Abstract
Mesoangioblasts are vessel-associated fetal stem cells that can be induced to differentiate into skeletal muscle, both in vitro and in vivo. Whether this is due to fusion or to transdifferentiation into bona fide satellite cells is still an open question, for mesoangioblasts as well as for other types of stem cells. The early steps of satellite cell myogenic differentiation involve MyoD activation, membrane hyperpolarization and the appearance of ACh sensitivity and gap junctional communication. If mesoangioblasts differentiate into satellite cells, these characteristics should be observed in stem cells prior to fusion into multinucleated myotubes. We have investigated the functional properties acquired by mononucleated green fluorescent protein (GFP)-positive mesoangioblasts co-cultured with differentiating C2C12 myogenic cells, using the patch-clamp technique. Mesoangioblasts whose membrane contacted myogenic cells developed a hyperpolarized membrane resting potential and ACh-evoked current responses. Dye and electrical coupling was observed among mesoangioblasts but not between mesoangioblasts and myotubes. Mouse MyoD was detected by RT-PCR both in single, mononucleated mesoangioblasts co-cultured with C2C12 myotubes and in the total mRNA from mouse mesoangioblasts co-cultured with human myotubes, but not in human myotubes or stem cells cultured in isolation. In conclusion, when co-cultured with muscle cells, mesoangioblasts acquire many of the functional characteristics of differentiating satellite cells in the absence of cell fusion, strongly indicating that these stem cells undergo transdifferentiation into satellite cells, when exposed to a myogenic environment.
Over 40 years ago, skeletal muscle was found to be endowed with a resident population of stem cells (Mauro, 1961), termed satellite cells, needed for postnatal tissue growth and repair upon injury. Much more recently, stem/progenitor cells derived from embryologically unrelated tissues such as bone marrow, dorsal aorta, brain, muscle connective tissue and sinovia have been shown to be capable of differentiating into muscle cells (Ferrari et al. 1998; De Angelis et al. 1999; Galli et al. 2000; Qu-Petersen et al. 2002; Asakura et al. 2002; Tamaki et al. 2002; De Bari et al. 2003). Some of these cells can be expanded in vitro while remaining pluripotent. In particular, mesoangioblasts are vessel-associated fetal stem cells capable of expressing myosin and differentiating into skeletal muscle cells, both in vitro, upon co-culture with myogenic cells, and in vivo upon transplantation into chick embryos or dystrophic mouse muscle (Minasi et al. 2002; Sampaolesi et al. 2003). Whether mesoangioblasts or other types of stem cells acquire a myogenic satellite cell-like phenotype before fusion or rather differentiate as a consequence of fusion and exposure of donor nuclei to MyoD is still a matter of controversy. It has been reported that adult bone marrow-derived side population (SP) cells undergo a conversion to satellite cells before fusing with multinucleate muscle fibres, both in culture and in vivo (LaBarge & Blau, 2002). However others have proposed that fusion of a myeloid intermediate is the only mechanism of recruitment of non-muscle-derived donor cells to regenerating skeletal muscle (Camargo et al. 2003). As fusion is the natural fate of myogenic cells, this issue is particularly difficult to approach for skeletal muscle in vivo, where results are retrospective and open to different interpretations. Thus we addressed this question in vitro, with the aim of understanding whether mesoangioblasts differentiate into satellite cells before fusing with muscle cells, or fuse with muscle cells in the absence of a preliminary differentiation.
Many years ago we demonstrated that satellite cells and committed myoblasts express low levels of functional nicotinic acetylcholine receptors (nAChR) before myosin becomes detectable (Cossu et al. 1987), so that their expression can be considered an early marker of myogenic differentiation. As these receptors can be studied with high sensitivity at the single cell level, by means of electrophysiological recordings, we performed patch-clamp experiments on mesoangioblasts co-cultured with C2C12 mouse or human myogenic cells to define the timing of nAChR expression during the fusion process with myotubes. To ascertain whether nAChR expression is under the canonical control of myogenic regulatory factors, such as MyoD, the expression of the latter factor was also investigated.
Methods
Cell culture and membrane preparation
C2C12 myoblasts were cultured using Dulbecco's modified Eagle's medium (DMEM, Gibco) supplemented with 20% fetal calf serum (FCS, Gibco) in a humidified incubator with 5% CO2, at 37°C. Two different clones (B13 and D16) of mesoangioblasts, previously transduced with a lentiviral vector expressing the green fluorescent protein (GFP, Sampaolesi et al. 2003), were cultured on collagen-coated dishes using DMEM plus 10% FCS under the same conditions. Co-cultures were obtained by plating in 35-mm Petri dishes (Falcon) 35 000 C2C12 myoblasts plus 50 000 mesoangioblast of either clone, with comparable results. After 48 h, growth medium was replaced by a low-serum differentiating medium (DM; DMEM plus 2% horse serum, Gibco), to induce myotube formation, as previously described (Yaffe & Saxel, 1977). In order to establish how promptly mesoangioblasts become responsive to ACh, in some experiments the stem cells were added to fully differentiated C2C12 myotubes, already maintained in DM for 48 h. Human myotubes were obtained from frozen satellite cells derived from muscle biopsies, with the informed consent of the patients, as previously described (Broccolini et al. 2004), and differentiated in the same medium used for C2C12 myotubes. Mesoangioblasts were added to differentiated human myotubes (72 h in DM) and allowed to differentiate for 48 h.
Membranes of C2C12 cells were obtained as described elsewhere (Palma et al. 2003), except that a hypotonic buffer was used for cell lysis instead of glycine buffer. Membranes were resuspended in DM and used fresh or stored at −80°C until use.
