Abstract
Clinical studies have shown that sinoatrial node dysfunction occurs at the highest incidence in the elderly population. Guinea-pigs were studied throughout their lifespan (i.e. birth to 38 months) to investigate the possible mechanism leading to nodal dysfunction. Using immunofluorescence with confocal microscopy, Cx43 protein expression was shown at birth to be present throughout the sinoatrial node and atrial muscle, however, at one month Cx43 protein was not expressed in the centre of the sinoatrial node. Throughout the remainder of the animal's lifespan the area of tissue lacking Cx43 protein progressively increased. Western blot provided verification by quantitative analysis that Cx43 protein expression within the sinoatrial node decreased with age; however, the expression of other cardiac connexins, Cx40 and Cx45, did not differ with age. Analysis of conduction maps showing propagation of the action potential across the sinoatrial node, from the initiation point to the crista terminalis, found that the action potential conduction time taken and conduction distance increased proportionally with age; conversely the conduction velocity decreased with age. We have shown ageing induces degenerative changes in action potential conduction, contributed to by the observed loss of Cx43 protein. Our data identify Cx43 as a potential therapeutic target for quashing the age-related deterioration of the cardiac pacemaker.
Cardiovascular disease is at epidemic proportions within the elderly. It is predicted that by 2040 nearly 30% of the population in developed countries will be elderly, i.e. over 65 years of age (Lakatta & Sollott 2002; Laurent 2002). Functional problems associated with the sinoatrial (SA) node, the pacemaker of the heart, are at their highest incidence in the elderly population (Rodriguez & Schocken 1990). The clinical symptoms of SA node dysfunction range from dizziness, fatigue and palpitations, clinically observed as rhythm disturbance, bradycardia, sinus pauses, sinus arrest, sinus exit block and re-entrant arrhythmias, resulting, without treatment, in sudden death (Mandel et al. 1999; Ross & Kenny 2000). The quality of life and longevity of these patients can be increased by implantation of an artificial pacemaker (Pyatt et al. 2002).
The cardiac action potential originates within the centre of the SA node, from whence it propagates across the remaining heart tissue assisted by the presence of specialized cellular junctions. Desmosomes are responsible for the intercellular adhesion of the cardiac myocytes, consisting of desmoplakin and desmin protein, and electrical junctions or ‘gap junctions’ consist of connexin (Cx) proteins; predominantly formed from Cx43, but also Cx40 and Cx45 (Honjo et al. 2002). Cx proteins not only have different spatial distributions throughout the heart, but different voltage dependence, single-channel conductance and permeability properties, ensuring propagation of the action potential through the heart in the correct manner (Jongsma & Wilder 2000).
The conduction velocity of the action potential through the cardiac tissues is dependent on the number and type of gap junctions present between adjacent cells, controlled by the rate of Cx protein synthesis versus the rate of degradation (Beardslee et al. 1998). Other defining factors of conduction are the upstroke velocity of the action potential and tissue architecture. Studies of Cx43-deficient mice have shown that reduced Cx43 protein expression; reduces electrical coupling, slows conduction and accelerates the onset, frequency and duration of arrhythmias. Heterozygous Cx43+/− mice, in comparison with wild-type mice, demonstrate a 50% reduction in Cx43 protein expression, leading to a significant slowing of action potential conduction (Thomas et al. 1998; Eloff et al. 2001).
Here we report our results from animals aged from the neonate to the senescent, i.e. birth to 38 months. These animals show age-related changes within the SA node in terms of Cx43 protein expression, SA node action potential conduction time and conduction distance, also the velocity at which the action potential propagates across the SA node. Our data provide a further understanding of the development of the SA node and a mechanistic insight into its deterioration with age.
Methods
Sample acquisition
Healthy Duncan Hartley guinea-pigs were obtained at several ages; neonates at less than 1 day, young at 1 month, adults at 18 months, old at 28 months and senescent at 38 months. In accordance with UK Home Office guidelines, all animals were killed by anaesthetic overdose. The body weight was recorded, the heart removed and rinsed in normal Tyrode solution (pH 7.4), blotted dry and weighed. From the right atrium, the SA node was isolated for further analysis.
