Abstract
After exocytosis, chromaffin granules release essentially all their catecholamines in small fractions of a second, but it is unknown how fast they release stored peptides and proteins. Here we compare the exocytic release of fluorescently labelled neuropeptide Y (NPY) and tissue plasminogen activator from single granules. Exocytosis was tracked by measuring the membrane capacitance, and single granules in live cells were imaged by evanescent field microscopy. Neuropeptide Y left most granules in small fractions of a second, while tissue plasminogen activator remained in open granules for minutes. Taking advantage of the dependence on pH of the fluorescence of green fluorescent protein, we used rhythmic external acidification to determine whether and when granules re-sealed. One-third of them re-sealed within 100 s and retained significant levels of tissue plasminogen activator. Re-sealing accounts for only a fraction of the endocytosis monitored in capacitance measurements. When external [Ca2+] was raised, even neuropeptide Y remained in open granules until they re-sealed. It is concluded that a significant fraction of chromaffin granules re-seal after exocytosis, and retain those proteins that leave granules slowly. We suggest that granules vary the stoichiometry of release by varying both granule re-sealing and the association of proteins with the granule matrix.
After exocytosis, most secretory granules in chromaffin cells release their catecholamines completely and within tens of milliseconds (Wightman et al. 1991; Chow et al. 1992; but see also Graham & Burgoyne, 2002). However, chromaffin granules also contain and release a cocktail of proteins, including the granule matrix protein chromogranin, peptide hormones such as NPY (Kataoka et al. 1985), and enzymes such as dopamine-β-hydroxylase and the serine protease, tissue plasminogen activator (tPA) (Parmer et al. 1997). The different proteins may be released at different rates, depending on their size and on their propensity to aggregate. In PC12 cells, for example, neuropeptide Y-fused to enhanced green fluorescent protein (NPY-EGFP) is released more rapidly than tissue plasminogen activator-EGFP (tPA-EGFP) (Taraska et al. 2003), although the extent of the difference was unclear because that study could not temporally resolve the release of NPY-EGFP. In lactotropes, the granule matrix was often not released at all and instead retrieved along with the granule membrane when granules re-sealed (Angleson et al. 1999).
Unlike neurotransmitters that open ion channels in milliseconds, proteins and peptide hormones generally act more slowly, because they must first reach cells located at a distance and then mediate their effects by signalling cascades. Therefore, it may not matter physiologically whether a granule takes milliseconds or seconds to release its hormone, provided exocytosis ultimately goes to completion. However, if granules re-seal (‘cavicapture’, Henkel & Almers, 1996; Taraska & Almers, 2004) then the fraction of material released will depend both on the rate at which material is lost, and on the time the granule cavity stays open. Hence re-sealing granules are potentially capable of selective retention of secretory cargo.
Previous work has shown that granules readily undergo cavicapture in PC12 cells, a cell line derived from chromaffin cells (Holroyd et al. 2002; Taraska et al. 2003; Taraska & Almers, 2004). Here we explore the relationship between cavicapture and protein release in chromaffin cells, for the following reasons. First, cell lines do not always faithfully recapitulate the behaviour of the somatic cells from which they are derived. For example, the only direct observation of cavicapture in chromaffin granules was made in spontaneously secreting, cell-attached patches, where it was a rare event at physiological external [Ca2+] (7%, Ales et al. 1999). Second, while PC12 cell granules retain the membrane protein phogrin when they re-seal, it is unknown whether chromaffin or PC12 granules also retain soluble proteins. Finally, the time course of cavicapture has not been studied in any cell. Capacitance measurements have shown that intact chromaffin cells complete membrane retrieval in a few seconds (Henkel & Almers, 1996; Palfrey & Artalejo, 1998). However, when using amperometry in permeabilized chromaffin cells, it was found that inhibitors of dynamin increased the amount of catecholamine released from single vesicles (Graham et al. 2002). The finding implies that without such inhibitors, granules close within the 10 ms duration of an amperometric spike. Such short-lived connections with the external space would be missed in most capacitance measurements. Clearly, it seemed of interest to measure the time course of cavicapture directly.
Here we explore whether and under what conditions cavicapture leads to the retention of proteins. We first explored the release of NPY-EGFP and tPA-EGFP from single chromaffin granules after electrical stimulation. We found that tPA-EGFP escaped 40- to 50-fold more slowly than NPY-EGFP. Using a novel method, we next determined the time course of cavicapture, and compared it to the time course of endocytosis as assayed in capacitance measurements. At least one-third of all granules re-sealed within 100 s in electrically stimulated cells, and these retained tPA while releasing NPY. Surprisingly, cavicapture occurred more slowly than endocytosis as measured by membrane capacitance. Finally, an intervention known to inhibit the disassembly of the granule matrix caused NPY-EGFP to be retained in open granules for minutes.
Methods
Cells, plasmids and transfection
Bovine chromaffin cells were prepared (Parsons et al. 1995) with the following modifications. Cells were plated on 10 cm diameter Petri dishes at 107 cells per dish and fibroblasts were allowed to adhere overnight. The weakly adhering cells, mostly chromaffin cells, were gently washed off the dish with a serological pipette and centrifuged at 40 g for 15 min. They were re-suspended in ice-cold Ca2+- and Mg2+-free phosphate-buffered saline (Life Technologies), at 106 cells ml−1. The cell suspension (0.8 ml) was placed in an electroporation cuvette (0.4 mm gap size, BTX) with 10–40 μg of plasmid DNA, and electroporated in a T820 Electrosquare Porator (BTX) with a 20 ms 220 V voltage pulse. Cells were plated 5 min later onto high refractive index glass coverslips (n488 = 1.80, Plan Optik, Germany) coated with poly d-lysine (0.1 mg ml−1) and placed in plastic dishes containing pre-equilibrated culture medium (Parsons et al. 1995). The medium was renewed after 1 day, and the cells were used 2–4 days after transfection.
Human pro-neuropeptide Y (Lang et al. 1997) was placed in the pEGFP-N1 vector (Clontech). hNPY-Venus (Nagai et al. 2002) was a kind gift from Dr A. Miyawaki. Rat tissue plasminogen activator fused with EGFP (Lochner et al. 1998) and with cyan fluorescent protein (CFP) were gifts of Dr B. Scalettar (Lewis and Clark College, Portland, OR, USA). Transfected cells were identified by their punctate fluorescence under epifluorescence, and were selected for recording if they fluoresced also under evanescent field illumination. In some experiments, untransfected cells were stained with acridine orange during a 3 min incubation in normal recording solution (see later) plus 1 μm dye. Before recording, cells were washed with dye-free recording solution. Ascorbic acid (10 μm) was present throughout, both in the recording and pipette solution (see later).
During experiments, cells were bathed in normal recording solution (mm: 130 NaCl, 3 KCl, 5 CaCl2, 1 MgCl2, 10 glucose, 10 Hepes, pH 7.5) or in high Ca2+ solution (62.5 NaCl, 3 KCl, 50 CaCl2, 1 MgCl2, 10 glucose, 10 Hepes, adjusted to pH 7.5 with NaOH). In some experiments normal recording solution was quickly exchanged for various test solutions, as follows. Low pH solution (pH 5.5) was identical to normal recording solution except that Mes replaced Hepes. Ammonium chloride solution contained 50 mm NH4Cl replacing NaCl on an equimolar basis. A high K+ solution contained 75 mm KCl replacing NaCl on an equimolar basis. A high Ca2+ solution contained 50 mm Ca2+ that replaced Na+ on an equimolar basis. In some experiments, the high Ca2+ solution was buffered with Mes (pH 5.5) in place of Hepes. The osmolarity of all solutions was measured and was around 300 mosmol l−1.
For fast solution changes, a theta glass capillary (World Precision Instruments) was pulled to ∼100 μm tip size, and two lines containing control or test solution were inserted. The change in solution was controlled by electromagnetic valves (Lee Company) actuated manually or by the patch-clamp amplifier. The tip of the perfusion pipette was positioned within 100 μm of the cell. The solution exchange under the cell was generally complete in 1 s or less (e.g. Fig. 8). All chemicals were purchased from Sigma.