Drugs, chemicals and solutions
Analytical grade reagents were purchased from Sigma (St Louis, MO, USA), except for Alexa Fluor 594 hydrazide (Molecular Probes, Eugene OR, USA). Cells were bathed in an external solution composed of (mm): NaCl 140, KCl 2.8, CaCl2 2, MgCl2 2, Hepes/NaOH 10 and glucose 10; pH 7.3. Patch pipettes were filled with an internal solution containing (mm): CsCl 130, BAPTA 5, Hepes/CsOH 10 and MgATP 2; pH 7.3. For single-cell RT-PCR experiments and for resting potential determinations, the internal solution contained (mm): KCl 130, BAPTA 5 and Hepes/KOH 10; pH 7.3.
Polymerase chain reaction analysis
Total RNA was extracted from cells cultured on 60-mm Petri dishes using the Absolutely RNA kit (Stratagene, La Jolla, CA, USA) and on-column RNase-free DNase treatment, according to the manufacturer's instructions. The cDNA was synthesized from 1 μg of total RNA, using ThermoScript reverse transcriptase (Invitrogen, Carlsbad, CA, USA) primed with random examers. Primer pairs (Table 1), selected according to NCBI mRNA sequences, were designed to hybridize only with mouse MyoD, mouse muscle nAChR α1 subunit, or human muscle nAChR α1 subunit cDNA and to be intron-spanning. The cDNA product (2 μl) was amplified by PCR in a 50 μl reaction mixture for 25 cycles (see Table 1) using Taq DNA Polymerase recombinant (Invitrogen) with the ‘OUT’ primer pairs. The amplification product (1 μl) was diluted 1: 250 and PCR-amplified for 30 cycles with the ‘INT’ primers (Table 1). The final products (10 μl) were run (5 V cm−1) on a 2% agarose gel stained with ethidium bromide together with a 100 bp marker (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Table 1. Primer pairs and conditions for PCR.
| Gene | Acc. no. | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) | bp | |
|---|---|---|---|---|---|
| Total mRNA | |||||
| MyoD | OUT | NM_010866 | GCCCGCGCTCCAACTGCTCTGAT | TCTTTTGGGCGTGAAGAACCAG | 438 |
| (mouse) | INT | GAATGGCTACGACACCGCCTACTAC | CCTACGGTGGTGCGCCCTCTGC | 331 | |
| nAChR α1 | OUT | NM_007389 | TTTCACTCTCCGCTGATCAAGC | GAATACAGCCGTGTGAGCAGAG | 397 |
| (mouse) | INT | GCAGAGACCATGAAGTCAGACCAG | CTGCATGTTTGGTAAGTGTGAAAGG | 278 | |
| nAChR α1 | OUT | NM_000079 | ATTTGTCTACTTGCTCCACTCGC | TTCACTCTTCAGGGAGACAGCAG | 150 |
| (human) | |||||
| GAPDH | OUT | NM_008084 | GTGGCAAAGTGGAGATTGTTGC | TTTCTCGTGGTTCACACCCATC | 344 |
| Single cell PCR | |||||
| MyoD | OUT | NM_010866 | GATGGCATGATGGATTACAGCG | TCTTTTGGGCGTGAAGAACCAG | 419 |
| (mouse) | INT | GAATGGCTACGACACCGCCTACTAC | GGTCTGGGTTCCCTGTTCTGTGT | 223 | |
| GAPDH | OUT | NM_008084 | GTGGCAAAGTGGAGATTGTTGC | TTTCTCGTGGTTCACACCCATC | 344 |
| (mouse) | INT | ATCAACGACCCCTTCATTGACC | AGATGATGACCGTTTGGCTC | 268 | |
Forward and reverse primers used for the nested RT-PCR, either from total mRNA extraction or from single-cell cytoplasm. Acc. no., access number in the NCBI data base; bp, expected size of the product. The same primers were used for human and mouse GAPDH. The PCR mixture consisted of: 0.2 μm each dNTP (Invitrogen), 0.5 μm of each primer pair (0.25 μm for the ‘OUT’ amplification of single-cell PCR), 1.5 mm MgCl2 and 2.5 U of Taq DNA Polymerase recombinant (Invitrogen). Following one denaturation step at 94°C (120 s), cycling conditions were: for total mRNA, 30 s at 94°C (denaturation), 35 s at 61°C (annealing), 40 s at 72°C (extension). For single-cell PCR, times were 20 s at 94°C, 35 s at 61°C, 30 s at 72°C. Final elongation: 5 min at 72°C.
For single-cell RT-PCR experiments, the cell cytoplasm was harvested through the patch pipette after recording the cell response to ACh. The cDNA was synthesized using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's instructions. The content of the pipette (4 μl solution plus the cytoplasm) was expelled by a positive pressure into a PCR thin-layer tube, containing random examers plus dNTPs (1 μl) for the initial step of first-strand synthesis (65°C for 5 min). After cooling on ice, the enzyme-containing mixture (5 μl) was added. The reaction was performed at 42°C for 50 min, then the enzyme was inactivated at 70°C for 15 min. For the amplification of the cDNA products, a nested PCR amplification was performed, using primer pairs designed with Oligo Analyser (Integrated DNA Technologies, Coralville, IA, USA) or Oligo Explorer (Gene Link, Westchester, NY, USA). In the first PCR step, all the cDNA obtained for each cell (10 μl), was amplified using all the ‘OUT’ primers (Table 1) simultaneously for 30 cycles. The amplification product (1 μl) was diluted 1: 150 and PCR-amplified for 30 cycles with the ‘INT’ primers (Table 1). The final products (10 μl) were visualized as above.
As a positive control and for an internal standard we used the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). For negative controls, amplifications were performed using water as PCR template or omitting the reverse transcriptase step in the first strand synthesis reaction. For single-cell PCR, reactions were also performed on the content of patch pipettes dipped into the solution bathing the cells, to rule out contaminations from extracellular material. Of four tested, none was found positive.