Extracellular electrode recording
The SA node was mounted endocardial surface-up in bicarbonate buffered Tyrode solution, maintained at 37°C. An extracellular modified bipolar electrode was used to measure the extracellular potential at 1 mm intervals (except in the neonate where this was performed at 0.5 mm interval) (Yamamoto et al. 1998), and a map across the SA node was constructed (SigmaPlot V8.0, Chicago, USA). The location of the crista terminalis (CT) was identified anatomically with 0.1 mm resolution, and the leading pacemaker site at the centre of the SA node was precisely defined with 0.1 mm resolution by re-scanning the action potential initiation site with the bipolar electrode. We also measured the intrinsic heart rate (i.e. the rate manifested by the autonomically denervated SA node), the action potential conduction time and the distance from the centre SA node to the CT.
Quantitative fluorescent in situ hybridization (QFISH)
Atrial tissue sections were washed (in TBS) for 2 min and fixed in 3.7% formaldehyde for 2 min exactly. Following two washes, the tissue was pre-treated with proteinase K for 10 min and washed twice. The tissue was dehydrated using an increasing ethanol series (70%, 85% and 95%, at −20°C) and air dried. Peptide nucleic acid (PNA) telomere-specific probe was added to each section (10 μl of K5325; Dako, Denmark) which was covered with a coverslip. The probe was incubated with the tissue at 80°C for 3 min exactly, and then at room temperature for 2 h in the dark. The coverslip was removed and the sections washed at 65°C for 5 min to eliminate any trace of unhybridized probe. Tissue was rehydrated by decreasing the ethanol series, air dried and vectashield mounted. Fluorescent telomere labelling from single nuclei was observed by laser scanning confocal microscope (same settings for all images). Fluorescence was quantified using Scion Image (Scion Corporation, MD, USA). No fluorescent telomere labelling was observed when no probe was present at the hybridization stage of the QFISH protocol.
Immunofluorescence
Tissue was mounted in cyro-media and frozen in isopentane cooled by liquid nitrogen, sectioned at 14 μm and placed on polysine-coated slides. Tissue sections were labelled with antibodies according to protocols previously described (Jones et al. 2002). Slides were then examined by laser scanning confocal microscopy (Leica, Milton Keynes, UK) and images collected using identical settings.
Analysis of protein expression
Tissue was snap-frozen and processed for analysis as previously described (Jones et al. 2002). Samples (50 (μg total protein) lane−1) were separated by electrophoresis under reducing conditions by 10% SDS-PAGE, followed by transfer to nitrocellulose membrane. The membrane was probed by antibodies as previously described (Jones et al. 2002). Bands were detected by SuperSignal, West Pico (Pierce, Cheshire, UK) and analysed by Scion Image.
Antibodies
All connexin antibodies were purchased from Chemicon International, Inc., USA. Anti-Cx43, the mouse monoclonal antibody raised against rat cardiac Cx43 (cat. no. MAB3068) was applied to tissue sections at 1 μg ml−1 and membranes at 0.1 μg ml−1. Anti-Cx40, the rabbit polyclonal antibody raised against mouse Cx40 (cat. no. AG634) was applied to membranes at 10 μg ml−1. Anti-Cx45, the mouse monoclonal antibody raised against human Cx45 (cat. no. MAB3100) was applied to membranes at 10 μg ml−1. Monoclonal mouse anti-human desmin, clone D33, antibody (cat. no. M0760, DAKO) was applied to membranes at 6 μg ml−1.
Masson's trichrome-derived collagen stain
The tissue section was immersed in PBS for 5 min, then placed in phosphomolybdic acid (1 g (l water)−1) for 5 min and drained. It was immersed in methyl blue (2 g (l water)−1), 1 ml glacial acetic acid) for 1.5 min and rinsed in water. If the collagen was stained too dark, rinsing was repeated as required.
