Abstract
Primary hepatocellular carcinoma (HCC) is a significant tumor worldwide and represents the most common primary hepatic neoplasm. Staging criteria are important for appreciation of timely work up of these neoplasms in contradiction with surgical colleagues. This article demonstrates the appearance of HCC on multiphasic, multidetector CT (MDCT) and relates these findings to current staging criteria. The variable appearance on different planes of contrast is critical to appreciate in staging this neoplasm. The hypervascular nature of the primary tumor makes MDCT and three-phase imaging a critical feature in the detection and characterization of this tumor. This is especially critical in the patients who are candidates for surgical resection. Additionally, MDCT has allowed arterial phase imaging to define the vascular supply of the tumor. An accurate representation of the size and number of lesions is critical in not only the initial staging but also the follow-up of hepatocellular carcinoma. The post-treatment features including the appearance post-surgically and after radiofrequency ablation can be well appreciated on MDCT.
Keywords: Hepatocellular carcinoma, liver, multidetector CT
Introduction
Hepatocellular carcinoma (HCC) is the eighth most common malignancy worldwide and the most common malignant neoplasm of the liver. Epidemiologically, HCC is most common in Asia and sub-Saharan Africa [1, 2]. The most common risk factors identified include cirrhosis and hepatitis B and C. Additional risk factors include: hemochromatosis, α1-antitripsin deficiency, exposure to aflatoxins, Thorotrast administration, oral contraceptives, and vinyl chloride exposure [1, 3]. Hepatitis B is considered the primary cause of approximately 80% of cases worldwide. The peak age group for development is in the 6th to 8th decades with a definite male predominance of 4:1. The median survival rate remains dismal being less than 1 year and a 5-year survival rate of only 2%–5%. [4] There has been some improvement in 1-year survival over the past 5 years, thought to be a reflection of earlier detection of smaller resectable tumors and more aggressive surgical approaches.
Clinical presentation
The most common symptomatology includes: abdominal pain, malaise, fatigue, and weight loss [5]. Findings on physical examination often include an enlarged, irregular, and nodular liver as a result of cirrhotic change. Jaundice and abnormal liver function tests may only be evident later in the course because of the significant functional reserve of the liver parenchyma. Paraneoplastic syndromes are not uncommon including hypercalcemia and hyperglycemia as well as polycythemia which occurs in less than 10% of patients [6].
Pathology
HCC is the result of a series of events that cause the development of frank malignancy from smaller lesions. A number of stages have been identified including: cirrhosis and regenerative nodules, dysplastic nodules, early HCC, and advanced HCC [7, 8]. The earliest phase i.e., regenerative nodules are areas of hepatocyte proliferation surrounded by fibrous septa that develop as a non-specific response to liver injury. In contrast to regenerative nodules, the dysplastic nodule is pre-malignant and represents proliferation of hepatocytes with nuclear variation that can be identified as low-grade to high-grade dysplasia. Early HCC is those lesions that are less than 2 cm in diameter with well-differentiated cells and a preserved trabecular appearance [8]. These early tumors usually have a well-defined margin with a thin fibrous capsule but may demonstrate extra-nodular growth into surrounding liver sinusoids. Large HCCs, those greater than 2 cm, are classified as advanced HCC. These may be nodular, massive, and diffuse. Diffuse HCC results in tumor nodules dispersed within the liver parenchyma.
Staging
Staging of HCC is critical to clinical management and is assessed through the TNM system. The primary lesion is defined by tumor size, number, and location of lesions, invasion of local vascular structures, and extension into the biliary system. Additionally, the staging system evaluates the location of regional, nodal disease and the presence or absence of distant metastases (Table 1) [5, 9–11]. T1 lesions are those that are solitary tumors without vascular invasion, T2 lesions are solitary tumors with vascular invasion or multiple tumors, none larger than 5 cm; T3 represents multiple tumors larger than 5 cm, tumor invading major portal or hepatic venous structures, and T4 lesions are tumors with direct invasion of adjacent organs. Using a binary method, lymph node staging consists of N0 without regional metastases and N1 with regional lymph node metastasis. Distant metastases are defined with M0 being no distant metastasis and M1 being the presence of distant metastasis.
Table 1.