Patch-clamp recordings and fluorescence analysis
Whole-cell and outside-out current responses were recorded at room temperature (23–26°C), using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) and a gravity-driven fast perfusion system (RSC-200, Bio-Logic, Claix, France), as previously described (Grassi et al. 2003). Data were sampled and analysed using pCLAMP 9.0 (Axon Instruments). Patch pipettes of borosilicate glass had a tip resistance of 3–5 MΩ, compensated for by 85–90%. ACh dose–response curves were constructed by applying ACh at different concentrations to each cell, with 30–60 s interval between applications (holding potential, −70 mV). The amplitude of ACh-evoked whole-cell currents (ACh-currents) was normalized to the values obtained at 300 μm ACh in each cell and averaged. Data were best fitted to a Hill equation using Origin 7.0 software. ACh current–voltage relationships were constructed by measuring the cell responses elicited by ACh (10 or 30 μm) at different test potentials, and holding the cell at −70 mV between tests. To average data from different cells, ACh-currents from each cell were normalized taking the response at −70 mV as −100%. Voltage-gated Ca2+ currents were studied by delivering 300 ms-long depolarizing pulses from a resting potential of −70 mV. 4-aminopyridine (2 mm) was added to the extracellular solution in some experiments, with no appreciable change.
Membrane capacitance (Cm) was measured from capacitative transients evoked by a 10 mV depolarizing step (Vstep). The total charge mobilized by the voltage step (Qstep) was calculated from transient integral, whereby Cm=Qstep/Vstep was obtained (de Roos et al. 1996).
Alexa Fluor 594 hydrazide (100 μm) was added to the patch pipette solution. Fluorescence signal at 640 nm (excitation, 550 nm), sampled using a high-sensitivity digital camera (SensiCam, PCO, Kelheim, Germany) connected to an Axioskop FS2 microscope (Zeiss, Oberkochen, Germany), was averaged over the cell soma using the Axon Instruments Workbench 2 software. The fluorescence intensity measured at different times in the ‘coupled’ cell was divided by that of the patched cell, to obtain the relative intensity.
All results are given as mean ± s.e.m. Statistical significance was tested using one-way ANOVA. Images of all the cells used in this work have been taken during electrophysiological recordings with the above equipment.
Immunocytochemistry
Immunofluorescence analysis was carried out as described (Tajbakhsh et al. 1994) using a rabbit anti-MyoD polyclonal antibody (Koishi et al. 1995). Briefly, cell cultures were washed with PBS (Gibco) and fixed with paraformaldehyde. After careful rinsing with PBS, cells were permeabilized with Triton X-100 and incubated at 4°C overnight with the primary antibody in PBS containing 1% bovine serum albumin (BSA). At the end of incubation, the cells were washed with PBS and incubated for 1 h at room temperature with rhodamine-conjugate goat anti-rabbit IgG (1: 100 dilution; Sigma). Finally, cultures were washed, mounted in 75% glycerol/PBS (pH 7.5), and observed under a Zeiss Axiophot epifluorescence microscope.
To measure fusion index, mesoangioblasts–myotube co-cultures were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 and nuclei were stained with Hoechst 33342 (2 min at 25°C). Fusion index, defined as the ratio of the number of nuclei in multinucleated cells to the total number of nuclei in the field, was measured counting GFP-positive and negative cells separately in 15 fields (400 × magnification) for each 35-mm Petri dish.
Results
Mesoangioblasts in close contact with myotubes respond to ACh
When co-cultured with C2C12 myogenic cells, GFP-positive mesoangioblasts showed a non-uniform morphology, with many cells exhibiting processes growing away from the soma (Fig. 1A and B). After 4–5 days in vitro, most GFP-positive cells were close to, or touched with their processes, myotubes or other mesoangioblasts, while others were isolated (Fig. 1B). The cells were flat and nuclei usually occupied a large portion of the cytoplasm. Although some GFP-positive myotubes, probably derived from the fusion with mesoangioblasts, were observed in 10 out of 38 preparations used in this study, most mesoangioblasts were mononucleated. In four dishes from two different preparations (4 days in vitro), we counted four GFP-positive cells with two nuclei, and one with three nuclei, out of 1608 scored, corresponding to a fusion index of about 0.7%. Therefore, our analysis focused on mononucleated GFP-positive cells, as visually determined under phase-contrast microscope (see example in Fig. 1A).
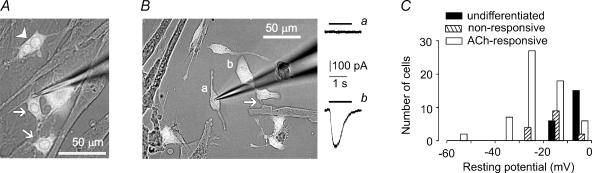
Figure 1. Mesoangioblasts contacting C2C12 myotubes respond to ACh.
A, representative field of a co-culture of mesoangioblasts (GFP-positive, white cells) and C2C12 myotubes, as viewed under transmitted light and epifluorescence simultaneously. Arrowhead points to a binucleated mesoangioblast, arrows to mononucleated cells. The patch pipette is on the right. B, the mesoangioblast marked a is isolated, that marked b contacts a myotube through the process indicated by the arrow. Right panel, cell a does not respond to ACh (10 μm, black line), cell b shows an inward current (downward deflection). Holding potential, −70 mV. C, histogram of membrane resting potential of mesoangioblasts cultured alone or with C2C12 myotubes. Values were obtained with a CsCl-based intracellular solution, immediately after membrane rupture.
