Abstract
Corelease of glycine and GABA from the single synaptic terminal (synaptic bouton) is well accepted in immature rat spinal cord and brainstem. However, it raises the question of how glycine and GABA are accumulated in the same synaptic vesicles and coreleased. To address this issue, spontaneous miniature inhibitory postsynaptic currents (mIPSCs) and focally evoked IPSCs (eIPSCs) mediated via a single synapse were recorded from synaptic bouton preparations of the rat immature sacral dorsal commissural nucleus (SDCN) neurones by whole-cell patch recording. Focal stimulation of a single synaptic bouton revealed that three different quantal releases occur from a single synaptic bouton: i.e. pure glycine, pure GABA, and mixed. Prolonged treatment with bafilomycin A1, a vacuolar-type H+/ATPase inhibitor, to the SDCN neurone greatly suppressed frequency and amplitude of the mIPSCs. During washing out of bafilomycin A1, complete recovery in the amplitude of glycinergic mIPSCs was observed, while that of GABAergic and mixed mIPSCs was incomplete. These observations indicate that three types of vesicles coexist in single synaptic terminals, and that refilling of glycine into the synaptic vesicle predominantes over GABA after pretreatment with bafilomycin A1 in immature rats. This could be explained by the decrease in the cytosolic concentration of GABA, or by the presence of subtypes of vesicular inhibitory amino acid transporter in the synaptic vesicle membrane.
Glycine and GABA are fast inhibitory neurotransmitters in the mammalian central nervous systems. In some regions of the spinal cord and brainstem, inhibitory inputs change from predominantly GABAergic to glycinergic over the first two postnatal weeks (Gao et al. 2001; Kim & Kandler, 2003; Nabekura et al. 2004). During this period, it is well documented that glycine and GABA are coreleased from the same synaptic vesicles (Jonas et al. 1998; O'Brien & Berger, 1999; Keller et al. 2001), and it is generally considered that glycine and GABA are accumulated via a common vesicular inhibitory amino acid transporter (VIAAT) located in the synaptic vesicle membrane (Burger et al. 1991; Dumoulin et al. 1999; Raiteri et al. 2001). Synaptic vesicles are acidified by a vacuolar-type H+/ATPase, which provides a driving force for the uptake of neurotransmitter (Gasnier, 2004). The original studies of vesicular uptake of GABA and glycine show that there is no difference between the proton pump in the two cases (Fykse & Fonnum, 1988; Christensen et al. 1990). Although the uptake of glycine and GABA is well documented in biochemical studies (Fykse & Fonnum, 1988; Christensen et al. 1990; Burger et al. 1991; Christensen & Fonnum, 1991; McIntire et al. 1997; Sagnéet al. 1997; Chaudhry et al. 1998; Raiteri et al. 2001), the properties of filling glycine and GABA into the synaptic vesicles have not been elucidated from physiological or pharmacological points of view.
The sacral dorsal commissural nucleus (SDCN) is located in the dorsal area of the central canal in the lower lumbar and sacral spinal cord, and is known to receive glycinergic, GABAergic, and mixed synaptic inputs (Katsurabayashi et al. 2001; Jang et al. 2002; Wu et al. 2002). To study the mechanisms involved in the three different synaptic inputs into the SDCN neurones, we recorded spontaneous miniature inhibitory post synaptic currents (mIPSCs), before and after the application of bafilomycin A1, a vacuolar-type H+/ATPase inhibitor, to examine the refilling profile of glycine and GABA into the synaptic vesicles in acutely isolated SDCN neurones with functional synaptic boutons remaining (so called ‘synaptic bouton’ preparations) (Rhee et al. 1999; Katsurabayashi et al. 2001; Jang et al. 2002; Akaike & Moorhouse, 2003). We report the differential profiles for glycine and GABA refilling into the synaptic vesicles in SDCN neurones after pretreatment with bafilomycin A1.
Methods
Mechanical dissociation of the SDCN neurones
The spinal cords of 8- to 12- day old (P8–12) Wistar rats were quickly removed during deep anaesthetization by intraperitioneal (i.p.) injection of pentobarbital (50 mg kg−1). Then spinal cord slices of 370 μm thickness were prepared from the lumbosacral (L5–S4) segment. Single sacral dorsal commissural nucleus (SDCN) neurones were mechanically dispersed from fthe resh spinal cord slice preparation to preserve functional presynaptic terminals as previously described (Katsurabayashi et al. 2001). The ionic composition of the internal (patch pipette) solution was (mm): 43 CsCl, 92 Cs-methanesulphonate, 5 TEA-Cl, 2 EGTA, 4 ATP-Mg, and 10 Hepes, which was adjusted to pH 7.2 with Tris-OH. The ionic composition of the external standard solution was (mm): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 Hepes, which was adjusted to pH 7.4 with Tris-OH.