TNM staging system devised by the American Joint Committee on Cancer
| Classification | Meaning |
|---|---|
| Tumor | |
| TX | Tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Solitary tumor 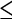 2 cm without vascular invasion 2 cm without vascular invasion |
| T2a | Solitary tumor 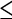 2 cm with vascular invasion 2 cm with vascular invasion |
| T2b | Multiple tumors <2 cm limited to one lobe without vascular invasion |
| T2c | Solitary tumor >2 cm without vascular invasion |
| T3a | Solitary tumor >2 cm with vascular invasion |
| T3b | Multiple tumors <2 cm limited to one lobe with vascular invasion |
| T3c | Multiple tumors >2 cm limited to one lobe with or without vascular invasion |
| T4a | Multiple tumors in more than one lobe |
| T4b | Tumors involving a major branch of the portal vein or hepatic vein(s) |
| T4c | Invasion of adjacent organs other than the gallbladder |
| Nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph nodes |
| N1 | Regional lymph node metastasis |
| Metastases | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present (most common to lung and bones) |
| Stage | |
| I | T1 N0 M0 |
| II | T2 N0 M0 |
| IIIA | T3 N0 M0 |
| IIIB | T1 N1 M0 |
| T2 N1 M0 | |
| T3 N1 M0 | |
| IVA | T4 N [any] M0 |
| T [any] N0 M1 | |
| IVB | T [any] N [any] M1 |
Imaging findings
Computed tomography (CT) and magnetic resonance (MR) are the primary modalities used to obtain imaging studies for the diagnosis and staging of HCC [12–24]. In this manuscript we specifically address the use of multidetector CT (MDCT) in the assessment of hepatocellular carcinoma [5, 10]. Although MR imaging may be more sensitive in detecting small lesions, MDCT is highly effective in the detection and staging of HCC. This is, however, significantly dependent upon technique, which must be meticulous with new MDCT scanners. There are three CT-defined phases of contrast enhancement that should be exploited for the accurate detection and staging of HCC.
The first phase, the hepatic arterial phase (HAP) occurs at 20–30 s following the administration of contrast material. Contrast material should be given with a bolus injection and power injector and rates should be fast in the range of 4–6ml/s. Scanning in the second phase should ideally be performed as an early parenchymal phase in the range of 40–55 s, and the third phase, now termed the hepatic venous phase (HVP), previously termed the portal venous phase (PVP) on single detector CT occurs from 65 to 80 s following contrast administration (Fig. 1). Hypervascular lesions are best imaged during the early arterial phase when they appear as areas of increased enhancement relative to the unenhanced liver parenchyma [5, 11, 12]. Although hypervascular lesions are usually best seen during this phase, they are often also seen during the early parenchymal phase and are usually less well seen during the standard HVP/PVP phases. MDCT also allows for high-quality, thin-section imaging. Scans are ideally performed at less than 1 cm, specifically 5 mm sections. The early phase images can be used to develop a three-dimensional (3D) vascular map. When a capsule is present, it is usually hypodense on the HAP or mixed density on the PVP and showing some enhancement on delayed images. The use of MDCT has essentially replaced CT arterial portography and allows a non-invasive approach to imaging HCC. Multiphasic imaging allows the detection of tumor thrombus within vascular structures most efficiently on the later phase images. One may be able to distinguish between bland tumor thrombus and tumor thrombus by identifying the enhancement of clot in earlier phases. The sensitivity and specificity of MDCT are still evolving with changes in the equipment. This should erode the previously documented limitations of detection although in the background of the cirrhosis, there still are significant limitations in detection. The following images demonstrate the appearance of HCC on MDCT for the various stages using the TNM system as referenced in Table 1 (Figs 2–8). The variables of detectability of HCC depend on the phase of contrast administration and each individual tumor is important to appreciate (Figs 9–11).
Figure 1.
Demonstration of optimal image appearance in the three major phases: (a) hepatic arterial phase (HAP), (b) late arterial phase (LAP), (c) hepatic venous phase (HVP). MDCT allows the ability to acquire images within each of these three distinct phases for optimal imaging of hypervascular lesions.
Figure 2.
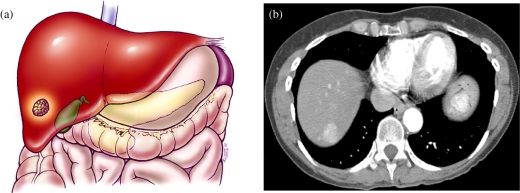
Hepatocellular carcinoma TNM system—T1. (a) Color diagram demonstrating a T1 lesion: solitary <2 cm without the presence of vascular invasion. (b) MDCT at the dome of the liver in the hepatic arterial phase (HAP) demonstrating a 2 cm hypervascular lesion in the dome of the liver.
