head Neck Surg. 1983;5:508–13. doi: 10.1002/hed.2890050610. [DOI] [PubMed] [Google Scholar]
- 41.Harbo G, Grau C, Bundgaard T, et al. Cancer of the nasal cavity and paranasal sinuses. A clinico-pathological study of 277 patients. Acta Oncol. 1997;36:45–50. doi: 10.3109/02841869709100731. [DOI] [PubMed] [Google Scholar]
- 42.Barton RT. Nickel carcinogenesis of the respiratory tract. J Otol. 1977;6:412–22. [PubMed] [Google Scholar]
- 43.Wiseman SM, Popat SR, Rigual NR, et al. Adenoid cystic carcinoma of the paranasal sinuses or nasal cavity: a 40-year review of 35 cases. Ear Nose Throat J. 2002;81:510–4, 516–7. [PubMed] [Google Scholar]
- 44.Chummun S, McLean NR, Kelly CG, et al. Adenoid cystic carcinoma of the head and neck. Br J Plast Surg. 2001;54:476–80. doi: 10.1054/bjps.2001.3636. [DOI] [PubMed] [Google Scholar]
- 45.Raux-Rakotomalala F, Houliat T, Martel J, Stoll D, Bebear JP, Darrouzet V. Adenoid cystic carcinoma of the head and neck: a review of 30 cases. Rev Laryngol Otol Rhinol (Bord) 2003;124:235–41. [PubMed] [Google Scholar]
- 46.Hallacq P, Labrousse F, Roullet B, Orsel S, Bessede JP, Moreau JJ. Adenoid cystic carcinomas invading the skull base. Apropos of 4 cases and review of the literature. Neurochirurgie. 2001;47:542–51. [PubMed] [Google Scholar]
- 47.Tran L, Sidrys J, Horton D, Sadeghi A, Parker RG. Malignant salivary gland tumors of the paranasal sinuses and nasal cavity. The UCLA experience. Am J Clin Oncol. 1989;12:387–92. doi: 10.1097/00000421-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Jones AS, Beasley NJ, Houghton DJ, Helliwell TR, Husband DJ. Tumours of the minor salivary glands. Clin Otolaryngol Allied Sci. 1998;23:27–33. doi: 10.1046/j.1365-2273.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- 49.Orvidas LJ, Lewis JE, Weaver AL, Bagniewski SM, Olsen KD. Adenocarcinoma of the nose and paranasal sinuses: a retrospective study of diagnosis, histologic characteristics, and outcomes in 24 patients. Head Neck. 2005;27:370–5. doi: 10.1002/hed.20168. [DOI] [PubMed] [Google Scholar]
- 50.Roux FX, Behm E, Page P, Laccourreye O, Pages JC, Brasnu D. Adenocarcinomas of the ethmoid sinuses. Epidemiological data. Ann Otolaryngol Chir Cervicofac. 2002;119:271–80. [PubMed] [Google Scholar]
- 51.Stoll D, Bebear JP, Truilhe Y, Darrouzet V, David N. Ethmoid adenocarcinomas: retrospective study of 76 patients. Rev Laryngol Otol Rhinol (Bord) 2001;122:21–9. [PubMed] [Google Scholar]
- 52.Claus F, Boterberg T, Ost P, et al. Postoperative radiotherapy for adenocarcinoma of the ethmoid sinuses: treatment results for 47 patients. Int J Radiat Oncol Biol Phys. 2002;54:1089–94. doi: 10.1016/s0360-3016(02)02985-1. [DOI] [PubMed] [Google Scholar]
- 53.Knegt PP, Ah-See KW, vd Velden LA, Kerrebijn J. Adenocarcinoma of the ethmoidal sinus complex: surgical debulking and topical fluorouracil may be the optimal treatment. Arch Otolaryngol Head Neck Surg. 2001;127:141–6. doi: 10.1001/archotol.127.2.141. [DOI] [PubMed] [Google Scholar]
- 54.Franchi A, Gallo O, Santucci M. Clinical relevance of the histological classification of sinonasal intestinal-type adenocarcinomas. Hum Pathol. 1999;30:1140–5. doi: 10.1016/s0046-8177(99)90029-1. [DOI] [PubMed] [Google Scholar]
- 55.Van Prooyen Keyzer S, Eloy P, Delos M, Doyen C, Bertrand B, Rombaux P. Sinonasal lymphomas. Case report. Acta Otorhinolaryngol Belg. 2000;54:45–51. [PubMed] [Google Scholar]
- 56.Abbondanzo SL, Wenig BM. Non-Hodgkin’s lymphoma of the sinonasal tract. A clinicopathologic and immunophenotypic study of 120 cases. Cancer. 1995;75:1281–91. doi: 10.1002/1097-0142(19950315)75:6<1281::aid-cncr2820750610>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 57.Banthia V, Jen A, Kacker A. Sporadic Burkitt’s lymphoma of the head and neck in the pediatric population. Int J Pediatr Otorhinolaryngol. 2003;67:59–65. doi: 10.1016/s0165-5876(02)00283-5. [DOI] [PubMed] [Google Scholar]