Focal stimulation of a single synaptic terminal
Focal electrical stimulation of a single bouton adherent to a mechanically isolated neurone has been previously described (Akaike et al. 2002). Briefly, 100 μs voltage pulses (30 V) were applied to a glass stimulating pipette (inner diameter about 0.5 μm) at a frequency of 0.2 Hz, using a stimulator (SS-202 J, Nihon Koden, Tokyo, Japan). The stimulating pipette was filled with standard external solution and was placed close to the surface of a single SDCN neurone from which a whole-cell recording was being made. The stimulating pipette was then carefully moved along the surface membrane of the soma or dendrites until an inward current appeared in an all-or-nothing fashion, indicating that the stimulating pipette was positioned just above a single bouton. It was confirmed that the current was evoked from a single bouton by observing whether it disappeared in an all-or-nothing fashion when the stimulus strength was reduced or when the stimulation pipette was shifted horizontally (see Akaike et al. 2002; Akaike & Moorhouse, 2003). When the evoked responses were glycinergic or GABAergic only, they were discarded from the present study.
Data analysis
Synaptic currents were counted and analysed using Mini-Analysis software (version 5.01, Synaptosoft Inc., GA, USA). The threshold for detection of mIPSCs was set at 3 pA above the background noise. The currents were filtered at 2 kHz and digitized at 10 kHz. Events were rejected or accepted on the basis of their rise and decay times. Rise times were determined between 10% and 90% of the peak amplitude of the IPSCs. The decay time constant of mIPSCs was initially obtained by fitting a double exponential function to the mIPSC decay from the time period corresponding to between 90% and 10% of the peak mIPSC amplitude. Individual events were fitted (with >200 iterations) to the function:
where, y0 is a baseline average amplitude and yfast and yslow are amplitudes of fast and slow decay time constants, respectively. τfast and τslow are decay time constants. When the relative contribution of one of the exponential distributions was <1%, the mIPSCs were considered to have a single exponential decay. Thus, the decision on whether a single mIPSC decayed with a single or with dual components was completely objective. Decay time constants of pure glycinergic and pure GABAergic mIPSCs pharmacologically isolated by 5 μm bicuculline and 100 nm strychnine were best fitted by a single exponential equation (Fig. 2) (See also Jonas et al. 1998; Nabekura et al. 2004). Differences in the amplitude distribution of mIPSCs were compared by non-parametric statistical analysis (Kolmogorov–Smirnov test). Differences were considered significant if P < 0.05. All results are expressed as means ± s.e.m.
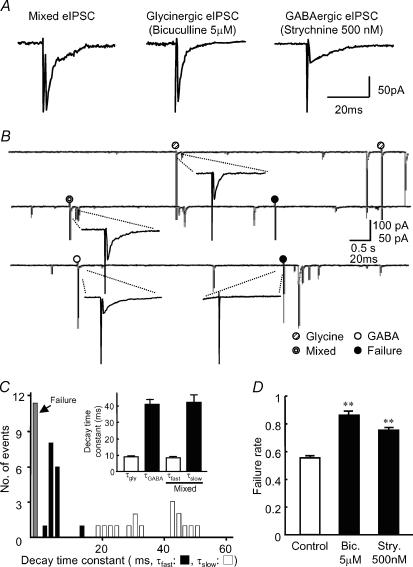
Figure 2. Focal stimulation of a single bouton.
A, glycinergic and GABAergic eIPSCs were pharmacologically isolated from the mixed focal eIPSCs. The data were obtained from the same bouton shown in B. B, typical traces of focal eIPSCs successively elicited from a single synapse at 0.2 Hz. Note that spontaneous mIPSCs are evoked by either glycine or GABA release or both. C, histograms of the decay time constants (τfast and τslow) of the mixed focal eIPSC in a single synapse (16 events). Bin size, 2 ms. Inset shows summary data of the averaged decay time constants of focal eIPSCs (n = 4). D, the failure rate (the frequency of failure events in the total stimuli) of focal eIPSCs was increased in the presence of bicuculline or strychnine. Each column represents the mean + s.e.m. from four neurones; **P < 0.01.
Drugs
Throughout all the experiments, the external bath solution contained 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 10 μmd-2-amino-5-phosphonopentanoic acid (d-AP5) to block AMPA- and NMDA-type glutamatergic responses, respectively. All drugs were applied to the recording cell via a Y-tube microperfusion system. With this technique, the external solution surrounding a neurone could be exchanged within 20–50 ms (Murase et al. 1990; Katsurabayashi et al. 2001). Bafilomycin A1 (Tocris, USA) was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 500 μm, and this was added to the solution to give a final concentration of 1 μm. In order to almost exhaust the transmitters in the synaptic vesicles (Williamson & Neale, 1994; Zhou et al. 2000), some slices were incubated with bafilomycin A1 for 2 h at 37°C. The incubation solution contained (mm): 124 NaCl, 5 KCl, 1.2 KH2PO4, 24 NaHCO3, 2.4 CaCl2, 1.3 MgSO4 and 10 glucose, at pH 7.4 (equilibrated with 95% O2 and 5% CO2).