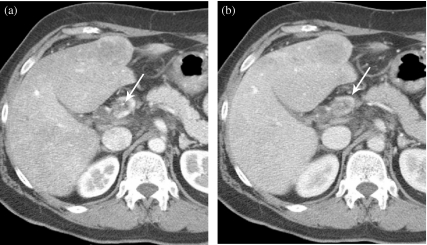
Figure 3.
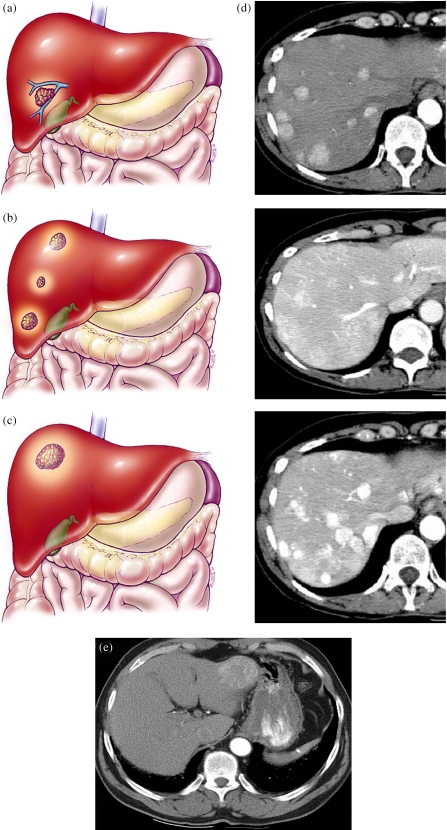
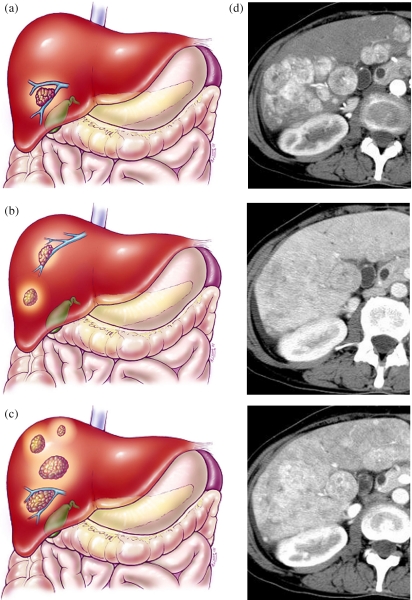
Hepatocellular carcinoma TNM system—T2. (a) A T2a lesion represents a solitary tumor <2 cm with vascular invasion. (b) A T2b lesion is multiple tumors <2 cm limited to one lobe without vascular invasion. (c) A T2c lesion is a solitary tumor >2 cm without vascular invasion. (d) CT scan showing multiple phases with T2b lesions: HAP, LAP, HVP. (e) Example of T2c lesion in left lobe.
Figure 4.
Hepatocellular carcinoma TNM system—T3. Color diagrams demonstrating T3 lesion staging. (a) T3a is a solitary tumor >2 cm with vascular invasions. (b) A T3b lesion consists of multiple tumors limited to one lobe <2 cm with vascular invasion. (c) A T3c lesion consists of multiple tumors limited to one lobe of the liver measuring >2 cm with or without vascular invasion. (d) Multiphasic CT scan showing T3c lesions: HAP, LAP, HVP.
Figure 5.
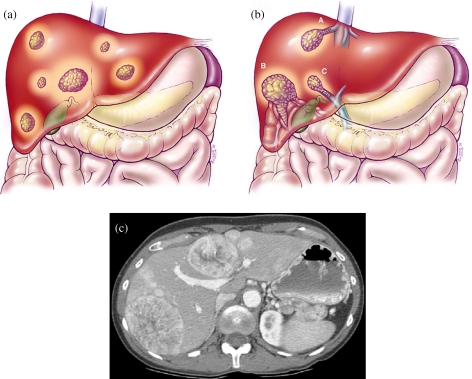
Hepatocellular carcinoma TNM system—T4. (a) T4a demonstrating multiple tumors in more than one lobe of the liver. (b) T4b multiple tumors involving a major branch of the portal venous or hepatic venous system. (c) CT scan at the level of the portal vein during the late arterial phase (LAT) demonstrating multiple tumors in a bilobar distribution.