Statistical analysis
Data are expressed as means ± s.e.m. and statistical differences assessed by two-way ANOVA post hoc analysis made using a Holm–Sidak comparative test. Correlations were assessed by linear regression. Differences and correlations were taken as significant if P < 0.05, n corresponds to the number of animals used at each age, except for fluorescent in situ hybridization where n corresponds to the number of nuclei examined.
Results
Physical changes of the guinea-pig during ageing
The guinea-pig body weight significantly increased from the neonate to the young and to the adult animals (Fig. 1A). The guinea pig heart weight significantly increased from the neonate to the young and to the adult animals (Fig. 1B). The adult heart and body weights were maintained throughout the remainder of the animal's life without any significant change. The heart-to-body weight ratio did not change significantly at any point during the animal's life (Fig. 1C).
Figure 1. Physical changes of the guinea-pig during ageing.
A, mean body weight (neonate versus young P < 0.0001, young versus adult P < 0.0001), B, mean heart weight (neonate versus young P < 0.0001, young versus adult P < 0.0001) and C, mean heart-to-body weight ratio are shown versus age (n = 5). D, FISH-labelled telomeric DNA from atrial nuclei of different aged animals plus the ‘no probe’ control (scale bar, 1 μm). E, the mean percentage telomere labelling per nuclei versus age (n = 100 nuclei from n = 5 animals; linear regression, y = −3.6049x + 57.04, x is the age in months). Error bars show s.e.m.
Tissue from the right atrium was analysed by QFISH for telomeric DNA, a known marker of ageing (Fig. 1D). The magnitude of fluorescent telomere labelling in the nuclei did not differ between the neonatal or young animal. By contrast, telomere labelling decreased to 93 ± 6% in the adult, 62 ± 3% in the old and 19 ± 1% in the senescent animal, when normalized to the young (Fig. 1E). Data were fitted by linear regression and the age at which telomere labelling declined to zero determined. Using linear regression, the maximum lifespan of the guinea-pig model was predicted to be 44 months. This is consistent with our observations of >50% mortality at the old age and >99% mortality past senescent age.
Expression of Cx43 protein
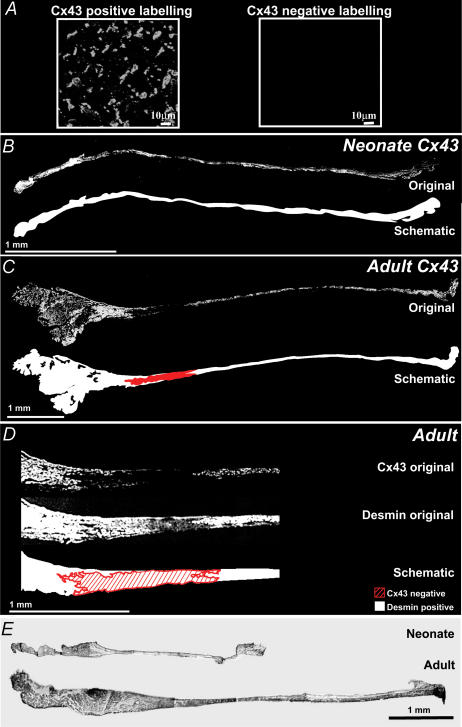
An example of positive- and negative-labelled Cx43 protein from atrial tissue is shown (Fig. 2A). In the neonate animal, an original confocal image of a section through the SA node showed Cx43 protein was labelled uniformly across the whole intercaval region, illustrated by the schematic of detected Cx43 protein in white (Fig. 2B). By comparison, in the adult animal there was an area in the original confocal image where there was no labelled Cx43 protein, highlighted in red in the representational schematic (Fig. 2C). Adjacent sections were labelled with anti-Cx43 and anti-desmin to demonstrate that the area without Cx43 labelling does contain desmin and therefore myocytes (Fig. 2D). Furthermore, adjacent tissue sections to the neonate (Fig. 2B) and adult (Fig. 2C) sections were stained, and collagen was demonstrated to be present in a continuous manner across the SA node, CT and atrial muscle.
Figure 2. Expression of Cx43 protein.