Figure 8. Display of NPY-GFP labelled granules at elevated [Ca2+].
A1 and A2, voltage and pH changes at 50 mm external [Ca2+]. B1, fluorescence at the granule site, showing a slow increase on exocytosis and a slow but transient decrease when the pH was lowered and raised again. B2 continues recording at lower imaging frequency, with voltage and pH indicated in A2. For 300 s fluorescence fell and rose as the pH was lowered and raised, indicating an open fusion pore. Downward arrow indicates presumed time of cavicapture. C1 and C2 as in B1 and B2 but for a granule that re-sealed within 5 s after opening. Vertical arrow indicates when the granule opened a second time. C3, section of C2 marked by the arrow, shown at an expanded time scale. D1 and D2 from a third granule that re-sealed within 2 s and before the pH could be changed. It was soon lost from view (downward triangle). Throughout, horizontal dotted lines indicate fluorescence of the granule before its first fusion. Dashed lines and shaded areas interrupt the traces and indicate times when measurements were suspended. Note that the timing in A2 applies to B2 but not precisely to C2. E, time course of cavicapture estimated as the latency before first closure, as in Fig. 6D. Open circles and horizontal lines plot the fraction of granules remaining open (38 granules in 7 cells). Granules were counted as open unless they were seen to close; the count includes 9 granules that underwent discharge as in Fig. 1, and four granules that disappeared without becoming demonstrably pH resistant. The black trace indicates what remains of the capacitance increase caused by the stimulus, and was determined as in Fig. 7A (19 recordings in 7 cells). F, capacitance changes (upper) and membrane currents (lower) in a single cell at 5 mm (black) and 50 mm external [Ca2+] (grey). Capacitance was not measured during 0.5 s voltage steps, hence the gap in the trace and the upward jump in the trace when the capacitance measurement is resumed.
Experiments were carried out at 22–25°C; results are given ± s.e.m.
Imaging
Cells were viewed with an Zeiss Axiovert 135 microscope using through-the-lens evanescent field illumination as described previously (Zenisek et al. 2000), using a 1.65 numerical aperture objective (Apo × 100 O HR, Olympus). Images were magnified 2.5 times and recorded by an intensified digital camera (Pentamax, Princeton Instruments) as 50 ms duration frames at 20 Hz. Some experiments were recorded with an image intensifier and a video camera, and stored on an optical disk recorder (Zenisek et al. 2000). In some experiments we alternated between viewing proteins carrying the fluorescent proteins CFP and Venus (a yellow fluorescent protein) (Nagai et al. 2002). These were done with a back-illuminated camera (Micromax, Roper Scientific) as described previously (Taraska et al. 2003).
Electrical and optical recordings
We recorded from cells in the whole cell configuration with a patch pipette (2–3 MΩ resistance) coated with dental wax, in voltage clamp mode using an EPC-9 amplifier running PULSE (Heka, Germany). The pipette solution contained 140 mm CsCl, 10 mm Hepes, 2 mm MgCl2, 4 mm ATP, 0.4 mm GTP and 0.1 mm EGTA. In experiments with NPY-EGFP and tPA-EGFP, the cell capacitance was monitored with the ‘Sine + DC’ feature of the PULSE lock-in mode. A 1 kHz, 20 mV peak amplitude sine wave was applied to a holding potential of −70 mV. The cell was stimulated with a 500 ms voltage jump to +20 mV. At the same time, images were acquired at 20 Hz running Metamorph (Universal Imaging) at 20 Hz in stream (continuous) mode for 5 s. The stream started 500 ms before the voltage pulse. Sometimes the 100 frame stream was followed by a sequence of 60 exposures (50 ms) taken at 1 Hz (called time lapse sequence hereafter). The interval between stream and time lapse depended on the size of the recorded image, and was less than a second. The entire sequence (a voltage pulse accompanied by stream imaging and followed by time lapse recording) repeated at variable intervals of 0.5–5 min. For the highest time resolution, images were acquired with a video camera and later digitized and de-interlaced to improve time resolution (Zenisek et al. 2002).
Image analysis
Granules changing their appearance during a movie (‘events’) were identified by eye while the movie was played. Movies of acridine orange-stained cells were populated so densely with granules that identifying events by eye became difficult. To highlight events in acridine orange movies, we formed a 10-frame rolling average of such movies, subtracted it from the original while adding a constant, and viewed the result. In cells expressing NPY-EGFP, events were classified as either exocytic discharge or display (see Results). Events were analysed using routines programmed in Matlab.
Our background subtraction procedure was designed to measure the fluorescence of a granule accurately even if the granule had a close neighbour. First, a 1.6–2.5 μm square region centred on the granule of interest was excised from an image of the chromaffin cell, and 5 to 10 frames before the start of the event were averaged (Fig. 1A). The result was plotted as a three-dimensional landscape giving fluorescence intensity as a function of position (Zenisek et al. 2000). The landscape was then fitted by the sum of (a) an inclined plane, (b) a radially symmetric first Gaussian function centred on the granule of interest, and (c) a second Gaussian function centred on the granule's nearest neighbour. The parameters of the inclined plane, the centre positions of the Gaussian functions, their amplitudes and widths were free parameters. Gaussian functions provided a good fit to the image of a granule. The centroids of the two Gaussian functions are indicated in Fig. 1A; that for the granule of interest by a cross and that for its neighbour by a dagger. The more centrally located Gaussian function (Fig. 1B) was subtracted from the pre-fusion average and yielded an image of the local background (Fig. 1C). The background image was subtracted from the remainder of the movie. Images shown are before background subtraction unless indicated otherwise.
Figure 1. Background subtraction.
A, small section of a fluorescence image of a chromaffin cell. The centroids of the Gaussian functions fitted are indicated by a cross for the granule of interest, and by a dagger for its nearest neighbour. B, Gaussian functions fitted to the granule of interest, shown separately. C, difference image A minus B provides an estimate of the local background.
In most experiments, the fluorescence from the granule was analysed by re-fitting each frame with a Gaussian function on an inclined plane. Its amplitude was taken as the fluorescence immediately at the granule site, and the value of the inclined plane at the centre of the Gaussian function was taken as an updated version of the local background. The amplitude times the squared width of the Gaussian function was used as an estimate of the integrated light, or the total light emitted by chromophores in the granule, even after the fluorophores have spread during release from granules.
Immunostaining
After being washed in recording solution, cells were incubated with sheep anti-tPA antibody (affinity purified anti-human tPA, Enzyme Research, South Bend, IN, USA) at a concentration of 10 μg ml−1 for 10 min. Then 2 μl of concentrated nicotine was added to some cells (final concentration of 10 μm) while other cells remained undisturbed. After a further 1 min, cells were washed for 5 min in recording solution, then fixed with 5% paraformaldehyde in phosphate buffered saline (PBS, 20 min) and rinsed twice in PBS. Cells were blocked with 50 mm glycine and 0.3% bovine serum albumin, then permeabilized with 0.05% saponin for 30 min. AlexaFluor-488 conjugated donkey anti-sheep antibody (Molecular Probes) was added for 1 h at 2 μg ml−1. After extensive washing, cells were mounted in Mowiol. They were observed with a laser scanning confocal microscope (Leica DMR TCS SP2, Leica Microsystems, Germany) with a 63 ×, 1.4 NA objective and 4 × zoom. The pinhole aperture was 1 Airy. Images were recorded at vertical intervals of 0.4–0.8 μm (8–10 images per cell); laser intensity, photomultiplier gain and other parameters were kept constant for all conditions.