When cultured in isolation for 4–5 days (48 h in DM) on collagen-coated Petri dishes (non-differentiated cells), mesoangioblasts never showed current responses to ACh (20 cells tested, data not shown). In co-cultures, none of the 20 mesoangioblasts grown away from myotubes that we tested responded to ACh (Fig. 1B). Conversely, ACh-currents were observed in 130 out of 190 mesoangioblasts that contacted C2C12 cells, independent of myotube size and density (e.g. Fig. 1B). Membrane resting potential was also affected by co-culture with C2C12 myotubes (Fig. 1C). In undifferentiated mesoangioblasts, membrane potential (measured using a KCl-based intracellular solution) was −7.5 ± 2.5 mV (n = 10), while it increased in co-cultures, to −12.6 ± 2.7 mV (n = 10) in cells non-responsive to ACh and to −30.8 ± 2.3 mV (n = 14) in responsive cells. These values are significantly different (P < 0.03), indicating that there is a progressive hyperpolarization of mesoangioblasts in co-culture. For comparison, the resting potentials of mononucleated myoblasts and myotubes were −29.9 ± 5.1 mV (n = 17) and −50.4 ± 7.3 mV (n = 5), respectively, indicating that myoblasts and ACh-responsive mesoangioblasts have comparable resting potentials (P = 0.9). Values measured using a CsCl-based intracellular solution, routinely taken together with ACh-evoked responses, were lower, but clearly demonstrated, in a larger set of cells, the hyperpolarization of differentiating mesoangioblasts (see Fig. 1C). Voltage-activated Ca2+ currents were not observed in 20 cells tested (data not shown), although all were responsive to ACh.
The differentiation process was fast, as membrane hyperpolarization and ACh-currents were observed in eight out of nine mesoangioblasts tested 16–24 h after being added to fully differentiated myotubes. In these cells, the mean resting potential increased up to −20 mV (n = 3) at 24 h of co-culture, while ACh-current ranged between −20 and −400 pA, as in standard conditions (see below).
When mesoangioblasts were co-cultured for 4 days with cells of the tumour cell line BC3H1, which express nAChR but do not fuse, none of 13 patched cells that contacted BC3H1 cells showed a response to ACh (data not shown), suggesting that non-myogenic cells are not able to induce myogenic differentiation of mesoangioblasts.
To test whether a putative membrane factor present on myotube sarcolemma was in itself sufficient to induce mesoangioblast differentiation, stem cells were incubated with C2C12 membranes for 2–8 days, in DM. Membranes form vesicles in aqueous solution and retain a full complement of proteins, including transmitter-gated receptors and possibly G protein-coupled receptors, that can be functionally expressed upon injection in Xenopus oocytes (Miledi et al. 2002). Within a few hours of addition to the cultures, membranes settled over the mesoangioblast monolayer. For experiments, we selected stem cells under membrane aggregates. However, at no time were responsive cells found (16 cells patched), indicating that intact, alive myotubes are required for mesoangioblast differentiation (data not shown).
Mesoangioblasts are coupled through gap junctions to other mesoangioblasts, but not to myotubes
As ACh-currents were only observed in mesoangioblasts in contact with C2C12 cells, we considered the possibility that the responses actually originated from myogenic cells and spread to mesoangioblasts through gap junctions.
Alexa Fluor 594, a red-fluorescent dye permeating through gap junctions, was injected into mesoangioblasts via the patch pipette and its diffusion to neighbouring cells monitored for 5–15 min. The dye diffused to coupled mesoangioblasts in two out of 10 tested cells (e.g. Fig. 2A). In the ‘coupled’ cells, the fluorescence intensity reached half of the value measured in the ‘patched’ cell within 10 min (Fig. 2B). When cells were bathed with an acidic salt solution (pH 5.3), the percentage of coupled cells remained the same (two out of nine), but a much reduced dye transfer was observed (Fig. 2B), possibly because gap junctions were physiologically modulated by pH.
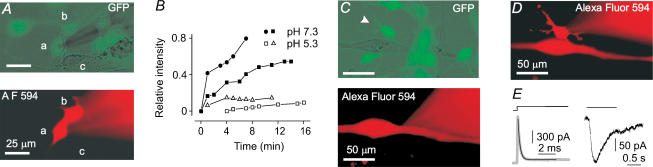
Figure 2. Mesoangioblasts are connected through gap junctions among them but not to C2C12 myotubes.
A, mesoangioblast a (patched cell, patch pipette on the right, out of focus) is dye-coupled to mesoangioblast b but not to C2C12 myoblast c. The GFP-positive cells are green when simultaneously viewed under phase contrast and epifluorescence (top). At 7 min after membrane patch rupture, cell b but not cell c shows a strong Alexa Fluor 594 epifluorescence signal (in red, bottom panel). B, time course of dye loading in four mesoangioblast pairs, including that shown in A (•) at pH 7.3 or 5.3. C none of the GFP-positive mesoangioblasts (green) juxtaposed to a myotube became loaded by Alexa Fluor 594 within 12 min of myotube dialysis through the patch pipette (red halo on the right, bottom). D, the mesoangioblast indicated by the arrowhead in C is not coupled to the other mesoangioblasts. Picture taken 38 min after membrane patch rupture (patch pipette on the right). The myotube was still fluorescent. E, capacitative transient (grey trace), superimposed with the best fitting exponential curve (black line, τ = 0.22 ms) and ACh-response (30 μm) in the same cell as in D. Holding potential, −70 mV. All pseudocolor images were obtained digitally.
By contrast, when Alexa Fluor 594 was injected into myotubes (10 cells tested) via the patch pipette, no fluorescence appeared in the juxtaposed mesoangioblasts after 15–30 min of membrane patch rupture (e.g. Fig. 2C), although subsequent recordings performed on mesoangioblasts showed that they were responsive to ACh (Fig. 2D and E). High intracellular Ca2+ concentration is known to induce the closure of gap junctions. To prevent any leakage of Ca2+ ions into the patched myotube, which would decrease junctional coupling, the concentration of extracellular CaCl2 was reduced to 0.1 mm in three of these experiments. These data indicate that mesoangioblasts are occasionally dye-coupled among themselves, but not to C2C12 cells.