All experiments conformed to the Guiding Principles for the Care and Use of Animals approved by The Council of The Physiological Society of Japan and all efforts were made to minimize the number of animals used.
Results
Isolation of glycinergic and GABAergic mIPSCs in the SDCN neurones
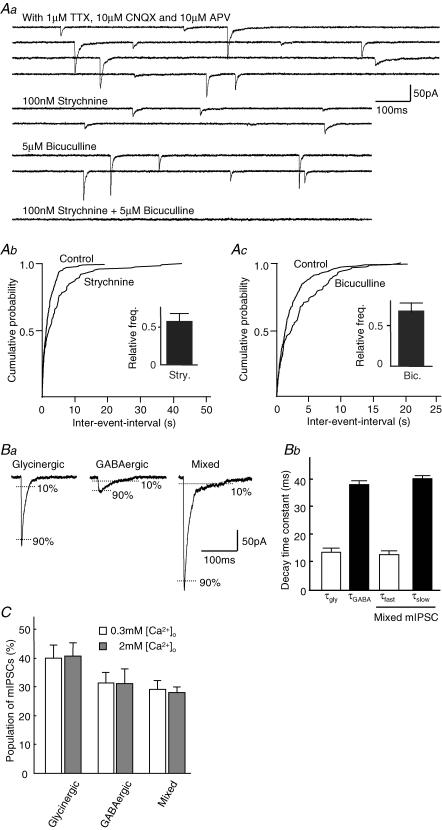
To record mIPSCs from the SDCN neurone, a whole-cell patch recording technique was used at a holding potential (VH) of −60 mV in the presence of 1 μm TTX, 10 μm CNQX and 10 μm d-AP5. Strychnine (100 nm), a glycine receptor antagonist, halved the mean amplitude and slightly decreased the frequency of mIPSCs (before strychnine: amplitude: 42.0 ± 4.1 pA, frequency: 0.8 ± 0.1 Hz; during strychnine: amplitude: 18.9 ± 3.4 pA, frequency: 0.6 ± 0.1 Hz, n = 4, Fig. 1Aa and Ab). Additional application of the selective GABAA receptor antagonist, bicuculline (5 μm), completely suppressed these remaining mIPSCs (Fig. 1Aa). After washing out strychnine and bicuculline, 5 μm bicuculline was again applied in order to pharmacologically isolate the pure glycinergic mIPSCs in the same neurone. Bicuculline also reduced the mean amplitude and the frequency of mIPSCs (before bicuculline: amplitude: 50.8 ± 5.4 pA, frequency: 0.6 ± 0.1 Hz; during bicuculline: amplitude: 33.9 ± 1.9 pA, frequency: 0.4 ± 0.1 Hz, Fig. 1Ac), and additional application of 100 nm strychnine completely blocked the remaining mIPSCs (data not shown). Decrease in the frequency of mIPSCs observed in the present experiments on the application of strychnine (Fig. 1Ab) or bicuculline (Fig. 1Ac) implies that glycine or GABA are accumulated into separate synaptic vesicles, and are spontaneously released from the presynaptic nerve terminal(s).
Figure 1. Pharmacological isolation of glycinergic and GABAergic mIPSCs.
All experiments were performed in the presence of 1 μm TTX, 10 μm CNQX and 10 μm AP5 (APV) at a VH of −60 mV. Aa, typical traces of mIPSCs with or without glycine receptor antagonist, strychnine (100 nm) and/or GABAA receptor antagonist, bicuculline (5 μm). Ab, Ac, cumulative histograms of mIPSCs frequency in the presence and absence of strychnine (Ab) or bicuculline (Ac). Inset shows the averaged relative mIPSC frequency in the presence of strychnine (Ab) or bicuculline (Ac) from four neurones. Ba, typical traces of glycinergic and GABAergic mIPSCs are shown. Decay time constants were defined as the time for the mIPSC to decay from 90% to 10% of the peak amplitude. Note the different decay time constants between these mIPSCs. Bb, summary data of the averaged decay time constants of mIPSCs are shown (n = 11). Note that the τfast of the mixed mIPSCs corresponds to τgly, whereas the τslow of the mixed mIPSCs corresponds to τGABA. C, population of glycinergic, GABAergic and mixed mIPSCs in normal and low [Ca2+]o. The results were expressed as percentage of all events and represent the mean + s.e.m. from 11 neurones.