Results
Rapid discharge of granule contents after a stimulus
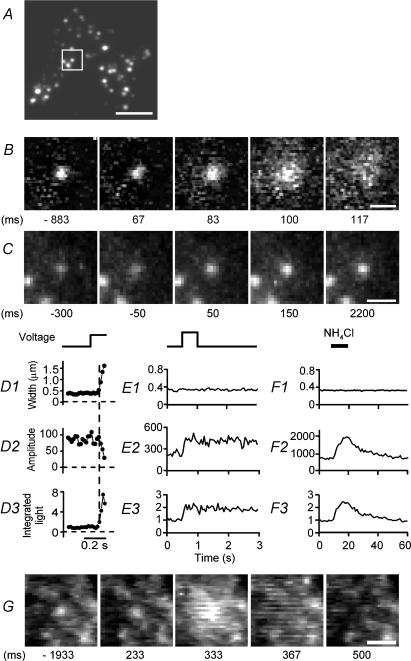
To watch exocytic protein release from single granules, we transfected bovine chromaffin cells with pro-NPY-EGFP. NPY is normally stored in chromaffin granules (Kataoka et al. 1985), and NPY-EGFP has been used as a marker of dense core granules in PC12 cells (Lang et al. 1997; Nagai et al. 2002; Holroyd et al. 2002; Taraska et al. 2003) and chromaffin cells (Steyer & Almers, 2001). Cells were placed under voltage clamp and observed by evanescent field fluorescence microscopy, a method well suited to observe exocytosis as it selectively images the plasma membrane and the adjacent 100 nm of cytoplasm. Figure 2A shows the ‘footprint’ where a cell adhered tightly to the coverslip. Single granules containing NPY-EGFP appear as fluorescent spots. They populate the footprint more sparsely than in previous work in PC12 cells, probably because the longer turnover time for granules (18 days in chromaffin cells, Corcoran et al. 1984), and because most granules were generated before the cell was transfected. The sparse population facilitates fluorescence measurements from a single granule without interference from its neighbour. To stimulate exocytosis, 0.5 s voltage steps to +20 mV opened voltage-gated Ca2+ channels and elicited Ca2+ inward currents (average 395 ± 56 pA in 27 transfected cells). The voltage steps increased the membrane capacitance by an average of 185 ± 20 fF. Hence our cells responded to Ca2+ inward current with exocytosis.
Figure 2. Discharge and display of NPY.
A, evanescent field image showing the ‘footprint’ of an NPY-EGFP-expressing chromaffin cell where the cell adheres to a coverslip. B, sequence of de-interlaced video frames taken at 60 Hz: same region as marked by the square in A. Times relative to the start of a 0.5 s voltage pulse. The NPY-EGFP discharged from the granule was visible as a fluorescent cloud. Images shown after background subtraction as described in Methods. C, granule undergoing exocytosis without discharging NPY in another cell. D, results of fitting a Gaussian function to the granule in B. Width (D1), amplitude (D2) and volume integral of the Gaussian function (D3), assumed to be proportional to the total light from the granule's NPY-EGFP. D3 is plotted relative to the pre-fusion average. Dashed vertical line indicates the presumed moment of fusion. Granules could be fitted with Gaussian functions for only a few frames after fusion. Pixel values in D2 are lower than in E2, F2 and elsewhere in this paper because images were taken with an intensified video camera that digitized light values on an 8-bit scale. E, similar analysis based on background-subtracted versions of the images in C. F, analysis as in D and E of an image sequence from a quiescent granule in a resting cell. A 10 s perfusion with 50 mm NH4Cl (horizontal bar) caused the pH in the granule lumen to rise transiently and the granule to brighten and then dim again. G, granule in a cell stained with acridine orange; images shown before background subtraction. As in B, a fluorescent cloud indicated release of the dye from the vesicle at the centre. Times in B, C and G relative to the start of voltage pulses. Calibration bars 5 μm in A and 1 μm elsewhere.
Aside from causing an increase in cell surface area, exocytosis can be observed by imaging. After a voltage pulse, an average of about two NPY-EGFP expressing granules per footprint underwent a detectable change. Most behaved as in Fig. 2B (152 of 226 events in 43 cells) where a fluorescent cloud appeared briefly at the site of the granule and left darkness behind (Steyer et al. 1997; Oheim et al. 1998; Tsuboi et al. 2000; Steyer & Almers, 2001; Barg et al. 2002; Taraska et al. 2003). We will refer to such events as ‘discharge’, because the cloud indicates the exocytic discharge of fluorescent cargo from the granule. Figure 2D analyses results in Fig. 2B. NPY-EGFP left the granule rapidly after exocytosis, and diffused away in the narrow cleft between the glass and the adherent cell. The fluorescence profile increased in width and declined in peak intensity. However, the light integrated over the entire region briefly increased nearly 8-fold, indicating that NPY-EGFP strongly increased its fluorescence. Two factors contribute to this increase. First, when NPY-EGFP is released it inevitably moves closer to the glass where it is more intensely illuminated by the evanescent field. Second, the pH sensed by EGFP rises as acid escapes from the granule, resulting in an increase of the EGFP brightness (Patterson et al. 1997; Kneen et al. 1998; Llopis et al. 1998; see later).
Other types of behaviour were occasionally observed with NPY-EGFP. Sometimes (4% of 226 events) a dimmer dot remained after the cloud of NPY-EGFP had passed, suggesting that the discharge of NPY-EGFP was incomplete. In other cases (7%) a cloud of released NPY-EGFP appeared where no granule was visible before, perhaps through exocytosis of a granule some distance from the glass–cell interface. Granules are sometimes seen to contact funnel-shaped invaginations of the plasma membrane (Plattner et al. 1997).
Some granules retain NPY-EGFP after exocytosis
An unexpected behaviour was observed in 22% of events (Fig. 2C) and is referred here as ‘display’. The NPY-EGFP-containing granule brightened, but its fluorescence did not spread significantly and remained at the granule site throughout the recording. Evidently NPY-EGFP left the granule only slowly, if at all. The event in Fig. 2C is analysed in Fig. 2E. The integrated fluorescence increased by 3.5 ± 0.3-fold (38 granules in 21 cells). Rarely (4%), granules underwent display for a few seconds and then discharged their NPY.
Why does fluorescence increase in display? When fusion pores open, granules will de-acidify and thus relieve the quenching of EGFP fluorescence. For instance, with a pH of 5.5 inside granules (Rottenberg, 1979) and an extracellular pH of 7.5, this effect will raise fluorescence 2- to 4-fold (Patterson et al. 1997; Kneen et al. 1998; Llopis et al. 1998). Figure 2F further examines this possibility. Cells were locally superfused with a solution containing NH4Cl. Ammonium ions are in equilibrium with membrane permeant NH3, a proton carrier that dissipates pH gradients across membranes (Miesenbock et al. 1998). Most granules (52 of 66 in 9 cells) brightened transiently while NH4Cl was applied and withdrawn, with the integrated light increasing by a factor of 3.5 ± 0.2 (n = 52 of 66 granules). This change is similar or identical in size to the brightening observed during display. Evidently, display in most granules could be explained entirely if the pH inside the granule became equal to that of the external medium.
About 21% of granules (14 of 66) did not brighten in response to NH4Cl, presumably because their lumen already had a neutral pH. Granules that do not accumulate protons will also not accumulate catecholamines from the cytosol. Exocytosis of such empty granules has been observed in patch amperometric measurements (Tabares et al. 2001). We do not know why some granules fail to accumulate protons.
In an alternative explanation of display, granules might brighten as they approach the plasma membrane and move into the evanescent field. Indeed, for minutes after a stimulus, granules appear and brighten as they dock to replace those lost by exocytosis (Steyer et al. 1997; Oheim et al. 1998). This possibility was tested in cells stained with acridine orange. Although the low pH in granules causes strong accumulation of the dye in granules, it does not, in itself, change the fluorescence of the dye. In a fluorimeter, fluorescence varied by < 15% (emission 525 and 700 nm, concentration 0.01, 0.1 or 1 mm) when pH was varied between pH 5.0 and 7.35 (data not shown). Hence, acridine orange-loaded granules will brighten when they approach the membrane but not immediately when their internal pH increases.