- 58.Quraishi MS, Bessell EM, Clark DM, Jones NS, Bradley PJ. Aggressive sino-nasal non-Hodgkin’s lymphoma diagnosed in Nottinghamshire, UK, between 1987 and 1996. Clin Oncol (R Coll Radiol) 2001;13:269–72. doi: 10.1053/clon.2001.9266. [DOI] [PubMed] [Google Scholar]
- 59.Logsdon MD, Ha CS, Kavadi VS, Cabanillas F, Hess MA, Cox JD. Lymphoma of the nasal cavity and paranasal sinuses: improved outcome and altered prognostic factors with combined modality therapy. Cancer. 1997;80:477–88. doi: 10.1002/(sici)1097-0142(19970801)80:3<477::aid-cncr16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 60.Cavalot AL, Ricci E, Nazionale G, Palonta F, Fadda GL. Primary non-Hodgkin’s lymphoma of the nasal cavity. Clinical case report and discussion. Acta Otolaryngol. 2000;120:545–50. doi: 10.1080/000164800750046081. [DOI] [PubMed] [Google Scholar]
- 61.Jia H, Sun T. Extranodal NK/T-cell lymphoma mimicking cellulitis. Leuk Lymphoma. 2004;45:1467–70. doi: 10.1080/10428190310001652313. [DOI] [PubMed] [Google Scholar]
- 62.Cheeseman AD, Jani P. Scott-Brown’s Otolaryngology. Laryngology and head and neck surgery. 6th ed. vol. 5. 1997. Cysts, granulomas and tumours of the jaws, nose and sinuses; pp. 5/23/35–36. [Google Scholar]
- 63.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Kirkwood JM. Systemic adjuvant treatment of high-risk melanoma: the role of interferon alfa2b and other immunotherapies. Eur J Cancer. 1998;34(Suppl 3):S12–17. doi: 10.1016/s0959-8049(97)10159-9. [DOI] [PubMed] [Google Scholar]
- 65.Vasan NR, Medina JE, Canfield VA, Gillies EM. Sinonasal neuroendocrine carcinoma in association with SIADH. Head Neck. 2004;26:89–93. doi: 10.1002/hed.10345. [DOI] [PubMed] [Google Scholar]
- 66.Cullen MJ, Cusack DA, O’Briain DS, Devlin JB, Kehely A, Lyons TA. Neurosecretion of arginine vasopressin by an olfactory neuroblastoma causing reversible syndrome of antidiuresis. Am J Med. 1986;81:911–6. doi: 10.1016/0002-9343(86)90368-2. [DOI] [PubMed] [Google Scholar]
- 67.Yu J, Koch CA, Patsalides A, et al. Ectopic Cushing’s syndrome caused by an esthesioneuroblastoma. Endocr Pract. 2004;10:119–24. doi: 10.4158/EP.10.2.119. [DOI] [PubMed] [Google Scholar]
- 68.Tamase A, Nakada M, Hasegawa M, Shima H, Yamashita J. Recurrent intracranial esthesioneuroblastoma outside the initial field of radiation with progressive dural and intra-orbital invasion. Acta Neurochir (Wien) 2004;146:179–82. doi: 10.1007/s00701-003-0179-y. [DOI] [PubMed] [Google Scholar]
- 69.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37:1571–6. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 70.Dias FL, Sa GM, Lima RA, et al. Patterns of failure and outcome in esthesioneuroblastoma. Arch Otolaryngol Head Neck Surg. 2003;129:1186–92. doi: 10.1001/archotol.129.11.1186. [DOI] [PubMed] [Google Scholar]
- 71.Bradley PJ, Jones NS, Robertson I. Diagnosis and management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg. 2003;11:112–8. doi: 10.1097/00020840-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Lund VJ, Howard D, Wei W, Spittle M. Olfactory neuroblastoma: past, present, and future? Laryngoscope. 2003;113:502–7. doi: 10.1097/00005537-200303000-00020. [DOI] [PubMed] [Google Scholar]
- 73.Frierson HF Jr, Ross GW, Mills SE, Frankfurter A. Olfactory neuroblastoma. Additional immunohistochemical characterization. Am J Clin Pathol. 1990;94:547–53. doi: 10.1093/ajcp/94.5.547. [DOI] [PubMed] [Google Scholar]