In addition, mIPSCs could be classified into three types according to the different decay time constants: fast decaying, slow decaying, and biphasic mIPSCs with both fast and slow decay time constants (Fig. 1B). In the presence of 5 μm bicuculline, all mIPSCs exhibited a fast decay time constant (τgly = 13.9 ± 0.3 ms, n = 11) and were sensitive to strychnine, whereas, in the presence of 100 nm strychnine, all mIPSCs exhibited slow decay time constants (τGABA = 39.2 ± 0.4 ms, n = 11) and were sensitive to bicuculline. In addition, mIPSCs with biphasic fast and slow decay time constants were also observed in the control (Fig. 1Aa). The decay time constants of these biphasic mIPSCs were well fitted by double exponential equations (τfast and τslow were 13.1 ± 0.6 ms and 41.0 ± 1.2 ms, respectively, Fig. 1Bb). The fast and slow time constants were very similar to those of the pharmacologically isolated τgly and τGABA, and the biphasic mIPSCs were abolished by the combined application of strychnine and bicuculline.
The biphasic mIPSCs were still observed at a low concentration of extracellular Ca2+ solution (0.3 mm) without changing the proportion of mIPSCs (2 mm[Ca2+]o: 28.0 ± 1.9%, 0.3 mm[Ca2+]o: 29.1 ± 3.1%, n = 11, Fig. 1C). These data rule out the possibility that the biphasic mIPSCs could have arisen from superimposition of the individual fast- and slow-decaying mIPSCs. Therefore, it seems that the biphasic mIPSCs are mediated by corelease of both glycine and GABA from a single vesicle. There is strong evidence from these observations that the recorded mIPSCs are composed of three types of mIPSCs: i.e. pure glycinergic, pure GABAergic and mixed (glycinergic/GABAergic) mIPSCs.
To investigate whether the three types of mIPSCs are generated at specific synapses independently or concomitantly, we performed focal stimulation of a single synaptic bouton onto the SDCN neurone (Akaike et al. 2002; Jang et al. 2002; Murakami et al. 2002). Stimulation of a single synaptic bouton at 0.2 Hz elicited evoked IPSCs (eIPSCs) at a VH of −60 mV. The eIPSCs were all-or-nothing in response to a gradual increase in the stimulus intensity (see also Akaike & Moorhouse, 2003). As shown in Fig. 2A and B, three types of eIPSCs with distinct decay time constants were elicited irregularly, which were pharmacologically classified as glycinergic, GABAergic and mixed, as in the case of mIPSCs. The decay time constants for glycinergic and GABAergic eIPSCs (τgly and τGABA) were 9.0 ± 0.2 ms and 41.1 ± 3.2 ms, respectively, whereas those of the mixed eIPSCs (τfast and τslow) were 8.4 ± 0.6 ms and 42.2 ± 4.6 ms (n = 4, Fig. 2C), which were consistent with the values obtained with mIPSCs. We repeated the similar experiments and observed three types of eIPSCs in four other synaptic boutons, thereby indicating that this is a general feature at SDCN inhibitory synapses. In addition, application of 5 μm bicuculline or 500 nm strychnine also increased the failure rate of eliciting eIPSCs from 0.55 ± 0.02 (control) to 0.86 ± 0.03 and 0.75 ± 0.02, respectively (Fig 2D, n = 4). These observations, taken together, indicate that a single synaptic bouton contains three types of vesicles filled with pure glycine, pure GABA and a mixture of glycine and GABA, and could release them, in an independent manner.
Profiles of glycine and GABA refilling into the synaptic vesicles after bafilomycin A1
In order to study mechanisms involved in the three types of vesicles, corresponding to the three types of mIPSCs and eIPSCs, mIPSCs were recorded before and after the application of bafilomycin A1, a vacuolar-type H+/ATPase inhibitor.
Direct application of bafilomycin A1 to the synaptic bouton preparation dramatically increased the frequency of all mIPSCs, which then slowly decreased. However, the treatment with bafilomycin A1 for up to 1 h did not abolish the mIPSCs completely (data not shown). Therefore, the acute spinal slice preparations including SDCN were incubated in a solution containing bafilomycin A1 for 2 h at 37°C, so that the uptake of neurotransmitters into the synaptic vesicles would be expected to be completely inhibited (Williamson & Neale, 1994; Zhou et al. 2000). After incubation for 2 h, we obtained synaptic bouton preparations of SDCN neurones from these slices. Figure 3Aa shows a typical trace of mIPSCs recorded from a SDCN neurone, just after setting up the neurone in the recording system in normal external solution. During the recording period, over 50 min, the input membrane resistance of neurones was not changed. As shown in Fig. 3A, prolonged treatment with bafilomycin A1 had almost completely suppressed the mIPSCs, and then the frequency and amplitude of the mIPSCs gradually recovered during rinsing with normal external solution.
Figure 3. Recovery of amplitude and frequency of mIPSCs after washing out bafilomycin A1.