Chromaffine cells are electrically coupled by low-conductance pathways that do not allow dye transfer (Moser, 1998). In these cells, capacitative transients can be fitted by a double exponential, whereas a single exponential is required to fit the transients in isolated cells. We examined whether a similar electrical coupling occurs in mesoangioblasts, in spite of the absence of dye transfer. The capacitative transients elicited by a 10 mV depolarizing step were fitted by a single exponential in 31 out of the 76 mesoangioblasts responsive to ACh examined (e.g. Fig. 2E), whereas two exponentials were required to adequately fit all the other transients (data not shown). By contrast, a single exponential fitted the capacitative transients of the majority of non-responsive cells (21 out of 29), as well as of non-differentiated mesoangioblasts (24 out of 28). These findings indicate that ‘electrically isolated’ cells, that is those with a mono-exponential capacitative transient, are responsive to ACh, although the appearance of ACh sensitivity correlates with an enhanced cell coupling (i.e. the appearance of bi-exponential transients), an event possibly preliminary to mesoangioblast–myotube fusion. Cm increased during differentiation of mesoangioblasts as well as myoblasts (Table 2), as previously reported for human satellite cells (Liu et al. 1998). However, there was a large overlapping of the values, even between small myotubes and mesoangioblasts cultured in isolation, so that the best criterion to define the number of nuclei of a cell remained the visual inspection. To strengthen the visual data, and be sure that additional nuclei had not been overlooked, mesoangioblasts with a Cm exceeding 100 pF (11 out of 104 tested), which is larger than any mononucleated C2C12 cell encountered (Table 2), were discarded.
Table 2.
Cell membrane capacitance (Cm)
| Mean Cm (pF) | Range (pF) | n | |
|---|---|---|---|
| Undifferentiated mesoangioblasts | 32.4 ± 3.5 | 10–75 | 26 |
| Non-responsive mesoangioblasts | 38.3 ± 4.0 | 10–129 | 45 |
| ACh-responsive mesoangioblasts | 49.4 ± 2.5 | 10–93 | 93 |
| Mononucleated C2C12 | 32.0 ± 4.1 | 15–96 | 20 |
| C2C12 myotubes | 81 ± 11 | 35–190 | 16 |
Values are mean ± s.e.m. of Cm in the given number of cells (n), averaged over the indicated range. ACh-responsive mesoangioblast with Cm > 100 pF have been omitted. Cm values for myotubes and ACh-responsive mesoangioblasts are significantly different between them and from all other sets (P < 0.02). The other values are not different (P > 0.3).
Characterization of ACh-current
The characterization of ACh-current was carried out in mononucleated mesoangioblasts co-cultured for 4–5 days with C2C12 myotubes, including cells with a bi-exponential decay of the capacitative transient. The amplitude of ACh-current depended on ACh concentration and on membrane test potential (e.g. Fig. 3). With an ACh concentration of 10 μm at −70 mV, the average response was −210 ± 30 pA (n = 50), ranging between −20 and −1000 pA. A similar scattering was observed also when only the 16 mesoangioblasts with capacitance below 35 pF, that is smaller than any myotube, were considered. In these cells, ACh-current density ranged from 0.9 to 21 pA pF−1 (mean, 5.9 ± 1.4 pA pF−1, n = 16) with an ACh concentration of 10 μm, indicating that mesoangioblasts express a variable amount of functional nAChRs. Nicotine mimicked the effect of ACh in 10 cells tested, although with a lower potency, as a larger concentration of nicotine as compared to ACh was required to elicit equivalent currents (Fig. 3A). When tested on myotubes in the same culture dish, the relative potency of ACh and nicotine was the same as in mesoangioblasts (data not shown). Current run-down was limited, as 4–8 min after patch membrane rupture, the amplitude of ACh-current was 62 ± 8% (n = 41) of the first response, independent of the ACh concentration used.
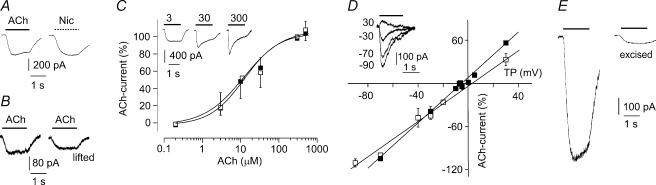
Figure 3. Nicotinic responses of mesoangioblasts co-cultured with C2C12 myotubes.
A, responses to ACh (10 μm) and nicotine (100 μm) in the same mesoangioblast. B, currents evoked by ACh (10 μm) in a mesoangioblast, before (left) and after (right) lifting the cell. C, ACh dose–response relationships in mesoangioblasts (□, n = 3–7 cells) and C2C12 myotubes (▪, n = 4 cells), best fitted to Hill equations (lines). Inset: ACh-currents at the indicated concentrations in a single mesoangioblast. Note the acceleration in current decay increasing ACh concentrations. D, ACh-current response versus test potential (TP) in mesoangioblasts (□, n = 2–7 cells) and C2C12 myotubes (▪, n = 3 cells), with linear best fits. Inset: ACh-currents at the indicated test potentials in a mesoangioblast. Note the clear outward current evoked at +30 mV. ACh, 30 μm. E, responses to ACh (10 μm) in a mesoangioblast under whole-cell recording condition (left) and after excising an outside-out patch (right). In all panels, holding potential, −70 mV; current digital filtering, 200 Hz. Data in C and D: mean ±s.e.m., with error bars omitted if n = 2 or if smaller than symbol.
To make sure that current responses arose from mesoangioblasts, some responsive cells were lifted from the dish and ACh-current recorded after the cell had been isolated. In the three cells tested, ACh-current amplitude was not greatly altered by lifting (e.g. Fig. 3B). This unequivocally shows that ACh-activated receptors are expressed by the mesoangioblasts.