A, high magnification examples of positive- and negative-labelled Cx43 protein from atrial tissue are shown. B, an original confocal image of a neonate section labelled with anti-Cx43 antibody showed Cx43 protein was expressed throughout the SA node and surrounding atrial muscle, further illustrated by the schematic image where detected Cx43 protein is shown in white. C, an original confocal image of an adult section labelled with anti-Cx43 antibody identified an area lacking Cx43 protein, shown in the schematic image in red with the remaining tissue expressing Cx43 protein in white. D, high magnification confocal images of sequential serial sections of the SA node region showed the area lacking Cx43 protein was positive for desmin. Desmin protein was continuous and uniformly labelled across the SA node and atrial muscle. A schematic image shows desmin protein in white and the area lacking Cx43-labelled protein hashed in red. E, original images from a light microscope show collagen is present throughout the SA node at all ages studied.
Progressive increase in the area of tissue absent of Cx43 protein during ageing
For each age, SA node sections were labelled with anti–Cx43 at 0.5 mm intervals to permit a two-dimensional assessment of Cx43 distribution with age. Schematic images of Cx43 labelling are shown on the right and a photograph of the intact SA node with the area lacking Cx43-labelled protein outlined on the left of Fig. 3A. These panels show that the area lacking Cx43 increased in length from 2.0 mm in the young to 9.5 mm in the senescent animal. Overall, the area lacking Cx43 increased from 3.5 ± 0.6 mm2 in the young animal to 22.4 ± 2.5 mm2 in the adult, compared with 34.1 ± 2.1 mm2 in the old animal and reaching an area of 47.6 ± 2.0 mm2 in the senescent animal (Fig. 3B). Comparing the young to the senescent animal, the area lacking Cx43 protein increased from 3.5 ± 0.6 to 47.6 ± 2.0 mm2 (P < 0.0001, n = 5). Linear regression to the total data set indicated a significant and proportional correlation between the area lacking Cx43 protein and age (linear regression: y (mm2) = 1.031 +. 235x, where x is the age in months; correlation coefficient = 0.98, P < 0.001). Overall, the area lacking Cx43 increased 14-fold with age.
Figure 3. Progressive increase in the area of tissue absent of Cx43 protein during ageing.
A, for each age group, schematic images at 0.5 mm intervals are shown on the right and the intact SA node on the left. At each interval, the exact location of the area lacking Cx43 protein has been translated to the intact SA node (SAN) image. The total area where Cx43 protein has not been detected is outlined in red on the intact SA node. B, the mean area of SA node where Cx43 protein expression was not detected is shown versus age. (n = 5; ANOVA P < 0.0001; every group is significantly different from the other P < 0.001). Error bars show s.e.m.
Total connexins versus age
To confirm our findings, the SA node area was further analysed for Cx40, Cx43 and Cx45 protein expression (Fig. 4). Samples of SA node tissue were analysed by Western blot. Illustrative membranes are shown for desmin, total Cx40, total Cx43 and total Cx45 protein expression. Only total Cx43 expression significantly changed with age (Fig. 4A). Equal protein loading was confirmed by uniform bands of desmin expression at 53 kDa, therefore total Cx43 protein was standardized to desmin content. There was a significant decrease in total Cx43 expression (in arbitrary units from densitometry) from the neonate (2.21 ± 0.33) to the young (1.36 ± 0.28), from the young to the old (0.14 ± 0.02) and from the adult (0.83 ± 0.04) to the senescent (0.05 ± 0.01). By linear regression there is a significant correlation between age and Cx43 expression (P < 0.001; correlation coefficient = 0.84). Expressing the data as a percentage of the mean expression identified in the neonate, in the young animal the expression falls to 61 ± 28% of the average initial expression, 38 ± 2% in the adult, 6 ± 1% in the old and 2 ± 0.4% in the senescent (n = 5 in each case) (Fig. 4B).
Figure 4. Total content of connexins versus age.
