Abstract
pHi affects a number of cellular functions, but the influence of pHi on mammalian ciliary beat frequency (CBF) is not known. CBF and pHi of single human tracheobronchial epithelial cells in submerged culture were measured simultaneously using video microscopy (for CBF) and epifluorescence microscopy with the pH-sensitive dye BCECF. Baseline CBF and pHi values in bicarbonate-free medium were 7.2 ± 0.2 Hz and 7.49 ± 0.02, respectively (n = 63). Alkalization by ammonium pre-pulse to pHi 7.78 ± 0.02 resulted in a 2.2 ± 0.1 Hz CBF increase (P < 0.05). Following removal of NH4Cl, pHi decreased to 7.24 ± 0.02 and CBF to 5.8 ± 0.1 Hz (P < 0.05). Removal of extracellular CO2 to change pHi resulted in similar CBF changes. Pre-activation of cAMP-dependent protein kinase (10 μm forskolin), broad inhibition of protein kinases (100 μm H-7), inhibition of PKA (10 μm H-89), nor inhibition of phosphatases (10 μm cyclosporin + 1.5 μm okadaic acid) changed pHi-mediated changes in CBF, nor were they due to [Ca2+]i changes. CBF of basolaterally permeabilized human tracheobronchial cells, re-differentiated at the air–liquid interface, was 3.9 ± 0.3, 5.7 ± 0.4, 7.0 ± 0.3 and 7.3 ± 0.3 Hz at basolateral i.e., intracellular pH of 6.8, 7.2, 7.6 and 8.0, respectively (n = 18). Thus, intracellular alkalization stimulates, while intracellular acidification attenuates human airway CBF. Since phosphorylation and [Ca2+]i changes did not seem to mediate pHi-induced CBF changes, pHi may directly act on the ciliary motile machinery.
pHi is an important element of cellular homeostasis and affects a number of cellular functions (for review see Roos & Boron, 1981). Variations in pHi of airway epithelia may occur in vivo in response to shifting luminal CO2 tension (PCO2) during a full breathing cycle (Willumsen & Boucher, 1992); however, neither the extent of such a possible pHi change nor its effect on cellular functions are known. There are only data available on the relationship between extracellular pH and CBF of mammalian airway epithelial cells (van de Donk et al. 1980; Luk & Dulfano, 1983; Clary-Meinesz et al. 1998): alkaline solutions up to pH 9–10 had no effect on CBF, while acidic solutions with a pH < 7.0 attenuated ciliary beating. Similar results were found when cell cultures were exposed to SO2, making the bathing solutions extremely acidic (Kienast et al. 1994). In another study, pH of the medium between 6.5 and 7.5 did not influence CBF (Ingels et al. 1991). It remains unclear, however, by how much extracellular solutions actually changed pHi in any of these experiments. Changes of mammalian CBF due to pHi would not only affect cilia during the breathing cycle but also during exacerbations of airway diseases with airway acidification (e.g. asthma).
Surprisingly little information is available on pH-induced changes in ciliary/flagellar beat frequency in non-mammalian systems. Reactivation of isolated newt lung axonemes suggested a bell-shaped reactivation optimum at pH 7.0 and the absence of outer dynein arms, while influencing overall beating frequency, did not affect the bell-shaped pH responsiveness (Hard et al. 1992). However, studies on demembranated sperm suggested that mild alkalization increased flagellar beat frequency (Gibbons & Gibbons, 1972; Brokaw & Kamiya, 1987; Keskes et al. 1998) with the exception of one study using high Ca2+ concentrations (Ho et al. 2002). Human spermatozoa lacking outer dynein arms, the arms that mainly determine ciliary frequency (Brokaw & Kamiya, 1987), failed to show higher beat frequency during mild alkalization (Keskes et al. 1998), suggesting that, in contrast to newt lung cilia (Hard et al. 1992), outer dynein arms are involved in the human flagellar response to changing pHi.
Hypothetically pH changes could have direct effects on the outer dynein arm or influence the activity of axonemal kinases and phosphatases that are sensitive to pH (Cox & Taylor, 1995; Reddy et al. 1998). Of particular interest is the cAMP-dependent protein kinase (PKA), an important regulator of mammalian CBF (Wyatt et al. 1998; Lieb et al. 2002; Zagoory et al. 2002), and phosphatases shown to control ciliary beating in protozoa (Klumpp et al. 1990; Momayezi et al. 1996; Noguchi et al. 2003; Deckman & Pennock, 2004). Another important regulator of CBF, [Ca2+]i, was also found to be regulated by pHi in several cell types (Thomas et al. 1979; Browning & Wilkins, 2002).
Thus, the purpose of this study was to define the extent and mode of pHi action on CBF of human tracheobronchial epithelial cells. Our results suggest that pHi between 6.8 and 8.0 influences ciliary beating perhaps directly at the axonemal level as pH-mediated CBF changes did not seem to be mediated via kinase/phosphatase systems or [Ca2+]i.
Methods
Chemicals
LHC basal medium, Trace elements 100 ×, Stock 4100 ×, and Stock 11 100 × were purchased from Biosource International (Rockville, MD, USA); Ham's nutrient F-12 and gentamicin from Gibco BRL Laboratories (Grand Island, NY, USA); the acetoxymethyl ester form of the pH-sensitive dye BCECF and fura-2 from Molecular Probes (Eugene, OR, USA); nigericin from Molecular Probes (Eugene, OR, USA) and Calbiochem (La Jolla, CA, USA); thapsigargin and H-89 from Calbiochem (La Jolla, CA, USA); cyclosporin A from Fluka (Buchs, Switzerland); and okadaic acid from Research Biochemicals International (Natick, MA, USA). All other reagents were from Sigma Chemicals (St Louis, MO, USA).
Solutions
Table 1 lists the compositions of solutions used. The free Ca2+ and Mg2+ concentration of EGTA- and ATP-containing solutions was estimated using WebMAXC Standard software by Chris Patton from Stanford University, available at http://www.stanford.edu/~cpatton/webmaxcS.htm (constants used: CMC1002. TCM).
Table 1.
Composition of solutions
| Solution | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Solution name | Standard Hepes | NH4Cl-Hepes | High K+ Hepes | Ca2+-free Hepes | Ca2+-free NH4Cl-Hepes | 25 mm gluconate Hepes | 25 mm HCO3− | Standard internal | Standard internal w/o ATP |
| NaCl | 142 | 132 | 17.3 | 142 | 132 | 117 | 117 | 10 | 10 |
| NH4Cl | — | 10 | — | — | 10 | — | — | — | — |
| KCl | 5.3 | 5.3 | 130 | 5.3 | 5.3 | 5.3 | 5.3 | — | — |
| Na-gluconate | — | — | — | — | — | 25 | — | — | — |
| NaHCO3 | — | — | — | — | — | — | 25 | — | — |
| K-gluconate | — | — | — | — | — | — | — | 140 | 150 |
| CaCl2 | 1.3 | 1.3 | 1.3 | — | — | 1.3 | 1.3 | 0.33† | 0.1 |
| MgCl2 | 0.5 | 0.5 | 0.5 | 0.5* | 0.5* | 0.5 | 0.5 | — | — |
| MgSO4 | 0.4 | 0.4 | 0.4 | 0.46* | 0.46* | 0.4 | 0.4 | — | — |
| Na2HPO4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | — | — |
| KH2PO4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | — | — |
| Glucose | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | 5.6 | — | — |
| Hepes | 10 | 10 | 20 | 10 | 10 | 10 | — | 20 | 20 |
| Nigericin | — | — | 0.015 | — | — | — | — | — | — |
| EGTA | — | — | — | 1 | 1 | — | — | — | — |
| MgATP | — | — | — | — | — | — | — | 5 | — |
| CPK | — | — | — | — | — | — | — | 50 U ml−1 | — |
| CrP | — | — | — | — | — | — | — | 10 | — |
| Equilibrated with | air | air | air | air | air | air | 5% CO2/95% O2 | air | air |
| pH ‡ | 7.4 | 7.4 | 6.8–7.2–7.5–7.8 (adjusted with KOH) | 7.4 | 7.4 | 7.4 | 7.4 (adjusted with NaHCO3) | 6.8–7.2–7.6–8.0 (adjusted with KOH) | 7.2 (adjusted with KOH) |
All concentrations are given in mm unless otherwise indicated.
Approximate free [Mg2+] after chelation by EGTA is 0.9 mm:
approximate free [Ca2+] after chelation by ATP is 0.1 mm:
pH was adjusted with concentrated NaOH solution unless otherwise indicated. CPK, creatine phosphokinase; CrP, creatine phosphate disodium salt.