When acridine orange-stained cells were stimulated, exocytosis resulted in striking flashes of light (Fig. 2G) (Steyer et al. 1997; Oheim et al. 1998; Tsuboi et al. 2000). The flash resulted because the fluorescence is strongly quenched while the dye is concentrated in the granule, and this quenching is relieved as dye dilutes into the external medium. Sixty-one events as in Fig. 2G were observed in five cells. Even if we ignore the 30 discharge events at sites where no granule was visible before the stimulus, only 1 of 31 granules brightened without spreading. This one granule brightened by only 30% and did so before the stimulus. Evidently, granules rarely approach the plasma membrane over the 5-s recording intervals used here, and the display phenotype is rarely or never seen when the chromophore in the granule cannot brighten as the pH rises. We conclude that display generally results from a rise in intragranule pH.
The following results indicate that display results from exocytosis. First, if display and discharge both start when the fusion pore opens, then both should start with the same delay after a stimulus. This was observed (Fig. 3A). Furthermore, external acidification should reverse the increase in fluorescence seen during display. Indeed, 7 of 8 granules dimmed again after display when the external pH was lowered (Fig. 3B, C). In the same images, granules that had not undergone display did not dim. Similarly, granules did not dim if the pH was not changed after exocytosis (Fig. 3D). Clearly, display generates an aqueous connection between the interior of the granules and the external space and therefore indicates exocytosis. Surprisingly, NPY-EGFP failed to escape from these granules even though they stayed open. One granule in Fig. 3C failed to dim when the pH was lowered after display; this granule may have re-sealed before the pH change.
Figure 3. Display represents exocytosis.
A, number of discharge and display events plotted as cumulative latency histograms as a function of time. B, response to external acidification. Images of a granule before the voltage pulse (1) and after display, first at pH 7.5 (2) and then at pH 5.5 (3). Analysis of experiments as in B. C, timing of voltage pulse and pH change. D (top), fluorescence amplitude of granules after display (•, 8 granules in 4 cells) and of other granules in the same images that did not change appearance during the movie (○, 12 granules). Values for each granule were normalized to the mean of the last 7–10 measurements before the pH change, then the results for all granules were averaged. Bottom, from display events as in (•) but without a pH change. For each granule, fluorescence was normalized to the mean recorded 1.5–2.0 s after the stimulus; the results were then averaged (19 granules in 10 cells).
It seemed possible that display is a consequence of over-expressing NPY-EGFP and of overloading the granule with cargo or with EGFP. Therefore, we used the brightness of granules before exocytosis as an indicator of how much NPY-EGFP they contained, and compared, separately for each cell, the pre-exocytic brightness of granules undergoing display with that of granules undergoing discharge. The brightness ratio (display/discharge), was not significantly different from 1 in 4 cells where both types of events were recorded (1.4 ± 1.4, n = 4). Therefore, display is unlikely to result from the over-expression of NPY-EGFP.
Release of tissue plasminogen activator
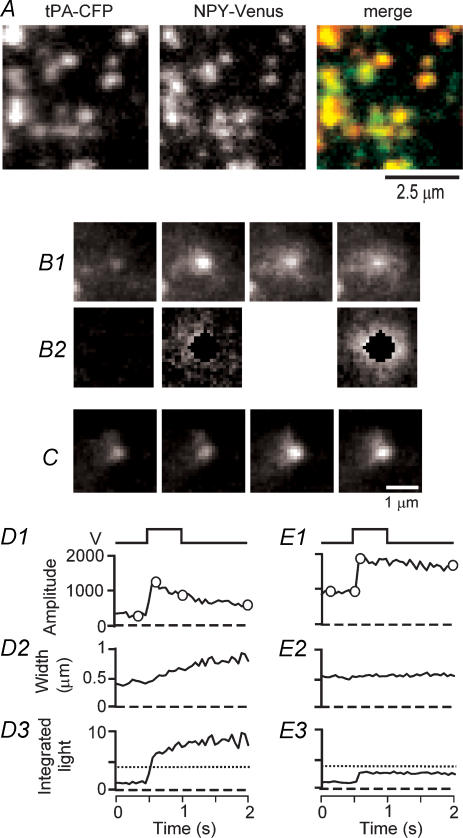
We next explored the release of tissue plasminogen activator (tPA), another protein contained in secretory granules of chromaffin (Parmer et al. 1997) and PC12 cells (Taraska et al. 2003). To confirm that tPA is targeted towards chromaffin granules when conjugated with a fluorescent protein, cells were cotransfected with tPA-CFP and with NPY fused to Venus, a yellow fluorescent protein (Nagai et al. 2002). Although the proteins inhabited individual granules in strongly varying proportions, it was rare to find tPA-CFP without NPY-Venus and vice versa (Fig. 4A). Of the labelled granules, 90 ± 3% contained detectable amounts of both fluorophores (3 cells). Similar results were obtained in PC12 cells (Taraska et al. 2003).
Figure 4. Slow release of tPA-EGFP.
A, portion of a chromaffin cell co-expressing tPA-CFP (left) and NPY-Venus (middle); the two images are merged on the right. B1, exocytosis after a voltage pulse. The granule brightens and its fluorescence spreads. B2, same granule after subtracting background. To highlight the fluorescent cloud, the brightness was increased and the centre was blanked out. Note that the cloud spreads while the peak brightness in B1 diminishes. C, another granule brightened but failed to spread, resembling display of NPY-EGFP. The granules in B (D1–3) and C (E1–3) were analysed as in Fig. 2. Plots show the fluorescence at the granule site (amplitude), the width, and the total light from the granule (integrated fluorescence). Voltage pulses are shown above. Horizontal dotted lines in D3 and E3 show the brightening accounted for by the expected change in intragranule pH.
Granules showed strikingly varied behaviours during exocytosis. However, whereas the events with NPY fell into two easily distinguishable categories, those with tPA showed a more graded variation. Figure 4 shows extremes on a spectrum. In Fig. 4B, tPA-EGFP was observed as a cloud of fluorescence spreading from the granule. The fluorescence at the granule site rose and then declined as fluorescence spread, while the width of the fluorescent patch increased throughout the recording (Fig. 4D1–3). As in the discharge of NPY-EGFP (Fig. 3D), there was a large increase in integrated light, reflecting both de-acidification of the granule and release of tPA-EGFP towards the glass substrate. Most granules (30 of 39), however, behaved more similarly to the display events seen with NPY-EGFP, in that they brightened but spread little over the subsequent 4 s. An example is shown in Fig. 4C and analysed in Fig. 4E. The fluorescence at the granule site (amplitude) doubled abruptly while the granule remained compact and its width increased only slightly. As with display of NPY-EGFP, pH changes indicated that tPA was connected to the external space (see later).
Differential release of tPA and NPY
To compare the release of the two proteins, the fluorescence at the granule site was plotted against time. We first discuss Fig. 5A where discharge and display events are averaged together. The tPA-EGFP fluorescence brightened 4.2-fold and declined slowly. NPY-EGFP fluorescence brightened by much less, in part because NPY-EGFP diffused away too rapidly once it escaped the granule, and in part because the fluorescence change was too fast to be tracked well with our camera. To estimate the difference in release rates nonetheless, we assume that the choice of which protein is labelled affects neither the intragranule pH, the size of docked granules nor their distance from the plasma membrane. The fluorescence after exocytosis should then be proportional to the fraction of labelled protein remaining in the granule. For example, 67 ms after exocytosis, the fluorescence of NPY-EGFP was 1.6 times brighter than before, while that of tPA-EGFP was 4.2 times brighter. It follows that no more than 1.6/4.2 = 38% of the NPY remained in the granule after 67 ms. This corresponds to a time constant of 69 ms inasmuch as the decline in fluorescence is exponential with NPY-EGFP. When an exponential function was fitted to the final 1 s of the tPA-EGFP data, the time constant was 3.0 s, a 40-fold larger value.
Figure 5. Differential release of tPA-EGFP and NPY-EGFP.