A, paired samples of atrial (a) and SA node (s) tissue from animals of different ages were analysed for protein expression. Typical blots for desmin (53 kDa), Cx45 (45 kDa), Cx43 (43 kDa) and Cx40 (40 kDa) are shown. B, Cx43 protein expression in the SA node expressed as a percentage of the mean amount identified in the neonate. Cx43 expression within the SA node significantly fell in a progressive manner correlating with age to reach 2 ± 0.4% of that identified in the neonate animals (n = 5 in each case) by senescence, a 98% decline in Cx43 protein expression within the SA node region. Error bars show s.e.m.
Cx40 expression did not significantly correlate with age (P = 0.21) and demonstrated no significant change with age from the neonate (0.19 ± 0.02, in arbitrary units from densitometry) to the senescent animal (0.18 ± 0.02). Cx45 expression also remained stable throughout the lifespan of the animals, showing no correlation with age (P = 0.74) or change from the neonate (0.1 ± 0.02) to the senescent animal (0.1 ± 0.02).
The intrinsic heart rate changes with age
The intrinsic heart rate, i.e. the spontaneous rate manifested by the autonomically denervated SA node, was measured for each age (Fig. 5A). The rate of generated spontaneous action potentials decreased from 249 ± 13 beats min−1 in the neonate to 177 ± 5 beats min−1 in the young, 157 ± 5 beats min−1 in the adult to 152 ± 5 beats min−1 in the senescent animal. There was a significant correlation between age and intrinsic heart rate (P < 0.001, correlation coefficient = 0.6). This correlation was also present if the large change observed from the neonate to the young, perhaps the consequence of a developmental effect, was excluded (P = 0.001, correlation coefficient = 0.6). This decline is a comparable effect to that observed in humans from 20 to 71 years of age (Jose & Collison 1970; Opthof 2000).
Figure 5. Conduction of the action potential changes with age.
A, mean intrinsic heart rate versus age (n = 5; ANOVA P < 0.0001; t test neonate versus young P < 0.0001, young versus adult P < 0.01). B–D, typical maps of action potential conduction in SA node preparations from neonate (B), adult (C) and senescent (D) animals. From the position of the leading pacemaker site (*), isochrones are shown at 2 ms intervals to the border of the crista terminalis (solid white line, CT), where the SA node tissue meets the atrial muscle. E–G, mean time taken for conduction of the action potential (E), the distance (F) and velocity from the leading pacemaker site in the SA node to the atrial muscle of the CT (n = 5; time taken, ANOVA P < 0.0001; distance, ANOVA P < 0.0001; velocity, ANOVA P < 0.005).
Conduction of the action potential changes with age
Conduction of the action potential was mapped from the leading pacemaker site identified within the centre of the SA node (*), to the junction between the SA node and atrial muscle, the crista terminalis (white line labelled CT), in the neonate, adult and senescent animals (Fig. 5B–D). The time taken for conduction of the action potential across the SA node significantly increased from the neonate (8.7 ± 0.5 ms) to the adult (15.8 ± 1.3 ms) and adult to senescent animal (21.2 ± 1.6 ms−1) (Fig. 5E). There was a significant correlation between nodal conduction time and age (P < 0.001, correlation coefficient = 0.87). The conduction time also significantly correlated with the expression of Cx43 as established by Western blot (P = 0.003, correlation coefficient = 0.71). The distance travelled by the action potential from the initiation site of spontaneous activity within the SA node to the CT increased significantly from the neonate (1.3 ± 0.1 mm) to the adult (2.2 ± 0.1 mm) and adult to senescent animal (2.6 ± 0.1 mm) (Fig. 5F), and there was a significant correlation between the conduction distance and age (P < 0.001, correlation coefficient = 0.89). There was also a significant correlation between age and conduction velocity (P < 0.001, correlation coefficient = 0.7), with conduction velocity significantly slowing from the neonate (0.15 ± 0.01 m s−1) to the senescent animal (0.12 ± 0.01 m s−1) (Fig. 5G).
Discussion
The findings of this study indicate that reduced SA node function has a higher incidence in the elderly as a direct result of changes in the organization and constitution of the SA node. Specific novel factors we have identified are changes in the conduction distance from the site of initiation of the cardiac action potential to the CT and the expression of Cx43 protein within the tissue, with consequent effects on the conductivity of the tissue.