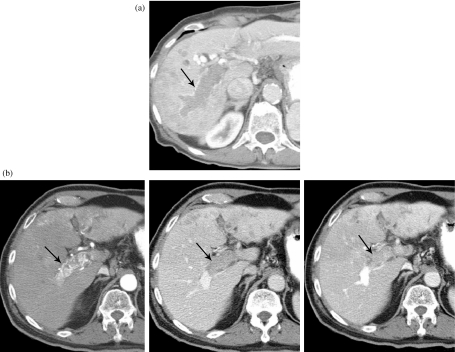
Figure 6.
MDCT of the liver demonstrating a T4b lesion with involvement of the main portal vein as well as multiple lesions in the liver. (a) Early phase (arrow) indicating a clot in the portal vein. (b) Later phase (arrow) with a clot in the portal vein.
Figure 7.
(a) An MDCT of a HCC T4 lesion demonstrating an extensive clot (arrow) within the main portal vein. (b) HCC, T4 lesion in a patient with a clot in the main portal vein (arrow) and extensive collaterals, three phases: HAP, LAP, HVP.
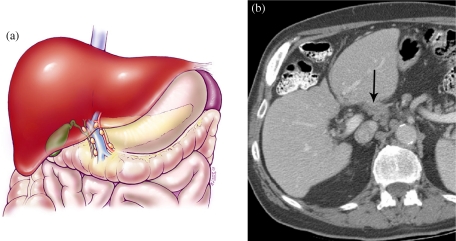
Figure 8.
Hepatocellular carcinoma TNM system demonstrating nodal disease. (a) Color diagram demonstrating nodes along the region of the porta hepatis. (b) MDCT scan at the level of the porta hepatis demonstrating nodal disease in the porta hepatis.
Figure 9.
Multifocal HCC demonstrating optimal enhancement noted in the HAP (a) and LAP (b). Lesions are seen as hypervascular against a low-density liver in the early hepatic arterial phase HAP and continue to enhance in the LAP. Note that the lesions are very poorly seen and have almost disappeared during the hepatic venous phase (HVP) (c).
Figure 10.
Hepatocellular carcinoma demonstrating best visibility in the late arterial phase (LAP) but also being able to be seen in the hepatic venous phase (HVP). Lesion not seen on HAP. (a) HCC not seen on HAP. (b) Hypervascular lesion in the dome of the liver during the LAP. (c) The lesion is still seen very well while in the HVP but now the low-attenuation necrosis is evident.
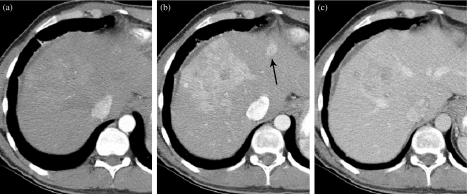
Figure 11.
Hepatocellular carcinoma with satellite lesion only seen on the late arterial phase (LAP). (a) Early hepatic arterial phase (HAP) imaging demonstrates the large lesion is only subtly seen but the satellite lesion is not clearly evident. (b) The primary large mass in the liver is now enhancing and the satellite lesion (arrow) can be seen in the left lobe of the liver (LAP). (c) Dominant lesion poorly seen and satellite lesion no longer seen.
Treatment options
The treatment of HCC includes surgery, chemotherapy, radiation therapy, and, most importantly, combination therapies. Data from the National Cancer Data Bank (NCDB) reported that approximately 17.7% of patients were treated with chemotherapy alone, 17% with surgery alone, and 3.2% with radiation therapy [25–29].
Surgery
Surgery is considered the primary treatment option for candidates in stages 1, 2, or 3a. In these cases, surgical removal can be performed by either immediate tumor resection or by orthotopic liver transplantation. The 5-year survival rates for these patients following liver transplantation ranges from 58% to 75% [3–7, 24–26]. For resection, 5-year survival ranges from 35% to 51% [25]. The recurrence rate after resection has been reported to be as high as 1/3 of patients with a recurrence rate of 3%–17% for orthotopic liver transplantation [30]. The 5-year survival rates for single lesions smaller than 5 cm has been reported as 63% [31]. Larger tumors, however, are associated with poorer outcomes especially in the absence of a tumor capsule, the presence of multiple nodules, satellite lesions, or the presence of vascular invasion.