Preparation of submerged tracheal epithelial cultures
Primary cultures of tracheal epithelial cells were prepared as previously described (Salathe & Bookman, 1995). Human tissue was obtained from organ donors whose lungs were rejected for transplant through the Life Alliance Organ Recovery Agency of the University of Miami. IRB-approved consents for use of these tissues for research were obtained by the Life Alliance Organ Recovery Agency. Cells from these cultures were used for measurements within 6 days after plating.
Preparation of air liquid interface cultures of tracheal epithelium
Human air–liquid interface (ALI) cultures were prepared according to published methods (Adler et al. 1990; Bernacki et al. 1999; Nlend et al. 2002), except that the cells were plated onto 24 mm diameter, 3 μm pore-sized Transwell collagen-coated inserts (Corning Costar Corporation, Cambridge, MA, USA). The ALI cultures were used for measurements after the cells fully re-differentiated (about 6–8 weeks).
Selective permeabilization of the basolateral membrane of cells grown at the ALI
The basolateral surface of the ALI culture was exposed to 10 000 U ml−1 Staphylococcus aureus alpha-toxin dissolved in solution 8 (Table 1) for 30 min at room temperature. Solutions 8 and 9 were composed to reflect physiological intracellular K+, Na+ and Cl− concentrations. Similar to the experience of others (Ostedgaard et al. 1992), however, we had to use 100 μm Ca2+ to avoid cell detachment.
Measurement of CBF
Cells grown on coverslips in submerged culture were mounted at room temperature onto the stage of a Nikon Eclipse E600FN upright water-immersion lens microscope in an open (Warner Instrument RC-25F with a working volume of 150 μl) or closed (Warner Instrument RC-21BR, working volume of 260 μl) perfusion chamber. They were perfused constantly. Ciliated cells were imaged with infrared differential interference contrast (DIC) optics with an optical gain of 600 ×. For online CBF measurements, the light path was directed to a CCD video camera (XC-7500 Sony) and a box of 3 × 3 pixels from the live, digitized, contrast-enhanced video image was selected (where one pixel samples an area of 180 nm × 180 nm). The magnitude spectra from a fast Fourier transform (FFT) of each of the pixel's intensity signals were computed online and displayed on the monitor for immediate adjustments. The intensity signals were recorded and later used for analysis according to published methods (Salathe & Bookman, 1999) using a sliding FFT window approach (128 frames per FFT, sliding the FFT window through the data set by 100 frames at a time), providing a frequency resolution of at least 0.23 Hz and a time resolution of ∼3 s. The individual FFT magnitude spectra were peak extracted for graph production (Salathe & Bookman, 1999).
In order to measure CBF in cells grown at the air–liquid interface (but imaged with the apical surface ‘submerged’), we used a holding chamber for the Transwell membranes allowing selective perfusion of the apical and basolateral sides of the cells. Data acquisition and processing was identical to the one described for submerged cultures.
Measurement of pHi
Coverslips without a confluent monolayer (usually 1 day after plating) were preferred for fluorescent measurement of pHi, because these ciliated cells could be more easily calibrated for pHi using nigericin (see below). Coverslips were rinsed in solution 1 (Table 1) and loaded with 2.5 μm BCECF-AM in solution 1 at 37°C for 15–30 min and again rinsed 3 times. For fluorescent measurements, a Lambda DG4 excitation system (Sutter, Novato, CA, USA) was used with 10 nm wide excitation filters centred on 495 and 440 nm (Chroma Technology Corp., Brattleboro, VT, USA). ‘Ratio-tool’ software from Isee Imaging (Raleigh, NC, USA) controlled the output of the lambda DG4. Ratiometric pH estimates were made by capturing the light (535 nm) emitted from the cells through a 60 × water immersion objective (Nikon Inc.) and directing it to a cooled CCD camera (CoolSnap Hq, Photometrics, Tucson, AZ, USA). Individual ciliated cells were identified as regions of interest (ROIs) and the BCECF ratio of emission intensity after excitation at 495 and 440 nm was computed within each ROI every 10–60 s on a pixel-by-pixel basis (after background fluorescence subtraction).
Calibration of pHi measurements
Preparation of the pH calibration curve
Nigericin was used to calibrate pHi measurements (Thomas et al. 1979). BCECF-loaded cells were perfused with calibration solutions containing 15 μm nigericin and 130 mm KCl at pH 6.8, 7.2, 7.5 and 7.8, respectively (solution 3, Table 1) while the fluorescence ratio was measured. The ratio data were normalized to the ratio at pH 7.2 (normalized fluorescence ratio: NFR; Fig. 1A). Each ratio value was accepted when the ratio reached a steady state level at a given calibration pH. Then, the calibration curve was constructed by plotting the average NFR values from 47 cells (5 different organ donors) against the corresponding pH values. The correlation between NFR and pH values was determined by linear regression analysis (r2 > 0.99, Fig. 1B) (Boyarsky et al. 1988; Osypiw et al. 1994; Paradiso, 1997; Evans et al. 2003).
Figure 1. Intracellular calibration of BCECF using nigericin.
A, a record of normalized fluorescence ratio (NFR) from a single, submerged, BCECF-loaded ciliated cell. The cell was first perfused with standard Hepes-buffered solution (solution 1, Table 1), which was switched to solutions containing 130 mm K+ and 15 μm nigericin (solution 3, Table 1), buffered at the indicated pH. Each fluorescence ratio value (I495/I440) was divided by the ratio obtained at pH 7.2 to obtain the NFR. B, pHi dependence of NFR. NFR data were obtained from 47 cells. Linear regression is shown with an r2 > 0.99.
pHi estimation
A one-point nigericin calibration was performed at the end of each experiment exposing the cells to the calibration solution with a pH of 7.2 (Boyarsky et al. 1988). NFR values were calculated by dividing the ratio data from the entire experiment by the ratio obtained in calibration solution at pH 7.2. NFR values were transformed to pHi by interpolation with the calibration plot.
Nigericin was delivered to the cells via separate tubing. After each experiment with nigericin, the mounting chamber and the manifold were washed with 100% ethanol and distilled water to avoid nigericin-induced changes in pHi in the following experiments (Richmond & Vaughan-Jones, 1997; Bevensee et al. 1999).
Measurement of [Ca2+]i
[Ca2+]i was measured with the same equipment used for pHi. Cells grown on coverslips were loaded with 5 μm fura-2 AM in solution 1 (Table 1) for 60 min at room temperature and were washed 3 times with solution 1. Cells were excited through 10 nm wide excitation filters centred on 340 and 380 nm (Chroma Technology Corp., Brattleboro, VT, USA), and the emitted light was captured at 535 nm. Fura-2 ratios were computed every 10–20 s, after background subtraction. Calibration of the calcium signal was done with in vitro measurements as described (Salathe & Bookman, 1995) according to Grynkiewicz et al. (1985); however, we re-estimated [Ca2+]i by adjusting fura-2 Kd values with changing pH (Lattanzio & Bartschat, 1991; Browning & Wilkins, 2002) as described below.
Simultaneous measurement of CBF and pHi or [Ca2+]i
By using a dual-image module and guiding the infrared signal for CBF measurements to the XC-7500 Sony CCD camera while sending all fluorescence signals (< 580 nm) to the cooled CCD camera, we were able to measure recordings of CBF and fluorescence (i.e. pHi or [Ca2+]i) of the same single cell simultaneously.
Experimental procedures
Three different methods were used to manipulate pHi: (a) ammonium pre-pulse; (b) removal of CO2 from the extracellular medium; and (c) permeabilization of the basolateral membrane with the pore-forming agent alpha-toxin (from Staphylococcus aureus). For method (a), bicarbonate-free solutions were used to avoid interference with soluble adenylyl cyclase, an enzyme activated by bicarbonate and possibly expressed in these cells (Chen et al. 2000; Litvin et al. 2003). All coverslips and ALI cultures used in these experiments were washed with bicarbonate-free solution, mounted into an open chamber and exposed to ambient air for at least 30 min before experiments to remove any remaining bicarbonate and CO2 from the cells. Measurements in open chambers were performed during continuous perfusion with the desired solution at a flow rate of 250 μl min−1 using a Harvard pump. After changing from one solution to another, flow rate was increased to 1000 μl min−1 for 1 min to accelerate the full exchange of the bathing solution (NH4Cl pre-pulse was applied for 2 min at 1000 μl min−1). These changes in flow rates did not influence CBF in control experiments, similar to our earlier findings (Lieb et al. 2002). For method (b), cells were loaded with BCECF in CO2/HCO3−-buffered solution at 5% ambient CO2 and then mounted into a closed chamber with delivery of all solutions via a closed system. Cells were perfused at a constant flow rate of 1000 μl min−1. Finally for method (c), ALI cultures were mounted into an open chamber with separate perfusion ports for the apical and basolateral side, maintaining perfusion at 500 and 1000 μl min−1 for the apical and basolateral side, respectively. Up to six cells per coverslip or ALI filter were measured in each experiment.