A marker of mammalian tissue ageing is the progressive shortening of telomeres (i.e. the protective caps at either end of chromosomes, which protect DNA from degradation and aberrant recombination). Our data show that telomere shortening is progressive during the ageing of the heart and confirm that our animals from birth to senescence (38 months) encompass the life-expectancy of the animal.
Others have previously shown that age does not affect heart rate in vivo, but does significantly reduce the intrinsic heart rate (i.e. the rate manifested by the heart in the absence of autonomic influences) (Di Gennaro et al. 1987). Our guinea-pig model is consistent with these observations. The guinea-pig intrinsic heart rate dropped from 177 ± 5 beats min−1 in the young to 152 ± 5 beats min−1 in the senescent animal. This is a comparable finding to the change in human intrinsic heart rate which drops from 107 beats min−1 at 20 years of age to 69 beats min−1 at 71 years (Jose & Collison 1970; Opthof 2000).
Immunofluorescence data showed Cx43 protein was evenly expressed across the right atrium, including the SA node region, in neonatal guinea-pigs. As cardiac crest cells migrate from the neural crest to the developing heart, they express an abundance of Cx43 protein critical to mammalian heart morphogenesis (Huang et al. 1998), and it is interesting that the location of the leading pacemaker site is not fixed during early development (Moorman et al. 1997). From the neonate to the young animal (i.e. birth to 1 month of age), immunofluorescence showed that an area lacking Cx43 protein develops within the SA node, which increased 14-fold during ageing of the animal. Further protein quantification by Western blot confirmed this finding, as the SA node in the young animal (1 month) contained 27-fold more Cx43 protein than that of the senescent animal (38 months). These two methods established the SA node of an aged animal has a substantial loss of Cx43 protein, in comparison with its young counterpart. Collagen and desmin were present in a continuous manner across the SA node for all ages (Opthof et al. 1985; Alings & Bouman 1993). Although, others have shown increased fibrosis and fat infiltration in the aged heart (Opthof et al. 1985; Song et al. 1999), these factors were shown not to affect SA node function (Alings & Bouman 1993).
Previously, the area lacking Cx43 protein in the SA node has been shown to conduct the action potential five-fold more slowly than in the surrounding tissue (Yamamoto et al. 1998). In conditions such as end-stage heart failure it has been suggested that in areas deficient of Cx43 protein, Cx40 becomes the principal conductor (i.e. Cx40 protein increases relative to total Cx43 within the SA node) (Thomas et al. 1998), but if this occurred the conduction velocity would decrease (Kanagaratnam et al. 2002). Our protein analysis demonstrated that in the aged guinea-pig, SA node, Cx40 and Cx45 protein expression did not change with age to compensate for Cx43 protein loss. Therefore, we predicted slowed action potential conduction in the SA node would occur when Cx43 protein expression was reduced.
Mapping of conduction of the action potential across the guinea-pig SA node showed the distance the action potential propagates from the leading pacemaker site in the SA node to the atrial muscle of the CT, and the time taken for conduction increased with age. The distance from the leading pacemaker site to the CT and the conduction time in the young guinea-pig are similar to those previously reported (Opthof et al. 1985). A study of the cat and rabbit showed SA node conduction time increased, and the intrinsic heart rate decreased with age in both species (Alings & Bouman 1993). In this present study we have shown a decline in the intrinsic heart rate and increase in conduction time, and identified two contributory factors; an increase in SA node conduction distance and a reduction in Cx43 protein. The distance and consequent conductivity changes combine to produce a decline in the function of the SA node. Both factors are important mechanisms contributing to the degeneration of the node and both distance and Cx43 expression closely correlate with the conduction time of the node (P < 0.003 in each case with correlation coefficients of 0.9 and 0.7, respectively). These combined factors would be anticipated, with an increasing probability with age, to eventually lead to disconnection of the SA node, rendering it unable to drive pacemaking of the heart.