A–C, granules were surrounded by 0.9 mm circles in background-subtracted video clips, and the average fluorescence within the circle was plotted against time. For each granule, fluorescence was normalized to the mean of the last 5 measurements before fusion. The results were then averaged. ○, tPA-EGFP; •, NPY-EGFP. A, average of all granules for NPY-EGFP (40 granules in 9 cells) and tPA-EGFP (35 granules in 8 cells). B, average of all discharge events in this NPY-EGFP dataset (n = 32) and all events with tPA-EGFP where fluorescence spread by more than 30% (n = 9). C, average of all display events with NP-EGFP (n = 8), and events with tPA-EGFP where fluorescence spread by 30% or less (n = 26). D, changes in tPA-CFP and NPY-Venus fluorescence in the same granule. Time in seconds relative to the start of perfusion with a solution of elevated [K+]. Because fluorescence was simultaneously excited at both 458 and 514 nm, some of the fluorescence attributed to NPY-Venus is due to tPA-CFP (equivalent to 26% of the fluorescence in the CFP channel). The faint fluorescence remaining at the granule site at the end of the NPY-Venus series may result from this effect.
The large difference may be thought to arise partly because display events are the minority with NPY-EGFP (20% in this dataset) but the majority with tPA (80%). However, even when tPA-EGFP did show signs of escaping from a granule, such escape was much slower than in discharge events with NPY-EGFP (Fig. 5B). We selected the subset of those tPA-EGFP containing granules that lost the most tPA-EGFP, and the subset of those NPY-EGFP containing granules that underwent discharge. When time constants of release were calculated for the two subsets as in Fig. 5A, the time constant for tPA-EGFP was 75-fold larger than for NPY-EGFP. Therefore, tPA-EGFP escaped from granules more than 40-fold slower than NPY-EGFP, no matter whether one compares entire datasets or subsets. Figure 5B also shows that the peak increase in fluorescence is 7-fold with tPA-EGFP but less than 3-fold with NPY-EGFP. Apparently, NPY-EGFP diffuses from the exocytic site nearly as rapidly as it emerges from the granule while tPA-EGFP lingers.
In display events, neither NPY-EGFP nor tPA-EGFP escaped significantly over the 1.3 s of recording (Fig. 5C), and the increase in fluorescence was entirely accounted for by pH increase sensed by the EGFP.
To test whether the slow release of tPA-EGFP is a consequence of over-expressing this protein, we co-expressed NPY-Venus and tPA-CFP in the same granules and imaged both proteins simultaneously. Cells were stimulated by raising external [K+] to 75 mm for 5 s while acquiring image pairs at 2 Hz. At this imaging frequency, we expect the discharge of NPY-EGFP or NPY-Venus to appear as a sudden loss of fluorescence, at least when these constructs are expressed alone. Indeed, even when granules contained both tPA-CFP and NPY-Venus, stimulation caused NPY-Venus to be lost in a manner that appeared instantaneous at 2 Hz. In 5 of 6 such events, tPA-CFP stayed behind; an example is shown in Fig. 5D. Evidently, the release of fluorescent NPY is rapid even when granules express and retain fluorescent tPA. Therefore, the reluctance of fluorescent tPA to leave open granules does not result because its over-expression inhibits the release of proteins in general.
Granules re-seal after exocytosis
While tPA-EGFP remains in granules it may be used to track their postexocytic fate, and fluorescence changes during pH jumps may reveal whether or not granules re-sealed. In Fig. 6A, for example, a granule brightened after the cell was stimulated. When the pH was temporarily lowered, the granule dimmed reversibly, indicating that its lumen had connected with the external space. Figure 6A2 also shows the timing of the stimulus and the pH change, and Fig. 6A3 the fluorescence changes in the granule. When a second pH change was imposed 10 s later in Fig. 6B, the granule fluorescence did not change significantly. We conclude that the granule re-sealed between 5 and 15 s after exocytosis. Figure 6C describes an experiment on another granule. As in Fig. 6A, the granule brightened during exocytosis, and then dimmed and brightened repeatedly during a train of rhythmic pH changes. However, it failed to respond to a later train of pH changes. This granule evidently remained connected for more than 1 min. It then became darker as it either lost some tPA-EGFP, acidified or retracted from the plasma membrane. At around 75 s (arrow in Fig. 6C2), the granule ceased to show fluorescence changes that were temporally correlated with pH changes. We conclude that the granule had re-sealed.
Figure 6. Tracking granule re-sealing by changes in external pH.
A1, granule undergoes exocytosis, then dims transiently on acidification. The background fluorescence decreased on acidification and was due to secreted tPA-EGFP on the cell surface or on the coverslip. Times of image acquisition indicated by reference to A2, which gives the timing of voltage (upper) and pH changes (lower). A3, changes in fluorescence on the same time scale as A2. Horizontal line (upper) marks episodes where the pH was lowered (1) and raised again (2). B1, failure of fluorescence to respond to a later pH change. Times of image acquisition as shown in B2. Scale bar (1 μm) applies to A1 and B1. B2–3, as in A2–3. B3 re-plots the data in A3 at a slower time scale, and continues with imaging frequency reduced from 20 to 1 Hz. Horizontal line marks episodes 1, 2 as well as a third including the first in a train of 5 subsequent pH changes. C1–2, as in B2–3 for another granule. C1, changes in voltage and pH; C2, fluorescence; it responded to the first train of pH pulses, but not to the pH pulse given after a subsequent stimulus (vertical arrow), nor to a later train of pH pulses. Later, the granule disappeared (▾). Horizontal line above trace marks episodes for determining granule re-sealing. This granule is assumed to have re-sealed during episode 6. For clarity, episodes 1 and 2 were not marked.
Most granules dimmed significantly during the first minute after fusion so that the signal to background ratio limited the accuracy of analysis. In order to determine the responsiveness to external pH even as the granules darkened, we divided the time after exocytosis into episodes of increasing length, as indicated above the fluorescence traces in Fig. 6A3, B3 and C2. Then we determined separately for each episode whether the granule was responsive to pH. The time of re-sealing was taken to be the midpoint of the first episode wherein the granule failed to respond to pH. Such experiments were performed successfully on 33 of 39 granules. All granules dimmed when the pH was first lowered (episode 1). Three failed to re-brighten during the subsequent rise in pH (episode 2), presumably because they re-sealed within the first 2–4 s after exocytosis. Nine closed during the next 90 s (median time 8 s). Five were still open and contained tPA-EGFP even after 150 s. The remaining 16 granules lost their fluorescence before re-sealing could be established by pH changes. These granules either re-sealed and then left the plasma membrane, or remained open and lost their tPA-EGFP.
Figure 7A (open circles and horizontal bars) plots the fraction of granules remaining open after exocytosis. The figure traces the time course of granule re-sealing under the assumption that no granules closed after they had lost their tPA-EGFP, and that every granule that closes also acidifies. Inasmuch as especially the first of the two assumptions is incorrect, the graph underestimates re-sealing. However, even this low estimate indicates that a third of all granules re-sealed within 1.5 min after fusion.
Figure 7. Cavicapture of chromaffin granules.
A, open circles and horizontal bars plot the fraction of granules that did not demonstrably re-seal in experiments as in Fig. 6. The response to pH changes was judged during each of 7 episodes as in Fig. 6B3 and C2. Successful pH changes were evident from the changes in background fluorescence around the granule site, as in Fig. 6A1 and B1. Thick trace, decline in membrane capacitance indicating membrane retrieval. For each recording, we first determined the amplitude of the rise in capacitance caused by the voltage pulse (see later in Fig. 8F for example), as the difference between the averages before and between 450 and 500 ms after the pulse. Values from the remainder of the recording were normalized to the amplitude thus determined, and the results from 14 recordings in 6 cells were averaged. For averaging, recordings were weighted in proportion to the granules observed to undergo exocytosis during each voltage pulse. B, chromaffin cells immunostained for surface-exposed tPA. Confocal images were taken at the ‘equators’ of cells where their outlines appeared largest. Outlines (red lines) are not visible at the brightness and contrast setting used for the two images, but were obvious when images were displayed at maximal brightness (not shown). Left, quiescent cells; right, single cell stimulated for 1 min with 10 μm nicotine. Punctate fluorescence is seen. The organelle indicated by the arrow (left) is well inside the outline and presumably moved into the cell after cavicapture. For analysis, bright regions were identified by thresholding the image, and counted as immunopositive organelles if they had diameters of at least 3 pixels (equivalent to 0.5 μm).