Statistics
All experimental data points were compared with date- and culture-matched controls. Two groups were compared using Student's unpaired t test, while Tukey-Kramer honestly significant difference test was used for comparison of more than two groups if an analysis of variance indicated a significant difference between groups (JMP software, SAS Institute Inc., Cary, NC, USA). A P < 0.05 was considered to be significant. Data were expressed as means ±s.e.m.
Results
The effect of changing pHi on CBF during ammonium pre-pulse
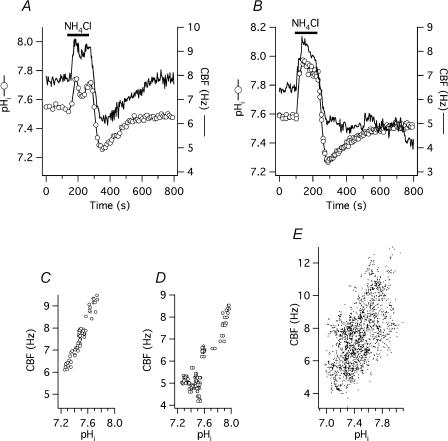
The ammonium pre-pulse technique was used to achieve a change in pHi of intact cells without changing the pH of the bathing solution (pHo). Baseline CBF was 7.2 ± 0.2 Hz and baseline pH was 7.49 ± 0.02 (n = 63 cells from 7 different organ donors). When the ammonium-free standard Hepes solution (solution 1, Table 1) was rapidly switched to one containing 10 mm NH4Cl (10 mm NaCl was replaced by 10 mm NH4Cl, solution 2, Table 1), CBF and pH increased at the same rate, within the time resolution of the measurements (10 s for pHi in these experiments). CBF reached a maximum of 9.4 ± 0.2 Hz (31 ± 1% above baseline; P < 0.001) within 52 ± 7 s, whereas pH reached a maximum of 7.78 ± 0.02 (P < 0.0001 compared with baseline) within 48 ± 6 s. Two minutes after the initial change to ammonium chloride, the medium was switched back to the standard Hepes solution. After removing extracellular NH4+, a rapid intracellular acidification took place and CBF and pH rapidly decreased to 5.8 ± 0.2 Hz (19 ± 2% below original baseline) and 7.24 ± 0.02, respectively.
Figure 2A and B shows two representative, simultaneous recordings of CBF and pHi during and after ammonium chloride exposure. To better visualize the correlation between pHi and CBF, these two parameters were plotted against each other in panels C–E. During the recovery from acid load, CBF either increased together with pHi (Fig. 2A and 35 of 63 cells), or remained at a low level during the rest of the experiment (Fig. 2B). In the 35 cells with recovering CBF, baseline CBF and pHi were 7.5 ± 0.2 Hz and 7.45 ± 0.03, respectively, not significantly different from the non-recovering 28 cells (6.9 ± 0.2 Hz and 7.53 ± 0.03, both P > 0.05). However, the maximum pHi following ammonium load was significantly higher in the latter group (7.84 ± 0.03) compared with the recovering cells (7.72 ± 0.03). The CBF peaks in both groups after ammonium load were not significantly different (9.7 ± 0.3 Hz in the recovering group versus 9.1 ± 0.3 Hz in the non-recovering group). During the following acidification phase, pHi in non-recovering cells was found to be significantly higher than in cells that recovered their CBF (7.27 ± 0.03 versus 7.20 ± 0.02, P < 0.05); on the other hand, CBF at pHi nadir was significantly higher in the recovering group (6.1 ± 0.2 Hz) compared with the non-recovering group (5.4 ± 0.2 Hz). Since the decrease of CBF during intracellular acidification was not significantly different between the two groups, it is possible that a too severe alkalization was responsible for the failure of these cells to recover their CBF after an acid load. This notion was supported by the fact that a lower pH could be reached in many other cells without any ill effects on CBF. However, other factors play a role as in other groups of cells, high pHi did not seem to influence the ability of CBF to recover after acidification (see inhibitor experiments below).
Figure 2. CBF responses to changing pHi during ammonium pre-pulse.
A, simultaneous CBF and pHi recording from a single, submerged, BCECF-loaded human tracheobronchial epithelial cell perfused with standard Hepes-buffered solution (solution 1, Table 1). Intracellular alkalization and acidification by transient exposure to 10 mm NH4Cl (solution 2, Table 1) results in pHi-coupled changes in CBF. During recovery from acid load, CBF again parallels pHi. B, traces from an experiment identical to panel A, but recorded from a different cell. Here, CBF fails to follow pHi during recovery from intracellular acid load. C, correlation between CBF and pHi plotted from the experiment shown in panel A. D, correlation between CBF and pHi plotted from the experiment shown in panel B. E, correlation between CBF and pHi plotted from 59 cells transiently exposed to 10 mm NH4Cl.
The effect of changing pHi on CBF during removal of extracellular CO2
An additional way to change pHi without changing pHo was to remove extracellular CO2 (Thomas, 1984; Willumsen & Boucher, 1992; Paradiso et al. 2003). For this purpose, cells in closed chambers were continuously perfused with CO2/HCO3−-buffered solution, pHo= 7.4 (25 mm NaCl of the standard Hepes solution was replaced with 25 mm NaHCO3 following equilibration with 5% CO2–95% O2, pH 7.4, no Hepes, solution 7, Table 1). Baseline pHi in this group of cells was 7.24 ± 0.02 (n = 13 cells from two donors), significantly lower than in cells bathed in Hepes-buffered, nominally CO2/HCO3−-free solution (7.49 ± 0.02, see above; P < 0.0001). Similarly, baseline CBF was significantly lower in CO2/HCO3−-buffered than in Hepes-buffered solution (6.1 ± 0.3 versus 7.2 ± 0.2 Hz, P < 0.005). When the CO2/HCO3−-buffered solution was switched to a nominally CO2/HCO3−-free buffer of the same pH (25 mm NaHCO3 was replaced by equimolar sodium gluconate, buffered with 10 mm Hepes; solution 6, Table 1), a rapid increase in pHi to 7.75 ± 0.03 was seen (P < 0.0001). When the CO2/HCO3−-buffered medium was reapplied and extracellular CO2 entered the cell forming carbonic acid, pHi fell below the original baseline to 7.04 ± 0.02 (P < 0.0001 compared with both baseline and peak pHi), followed by a slow pHi recovery. Similar to the ammonium pre-pulse experiments, CBF followed the changes in pHi, increasing from a baseline of 6.1 ± 0.3 Hz to 10.3 ± 0.5 Hz (or 71 ± 6% above baseline; P < 0.0001) and falling back to 5.7 ± 0.3 Hz (7 ± 3% below original baseline; P < 0.0001 compared with the peak CBF, but not significantly different from baseline). The time course of the intracellular alkalization and the parallel CBF increase was again within the resolution of the pHi measurement (10 s): pHi reached its maximum within 92 ± 6 s and CBF within 106 ± 13 s. Figure 3A shows a representative experiment.
Figure 3. CBF responses to changing pHi during removal of external CO2.
A, simultaneous CBF and pHi recording from a single, submerged, BCECF-loaded human tracheobronchial epithelial cell, mounted into a closed chamber and perfused with CO2/HCO3−-buffered solution (solution 7, Table 1) in exchange with CO2/HCO3−-free, Hepes-buffered solution (solution 6, Table 1). B, correlation between CBF and pHi plotted from the experiment shown in panel A. C, correlation between CBF and pHi plotted from 13 cells during and after removal of external CO2 identical to the experiment shown in panel A.
Even though removal of external CO2 both increased CBF and pHi significantly more than the alkalization phase of ammonium pre-pulse, the ratio of these two parameters (ΔCBF/ΔpHi) did not differ between the two methods (8.4 ± 0.6 s−1 (pH unit)−1versus 7.7 ± 0.4 s−1 (pH unit)−1 in the CO2 and NH4Cl group, respectively; P > 0.05). To better visualize the correlation between pHi and CBF, these two parameters were again plotted against each other (Fig. 3B and C).