The relation between ACh dose and ACh-current amplitude was virtually identical in C2C12 myotubes and mesoangioblasts (Fig. 3C). The data points were fitted to a Hill equation yielding an EC50 value of 12.0 μm and 12.4 μm and Hill coefficient (nH) of 0.84 and 0.97 for C2C12 myotubes and for mesoangioblasts, respectively, in good agreement with previously published data (Grassi et al. 1995). These findings indicate that mesoangioblast current responses arose through the activation of bona fide nAChRs. The voltage dependence of ACh-evoked responses was also very similar for myotubes and mesoangioblasts, although the reversal potential of ACh-current was more positive for the stem cells (+4.0 ± 1.9 mV, n = 5) than for myotubes (−3.5 ± 1.7 mV, n = 3) (Fig. 3D). This suggests that the nAChR expressed in differentiating stem cells is less permeable to Cs+ ions than the receptor of fully differentiated myotubes. Cs+-impermeant nAChR-channels have been observed in a rhabdomyosarcoma cell line (Grassi et al. 1993).
ACh-induced responses were observed in five of 10 outside-out patches (e.g. Fig. 3E), excised from cells with a whole-cell response exceeding 300 pA. Single channel activity was reliably recorded in two patches (Fig. 4). Channel conductances, estimated taking into account the positive reversal potential observed for whole-cell responses, were 36.5 and 37.3 pS (Fig. 4B). Channel openings were relatively short (Fig. 4C), but the paucity of the events prevented an accurate measurement.
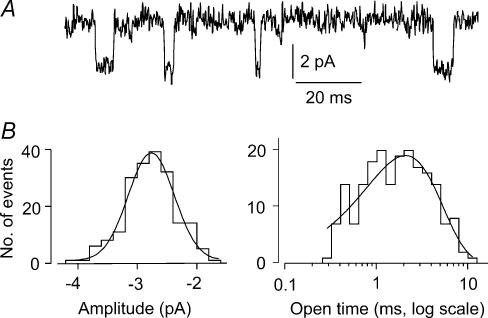
Figure 4. Unitary ACh-evoked events in mesoangioblasts.
A, channel openings recorded in an outside-out patch from a mesoangioblast, superfused with ACh (10 μm). Holding potential, −70 mV. B amplitude and open time distributions of the events from the same patch. Superimposed lines: best fitting Gaussian, with mean = 2.7 pA and exponential curve, with time constant, τ = 2.1 ms. Single channel conductance, estimated considering a reversal potential of +4 mV for ACh-current, was 36.5 pS.
Mesoangioblasts express nAChR and MyoD mRNAs when co-cultured with muscle cells
In order to study the myogenic determination/differentiation of those mesoangioblasts that express functional nAChRs when in contact with myotubes, we stained the culture with an antibody directed against MyoD. None of the GFP-positive mesoangioblasts, whether contacting myotubes or isolated, was labelled by the antibody, which strongly labelled the nuclei of multinucleated myotubes (data not shown). Immunocytochemistry has a relatively low sensitivity and MyoD must accumulate in a relatively large amount within the nucleus before it can be detected by the antibody. As in differentiating myogenic cells nAChR gene expression depends on the transcriptional activity of MyoD, we wondered whether MyoD might be expressed at levels sufficient to activate transcription of downstream genes but insufficient to be detected by immunocytochemistry. We therefore cultured mouse mesoangioblasts with human primary muscle cells and performed an RT-PCR with oligonucleotides that amplify murine but not human MyoD as well as the α1 subunit of the mouse muscle nAChR. In two co-culture dishes analysed in parallel, murine MyoD and the α1 subunit of the nAChR could be amplified, but not in mesoangioblasts cultured in isolation or in human myotubes (Fig. 5A). However, using primers that recognize the human α1 nAChR subunit, the amplification product was found in human but not C2C12 myotubes (data not shown), confirming that the reactions were specific.
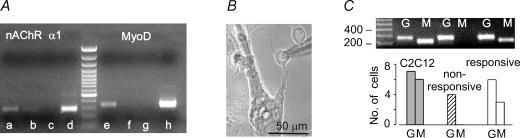
Figure 5. Expression of mRNAs for MyoD and nAChR α1 subunit in mesoangioblasts cultured with myogenic cells.
A, RT-PCR with mouse specific oligonucleotides for the α1 subunit of the muscle nAChR and MyoD, as indicated, amplified from the RNA extracted from co-cultures of mouse mesoangioblasts and human myogenic cells (a and e), from cultures of mouse mesoangioblasts alone (b and f) or human myogenic cells alone (c and g) and from mouse C2C12 myotubes (d and h), as positive control. Molecular markers are shown in the middle. B, typical mononucleated mesoangioblast selected for single-cell RT-PCR analysis. C, results of nested RT-PCR amplifications with oligonucleotides for GAPDH (G) and MyoD (M) on a single C2C12 myotube, a non-responsive and an ACh-responsive mesoangioblast (top) and summary of all the tested cells (bottom).
Although fusion index is very low, some mesoangioblasts may have fused with myogenic cells, and MyoD may be activated as a result of the fusion. We therefore performed single-cell RT-PCR on mesoangioblasts co-cultured with C2C12 myotubes. Mononucleated GFP-positive cells were carefully selected (e.g. Fig. 5B) and cytoplasm harvested after measuring ACh-current. A nested PCR amplification confirmed the presence of GAPDH in six out of seven ACh-responsive mesoangioblasts tested, indicating successful cytoplasm harvesting. In three of these cells, MyoD was also detected, whereas the transcript was not amplified in four mesoangioblasts unresponsive to ACh. For comparison, MyoD was amplified in six out of seven C2C12 cells tested (Fig. 5C). These data indicate that at the time of expression of functional nAChRs, mesoangioblasts have activated a myogenic programme and already express MyoD, although at very low levels.