We wondered to what extent granule re-sealing could account for the decline in membrane surface area measured in the capacitance assay. The continuous line in Fig. 7A plots the average membrane capacitance after the voltage pulse as it declines due to endocytosis. To facilitate comparison between capacitance and fluorescence data, the average weighted each capacitance trace in proportion to the granules seen to undergo exocytosis during that trace. Initially capacitance and fluorescence measurements agree, but later the cell capacitance declines more strongly than expected from the number of observed granule closures. The difference is due partly to granules losing their tPA-EGFP before they close, and partly because other membrane retrieval mechanisms operate after exocytosis. Such mechanisms may include clathrin-mediated endocytosis (Nagasawa, 1977; Patzak & Winkler, 1986; Artalejo et al. 2002) and other endocytic mechanisms.
Recent work implies that a significant percentage of granules re-seals rapidly enough to curtail the release even of a small molecule such as adrenaline (epinephrine) (Graham et al. 2002). In our assay, such granules would brighten as their pH rises, but become insensitive to external pH changes within the 10 ms duration of an amperometric spike. At physiological external [Ca2+], very few granules behaved in this way, and none did in the datasheet of Fig. 6.
We tested whether exocytosis of endogenous tPA could be detected by immunostaining. Live cells were incubated with antibody against tPA for 10 min, and then the antibody was washed away. Later, the cells were permeabilized and stained with a fluorescent secondary antibody. tPA-containing granules undergoing exocytosis during the 10 min incubation are expected to become visible as fluorescent dots if they re-seal before they release all their tPA. In resting cells, such immunostaining was rarely observed, and none was evident in Fig. 7B (left). However, stimulation with 10 μm nicotine not only evoked robust release of catecholamines in amperometric measurements (data not shown), but also caused the appearance of punctate immunofluorescence, both in the cell periphery and within the cell. Clearly, endogenous tPA became accessible to external antibody but remained associated with small organelles, presumably granules. Some of these were ultimately internalized. Our result is reminiscent of earlier work with dopamine β-hydroxylase (Wick et al. 1997). For quantitative analysis, optical sections were made through each cell at regular intervals, and fluorescent dots were counted. There were significantly more dots in stimulated (37.5 ± 4.9 dots per cell, n = 11) than in resting cells (3.4 ± 0.9 dots per cell, 10 cells). The result is consistent with the idea that granules of untransfected cells contain tPA (Parmer et al. 1997), that the tPA becomes exposed to the external space during exocytosis, and that some of it remains in granules when they re-seal.
High external calcium concentration favours display of NPY-EGFP labelled granules
In cell-attached patches, elevated external [Ca2+] promotes rapid cavicapture after spontaneous exocytosis (Ales et al. 1999), suggesting that it may also promote display. Indeed, raising external [Ca2+] from 5 mm to 50 mm raised the proportion of display events from 22% to 64% (74 of 115 events) in cells expressing NPY-EGFP.
Figure 8 shows recordings on three granules with the same experimental protocol as in Fig. 6. They illustrate the variation in behaviours observed. The granule in Fig. 8B1 brightened during display and then dimmed reversibly when the pH was transiently lowered. Fluorescence continued to respond to repeated changes in pH changes, indicating an open fusion pore (Fig. 7B2). After about 5 min the fluorescence stopped responding to pH, indicating cavicapture. Figure 8B1 is noteworthy in that the fluorescence took more than a second to rise, indicating slow escape of protons from the granule. Protons permeate rapidly even through some ion channels, hence the slow loss of protons seems surprising. Possibly, the pore opened only briefly and intermittently. A similar observation was made in Ins-1 cells (Barg et al. 2002).
Figure 8C illustrates the case of a granule that re-sealed and then underwent exocytosis a second time. As in Fig. 8B1, it brightened during display and then dimmed reversibly during a single pulse of low pH. Subsequent pH changes were without effect (Fig. 8C2), indicating that the granule had re-sealed. The fluorescence then declined slowly due to photobleaching and/or acidification. Minutes later, the granule re-opened (vertical arrow in Fig. 8C2). Figure 8C3 was recorded on an expanded timescale at the time indicated by the arrow in Fig. 8C2, and shows the granule brightening in response to the voltage pulse as it underwent display for a second time. Figure 8D1 shows an event of the kind expected from the work of Ales et al. (1999) or Graham et al. (2002) where the granule opened long enough to de-acidify, but closed before the external pH was lowered. This granule never re-opened and instead disappeared into the cytosol 25 s later (Fig. 8D2).
In a dataset on seven cells we observed 38 exocytic events. Among them were 29 display events, and these were analysed as in Fig. 8A–D. Nine granules re-sealed within < 1 s after exocytosis and before the pH could be changed. Fifteen others remained open for seconds to tens of minutes. The remaining four faded before pH resistance could be demonstrated. In total, 24 of 38 granules re-sealed. The fraction of granules re-sealing within 1 s (24%) was larger than at normal [Ca2+] with tPA (none), consistent with external Ca2+ promoting rapid cavicapture (Ales et al. 1999).
Figure 8E (open circles) plots the time course of re-sealing as in Fig. 6. The shape of the relationship suggests multiple kinetic components and hence multiple mechanisms for re-sealing, or multiple steps on the way to re-sealing. As a second indicator of membrane retrieval, the decline of the capacitance increase caused by the Ca2+ influx is also plotted in Fig. 8E. Over the first 30 s, results from both methods agreed therefore cavicapture could fully account for membrane retrieval. Later, open and closed circles diverged. As in Fig. 6, this probably resulted because of endocytic mechanisms other than cavicapture, and because some granules left, or lost their NPY-EGFP, at times when no pH changes occurred to test for re-sealing. Comparison with Fig. 6D shows that the capacitance decline was not strongly influenced by external [Ca2+].
Previous work attributed an increased incidence of rapid cavicapture to a larger flux through open Ca2+ channels, and to a resultant larger increase in cytosolic [Ca2+] (Ales et al. 1999). When [Ca2+] was applied directly to the cytosolic side, however, it failed to promote pore closures (Dernick et al. 2003), suggesting that Ca2+ acted externally. Consistent with this idea, raising external [Ca2+] did not increase Ca2+ influx during the voltage pulses to 20 mV used here to stimulate exocytosis (Fig. 8F). Peak Ca2+ current actually diminished when external [Ca2+] was raised (from −334 ± 42 pA at 5 mm [Ca2+] to −155 ± 29 pA at 50 mm [Ca2+], and the charge carried by that Ca2+ current stayed about the same (−40 ± 6 pC versus −44 ± 7 pC). These results do not support the idea that the increased incidence of display arose because the cytosolic [Ca2+] rose to higher levels, and suggest instead that the effect of [Ca2+] was external. [Ca2+] is known to aggregate chromogranins A and B in vitro (Gerdes et al. 1989; Yoo & Lewis, 1996) therefore the elevated [Ca2+] in our experiments probably slowed or prevented the expansion or disassembly of the granule matrix. Raising [Ca2+] to 50 mm also diminished the capacitance jump during a voltage pulse to 56 ± 18% in four cells where [Ca2+] was elevated and then lowered again.
An inhibition of granule matrix expansion could have promoted display by two mechanisms. First, matrix expansion may be required for the normal dilation of the fusion pore to diameters large enough to pass NPY-EGFP. Second, NPY-EGFP may readily desorb from the matrix only once the matrix has expanded. Both effects will tend to retard the escape of NPY-EGFP and hence promote display.
Discussion
We have combined capacitance recording and imaging while voltage pulses stimulated exocytosis. Functioning as both a spatial marker and as a pH indicator, EGFP in granules reported the release of granule contents in two ways. First, exocytosis caused granules to brighten as the release of protons relieved the quenching of EGFP. The rate of brightening was sometimes too fast to determine accurately even when images were taken at 30 Hz. Protons were probably released as rapidly as catecholamines in amperometric measurements.