Other treatment options
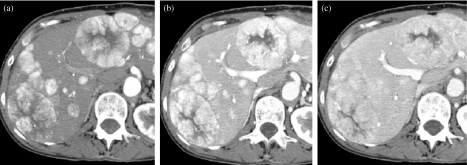
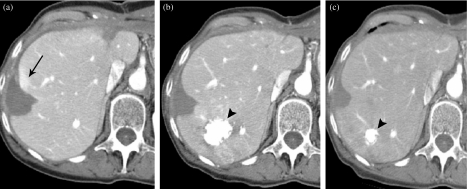
Percutaneous therapies such as ethanol injection, transarterial catheter embolization, cryoablation, and radiofrequency ablation (RFA), are secondary treatment options. RFA is a common therapeutic option for tumors smaller than 3 cm and results in 5-year survival rates of just less than 50% [32] (Fig. 12).
Figure 12.
Demonstration of radiofrequency ablation (RFA) of HCC. (a) The low attenuation peripherally in the right lobe is the result of RFA. There is some mild enhancement peripherally (arrow). (b) The low attention peripheral RFA defect is now slightly smaller and the area of enhancement has resolved, indicating it did not represent recurrent tumor but rather benign enhancement related to the RFA procedure on the scan done 4 months previously. Post-chemoembolization demonstrates the focal high-density area posteriorly in the right lobe (arrowhead). (c) MDCT 9 months later demonstrates the continued decrease in the size of the RFA defect without recurrence and the chemoembolized tumor is also smaller (arrowhead).
Adjuvent systemic chemotherapy has been used with single and multiple agents including 5-fluoro uracil, interferon, cisplatinum, thalidomide, octreotide, and tamoxifen.
References
- 1.el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 2.Tabor E. Hepatocellular carcinoma: global epidemiology. Dig Liver Dis. 2001;33:115–7. doi: 10.1016/s1590-8658(01)80062-1. [DOI] [PubMed] [Google Scholar]
- 3.Ward E, Boffetta P, Andersen A, et al. Update of the follow-up of mortality and cancer incidence among European workers employed in the vinyl chloride industry. Epidemiology. 2001;12:710–8. doi: 10.1097/00001648-200111000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Szklaruk J, Silverman PM, Tamm EP. Taveras and Ferrucci’s Radiology. Philadelphia, PA: Wolters Kluwer Health; 2004. Hepatocellular carcinoma. [Google Scholar]
- 5.Szklaruk J, Silverman PM, Charnsangavej C. A pictorial review: imaging in the diagnosis, staging, treatment, and surveillance of hepatocellular carcinoma. AJR. 2003;180:441–54. doi: 10.2214/ajr.180.2.1800441. [DOI] [PubMed] [Google Scholar]
- 6.Luo JC, Hwang SJ, Wu JC, et al. Paraneoplastic syndromes in patients with hepatocellular carcinoma in Taiwan. Cancer. 1999;86:799–804. doi: 10.1002/(sici)1097-0142(19990901)86:5<799::aid-cncr15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386–91. doi: 10.1097/00000421-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Monzawa S, Omata K, Shimazu N, Yagawa A, Hosoda K, Araki T. Well-differentiated hepatocellular carcinoma: findings of US, CT, and MR imaging. Abdom Imaging. 1999;24:392–7. doi: 10.1007/s002619900521. [DOI] [PubMed] [Google Scholar]
- 9.Feldman M. Gastrointestinal disease. In: Sleisenger MH, Feldman M, Scharnschmidt BF, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, and Management. 6th edn. Philadelphia, PA: Saunders; 1998. pp. 2046–50. [Google Scholar]
- 10.Noltenius H. Hepatocellular carcinoma. In: Noltenius H, editor. Human Oncology: Pathology and Clinical Characteristics. Baltimore, MD: Urban & Schwarzenberg; 1988. pp. 373–5. [Google Scholar]
- 11.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–43. doi: 10.1002/(sici)1097-0142(20000201)88:3<538::aid-cncr7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Murakami T, Oi H, et al. Detection of hypervascular hepatocellular carcinoma by dynamic MRI and dynamic spiral CT. J Comput Assist Tomogr. 1995;19:948–54. doi: 10.1097/00004728-199511000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Kanematsu M, Oliver JH 3rd, Carr B, Baron RL. Hepatocellular carcinoma: the role of helical biphasic contrast-enhanced CT versus CT during arterial portography. Radiology. 1997;205:75–80. doi: 10.1148/radiology.205.1.9314965. [DOI] [PubMed] [Google Scholar]