Why the SA node region continues to increase in size creating the observed increase in conduction distance is unknown and particularly perplexing, considering the apparent lack of overall further cardiac growth and hypertrophy. The resultant requirement of the initiated action potential to propagate through an extending distance of tissue with poor conductivity is a significant contributory factor to the age-dependent degradation of nodal function, but one that is difficult to target therapeutically.
Of greater interest as a potential therapeutic target is modulation of the conduction pathway. The observed age-correlated decline in conduction velocity will be moderated by two notable factors, the form of the action potential within the tissue, and the cell-to-cell coupling conductance within the tissue. Analysis of the action potential between ages is complicated by the large diversity of action potential forms that may be identified across the nodal structure. Consequently, despite ongoing efforts, it is still not possible at this stage to conclusively comment on the role of action potential changes in determining the age-related changes in SA node function. Further work utilizing selective isolation of tissue and cells from selected regions across the ageing nodal structure will yield the answers regarding what contribution any changes in action potential form may have in causing age-related changes in nodal function. Such changes, if identified, will act in concert with the alterations already noted by the present study to yield the described phenotype.
We have identified a significant age-related loss of Cx43 from the nodal structure. The identified loss (of 98% by senescence) is likely to be a notable influence on conduction (changes of 50% or more have been associated with a significant decline in conductivity in other studies of cardiac muscle (Thomas et al. 1998; Eloff et al. 2001)), and a significant contributor to the ageing-related increase in nodal dysfunction. The conductivity of the SA node tissue exists in a delicate balance. If the pacemaker cells of the node were perfectly coupled to the surrounding atrial muscle, the electrotonic load imposed would strongly suppress pacemaker function. On the other hand, if the conductivity were too low the node would not be able to drive the activation of the surrounding muscle. We hypothesize that this balance is instigated and eventually compromised with development and ageing. The loss of Cx43 with age will ultimately lead to disconnection of the pacemaker region, preceded by an increased conduction time and failure to be able to operate at high pacemaker rates, opening the door to cardiac initiation becoming dominant from latent pacemaker regions and other ectopic sites of activation. The decline in Cx43 protein expression identified is a potentially viable target for therapeutic intervention of the aged SA node. It will be interesting to note, perhaps by use of transfection, the effect of restoring Cx43 expression to levels observed in the young tissue. Could this reverse the ageing-associated degeneration of nodal function?
To summarize, our data have demonstrated that in the model of the aged guinea-pig, an area lacking Cx43 protein was identified in the SA node and the loss of Cx43 protein increased during ageing. Remodelling of the expression of gap junctions will alter the conduction properties of the SA node and contribute towards the reduced function of the aged SA node. Data in this study provide evidence that if the level of Cx43 protein expression remained stable throughout life, the conduction problems associated with SA node function in the elderly could be potentially prevented. Cx43 expression could be used as a future therapeutic target. Recent evidence has shown that activation of c-Jun N-terminal kinase (JNK) reduces Cx43 protein expression in vivo (Petrich et al. 2002), and therefore inactivation of JNK expression within the SA node could upregulate Cx43 expression, alternatively viral gene transfer therapy could be used to upregulate Cx43 expression and attempt to restore function in the SA node of elderly patients (Miake et al. 2002).
Acknowledgments
We would like to acknowledge the financial support of the British Heart Foundation and Medical Research Council.