Inhibition and stimulation of PKA does not prevent the effect of pHi on CBF
Kinases, specifically cAMP-dependent kinase (PKA), are important regulators of mammalian airway ciliary beating. Since the activity of kinase/phosphatase systems has been reported to depend on pH (Cox & Taylor, 1995; Reddy et al. 1998), H-7, a broad based serine–threonine kinase inhibitor, was used to inhibit PKA.
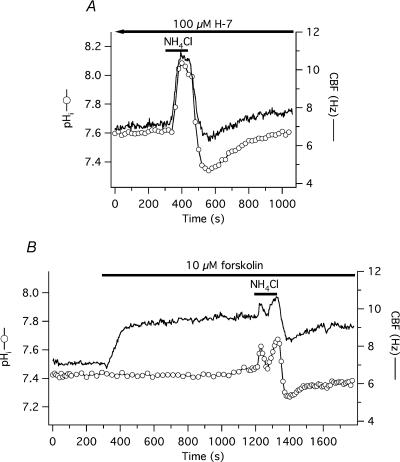
Cells were incubated with 100 μm H-7 for at least 20 min (estimated Ki of H-7 for PKA, PKG and PKC is 3.0, 5.8 and 6.0 μm, respectively, Hidaka et al. 1984) until a steady state CBF was achieved. Baseline CBF and pHi in the H-7 group was 7.2 ± 0.3 Hz and 7.45 ± 0.08, respectively (n = 10 cells from one donor), not significantly different from date- and culture-matched controls (6.4 ± 0.4 Hz, 7.56 ± 0.08 pH units, n = 6, both P > 0.05). Following H-7 exposure, CBF invariably decreased but average CBF was not significantly below original baseline after 20 min of H-7 exposure. Cells were exposed to 10 mm NH4Cl (solution 2, Table 1) in the continuous presence of H-7. During alkalization, pHi increased significantly to 7.82 ± 0.11 and 7.83 ± 0.07 in the H-7 and control group, respectively (P > 0.05). CBF also increased significantly in both groups: to 9.5 ± 0.4 Hz (or 49 ± 3% above H-7 baseline) in the H-7 group and to 8.7 ± 0.9 Hz (or 36 ± 6% above baseline) in the control group (P > 0.05). After removing external ammonium chloride, pHi fell in both groups: to 7.30 ± 0.04 in H-7-exposed and to 7.34 ± 0.06 in control cells (P > 0.05). Similarly, CBF decreased to the same extent in the H-7 and control groups, falling to 6.1 ± 0.3 Hz (or 96 ± 2% of the H-7 baseline) and to 5.8 ± 0.4 Hz (or to 91 ± 2% of the baseline; P > 0.05), respectively. A representative experiment is shown in Fig. 4A.
Figure 4. No effect of PKA inhibition or activation on CBF responses to changing pHi during ammonium pre-pulse.
A,simultaneous CBF and pHi recording from a single, submerged, BCECF-loaded human tracheobronchial epithelial cell pre-treated and continuously perfused with 100 μm H-7 and transiently exposed to 10 mm NH4Cl. Neither the CBF nor the pHi responses differed from control cells (see Fig. 2). B, simultaneous CBF and pHi recording from a single, submerged, BCECF-loaded human tracheobronchial epithelial cell continuously exposed to 10 μm forskolin followed by a transient exposure to 10 mm NH4Cl.
The efficacy of PKA inhibition by H-7 was confirmed in experiments using 1 μm forskolin to stimulate CBF, where H-7 (100 μm) significantly attenuated forskolin-induced stimulation of CBF. CBF increased by only 1.6 ± 0.2 Hz (or 31 ± 3%) above baseline in H-7 pre-treated cells compared with 4.8 ± 0.5 Hz (or 86 ± 11%) in control cells (all n = 6; P < 0.001 for both the absolute and percentage values).
These data were confirmed with a more specific inhibitor of PKA, namely 10 μm H-89 (Davies et al. 2000). The reported Ki values for PKA, CaM kinase II and PKC are 48 nm, 29.7 mm, and 31.7 mm, respectively. Cells were pre-incubated with H-89 for 20 min. As with H-7, H-89 did not influence the pH-mediated changes in CBF during ammonium pre-pulse experiments (n = 4) compared with date- and culture-matched control cells (n = 6). H-89 also inhibited the CBF increase upon exposure of cells to 10 μm forskolin (n = 7 for H-89/forskolin and n = 8 for forskolin control).
The possible role of PKA in mediating pHi-induced changes in CBF was also tested by fully stimulating the enzyme with 10 μm forskolin prior to applying the ammonium pre-pulse. Forskolin was continuously present during and after the ammonium exposure. Baseline CBF and pHi in the forskolin group before stimulation was 6.9 ± 0.4 Hz and 7.61 ± 0.07, respectively (n = 15 cells from 3 donors), not significantly different from controls (6.4 ± 0.5 Hz, 7.52 ± 0.04 pH units, n = 14). Following forskolin stimulation, CBF significantly increased by 42 ± 4% above baseline (up to 9.7 ± 0.5 Hz) and pHi remained unchanged (7.63 ± 0.08). During the alkalizing phase of the ammonium pre-pulse, pHi peaked at 7.79 ± 0.10 and 7.78 ± 0.03 in the forskolin and the control group, respectively (not significantly different from each other, but P < 0.05 compared with the baseline in both groups). In the forskolin group, peak CBF was 60 ± 6% above the original baseline (absolute CBF value was 10.9 ± 0.6 Hz) and 13 ± 4% above the post-forskolin plateau. The percentage change during alkalization of the control group cells was 30 ± 3% above baseline (absolute CBF value: 8.3 ± 0.3 Hz, significantly lower than peak CBF in the forskolin pre-treated cells, P < 0.001). These results show that even if PKA is already stimulated, increasing pHi still further stimulates ciliary beating. During acidification, pHi fell to 7.39 ± 0.07 in the forskolin pre-treated cells and to 7.26 ± 0.04 in the control cells (no significant difference between the two groups). As expected, CBF in the forskolin-stimulated cells was still 22 ± 4% above the original baseline (absolute value: 8.4 ± 0.5 Hz) while the beating frequency in the control group was 32 ± 4% below baseline (absolute value: 4.3 ± 0.2 Hz; P < 0.0001; Fig. 4B).
Inhibition of protein phosphatases does not influence the effect of pHi on CBF
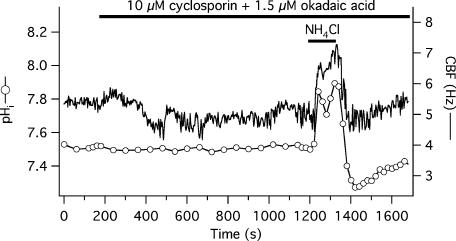
To inhibit protein phosphatases, a combination of inhibitors was used: 10 μm cyclosporin A (inhibitor of calcineurin, a type 2B protein phosphatase, at an IC50= 5 nm, Cohen et al. 1989) and 1.5 μm okadaic acid (inhibitor of type 1 at an IC50= 15–20 nm and type 2A protein phosphatase at an IC50= 0.1 nm, Cohen et al. 1989). Baseline CBF and pHi before application of the inhibitors was 6.7 ± 0.2 Hz and 7.46 ± 0.03, respectively (n = 11 cells from 2 donors), not significantly different from the date- and culture-matched controls (7.0 ± 0.3 Hz, 7.57 ± 0.06, n = 10). Cells in the phosphatase inhibitor group were perfused with the inhibitors for 17 min, resulting in a statistically insignificant CBF decrease (6.0 ± 0.3 Hz) and unchanged pHi (7.47 ± 0.02). During the alkalization phase of the ammonium pre-pulse, both pHi and CBF increased to the same level in both groups, significantly above baseline. Maximum pHi was 7.86 ± 0.01 in inhibitor-treated cells and 7.89 ± 0.06 in control cells (P > 0.05), while maximum CBF was 8.3 ± 0.3 Hz (40 ± 4% above post-inhibitor plateau frequency) in the inhibitor group and 9.4 ± 0.5 Hz (or 34 ± 3% above baseline) in the control group (P > 0.05 for both percentage and absolute changes). The nadir of pHi and CBF (as percentage decrease from baseline) during acidification did not differ between the treated and control groups: pHi was 7.20 ± 0.03 and 7.27 ± 0.06, while CBF fell to 90 ± 3% of the CBF plateau reached after adding inhibitors and to 89 ± 3% of the baseline in the two groups, respectively. A representative experiment is shown in Fig. 5.
Figure 5. CBF responses to changing pHi during ammonium pre-pulse – lack of effect by phosphatase inhibition.