Aa, typical traces of mIPSCs recorded just after washing out 1 μm bafilomycin A1. The synaptic bouton preparation was obtained from a slice incubated with 1 μm bafilomycin A1 for 2 h at 37°C. Ab, expanded traces of mIPSCs recorded at 1 and 10 min after washing out bafilomycin A1. B, the mean (± s.e.m.) frequencies of glycinergic, GABAergic and mixed mIPSCs were plotted against the time from washing out 1 μm bafilomycin A1. Each point represents mean mIPSC frequency for successive 5 min periods. Note that the uptake of glycine was faster than that of GABA and was transiently facilitated. Ca, the mean amplitude of the glycinergic, GABAergic and mixed mIPSCs at 0–5 min and 30–35 min after washing out bafilomycin A1 compared with controls. The results represent the mean + s.e.m. from 13 neurones (*, †P < 0.05, n.s: not significant). Cb, summary data of yfast and yslow of the mixed mIPSCs after washing out bafilomycin A1 for 30–35 min (**P < 0.01 versus control, n.s: not significant).
To estimate the time course of the recovery of glycinergic, GABAergic and mixed mIPSCs, we identified mIPSCs according to the decay time constants during the washing out of bafilomycin A1. Figure 3B shows the time course of recovery of the frequency of three mIPSCs. The frequency of glycinergic mIPSCs showed peak values (0.52 ± 0.09 Hz, n = 13) at 10–15 min after the start of washing, and then gradually decreased to a lower level, whereas, the frequency of GABAergic and mixed mIPSCs had more or less the same recovery, showing relatively stable increases without any remarkable peak. Finally, there were no significant differences between the rates of recovery of the frequency of the three types of mIPSCs. However, recovery of the amplitude of glycinergic mIPSCs was complete within 30–35 min after washing out bafilomycin A1 (control: 40.0 ± 4.8 pA; 30–35 min after washing: 36.6 ± 6.3 pA, n = 13, Fig. 3Ca), while those of GABAergic and mixed mIPSCs were incomplete. The mean amplitudes of GABAergic mIPSCs measured during the first 5 min and 30–35 min after the start of the washing were 9.0 ± 3.1 and 9.6 ± 3.4 pA, respectively, which were less than 55% of the normal value (control condition without bafilomycin A1, 17.4 ± 2.2 pA, Fig. 3Ca, n = 13). The mean amplitude of mixed mIPSCs also showed partial recovery at 30–35 min after washing out bafilomycin A1 (control: 55.5 ± 3.2 pA; 30–35 min after washing: 33.3 ± 5.7 pA, n = 13, Fig. 3Ca).
To further study the mechanisms for the partial recovery of the mixed mIPSCs, we divided the mixed mIPSCs into two components (yfast and yslow) and estimated the amplitude of each component during the washing out of bafilomycin A1, according to the method reported previously (Jonas et al. 1998; Nabekura et al. 2004). As shown in Fig. 3Cb, the mean amplitude of the yfast component at 30–35 min after washing out bafilomycin A1 showed almost complete recovery to the control level (control: 32.1 ± 2.3 pA, 30–35 min: 23.6 ± 3.6 pA, P = 0.16, Fig. 3Cb), whereas the amplitude of yslow at 30–35 min showed only partial recovery (control: 21.2 ± 1.6 pA, 30–35 min: 8.4 ± 1.9 pA, P = 0.004, Fig. 3Cb).
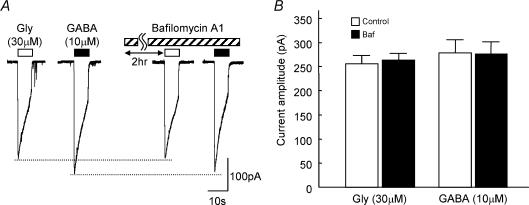
Next, we examined whether prolonged treatment with bafilomycin A1 affects the postsynaptic glycine and/or GABAA receptors. The sensitivities of the SDCN neurone to exogenously applied glycine or GABA were not affected by treatment with bafilomycin A1 (Fig. 4: glycinecontrol: 255.7 ± 19.0 pA, glycineBaf. 263.7 ± 14.5 pA; GABAcontrol: 278.7 ± 27.5 pA, GABABaf. 277.4 ± 24.1 pA, n = 5). These observations indicate that the effect of bafilomycin A1 is only on the presynaptic ending and that refilling of GABA into the synaptic vesicles is hampered after pretreatment with bafilomycin A1.
Figure 4. Effect of bafilomycin A1 on the postsynaptic glycine and GABAA receptors.
A, typical traces evoked by application of exogenous glycine (30 μm) or GABA (10 μm) for 10 s by using a Y-tube system in the presence or absence of 1 μm bafilomycin A1. Bafilomycin A1 was pre-incubated for 2 h at 37°C. B, summary data of amplitude of the response to exogenous glycine or GABA in the presence (▪) or absence (□) of bafilomycin Al. Each column is the mean + s.e.m. of data from five neurones.
Finally, we applied focal electrical stimulation to a single bouton in an attempt to record eIPSCs during washing of bafilomycin A1, but it was not possible to record eIPSCs in the present experiments.