References
- Alings AMW, Bouman LN. Electrophysiology of the ageing rabbit and cat sinoatrial node – a comparative study. Eur Heart J. 1993;14:1278–1288. doi: 10.1093/eurheartj/14.9.1278. [DOI] [PubMed] [Google Scholar]
- Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Di Gennaro M, Bernabei R, Sgadari A, Carosella L, Carbonin PU. Age-related differences in isolated rat sinus node function. Basic Res Cardiol. 1987;82:530–536. doi: 10.1007/BF01907222. [DOI] [PubMed] [Google Scholar]
- Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res. 2001;51:681–690. doi: 10.1016/s0008-6363(01)00341-8. [DOI] [PubMed] [Google Scholar]
- Honjo H, Boyett MR, Coppen SR, Takagishi Y, Opthof T, Severs NJ, Kodama I. Heterogeneous expression of connexins in rabbit sinoatrial node cells: correlation between connexin isotype and cell size. Cardiovasc Res. 2002;53:89–96. doi: 10.1016/s0008-6363(01)00421-7. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell–cell communication modulates mouse neural crest migration. J Cell Biol. 1998;143:1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Morton MJ, Hunter M, Boyett MR. Expression of TASK-1, a pH-sensitive twin-pore domain K+ channel, in rat myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H181–H185. doi: 10.1152/ajpheart.00963.2001. [DOI] [PubMed] [Google Scholar]
- Jongsma HJ, Wilder R. Gap junctions in cardiovascular disease. Circ Res. 2000;86:1193–1197. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4:160–167. doi: 10.1093/cvr/4.2.160. [DOI] [PubMed] [Google Scholar]
- Kanagaratnam P, Rothery S, Patel P, Severs NJ, Peters NS. Relative expression of immunolocalized connexins 40 and 43 correlates with human atrial conduction properties. J Am Coll Cardiol. 2002;39:116–123. doi: 10.1016/s0735-1097(01)01710-7. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A. 2002;132:699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- Laurent GJ. Molecular and cellular mechanisms of aging. Int J Biochem Cell Biol. 2002;34:1317. [Google Scholar]
- Mandel WJ, Jordan JL, Karagueuzian HS. Disorders of sinus function. Curr Treat Options Cardiovasc Med. 1999;1:179–186. doi: 10.1007/s11936-999-0021-9. [DOI] [PubMed] [Google Scholar]
- Miake J, Marban E, Nuss HB. Gene therapy: Biological pacemaker created by gene transfer. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- Moorman AFM, de Jong F, Denyn MFJ, Lamers WH. Development of the cardiac conduction system. Circ Res. 1997;82:629–644. doi: 10.1161/01.res.82.6.629. [DOI] [PubMed] [Google Scholar]
- Opthof T. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 2000;45:177–184. doi: 10.1016/s0008-6363(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Opthof T, de Jonge B, Mackaay AJ, Bleeker WK, Masson-Pevet M, Jongsma HJ, Bouman LN. Functional and morphological organization of the guinea pig sinoatrial node compared with the rabbit sinoatrial node. J Mol Cell Cardiol. 1985;17:549–564. doi: 10.1016/s0022-2828(85)80024-9. [DOI] [PubMed] [Google Scholar]
- Petrich BG, Gong X, Lerner DL, Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, Wang Y. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ Res. 2002;91:640–647. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- Pyatt JR, Somauroo JD, Jackson M, Grayson AD, Osula S, Aggarwal RK, Charles RG, Connelly DT. Long-term survival after permanent pacemaker implantation: analysis of predictors for increased mortality. Europace. 2002;4:113–119. doi: 10.1053/eupc.2002.0233. [DOI] [PubMed] [Google Scholar]
- Rodriguez RD, Schocken DD. Update on sick sinus syndrome, a cardiac disorder of aging. Geriatrics. 1990;45:26–36. [PubMed] [Google Scholar]
- Ross RA, Kenny RA. Pacemaker syndrome in older people. Age Ageing. 2000;29:13–15. doi: 10.1093/ageing/29.1.13. [DOI] [PubMed] [Google Scholar]
- Song Y, Yao Q, Zhu J, Luo B, Liang S. Age-related variation in the interstitial tissues of the cardiac conduction system; and autopsy study of 230 Han Chinese. Forensic Sci Int. 1999;104:133–142. doi: 10.1016/s0379-0738(99)00103-6. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Schuessler RB, Berul CB, Beardslee MA, Beyer EC, Mendelsohn ME, Saffitz JE. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction. Evidence for chamber-specific molecular determinants of conduction. Circulation. 1998;97:686–691. doi: 10.1161/01.cir.97.7.686. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Honjo H, Niwa R, Kodama I. Low-frequency extracellular potentials recorded from the sinoatrial node. Cardiovasc Res. 1998;39:360–372. doi: 10.1016/s0008-6363(98)00091-1. [DOI] [PubMed] [Google Scholar]