Discussion
Mesoangioblasts are a population of stem cells capable of fusing with muscle fibres in vivo and in vitro. In this work, we exploited the high sensitivity of the patch-clamp technique to examine to what extent mesoangioblasts acquire the functional characteristics of myogenic precursors prior to the fusion with differentiated C2C12 myotubes. We show that co-culture with myotubes promotes the activation of MyoD, increases cell resting potential and membrane capacitance, enhances electrical and dye coupling among mesoangioblasts, and induces the appearance of ACh-evoked responses via bona fide nAChRs, but not of voltage-gated Ca2+ currents. Each of these findings is consistent with the hypothesis that mesoangioblasts differentiate into satellite cells, which, in turn, may fuse with muscle fibres.
MyoD, together with Myf-5, is a primary myogenic regulatory factor, required for the determination of myoblasts (Seale & Rudnicki, 2000). The majority of satellite cells express MyoD and/or Myf-5 after 24 h in culture (Cornelison & Wold, 1997), in the early steps of the activation process. We show that, upon co-culture with myogenic cells, MyoD mRNA is expressed in mesoangioblasts, indicating the activation of a myogenic programme in these stem cells.
It has long been known that replicating mouse satellite cells have a membrane potential around −20 mV (Eusebi & Molinaro, 1984). The resting potential of human postnatal satellite cells in culture increases from −8 mV (replicating myoblasts) to −30 mV (fusion-competent myoblasts) to −65 mV (myotubes) (Liu et al. 1998). Comparable values were observed throughout the differentiation of rat embryonic myoblasts (Fambrough & Rash, 1971). The remarkable similarity of these values to those described in the present work indicates that mesoangioblasts co-cultured with myotubes acquire a membrane resting potential comparable to that of satellite cells. The capacitance of satellite cells increases by about 170% during the pre-fusion steps of myogenic differentiation (Liu et al. 1998). Our data demonstrate an equivalent increase (150%) between undifferentiated and ACh-responsive mesoangioblasts.
Cultured satellite cells from newborn rats are increasingly coupled by gap junctions before the formation of myotubes. The coupling is necessary for fusion, but virtually disappears after fusion (Constantin et al. 1997), so that myoblasts and newly formed myotubes are only occasionally coupled. Similar observations have been made for stable cell lines and embryonic myoblasts (Balogh et al. 1993; Proulx et al. 1997). The reported coupling among mesoangioblasts is in line with these data, although it raised the possibility that ACh-evoked responses actually originated from myotubes rather than mesoangioblasts.
ACh-current was recorded in cells not dye-coupled to myotubes and in electrically isolated mesoangioblasts, as defined by the single-exponential relaxation of capacitative transients. The recordings in excised patches confirmed that nAChRs are expressed by mesoangioblasts. Dose–response curves and current–voltage relations indicated that the same nAChR subtype is present in C2C12 myotubes and in stem cells. Indeed, ACh-evoked channel openings show a unitary conductance of about 37 pS and a mean open duration of 2–3 ms, typical of the fetal form of nAChR in outside-out patches (Ragozzino et al. 2000; Grassi et al. 2003). These data compare well with the established sensitivity to ACh of mouse and human satellite cells (Eusebi & Molinaro, 1984; Liu et al. 1998). Taking the different temperature into account, our findings also agree with the functional parameters of nAChRs described in cultured satellite cells (Cossu et al. 1987).
Rat satellite cells in culture do not express voltage-gated channels before fusion (Cognard et al. 1993), while in C2C12 cells, Ca2+ currents appear after about 3 days in DM, although tiny voltage-gated Na+ and T-type Ca2+ currents can be observed within 24 h of serum deprivation (Caffrey et al. 1989). These latter currents would be largely inactivated at the holding potential of −70 mV used in this work, and hence below detection threshold. Our experiments showed that mesoangioblasts do not express voltage-gated Ca2+ currents within 48 h of co-culture in DM, in line with the findings in myogenic cells. Together, our data unambiguously show that mesoangioblasts express at least two markers of myogenic differentiation, MyoD and nAChR, together with other functional properties of differentiating satellite cells, when they are in contact but not yet fused with C2C12 myotubes. Our findings are relevant to the current heated controversy related to cell fusion as the main if not only underlying mechanism of stem cell plasticity and ‘trans-differentiation’ (Wagers & Weissman, 2004). In the case of bone marrow SP cells, many reports claiming their ability to give rise to other cells types such as muscle, neurones or epithelia (Gussoni et al. 1999; Brazelton et al. 2000; Krause et al. 2001) have been challenged by other reports showing that this phenomenon is extremely rare and likely to be due to cell fusion. In the case of skeletal muscle, the situation is more complicated as fusion is the natural fate of determined myogenic cells during embryogenesis or in postnatal muscle regeneration. Two recent papers (Asakura et al. 2002; LaBarge & Blau, 2002) showed that genetically labelled SP cells may either fuse with regenerating fibres or acquire the identity and the anatomical location of bona fide satellite cells, suggesting that blood-borne cells may replenish their pool. However, more recently contrasting data have been reported (Camargo et al. 2003) showing that fusion of a bone marrow-derived, myeloid intermediate was the cause of muscle differentiation of SP cells and no genetically labelled satellite cells could be isolated from the regenerating muscle of transplanted animals. In the case of mesoangioblasts, differentiation into smooth muscle cells or osteoblasts occurs in response to transforming growth factor beta (TGF β) or bone morphogenetic protein-2 (BMP-2) and does not imply physical contact with other cell types (Minasi et al. 2002), thus making fusion an unlikely explanation. Differentiation into skeletal muscle cells only occurs in mesoangioblasts upon co-culture with differentiating myogenic cells and thus it is difficult to test whether it depends upon fusion or signals released by differentiating myogenic cells.
The data reported here show conclusively that activation of a myogenic programme occurs before and independently of fusion, which consequently cannot be invoked as the underlying mechanism. We have not determined which signal has to pass between muscle and stem cells to trigger the myogenic differentiation of the latter. Identifying this factor(s) is currently being attempted through the analysis of secreted/surface molecules present on C2C12 myogenic cells and the possible corresponding receptors expressed on the cell surface of mesoangioblasts.