Discussion
The major findings of this study were that (1) three types of mIPSCs were recorded from the ‘synaptic bouton preparation’ of mechanically isolated SDCN neurones: faster decaying events mediated by postsynaptic glycine receptors; slower decaying events mediated by postsynaptic GABAA receptors; and events with two components corresponding to GABA and glycine corelease; (2) in some single synaptic boutons tested, focal stimulation exhibited three types of eIPSCs, whose physiological and pharmacological properties were very similar to those of mIPSCs, indicating that release of pure glycine, pure GABA, and corelease of glycine and GABA could take place at a single terminal in SDCN neurones; (3) after pretreatment of SDCN neurones with bafilomycin A1, complete recovery in the amplitude of glycinergic mIPSCs was observed, while the recovery of GABAergic and mixed mIPSCs were incomplete.
Cotransmission of GABA and glycine was first demonstrated by showing biphasic decay kinetics of mIPSCs in the spinal cord and brainstem (Jonas et al. 1998; O'Brien & Berger, 1999), in addition to pure glycinergic and pure GABAergic transmission (Curtis et al. 1968; Schneider & Fyffe, 1992; Yang et al. 1997). In the rat cerebellum, it was also reported that a new connection exists between the cerebellar Lugaro cells and Golgi cells (Dieudonne & Dumoulin, 2000), and that Golgi cells receive both pure GABAergic inhibition from basket cells and mixed (GABAergic/glycinergic) inhibition from Lugaro cells (Dumoulin et al. 2001). Biphasic mIPSCs or eIPSCs observed in these tissues, which were sensitive to the combined application of bicuculline and strychnine, also indicate that GABA and glycine were coreleased from a single synaptic vesicle. These observations indicate that there exist three types of synaptic vesicles filled with pure GABA, pure glycine and a mixture of GABA and glycine at presynaptic nerve terminals in these preparations. It is generally considered that three types of mIPSCs, namely pure glycine, pure GABA and corelease of glycine and GABA, occur at individual synaptic contacts.
The present experiments confirmed the existence of three types of mIPSCs in SDCN neurones and showed that focal stimulation of a single synaptic bouton elicited three types of eIPSCs. This result indicates that three types of synaptic vesicles exist in a single synaptic bouton, which generates the three types of mIPSCs and eIPSCs. The focal stimulation used in the present experiments evoked eIPSCs in an all-or-nothing manner in response to the change in the stimulus intensity as reported previously (Akaike & Moorhouse, 2003), indicating that the focal stimulation activated a single synaptic contact. Therefore, it seems that one synaptic bouton in an SDCN neurone contains three types of vesicles, which exhibit three types of mIPSCs and eIPSCs.
In the present experiments, we found that the recovery of the amplitude of glycinergic mIPSCs was complete, while that of GABAergic and mixed mIPSCs was incomplete after pretreatment with bafilomycin A1. Bafilomycin A1 inhibits vacuolar H+/ATPase (Williamson & Neale, 1994; Zhou et al. 2000) and reduces the driving force for the uptake of neurotransmitters into the synaptic vesicle through VIAAT. Bafilomycin A1 greatly suppressed the amplitude of the three types of mIPSCs after prolonged treatment in the present experiments. The incomplete recovery of GABAergic and mixed mIPSCs could therefore be explained in two ways. First, cytosolic GABA concentration ([GABA]i) may be reduced by an unknown action of bafilomycin A1, since the loading of glycine and/or GABA into vesicles depends on their cytosolic concentrations (Dumoulin et al. 1999; Gasnier, 2000; 2004). GABA may require synthesis after treatment with bafilomycin A1, but it is known that glutamic acid decarboxylase 65 (GAD65), one of the GABA synthetic enzymes that resides mainly in GABAergic terminals, is low during development (Dumoulin et al. 1999). The fact that content of the vesicular transmitter depends on the presynaptic cytosolic concentration in the calyx of Held in rat brainstem (Ishikawa et al. 2002) and in cultured synapses (Dan et al. 1994) also supports this view. In addition, Christensen et al. (1991) reported that GABA has a higher affinity to the VIAAT than glycine, and therefore GABA would have appeared first if it was present in the cytoplasm (Christensen et al. 1991). Secondly, the packing process of GABA into the vesicles may be specifically hampered after pretreatment with bafilomycin A1 without any changes in [GABA]i and [glycine]i.
The cytosolic concentrations of GABA and glycine are controlled by the neuronal GABA transporters GAT1 and GAT4 (Radian et al. 1990; Guastella et al. 1991; Jursky & Nelson, 1996), and the neuronal glycine transporter GLYT2 (Liu et al. 1993) that are the plasma membrane uptake transporters for GABA and glycine, respectively. More recently, it has also been reported that GABA uptake via GAT1 modulates GABAergic transmission in the early postnatal rat hippocampus (Sipilä et al. 2004). Therefore, GABA accumulation into the nerve terminal by GATs may prevent glycine from significantly entering the vesicles by a common VIATT which recognizes both GABA and glycine as substrates. Likewise, the accumulation of glycine into the nerve terminal by GLYT2 may prevent the vesicular loading of other VIAAT substrates including trace amounts of GABA or β–alanine in the nerve terminal. Corelease of GABA and glycine at mixed inhibitory synapses may be achieved by a balance between the expression and activities of GATs and GLYT2 in the plasma membrane.