Second, EGFP-labelled granule cargo proteins were over-expressed, and their release from single granules observed directly. In principle, over-expression can cause mistargeting into membrane-bounded compartments other than secretory granules such as lysosomes, Golgi or trans-Golgi vesicles. However, such compartments are not known to undergo rapid exocytosis in response to the relatively gentle 0.5 s electrical stimuli given here, and would have been ignored in our analysis. We observed large differences in the release of the two proteins studied and even differences between granules in the way they release a single protein. These differences were not due to the over-expressed cargo, because they were still seen when the two proteins are co-expressed in single granules, and because the release behaviour of individual granules did not correlate with their content of labelled cargo.
Protein release from granules
NPY-EGFP left most granules so rapidly that it appeared outside the cell as a fluorescent cloud (‘discharge’). Evidently, the release from these granules was fast enough to temporarily overwhelm the dispersal of NPY-EGFP by diffusion in the external space. NPY-EGFP is either not bound to the granule matrix, or released from it faster than it can diffuse away. The finding sets a lower limit of 3 nm for the diameter of fusion pores in discharge events, because even GFP alone (Ormo et al. 1996) would barely fit through a pore that size.
The release of tPA-EGFP was more than 40-fold slower than of NPY-EGFP. This large difference was not apparent in earlier results on PC12 cells (Taraska et al. 2003), where the release of NPY-EGFP was not well resolved temporally. In most cases, tPA-EGFP remained at the granule site as a compact spot, with no visual indication that tPA-EGFP spread outside the granule. Instead, granules dimmed over tens of seconds as tPA was slowly released. Because tPA could not be seen outside these granules, we assume that tPA was released more slowly than it diffused away from the release site. When a fluorescent cloud did appear (20%) it spread more slowly than with granules containing NPY-EGFP. Therefore, the diffusion of tPA-EGFP is hindered even outside the granule, perhaps because tPA binds to the plasma membrane (Pittman et al. 1989).
It may be thought that the slow release of tPA is due to narrow fusion pores. Fusion pores in mast cells often pause their dilation at a semistable diameter before continuing it (Spruce et al. 1990; Curran et al. 1993), and similar observations were made in chromaffin cells (Albillos et al. 1997). Seventy-five per cent of granules released their NPY-EGFP in small fractions of a second, yet more than 75% of them released essentially no tPA-EGFP within 1.3 s. For fusion pores to accomplish this feat on their own, 50% of them must be large enough to release NPY-EGFP but too narrow to pass tPA-EGFP. Atomic structures are available for only fragments of tPA. The largest is the catalytic domain, a roughly spherical molecule of 5 nm diameter (Lamba et al. 1996). The molecular mass of tPA-EGFP (predicted 97 kDa) is 2.3–2.8 times larger than that of NPY-EGFP (predicted 34–42 kDa depending on the extent of intragranular cleavage of pre-pro-NPY). If molecular size varies with the cube root of molecular weight, a fusion pore excluding EGFP-tPA would be less than 1.4 times wider than one passing NPY-EGFP, and have a conductance that is (1.4)2 = 2 times higher. However, the conductance of semistable fusion pores is rarely maintained for seconds or tens of seconds, and varies over a factor of 10, with no indication that a narrow range of fusion pore conductances is preferred (Curran et al. 1993; Ales et al. 1999). Therefore, existing capacitance measurements do not clearly support the idea that fusion pores reproducibly maintain diameters intermediate between those of NPY-EGFP and tPA-EGFP. While this issue needs further study, it seems probable instead that tPA-EGFP binds to the granule matrix much more tightly than to NPY and unbinds slowly after exocytosis. Morphological studies have shown examples where granule matrices do not disperse rapidly and instead remain just outside or within the granule cavity, both in lactotropes (Angleson et al. 1999) and in chromaffin cells (Grynszpan-Winograd, 1975). Alternatively, tPA may be associated with the granule membrane.
The release of protein was surprisingly variable, most strikingly so with NPY-EGFP. In 20% of granules, NPY-EGFP was retained in the granule cavity for seconds after exocytosis (display). This result may be compared to amperometric recordings of catecholamine release from single granules. In such recordings, a ‘foot’ signal is generally followed by a fast current spike as a narrow fusion pore first retards release but then dilates rapidly to release the remaining contents in milliseconds. In a minority of recordings (3–13%; Ales et al. 1999; up to 20%, Zhou et al. 1996) the spike is absent and release continues slowly until the granule is empty. In such recordings, fusion pores apparently fail to dilate, at least while catecholamine remains in granules. It is tempting to suggest that display events are related to ‘stand-alone foot’ signals. The continued presence of NPY-EGFP in granules during display suggests that fusion pores in chromaffin cells can stay narrow for seconds, as also seen in spontaneously secreting cell-attached patches (Albillos et al. 1997).
Cavicapture and the selective retention of proteins
Capacitance measurements in chromaffin cells (Albillos et al. 1997; Ales et al. 1999; Henkel et al. 2001) have shown that some granules can re-seal after exocytosis, allowing the granule cavity to be retrieved intact. Using tPA-EGFP as a granule marker and pH changes to detect continuity with the external space, we now found that at least one-third of the granules ultimately re-sealed. The figure is a lower limit because our assay does not detect cavicapture when tPA-EGFP escapes before the granule re-seals. Evidently, cavicapture in electrically stimulated cells is more frequent than in spontaneously secreting cell-attached patches (Ales et al. 1999).
Cavicapture of dense core granules in endocrine cells was found also on previous occasions (Albillos et al. 1997; Ales et al. 1999; Angleson et al. 1999; Holroyd et al. 2002; Taraska et al. 2003), but its time course has not been explored. Here, we used rhythmic acidification to track the time course of cavicapture after a stimulus. Surprisingly, some granules remained open for tens of seconds before they re-sealed. This was unexpected given the idea (Thomas et al. 1994; Palfrey & Artalejo, 1998) that cavicapture mediates the fastest phase of endocytosis that retrieves membrane in seconds, both in chromaffin (Neher & Zucker, 1993; Artalejo et al. 1995; Smith & Neher, 1997; Engisch & Nowycky, 1998) and in other endocrine cells (Thomas et al. 1994; Tse et al. 1997). In our cells, capacitance measurements did not show prominent rapid endocytosis, yet significant cavicapture occurred, and it took as much as a minute in some granules. Clearly, cavicapture need not be fast, and it remains to be seen to what degree it accounts for the rapid membrane retrieval often seen after strong stimuli (Henkel & Almers, 1996; Palfrey & Artalejo, 1998). After cavicapture, granules sometimes remain in place to fuse a second time.
Because catecholamines leave granules in small fractions of a second, their release is rarely curtailed by cavicapture. The same appears to be generally true for NPY-EGFP, a much larger molecule. However, tPA-EGFP is released so slowly that about one-third of the granules re-sealed before releasing all of their tPA. In that sense, cavicapture caused the retention of proteins to be selective relative to NPY and catecholamines. Our findings echo earlier work on dopamine-β-hydroxylase (DbH). This protein, too, is incompletely released from chromaffin granules (Patzak et al. 1984; Wick et al. 1997), probably because some of it is bound to the granule membrane (Winkler et al. 1986) by an unknown mechanism (Lamouroux et al. 1987). Both DbH and tPA are enzymes. DbH is required to form noradrenaline and adrenaline, whereas tPA generates plasmin. Plasmin in turn can cleave chromogranin, thus generating inhibitors of noradrenaline release (Parmer et al. 2000). It is possible that the transient external exposure of granule-associated tPA aids yet other, unknown local signalling processes mediated by chromaffin granules.
Our results suggest that two mechanisms may co-operate in modulating the extent to which proteins are retained in single granules. One is the slow release from a granule matrix, an effect that will be physiologically regulated, at least in cells that regulate the disassembly or loss of granule matrices (Angleson et al. 1999). The other is cavicapture, a process that may well be regulated as well (Henkel et al. 2001; Artalejo et al. 2002). It will be interesting to learn whether and to what purpose other proteins are selectively retained in granules, and by what mechanisms their retention is regulated.