Simultaneous CBF and pHi recording from a single, submerged, BCECF-loaded human tracheobronchial epithelial cell perfused with 10 μm cyclosporine A plus 1.5 μm okadaic acid and transiently exposed to 10 mm NH4Cl.
pHi does not regulate CBF via changes in [Ca2+]i
[Ca2+]i plays a crucial role in regulating CBF (e.g. Salathe & Bookman, 1995, 1999; Salathe et al. 1997; Ma et al. 2002; Zagoory et al. 2002). It has been shown in several cell types that intracellular alkalization can increase, while intracellular acidification can decrease [Ca2+]i via regulation of Ca2+ release from intracellular stores (Thomas et al. 1979; Browning & Wilkins, 2002). We investigated whether CBF regulation by pHi depends on [Ca2+]i.
First, [Ca2+]i and CBF from the same single cells were measured simultaneously during ammonium pre-pulse (see Methods). The estimated baseline [Ca2+]i of cells perfused with standard Hepes-buffered solution (solution 1, Table 1) was 47.5 ± 10.3 nm, baseline CBF was 8.0 ± 0.3 Hz (n = 18 from 1 donor). Two minutes after starting the ammonium load (solution 2, Table 1), CBF increased as expected and significantly to 10.9 ± 0.4 Hz (37 ± 3% above the baseline). Estimated [Ca2+]i, however, did not change significantly (46.1 ± 7.6 nm). After the ammonium pre-pulse was completed and CBF returned to baseline, P2Y receptors were stimulated with 10 μm ATP for 1 min. Exposure to ATP resulted in a significant increase in both estimated [Ca2+]i (peak: 234.3 ± 36.4 nm) and CBF (peak: 12.6 ± 0.3 Hz or 61 ± 7% above baseline; see examples in Fig. 6A and B). To estimate [Ca2+]i, constant Kd (400 nm, Browning & Wilkins, 2002), Rmax, Rmin and β-values were used according to Grynkiewicz et al. (1985) using in vitro calibration procedures as outlined in material and methods. However, the affinity of fura-2 for Ca2+ has been reported to increase with increasing pH (i.e. Kd decreases at alkaline pH). In addition, Rmax as well as β were found to be pH dependent (Lattanzio & Bartschat, 1991; Browning & Wilkins, 2002). Therefore, Rmax, Rmin and β were measured in vitro in the pH range of 7.0–8.0. The changes in Rmin and β were negligible. The average maximum intracellular alkalization during ammonium pre-pulse in our hands was ∼0.30 pH units (from 7.50 to 7.80, see above). Based on our in vitro estimations and an approximate 12.5% decrease in Kd (400 nm to 350 nm) based on the study by Browning & Wilkins (2002), these changes would result in a 17% decrease in estimated [Ca2+]i. Thus, increases in [Ca2+]i are over-estimated and decreases are under-estimated by the ratio data upon alkalization.
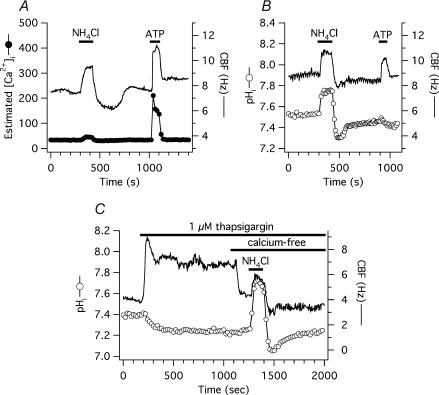
Figure 6. pHi-mediated changes in CBF are not due to [Ca2+]i variations.
A, simultaneous CBF and pHi recording from a single, submerged, fura-2-loaded human tracheobronchial epithelial cell, transiently exposed to 10 mm NH4Cl. Ammonium pre-pulse had a negligible effect on estimated [Ca2+]i, even if corrected for the pH-related changes in Kd after NH4Cl exposure. Purinergic stimulation with 10 μm ATP, however, resulted in an transient increase of both estimated [Ca2+]i and CBF. B, experiment shown in panel A was repeated in cells loaded with BCECF instead of fura-2. NH4Cl exposure caused a significant change in pHi, while ATP did not. C, simultaneous CBF and pHi recording from a single, submerged, BCECF-loaded human tracheobronchial epithelial cell, transiently exposed to 1 μm thapsigargin and nominally calcium free solution (solution 4, Table 1) before transient exposure to 10 mm NH4Cl. pHi-mediated CBF changes were not different from control cells.
To further show that changes in [Ca2+]i were not involved in the pHi-mediated changes in CBF, intracellular Ca2+ stores were emptied with 1 μm thapsigargin, an inhibitor of the endoplasmic reticulum Ca2+-ATPase (Thastrup et al. 1990). Upon thapsigargin exposure, baseline CBF of 6.4 ± 0.6 Hz (n = 8 cells from 3 donors) quickly increased and then declined until reaching a plateau at 8.2 ± 0.5 Hz or 34.5 ± 16.3% above baseline (P > 0.05; Fig. 6C). Three minutes before an ammonium load, extracellular Ca2+ was removed (solution 4, Table 1) and CBF decreased to 6.8 ± 0.6 Hz. Longer Ca2+-free medium exposure resulted in cell detachment. After 3 min in calcium-free, EGTA-buffered solution (nominal 0 [Ca2+]o), cells were exposed to 10 mm NH4Cl (solution 5, Table 1) containing 1 μm thapsigargin, resulting in a 28 ± 3% acceleration of ciliary beating above pre-NH4Cl baseline (8.6 ± 0.7 Hz), a value not significantly different from the 39 ± 5% increase from baseline of 6.3 ± 0.5 Hz to 8.7 ± 0.6 Hz seen in date-matched control cells (n = 5), neither exposed to thapsigargin nor calcium-free extracellular solution. Intracellular acid load after NH4Cl removal significantly decreased CBF in both groups. Here, however, CBF of cells in nominally free calcium solutions decreased significantly more than CBF of control cells: CBF decreased 22 ± 3% below pre-ammonium challenge baseline to 5.3 ± 0.5 Hz versus 5 ± 2% to 6.0 ± 0.5 Hz, respectively (P < 0.05 for percentage values; P > 0.05 for the absolute values). Baseline pHi was 7.38 ± 0.04 before ammonium exposure in cells bathed in nominally free calcium solutions versus 7.48 ± 0.04 baseline in control cells (P > 0.05). pHi peaks were the same in both groups: 7.77 ± 0.04 in nominally free calcium solutions versus 7.77 ± 0.05 in controls (P > 0.05); pHi nadir was 7.20 ± 0.04 versus 7.27 ± 0.1, respectively (P > 0.05).
Basolaterally permeabilized cells grown at the ALI
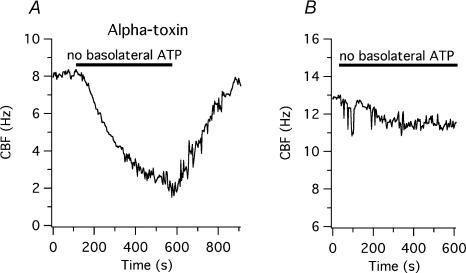
To control the cytoplasmic environment and to test whether the changes in CBF seen in intact cells were directly related to changes in pH, the basolateral membrane of human tracheobronchial epithelial cells re-differentiated at the ALI was selectively permeabilized with Staphylococcus aureus alpha-toxin as described in Methods. Staphylococcus aureus alpha-toxin makes the membrane permeable to molecules smaller than 5 kDa, including nucleotides (Bhakdi & Tranum-Jensen, 1991; Ostedgaard et al. 1992; Walev et al. 1993; Jonas et al. 1994; Reddy & Quinton, 1994; Bhakdi et al. 1996; Detimary et al. 1996; Watanabe & Takano-Ohmuro, 2002). To evaluate the efficacy of membrane permeabilization, the basolateral (i.e. intracellular) solution containing 5 mm MgATP and an ATP regenerating system (ARS, 50 U ml−1 creatine phosphokinase, 10 mm creatine phosphate disodium salt, solution 8 at pH 7.2, Table 1, Kakuta et al. 1985) was exchanged by a solution without ATP and ARS (solution 9, Table 1). Seven minutes after the removal of this system, CBF fell from a baseline of 6.7 ± 0.5 Hz by 4.2 ± 0.4 Hz (or by 62 ± 3%; P < 0.0001 for both absolute and percentage changes, n = 10 cells from 2 donors). These results suggested that the basolateral membrane was permeable to ATP, even though the cilia were able to maintain a low beating frequency of 2.5 ± 0.2 Hz (Fig. 7A). CBF returned to the original baseline when the ATP–ARS-containing basolateral solution was reapplied. Baseline CBF of non-permeabilized cells was 11.6 ± 0.6 Hz (significantly higher than that of permeabilized cells; P < 0.0001) and did not change upon removal of ATP–ARS from the basolateral solution (10.4 ± 0.4 Hz, P > 0.05, n = 4; Fig. 7B).