According to the current biochemical and immunocytochemical studies, it is generally considered that a common VIAAT is involved in the packing of both GABA and glycine into the synaptic vesicles (Gasnier 2000, 2004). The biochemical studies showed that GABA and glycine compete for uptake into purified synaptic vesicles. Furthermore, similar glycine-to-GABA uptake ratios were observed with vesicles from the brain or from the spinal cord where the glycinergic synapses are enriched (Christensen et al. 1991). These observations suggest the existence of similar, if not identical, transporters for the two amino acids (Burger et al. 1991; Christensen et al. 1991). Furthermore, in situ hybridization with a VIAAT probe showed that high levels of transcript can be detected in superior olive nuclei of the brainstem, which are known to be enriched glycinergic neurones. This indicates that a common VIAAT is responsible for the transport of GABA and glycine (Sagné et al. 1997). Moreover, immunocytochemical studies showed that VIAAT immunoreactivity can be observed in glycinergic axon terminals as well as GABAergic terminals in the brainstem and spinal cord (Chaudhry et al. 1998; Dumoulin et al. 1999).
Quantitative analysis, on the other hand, indicated that about 60% of the VIAAT-positive terminals lacked a detectable immunoreactivity for GAD65 (Dumoulin et al. 1999). VIAAT-positive and GAD-negative boutons were also found apposed to postsynaptic clusters of gephyrin, a protein responsible for clustering glycine receptors (Kuhse et al. 1995), as well as GABAA receptors (Essrich et al. 1998; Kneussel et al. 1999). These observations are considered to indicate that VIAAT-positive and GAD-negative puncta may represent glycinergic nerve terminals, assuming the presence of a common VIAAT (Dumoulin et al. 1999). However, these authors also noticed that a few presynaptic terminals enriched in GABA or glycine were immunonegative for VIAAT, leaving room for the possible existence of subtypes of VIAAT or an additional inhibitory amino acid transporter (Chaudhry et al. 1998; Dumoulin et al. 1999). This might indicate the possible existence of a subtype of VIAAT, which is more sensitive to bafilomycin A1 and has higher affinity for GABA. The existence of such a subtype of VIAAT would explain rationally the existence of three types of vesicles in a synaptic bouton, which induces pure glycinergic, pure GABAergic and mixed mIPSCs in the synaptic contact observed in the present experiments. However, further studies are definitely warranted to help understanding of the subtypes of VIAAT in central synapses.
In conclusion, the present study revealed that focal stimulation of a synaptic bouton results in three types of eIPSCs in SDCN neurones, and distinct refilling of glycine and GABA into the synaptic vesicles occurs after treatment with bafilomycin A1.
Acknowledgments
This study was supported by The Japan Health Sciences Foundation (No. 21279, Research on Brain Sciences) to Norio Akaike. We thank K. Creed and J. Nabekura for critical comments and for proofreading the manuscript.
References
- Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. doi: 10.1016/s0165-6147(02)00010-x. [DOI] [PubMed] [Google Scholar]
- Akaike N, Murakami N, Katsurabayashi S, Jin YH, Imazawa T. Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci Res. 2002;42:187–195. doi: 10.1016/s0168-0102(01)00320-0. [DOI] [PubMed] [Google Scholar]
- Burger PM, Hell J, Mehl E, Krasel C, Lottspeich F, Jahn R. GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron. 1991;2:287–293. doi: 10.1016/0896-6273(91)90267-4. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Fonnum F. Uptake of glycine, GABA and glutamate by synaptic vesicles isolated from different region of rat CNS. Neurosci Lett. 1991;129:217–220. [Google Scholar]
- Christensen H, Fykse EM, Fonnum F. Uptake of glycine into synaptic vesicles isolated from rat spinal cord. J Neurochem. 1990;54:1142–1147. doi: 10.1111/j.1471-4159.1990.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Christensen H, Fykse EM, Fonnum F. Inhibition of gamma-aminobutyrate and glycine uptake into synaptic vesicles. Eur J Pharmacol. 1991;207:73–79. doi: 10.1016/s0922-4106(05)80040-9. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Hosli L, Johnston GA. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6:1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Dan Y, Song HJ, Poo MM. Evoked neuronal secretion of false transmitters. Neuron. 1994;13:909–917. doi: 10.1016/0896-6273(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Dieudonne S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci. 2000;20:1837–1847. doi: 10.1523/JNEUROSCI.20-05-01837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Levi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999;112:811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieedonne S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fykse EM, Fonnum F. Uptake of gamma-aminobutyric acid by a synaptic vesicle fraction isolated from rat brain. J Neurochem. 1988;50:1237–1242. doi: 10.1111/j.1471-4159.1988.tb10599.x. [DOI] [PubMed] [Google Scholar]