Acknowledgments
We thank Ms S. Culazzu for excellent technical assistance and Dr S. Fucile for critical reading of the manuscript. Human satellite cells were kindly provided by Dr A. Broccolini (Department of Neuroscience, Catholic University, Rome, Italy). G.S. is supported by the PhD programme in Neurophysiology (Università La Sapienza). This work was supported by grants from Ministero Istruzione, Università, Ricerca to F.G. and has been presented in abstract form to the Fourth Forum of European Neuroscience (Lisbon, Portugal, 10–14th July 2004)
References
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh S, Naus SS, Merrifield PA. Expression of gap junctions in cultured rat L6 cells during myogenesis. Dev Biol. 1993;155:351–360. doi: 10.1006/dbio.1993.1034. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Broccolini A, Ricci E, Pescatori M, Papacci M, Gliubizzi C, D'Amico A, Servidei S, Tonali P, Mirabella M. Insulin-like growth factor I in inclusion-body myositis and human muscle cultures. J Neuropathol Exp Neurol. 2004;63:650–659. doi: 10.1093/jnen/63.6.650. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Brown AM, Schneider MD. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci. 1989;9:3443–3453. doi: 10.1523/JNEUROSCI.09-10-03443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Cognard C, Constantin B, Rivet-Bastide M, Imbert N, Besse C, Raymond G. Appearance and evolution of calcium currents and contraction during the early post-fusional stages of rat skeletal muscle cells developing in primary culture. Development. 1993;117:1153–1161. doi: 10.1242/dev.117.3.1153. [DOI] [PubMed] [Google Scholar]
- Constantin B, Cronier L, Raymond G. Transient involvement of gap junctional communication before fusion of newborn rat myoblasts. C R Acad Sci III. 1997;320:35–40. doi: 10.1016/s0764-4469(99)80084-5. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cossu G, Eusebi F, Grassi F, Wanke E. Acetylcholine receptor channels are present in undifferentiated satellite cells, but not in embryonic myoblasts in culture. Dev Biol. 1987;123:43–50. doi: 10.1016/0012-1606(87)90425-8. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, et al. Skeletal myogenic precursors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–877. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos ADG, van Zoelen EJJ, Theuvenet APR. Determination of gap junctional intercellular communication by capacitance measurements. Pflugers Arch. 1996;431:556–563. doi: 10.1007/BF02191903. [DOI] [PubMed] [Google Scholar]
- Eusebi F, Molinaro M. Acetylcholine sensitivity in replicating satellite cells. Muscle Nerve. 1984;7:488–492. doi: 10.1002/mus.880070613. [DOI] [PubMed] [Google Scholar]
- Fambrough D, Rash JE. Development of acetylcholine sensitivity during myogenesis. Dev Biol. 1971;26:55–68. doi: 10.1016/0012-1606(71)90107-2. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- Grassi F, Giovannelli A, Fucile S, Mattei F, Eusebi F. Cholinergic responses in cloned human TE671/RD tumour cells. Pflugers Arch. 1993;425:117–125. doi: 10.1007/BF00374511. [DOI] [PubMed] [Google Scholar]
- Grassi F, Palma E, Mileo AM, Limatola C, Eusebi F. The desensitization rate of embryonic muscle-type nicotinic acetylcholine receptors depends on the cellular environment. Pflugers Arch. 1995;430:787–794. doi: 10.1007/BF00386177. [DOI] [PubMed] [Google Scholar]
- Grassi F, Palma E, Tonini R, Amici M, Ballivet M, Eusebi F. Amyloidβ1–42 peptide alters the gating of human and mouse α-bungarotoxin-sensitive nicotinic receptors. J Physiol. 2003;547:147–157. doi: 10.1113/jphysiol.2002.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Koishi K, Zhang M, McLennan IS, Harris AJ. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn. 1995;202:244–254. doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang JA, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cells to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Liu J-H, Bijlenga P, Fischer-Lougheed J, Occhiodoro T, Kaelin A, Bader CR, et al. Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J Physiol. 1998;510:467–476. doi: 10.1111/j.1469-7793.1998.467bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cells of skeletal muscle fibres. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Eusebi F, Martinez-Torres A, Palma E, Trettel F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc Natl Acad Sci U S A. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Moser T. Low-conductance coupling between mouse chromaffin cells in situ. J Physiol. 1998;506:195–205. doi: 10.1111/j.1469-7793.1998.195bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Trettel F, Fucile S, Renzi M, Miledi R, Eusebi F. Microtransplantation of membranes from cultured cells to Xenopus oocytes: a method to study neurotransmitter receptors embedded in native lipids. Proc Natl Acad Sci U S A. 2003;100:2896–2900. doi: 10.1073/pnas.0438006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx A, Merrifield PA, Naus CG. Blocking gap junctional intercellular communication in myoblasts inhibits myogenin and MRF4 expression. Dev Genet. 1997;20:133–144. doi: 10.1002/(SICI)1520-6408(1997)20:2<133::AID-DVG6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates B, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Giovannelli A, Degasperi V, Eusebi F, Grassi F. Zinc permeates mouse muscle ACh receptor channels expressed in BOSC 23 cells and affects channel function. J Physiol. 2000;529:83–91. doi: 10.1111/j.1469-7793.2000.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D'Antona G, Pellegrino MA, et al. Cell therapy of α-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA. A new look at the origin, function, and ‘stem-cell’ status of muscle satellite cells. Dev Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Vivarelli E, Cusella-De Angelis MG, Rocancourt D, Buckingham M, Cossu G. A population of myogenic cells derived from the mouse neural tube. Neuron. 1994;13:813–821. doi: 10.1016/0896-6273(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, et al. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]