Figure 7. Basolateral permeabilization of human tracheobronchial epithelial cells re-differentiated at the air–liquid interface with alpha-toxin.
A, the basolateral surface of human airway epithelial cells grown and re-differentiated on 3 μm pore-sized inserts was permeabilized as described in Methods. Removal of ATP and the ATP-regenerating system (50 U ml−1 creatine phosphokinase, 10 mm creatine phosphate) caused a reversible CBF decrease. B, when re-differentiated cells were not permeabilized, removal of ATP and the ATP-regenerating system did not change CBF significantly.
When alpha-toxin was applied apically (in the same solution used for the basolateral permeabilization; solution 8, Table 1) while the basolateral membrane was perfused with the standard Hepes-buffered solution (solution 1, Table 1), removal of apical ATP–ARS (by changing to solution 9, Table 1) did not change CBF significantly. These results show that alpha-toxin permeabilizes specifically the basolateral membrane as previously reported in an epithelial cell line (Ostedgaard et al. 1992).
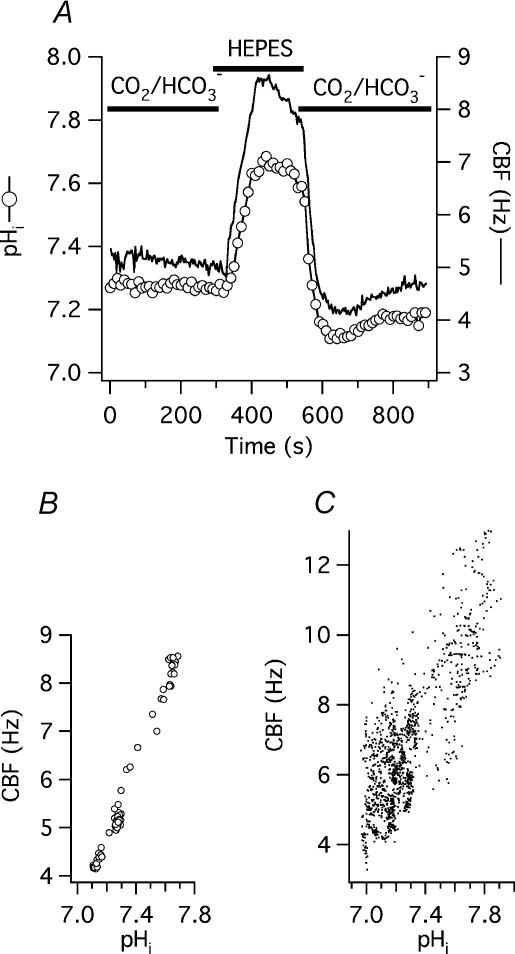
Correlation between pHi and CBF in permeabilized cells
After selective permeabilization of the basolateral membrane, the basal compartment of the ALI chamber was perfused with solutions titrated to pH 6.8, 7.2, 7.6 and 8.0 (solution 8, Table 1; see example in Fig. 8A). CBF was 3.9 ± 0.3, 5.7 ± 0.4, 7.0 ± 0.3 and 7.3 ± 0.3 Hz at pH 6.8, 7.2, 7.6 and 8.0, respectively (n = 18 each from 2 different organ donors; Fig. 8B). All these values were significantly different from each other except those measured at pH 7.6 and 8.0, suggesting that intracellular alkalization had a ceiling effect on stimulating CBF in permeabilized cells.
Figure 8. Correlation between pHi and CBF in basolaterally permeabilized human tracheobronchial epithelial cells.
A, representative measurement from a single re-differentiated cell permeabilized with alpha-toxin and basolaterally exposed to different solutions equilibrated at the shown pH. Increasing basolateral (i.e. intracellular) pH resulted in a CBF increase. B, data obtained as in panel A, but summarized for 18 measured cells. Values are the mean ±s.e.m.
Discussion
Previous studies showed that extracellular alkaline solutions up to pH 9–10.5 had no effect on mammalian airway CBF, while extracellular acidic solutions attenuated ciliary beating (van de Donk et al. 1980; Luk & Dulfano, 1983; Clary-Meinesz et al. 1998). However, the relationship between intracellular pH and CBF has been unclear. Here we demonstrate that relatively small changes in pHi result in significant changes in ciliary beating of human tracheobronchial epithelial cells. The observed changes in ciliary beating are large enough to alter a more macroscopic physiological parameter, i.e. mucociliary clearance: Seybold et al. (1990), for instance, found that a 16% increase in CBF can lead to a 56% increase in mucociliary transport velocity in isolated whole tracheas.
Our experiments suggest that changing pHi may directly act on the ciliary motile machinery, possibly the outer dynein arm. The exact mechanism of how pHi changes CBF remains unclear. One possibility is a change in the charge of histidine, possibly affecting dynein ATPase or a dynein light chain that influences dynein ATPase activity. To our knowledge, no data are available from the literature on the former hypothesis. However, a dynein light chain conformation change has been reported to depend on pH-induced changes in histidine ionization (Barbar et al. 2001). This dynein light chain, LC8, has been originally found on outer dynein arms (Piperno & Luck, 1979), has been reported to be important for flagellar beating and can be found as a dimer or monomer, depending on pH-induced histidine ionization (Barbar et al. 2001). Thus, although completely speculative, histidine charge changes on the outer dynein arm could be important in pH-dependent modulation of CBF.
The baseline pHi of human airway tracheobronchial ciliated cells submerged in nominally CO2/HCO3−-free solutions (pH adjusted to 7.4) at room temperature was surprisingly high at 7.49 ± 0.02. Poulsen & Machen (1996), however, reported a similarly high pHi (7.41 ± 0.09) in bovine tracheal epithelial cells bathed in CO2/HCO3−-free buffers. Similar pHi values were reported: 7.44 ± 0.01 in rat cardiac myocytes (Evans et al. 2003), 7.40 ± 0.02 in rat distal colon (Dagher et al. 1994), 7.42 ± 0.02 in rat ileum villus cells (Dagher et al. 1997), and 7.67 ± 0.05 in rat ileum crypt cells (Dagher et al. 1997). On the other hand, the pHi of human nasal airway epithelial cells bathed in CO2/HCO3−-free medium reported by another group was considerably less at 7.08–7.16 (Willumsen & Boucher, 1992; Paradiso, 1997; Paradiso et al. 2003). The reasons for these differences are not clear, but could be due to calibration issues, differences in experimental temperatures (Roos & Boron, 1981), disparate cell types and culturing methods (polarized cell culture). Our results using CO2/HCO3− -buffered solutions, however, agree with the published data: baseline pHi was 7.24 ± 0.02 in our experiments, 0.24 pH units lower than in nominally CO2/HCO3−-free solution (Paradiso et al. 2003). The pHi changes in response to ammonium pre-pulse were similar to those reported by others (Boyarsky et al. 1988; Singh et al. 1995; Paradiso, 1997; Ramirez et al. 2000; Brett et al. 2002). Although both the basolateral and apical membrane was available for NH4Cl in our submerged culture experiments, the shape of the pHi plot depicted in Fig. 2A and B suggested that the apical membrane responses were predominant (Willumsen & Boucher, 1992; Boron et al. 1994; Singh et al. 1995; Paradiso, 1997).
Our experiments in intact human tracheobronchial epithelial cells revealed a close kinetic relationship between pHi and CBF in the examined pH range of 7.0–7.8 (Figs 2 and 3). The changes occurred coincidentally at the beginning of the alkalization and acidification phase of ammonium pre-pulse or external CO2 removal, i.e. within the time resolution of the CBF measurements (3 s) and pH recordings (10–20 s).
The mode of pHi action on CBF is not clear and the possibility with histidine charge changes discussed above remains purely speculative. However, several possibilities could be excluded. Activation of soluble adenylyl cyclase (sAC), an enzyme distinct from the transmembrane adenylyl cyclase class by being insensitive to forskolin but activated by HCO3− (Chen et al. 2000; Litvin et al. 2003), was one possibility that was excluded. Although we have preliminary data from RT-PCR reactions suggesting that sAC is expressed in human tracheobronchial epithelial cells, the exact cellular distribution and localization of sAC in the airway epithelium is not known. However, all coverslips and ALI cultures in this study, except those used for the CO2 removal experiments, were perfused with CO2/HCO3−-free solutions and exposed to ambient air for at least 30 min before experimentation. Therefore, the intracellular [HCO3−] is expected to approach zero in these cells and changes in pHi should not have been able to change sAC activity via its HCO3− sensitivity.