- Gao BX, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. Neurophysiology. 2001;86:492–502. doi: 10.1152/jn.2001.86.1.492. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The loading of neurotransmitters into synaptic vesicles. Biochemical. 2000;82:327–337. doi: 10.1016/s0300-9084(00)00221-2. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Guastella J, Johnson CD, Stretton AO. GABA-immunoreactive neurons in the nematode Ascaris. J Comp Neurol. 1991;307:584–597. doi: 10.1002/cne.903070406. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Sahara Y, Takahashi T. A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron. 2002;34:613–621. doi: 10.1016/s0896-6273(02)00692-x. [DOI] [PubMed] [Google Scholar]
- Jang IS, Jeong HJ, Katsurabayashi S, Akaike N. Functional roles of presynaptic GABAA receptors on glycinergic nerve terminals in the rat spinal cord. J Physiol. 2002;541:423–434. doi: 10.1113/jphysiol.2001.016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Jursky F, Nelson N. Developmental expression of GABA transporters GAT1 and GAT4 suggests involvement in brain maturation. J Neurochem. 1996;67:857–867. doi: 10.1046/j.1471-4159.1996.67020857.x. [DOI] [PubMed] [Google Scholar]
- Katsurabayashi S, Kubota H, Wang ZM, Rhee JS, Akaike N. cAMP-dependent presynaptic regulation of spontaneous glycinergic IPSCs in mechanically dissociated rat spinal cord neurons. J Neurophysiol. 2001;85:332–340. doi: 10.1152/jn.2001.85.1.332. [DOI] [PubMed] [Google Scholar]
- Keller AF, Coull JA, Chéry N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I–II of the rat spinal dorsal horn. J Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr Opin Neurobiol. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features. J Biol Chem. 1993;268:22802–22808. [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Murakami N, Ishibashi H, Katsurabayashi S, Akaike N. Calcium channel subtypes on single GABAergic presynaptic terminal projecting to rat hippocampal neurons. Brain Res. 2002;27:121–129. doi: 10.1016/s0006-8993(02)03148-7. [DOI] [PubMed] [Google Scholar]
- Murase K, Randic M, Shirasaki T, Nakagawa T, Akaike N. Serotonin suppresses N-methyl-d-aspartate responses in acutely isolated rat dorsal horn neurons. Brain Res. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Radian R, Ottersen OP, Storm-Mathisen J, Castel M, Kanner BI. Immunocytochemical localization of the GABA transporter in rat brain. J Neurosci. 1990;10:1319–1330. doi: 10.1523/JNEUROSCI.10-04-01319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M, Bonanno G. Glycine is taken up through GLYT1 and GLYT2 transporters into mouse spinal cord axon terminals and causes vesicular and carrier-mediated release of its proposed co-transmitter GABA. J Neurochem. 2001;76:1823–1832. doi: 10.1046/j.1471-4159.2001.00159.x. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ishibashi H, Akaike N. Calcium channels in the GABAergic presynaptic terminals projecting to meynert neurons of the rat. J Neurochem. 1999;72:800–807. doi: 10.1046/j.1471-4159.1999.0720800.x. [DOI] [PubMed] [Google Scholar]
- Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. Fed Eur Biochem Soc Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Fyffe RE. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol. 1992;68:397–406. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- Sipilä S, Huttu K, Voipio J, Kaila K. GABA uptake via GABA transporter-1 modulates GABAergic transmission in the immature hippocampus. J Neurosci. 2004;24:5877–5880. doi: 10.1523/JNEUROSCI.1287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Neale EA. Bafilomycin A1 inhibits the action of tetanus toxin in spinal cord neurons in cell culture. J Neurochem. 1994;63:2342–2345. doi: 10.1046/j.1471-4159.1994.63062342.x. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Li Y, Xu TL. Co-release and interaction of two inhibitory co-transmitters in rat sacral dorsal commissural neurons. Neuroreport. 2002;13:977–981. doi: 10.1097/00001756-200205240-00016. [DOI] [PubMed] [Google Scholar]
- Yang HW, Min MY, Appenteng K, Batten TF. Glycine-immunoreactive terminals in the rat trigeminal motor nucleus: light- and electron-microscopic analysis of their relationships with motoneurones and with GABA-immunoreactive terminals. Brain Res. 1997;749:301–319. doi: 10.1016/S0006-8993(96)01326-1. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Petersen CC, Nicoll RA. Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurons. J Physiol. 2000;525:195–206. doi: 10.1111/j.1469-7793.2000.t01-1-00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]