Acknowledgments
We thank Morris Feldmann for writing many of the Matlab analysis programs, Linda Raymond for preparing chromaffin cells, and François Darchen for providing the chromaffin cells for the immunolabelling experiment. David Zenisek and Christien Merrifield provided helpful discussions, and David Machado insightful comments on the manuscript. D.P. was supported by fellowships from the French Ministry of Foreign Affairs and from the Human Frontier Science Program organization. This work was supported by, NIH MH-60600.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc Natl Acad Sci U S A. 2002;99:6358–6363. doi: 10.1073/pnas.082658499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renstrom E, Rorsman P. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Ruden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Corcoran JJ, Wilson SP, Kirshner N. Flux of catecholamines through chromaffin vesicles in cultured bovine adrenal medullary cells. J Biol Chem. 1984;259:6208–6214. [PubMed] [Google Scholar]
- Curran MJ, Cohen FS, Chandler DE, Munson PJ, Zimmerberg J. Exocytotic fusion pores exhibit semi-stable states. J Membr Biol. 1993;133:61–75. doi: 10.1007/BF00231878. [DOI] [PubMed] [Google Scholar]
- Dernick G, Alvarez de Toledo G, Lindau M. Exocytosis of single chromaffin granules in cell-free inside-out membrane patches. Nat Cell Biol. 2003;5:358–362. doi: 10.1038/ncb956. [DOI] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J Physiol. 1998;506:591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes HH, Rosa P, Phillips E, Baeuerle PA, Frank R, Argos P, Huttner WB. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989;264:12009–12015. [PubMed] [Google Scholar]
- Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci USA. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynszpan-Winograd O. Ultrastructure of the chromaffin cell. In: Greep RO, Astwood EB, editors. Hanbook of Physiology. Washington, DC: American Physiological Society; 1975. p. 295. [Google Scholar]
- Henkel AW, Almers W. Fast steps in exocytosis and endocytosis studied by capacitance measurements in endocrine cells. Curr Opin Neurobiol. 1996;6:350–357. doi: 10.1016/s0959-4388(96)80119-x. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Horstmann H, Henkel MK. Direct observation of membrane retrieval in chromaffin cells by capacitance measurements. FEBS Lett. 2001;505:414–418. doi: 10.1016/s0014-5793(01)02861-7. [DOI] [PubMed] [Google Scholar]
- Holroyd P, Lang T, Wenzel D, De Camilli P, Jahn R. Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proc Natl Acad Sci U S A. 2002;99:16806–16811. doi: 10.1073/pnas.222677399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Majane EA, Yang HY. Release of NPY-like immunoreactive material from primary cultures of chromaffin cells prepared from bovine adrenal medulla. Neuropharmacology. 1985;24:693–695. doi: 10.1016/0028-3908(85)90115-7. [DOI] [PubMed] [Google Scholar]
- Kneen M, Farinas J, Li Y, Verkman AS. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys J. 1998;74:1591–1599. doi: 10.1016/S0006-3495(98)77870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba D, Bauer M, Huber H, Fischer S, Rudolph R, Kohnert U, Bode W. The 2,3 A crystal structure of the catalytic domain of recombinant two-chain human tissue-type plasminogen activator. J Mol Biol. 1996;258:117–135. doi: 10.1006/jmbi.1996.0238. [DOI] [PubMed] [Google Scholar]
- Lamouroux A, Vigny A, Faucon Biguet N, Darmon MC, Franck R, Henry JP, Mallet J. The primary structure of human dopamine-beta-hydroxylase: insights into the relationship between the soluble and the membrane-bound forms of the enzyme. EMBO J. 1987;6:3931–3937. doi: 10.1002/j.1460-2075.1987.tb02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Wacker I, Steyer J, Kaether C, Wunderlich I, Soldati T, Gerdes HH, Almers W. Ca2+-triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent-wave microscopy. Neuron. 1997;18:857–863. doi: 10.1016/s0896-6273(00)80325-6. [DOI] [PubMed] [Google Scholar]
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci U S A. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner JE, Kingma M, Kuhn S, Meliza CD, Cutler B, Scalettar BA. Real-time imaging of the axonal transport of granules containing a tissue plasminogen activator/green fluorescent protein hybrid. Mol Biol Cell. 1998;9:2463–2476. doi: 10.1091/mbc.9.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Nagasawa J. Exocytosis: the common release mechanism of secretory granules in glandular cells, neurosecretory cells, neurons and paraneurons. Arch Histol Jpn. 1977;40(Suppl.):31–47. doi: 10.1679/aohc1950.40.supplement_31. [DOI] [PubMed] [Google Scholar]
- Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Oheim M, Loerke D, Stuhmer W, Chow RH. The last few milliseconds in the life of a secretory granule. Docking, dynamics and fusion visualized by total internal reflection fluorescence microscopy (TIRFM) Eur Biophys J. 1998;27:83–98. doi: 10.1007/s002490050114. [DOI] [PubMed] [Google Scholar]
- Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- Palfrey HC, Artalejo CR. Vesicle recycling revisited: rapid endocytosis may be the first step. Neuroscience. 1998;83:969–989. doi: 10.1016/s0306-4522(97)00453-3. [DOI] [PubMed] [Google Scholar]
- Parmer RJ, Mahata M, Gong Y, Mahata SK, Jiang Q, O'Connor DT, Xi XP, Miles LA. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest. 2000;106:907–915. doi: 10.1172/JCI7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmer RJ, Mahata M, Mahata S, Sebald MT, O'Connor DT, Miles LA. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J Biol Chem. 1997;272:1976–1982. doi: 10.1074/jbc.272.3.1976. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H, Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys J. 1997;73:2782–2790. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A, Bock G, Fischer-Colbrie R, Schauenstein K, Schmidt W, Lingg G, Winkler H. Exocytotic exposure and retrieval of membrane antigens of chromaffin granules: quantitative evaluation of immunofluorescence on the surface of chromaffin cells. J Cell Biol. 1984;98:1817–1824. doi: 10.1083/jcb.98.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A, Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: ultrastructural evaluation after immunolabeling. J Cell Biol. 1986;102:510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman RN, Ivins JK, Buettner HM. Neuronal plasminogen activators: cell surface binding sites and involvement in neurite outgrowth. J Neurosci. 1989;9:4269–4286. doi: 10.1523/JNEUROSCI.09-12-04269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H, Artalejo AR, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J Cell Biol. 1997;139:1709–1717. doi: 10.1083/jcb.139.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Meth Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J Cell Biol. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce AE, Breckenridge LJ, Lee AK, Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990;4:643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- Steyer JA, Horstmann H, Almers W. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- Tabares L, Ales E, Lindau M, Alvarez de Toledo G. Exocytosis of catecholamine (CA)-containing and CA-free granules in chromaffin cells. J Biol Chem. 2001;276:39974–39979. doi: 10.1074/jbc.M106498200. [DOI] [PubMed] [Google Scholar]
- Taraska JW, Almers W. Bilayers merge even when fusion is transient. Proc Natl Acad Sci U S A. 2004;101:8780–8785. doi: 10.1073/pnas.0401316101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci U S A. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Lee AK, Wong JG, Almers W. A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994;124:667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse FW, Tse A, Hille B, Horstmann H, Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Zhao C, Terakawa S, Rutter GA. Simultaneous evanescent wave imaging of insulin vesicle membrane and cargo during a single exocytotic event. Curr Biol. 2000;10:1307–1310. doi: 10.1016/s0960-9822(00)00756-9. [DOI] [PubMed] [Google Scholar]
- Wick PF, Trenkle JM, Holz RW. Punctate appearance of dopamine-beta-hydroxylase on the chromaffin cell surface reflects the fusion of individual chromaffin granules upon exocytosis. Neuroscience. 1997;80:847–860. doi: 10.1016/s0306-4522(97)00062-6. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H, Apps DK, Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986;18:261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Lewis MS. Effects of pH and Ca2+ on heterodimer and heterotetramer formation by chromogranin A and chromogranin B. J Biol Chem. 1996;271:17041–17046. doi: 10.1074/jbc.271.29.17041. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Feldman ME, Almers W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron. 2002;35:1085–1097. doi: 10.1016/s0896-6273(02)00896-6. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Misler S, Chow RH. Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys J. 1996;70:1543–1552. doi: 10.1016/S0006-3495(96)79718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]