Protein kinase/phosphatase systems could also be targets of pHi changes since the catalytic efficiency of PKA is optimal at near neutral pH and is inhibited at acidic pH (Cox & Taylor, 1995). Acidic pH not only inhibits PKA but also activates phosphatases to de-phosphorylate PKA targets, while alkaline pH has the opposite effect on both PKA and phosphatases (Reddy et al. 1998). However, neither inhibition of protein kinases with H-7, PKA with H-89 nor pre-stimulation of PKA by the transmembrane adenylyl cyclase activator forskolin could attenuate CBF responses to changing pHi. The same concentrations of the kinase inhibitors H-7 and H-89 significantly attenuated the CBF-stimulatory effect of forskolin, showing the efficacy of these agents. The use of H-7 as a broad inhibitor also excluded other kinases relevant for CBF (e.g. PKC). Similarly, pre-treatment with a mixture of two phosphatase inhibitors failed to show any significant difference in the coupling of CBF and pHi (Figs 4 and 5). Although it is more difficult to test the efficacy of phosphatase inhibition, the combination of two inhibitors at the concentrations used (at 100, 3000 and 2000 times the estimated IC50 for phosphatase 1, 2A and 2B, respectively) was probably sufficient to inhibit these enzymes.
Another important mediator of CBF regulation, [Ca2+]i, was also reported to be influenced by pHi in several cell types (Thomas et al. 1979; Browning & Wilkins, 2002). However, we could not modify the coupling of pHi and CBF by emptying internal Ca2+ stores with thapsigargin and inhibiting the influx of external Ca2+ using nominally Ca2+-free bathing solutions (Fig. 6C). Simultaneous measurements of [Ca2+]i and CBF during ammonium pre-pulse failed to show significant changes in estimated [Ca2+]i when CBF changed (Fig. 6A), even when the estimated [Ca2+]i was corrected for changes in the Kd of fura-2 due to pH (Lattanzio & Bartschat, 1991; Browning & Wilkins, 2002). Although measurement of the fura-2 fluorescence of the whole cell does not exclude the possibility of a localized rise in [Ca2+] in a subcellular compartment, this possibility is unlikely since the absence of calcium in intracellular stores as well as in the extracellular space did not change the results. The physicochemical effect of increasing pH to decrease the concentration of ionized calcium cannot account for the rise in CBF during alkalization as the opposite, namely a decrease in CBF, would have been expected.
Our experiments on permeabilized cells (Figs 7 and 8) further confirm that CBF is regulated by pHi and rule out the possibility that CBF changes during ammonium pre-pulse were coupled to changes in extra- or intracellular NH4+ and/or NH3 concentrations rather than to changes in pHi. That ciliary beating was not completely abolished in our permeabilized cells after 7 min perfusion with ATP and ARS-free bathing solution could be due to the presence of non-diffusible pools of nucleotides (Malaisse & Sener, 1987; Aprille, 1988; Detimary et al. 1996) and by residual production of ATP close to cilia.
Together, these data suggest that phosphorylation/dephosphorylation events and changes in [Ca2+]i are not responsible for the pHi-mediated changes in CBF and support a direct action of pHi on axonemal proteins. Studies performed on demembranated mammalian spermatozoa are of special interest in this regard because cilia and sperm flagella share considerable ultrastructural similarities as well as some similarities in their beat regulation (e.g. by PKA, for review see Urner & Sakkas, 2003). Most published experiments on demembranated spermatozoa demonstrate that mild alkalization augments flagellar beat frequency (FBF) of spermatozoa of different species (Gibbons & Gibbons, 1972; Brokaw & Kamiya, 1987; Keskes et al. 1998). One of these studies (Keskes et al. 1998) also showed that human spermatozoa that lack outer dynein arms failed to increase beat frequency during alkalization suggesting that outer dynein arms, that mainly determine the frequency of ciliary beating (Brokaw & Kamiya, 1987), might be directly involved in the response to changing pHi. Whether the effects of pH on spermatozoa are mediated through the same mechanisms, remains unclear since some of the pH effects in spermatozoa could be due to the stimulation of sAC.
Our results show that intracellular alkalization results in faster ciliary beating, while intracellular acidification attenuates CBF in human tracheobronchial epithelial cells. Variations in pHi of airway epithelia may occur in vivo in response to shifting luminal CO2 concentrations from 5 to 0.02% during a full breathing cycle (Willumsen & Boucher, 1992). It is intriguing to speculate that a possible pHi change during the breathing cycle might influence CBF in the large airways. If it occurs, it would result in a faster ciliary beating during inspiration. Furthermore, airway diseases associated with acidification of the surface liquid and possibly epithelium (e.g. asthma) could depress ciliary activity, possibly contributing to the decrease in mucociliary clearance seen in these diseases.
Acknowledgments
This work was supported by grants from the American Heart Association (Postdoctoral Fellowship to Z.S.) and the NIH (HL-66125 to G.E.C., and HL-60644 and HL-67206 to M.S.).
References
- Adler KB, Cheng PW, Kim KC. Characterization of guinea pig tracheal epithelial cells maintained in biphasic organotypic culture: cellular composition and biochemical analysis of released glycoconjugates. Am J Respir Cell Mol Biol. 1990;2:145–154. doi: 10.1165/ajrcmb/2.2.145. [DOI] [PubMed] [Google Scholar]
- Aprille JR. Regulation of the mitochondrial adenine nucleotide pool size in liver: mechanism and metabolic role. FASEB J. 1988;2:2547–2556. doi: 10.1096/fasebj.2.10.3290024. [DOI] [PubMed] [Google Scholar]
- Barbar E, Kleinman B, Imhoff D, Li M, Hays TS, Hare M. Dimerization and folding of LC8, a highly conserved light chain of cytoplasmic dynein. Biochemistry. 2001;40:1596–1605. doi: 10.1021/bi002278+. [DOI] [PubMed] [Google Scholar]
- Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol. 1999;20:595–604. doi: 10.1165/ajrcmb.20.4.3442. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Bashi E, Boron WF. Effect of trace levels of nigericin on intracellular pH and acid-base transport in rat renal mesangial cells. J Membr Biol. 1999;169:131–139. doi: 10.1007/s002329900525. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Waisbren SJ, Modlin IM, Geibel JP. Unique permeability barrier of the apical surface of parietal and chief cells in isolated perfused gastric glands. J Exp Biol. 1994;196:347–360. doi: 10.1242/jeb.196.1.347. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3. Am J Physiol. 1988;255:C844–856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Brett CL, Kelly T, Sheldon C, Church J. Regulation of Cl−–HCO3− exchangers by cAMP-dependent protein kinase in adult rat hippocampal CA1 neurons. J Physiol. 2002;545:837–853. doi: 10.1113/jphysiol.2002.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella. IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton. 1987;8:68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- Browning JA, Wilkins RJ. The effect of intracellular alkalinisation on intracellular Ca2+ homeostasis in a human chondrocyte cell line. Pflugers Arch. 2002;444:744–751. doi: 10.1007/s00424-002-0843-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J. 1998;11:330–333. doi: 10.1183/09031936.98.11020330. [DOI] [PubMed] [Google Scholar]
- Cohen P, Klumpp S, Schelling DL. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989;250:596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cox S, Taylor SS. Kinetic analysis of cAMP-dependent protein kinase: mutations at histidine 87 affect peptide binding and pH dependence. Biochemistry. 1995;34:16203–16209. doi: 10.1021/bi00049a036. [DOI] [PubMed] [Google Scholar]
- Dagher PC, Chawla H, Michael J, Egnor RW, Charney AN. Modulation of chloride secretion in the rat ileum by intracellular bicarbonate. Comp Biochem Physiol a Physiol. 1997;117:89–97. doi: 10.1016/s0300-9629(96)00281-2. [DOI] [PubMed] [Google Scholar]
- Dagher PC, Morton TZ, Joo CS, Taglietta-Kohlbrecher A, Egnor RW, Charney AN. Modulation of secretagogue-induced chloride secretion by intracellular bicarbonate. Am J Physiol. 1994;266:G929–934. doi: 10.1152/ajpgi.1994.266.5.G929. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckman CM, Pennock DG. Dephosphorylation of inner arm 1 is associated with ciliary reversals in Tetrahymena thermophila. Cell Motil Cytoskeleton. 2004;57:73–83. doi: 10.1002/cm.10158. [DOI] [PubMed] [Google Scholar]
- Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671–4676. doi: 10.1210/endo.137.11.8895332. [DOI] [PubMed] [Google Scholar]
- Evans RK, Schwartz DD, Gladden LB. Effect of myocardial volume overload and heart failure on lactate transport into isolated cardiac myocytes. J Appl Physiol. 2003;94:1169–1176. doi: 10.1152/japplphysiol.00778.2002. [DOI] [PubMed] [Google Scholar]
- Gibbons BH, Gibbons IR. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972;54:75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hard R, Blaustein K, Scarcello L. Reactivation of outer-arm-depleted lung axonemes: evidence for functional differences between inner and outer dynein arms in situ. Cell Motil Cytoskeleton. 1992;21:199–209. doi: 10.1002/cm.970210304. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250:208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- Ingels KJ, Kortmann MJ, Nijziel MR, Graamans K, Huizing EH. Factors influencing ciliary beat measurements. Rhinology. 1991;29:17–26. [PubMed] [Google Scholar]
- Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuta Y, Kanno T, Sasaki H, Takishima T. Effect of Ca2+ on the ciliary beat frequency of skinned dog tracheal epithelium. Respir Physiol. 1985;60:9–19. doi: 10.1016/0034-5687(85)90036-2. [DOI] [PubMed] [Google Scholar]
- Keskes L, Giroux-Widemann V, Serres C, Pignot-Paintrand I, Jouannet P, Feneux D. The reactivation of demembranated human spermatozoa lacking outer dynein arms is independent of pH. Mol Reprod Dev. 1998;49:416–425. doi: 10.1002/(SICI)1098-2795(199804)49:4<416::AID-MRD9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kienast K, Riechelmann H, Knorst M, Schlegel J, Muller-Quernheim J, Schellenberg J, Ferlinz R. An experimental model for the exposure of human ciliated cells to sulfur dioxide at different concentrations. Clin Invest. 1994;72:215–219. doi: 10.1007/BF00189317. [DOI] [PubMed] [Google Scholar]
- Klumpp S, Cohen P, Schultz JE. Okadaic acid, an inhibitor of protein phosphatase 1 in Paramecium, causes sustained Ca2+-dependent backward swimming in response to depolarizing stimuli. EMBO J. 1990;9:685–689. doi: 10.1002/j.1460-2075.1990.tb08160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio FA, Jr, Bartschat DK. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991;177:184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lieb T, Wijkstrom Frei C, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol. 2002;538:633–646. doi: 10.1113/jphysiol.2001.013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of ‘soluble’ adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin Sci. 1983;64:449–451. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- Ma W, Silberberg SD, Priel Z. Distinct axonemal processes underlie spontaneous and stimulated airway ciliary activity. J General Physiol. 2002;120:875–885. doi: 10.1085/jgp.20028695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse WJ, Sener A. Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim Biophys Acta. 1987;927:190–195. doi: 10.1016/0167-4889(87)90134-0. [DOI] [PubMed] [Google Scholar]
- Momayezi M, Wloga D, Kissmehl R, Plattner H, Jung G, Klumpp S, Schultz JE. Immunolocalization of protein phosphatase type 1 in Paramecium cells using antibodies against recombinant protein and peptides. J Histochem Cytochem. 1996;44:891–905. doi: 10.1177/44.8.8756761. [DOI] [PubMed] [Google Scholar]
- Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol. 2002;27:436–445. doi: 10.1165/rcmb.2002-0012OC. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Sasaki JY, Kamachi H, Inoue H. Protein phosphatase 2C is involved in the cAMP-dependent ciliary control in Paramecium caudatum. Cell Motil Cytoskeleton. 2003;54:95–104. doi: 10.1002/cm.10088. [DOI] [PubMed] [Google Scholar]
- Ostedgaard LS, Shasby DM, Welsh MJ. Staphylococcus aureus alpha-toxin permeabilizes the basolateral membrane of a Cl−-secreting epithelium. Am J Physiol. 1992;263:L104–112. doi: 10.1152/ajplung.1992.263.1.L104. [DOI] [PubMed] [Google Scholar]
- Osypiw JC, Gleeson D, Lobley RW, Pemberton PW, McMahon RF. Acid-base transport systems in a polarized human intestinal cell monolayer: Caco-2. Exp Physiol. 1994;79:723–739. doi: 10.1113/expphysiol.1994.sp003803. [DOI] [PubMed] [Google Scholar]
- Paradiso AM. ATP-activated basolateral Na+/H+ exchange in human normal and cystic fibrosis airway epithelium. Am J Physiol. 1997;273:L148–158. doi: 10.1152/ajplung.1997.273.1.L148. [DOI] [PubMed] [Google Scholar]
- Paradiso AM, Coakley RD, Boucher RC. Polarized distribution of HCO3- transport in human normal and cystic fibrosis nasal epithelia. J Physiol. 2003;548:203–218. doi: 10.1113/jphysiol.2002.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Luck DJ. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii. Purification of two dyneins. J Biol Chem. 1979;254:3084–3090. [PubMed] [Google Scholar]
- Poulsen JH, Machen TE. HCO3-dependent pHi regulation in tracheal epithelial cells. Pflugers Arch. 1996;432:546–554. doi: 10.1007/s004240050168. [DOI] [PubMed] [Google Scholar]
- Ramirez MA, Toriano R, Parisi M, Malnic G. Control of cell pH in the T84 colon cell line. J Membr Biol. 2000;177:149–157. doi: 10.1007/s002320001108. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Kopito RR, Quinton PM. Cytosolic pH regulates GCl through control of phosphorylation states of CFTR. Am J Physiol. 1998;275:C1040–1047. doi: 10.1152/ajpcell.1998.275.4.C1040. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM. Rapid regulation of electrolyte absorption in sweat duct. J Membr Biol. 1994;140:57–67. doi: 10.1007/BF00234486. [DOI] [PubMed] [Google Scholar]
- Richmond PH, Vaughan-Jones RD. Assessment of evidence for K+-H+ exchange in isolated type-1 cells of neonatal rat carotid body. Pflugers Arch. 1997;434:429–437. doi: 10.1007/s004240050417. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Salathe M, Bookman RJ. Coupling of [Ca2+]i and ciliary beating in cultured tracheal epithelial cells. J Cell Sci. 1995;108:431–440. doi: 10.1242/jcs.108.2.431. [DOI] [PubMed] [Google Scholar]
- Salathe M, Bookman RJ. Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol. 1999;520:851–865. doi: 10.1111/j.1469-7793.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathe M, Lipson EJ, Ivonnet PI, Bookman RJ. Muscarinic signaling in ciliated tracheal epithelial cells: dual effects on Ca2+ and ciliary beating. Am J Physiol. 1997;272:L301–310. doi: 10.1152/ajplung.1997.272.2.L301. [DOI] [PubMed] [Google Scholar]
- Seybold ZV, Mariassy AT, Stroh D, Kim CS, Gazeroglu H, Wanner A. Mucociliary interaction in vitro: effects of physiological and inflammatory stimuli. J Appl Physiol. 1990;68:1421–1426. doi: 10.1152/jappl.1990.68.4.1421. [DOI] [PubMed] [Google Scholar]
- Singh SK, Binder HJ, Geibel JP, Boron WF. An apical permeability barrier to NH3/NH4+ in isolated, perfused colonic crypts. Proc Natl Acad Sci U S A. 1995;92:11573–11577. doi: 10.1073/pnas.92.25.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular calcium stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984;354:3–22. doi: 10.1113/jphysiol.1984.sp015397. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. Protein phosphorylation in mammalian spermatozoa. Reproduction. 2003;125:17–26. doi: 10.1530/rep.0.1250017. [DOI] [PubMed] [Google Scholar]
- van de Donk HJ, Zuidema J, Merkus FW. The influence of the pH and osmotic pressure upon tracheal ciliary beat frequency as determined with a new photo-electric registration device. Rhinology. 1980;18:93–104. [PubMed] [Google Scholar]
- Walev I, Martin E, Jonas D, Mohamadzadeh M, Muller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immunity. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Takano-Ohmuro H. Extensive skinning of cell membrane diminishes the force-inhibiting effect of okadaic acid on smooth muscles of guinea pig hepatic portal vein. Jpn J Physiol. 2002;52:141–147. doi: 10.2170/jjphysiol.52.141. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J Physiol. 1992;455:247–269. doi: 10.1113/jphysiol.1992.sp019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Zagoory O, Braiman A, Priel Z. The mechanism of ciliary stimulation by acetylcholine: roles of calcium, PKA, and PKG. J General Physiol. 2002;119:329–339. doi: 10.1085/jgp.20028519. [DOI] [PMC free article] [PubMed] [Google Scholar]