- 14.Loyer EM, Chin H, DuBrow RA, David CL, Eftekhari F, Charnsangavej C. Hepatocellular carcinoma and intrahepatic peripheral cholangiocarcinoma: enhancement patterns with quadruple phase helical CT—a comparative study. Radiology. 1999;212:866–75. doi: 10.1148/radiology.212.3.r99se32866. [DOI] [PubMed] [Google Scholar]
- 15.Yu JS, Kim KW, Lee JT, Yoo HS. MR imaging during arterial portography for assessment of hepatocellular carcinoma: comparison with CT during arterial portography. AJR Am J Roentgenol. 1998;170:1501–6. doi: 10.2214/ajr.170.6.9609162. [DOI] [PubMed] [Google Scholar]
- 16.Murakami T, Kim T, Takamura M, et al. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology. 2001;218:763–7. doi: 10.1148/radiology.218.3.r01mr39763. [DOI] [PubMed] [Google Scholar]
- 17.Tublin ME, Dodd GD 3rd, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997;168:719–23. doi: 10.2214/ajr.168.3.9057522. [DOI] [PubMed] [Google Scholar]
- 18.Oi H, Murakami T, Kim T, Matsushita M, Kishimoto H, Nakamura H. Dynamic MR imaging and early-phase helical CT for detecting small intrahepatic metastases of hepatocellular carcinoma. AJR Am J Roentgenol. 1996;166:369–74. doi: 10.2214/ajr.166.2.8553950. [DOI] [PubMed] [Google Scholar]
- 19.Rummeny E, Weissleder R, Stark DD, et al. Primary liver tumors: diagnosis by MR imaging. AJR Am J Roentgenol. 1989;152:63–72. doi: 10.2214/ajr.152.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Onaya H, Itai Y. MR imaging of hepatocellular carcinoma. Magn Reson Imaging Clin N Am. 2000;8:757–68. [PubMed] [Google Scholar]
- 21.Winston CB, Schwartz LH, Fong Y, Blumgart LH, Panicek DM. Hepatocellular carcinoma: MR imaging findings in cirrhotic livers and noncirrhotic livers. Radiology. 1999;210:75–9. doi: 10.1148/radiology.210.1.r99ja1975. [DOI] [PubMed] [Google Scholar]
- 22.Ohtomo K, Matsuoka Y, Abe O, et al. High-resolution MR imaging evaluation of hepatocellular carcinoma. Abdom Imaging. 1997;22:182–6. doi: 10.1007/s002619900168. [DOI] [PubMed] [Google Scholar]
- 23.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–75. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 24.Rose AT, Rose DM, Pinson CW, et al. Hepatocellular carcinoma outcomes based on indicated treatment strategy. Am Surg. 1998;64:1128–34. discussion 1134–5. [PubMed] [Google Scholar]
- 25.Suarez Y, Franca AC, Llovet JM, Fuster J, Bruix J. The current status of liver transplantation for primary hepatic malignancy. Clin Liver Dis. 2000;4:591–605. doi: 10.1016/s1089-3261(05)70128-8. [DOI] [PubMed] [Google Scholar]
- 26.Fuster J, Garcia-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996;223:297–302. doi: 10.1097/00000658-199603000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueras J, Ibanez L, Ramos E, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl. 2001;7:877–83. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]
- 28.Livraghi T. Guidelines for treatment of liver cancer. Eur J Ultrasound. 2001;13:167–76. doi: 10.1016/s0929-8266(01)00129-x. [DOI] [PubMed] [Google Scholar]
- 29.Aguayo A, Patt YZ. Liver cancer. Clin Liver Dis. 2001;5:479–507. doi: 10.1016/s1089-3261(05)70175-6. [DOI] [PubMed] [Google Scholar]
- 30.Leung TW, Patt YZ, Lau WY, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–81. [PubMed] [Google Scholar]
- 31.Palma LD. Diagnostic imaging and interventional therapy of hepatocellular carcinoma. Br J Radiol. 1998;71:808–18. doi: 10.1259/bjr.71.848.9828792. [DOI] [PubMed] [Google Scholar]
- 32.Rust C, Gores GJ. Locoregional management of hepatocellular carcinoma: surgical and ablation therapies. Clin Liver Dis. 2001;5:161–73. doi: 10.1016/s1089-3261(05)70159-8. [DOI] [PubMed] [Google Scholar]