Abstract
The membrane potential changes following action potentials in thin unmyelinated cortical axons with en passant boutons may be important for synaptic release and conduction abilities of such axons. In the lack of intra-axonal recording techniques we have used extracellular excitability testing as an indirect measure of the after-potentials. We recorded from individual CA3 soma in hippocampal slices and activated the axon with a range of stimulus intensities. When conditioning and test stimuli were given to the same site the excitability changes were partly masked by local effects of the stimulating electrode at intervals < 5 ms. Therefore, we elicited the conditioning action potential from one axonal branch and tested the excitability of another branch. We found that a single action potential reduced the axonal excitability for 15 ms followed by an increased excitability for ∼200 ms at 24°C. Using field recordings of axonal action potentials we show that raising the temperature to 34°C reduced the magnitude and duration of the initial depression. However, the duration of the increased excitability was very similar (time constant 135 ± 20 ms) at 24 and 34°C, and with 2.0 and 0.5 mm Ca2+ in the bath. At stimulus rates > 1 Hz, a condition that activates a hyperpolarization-activated current (Ih) in these axons, the decay was faster than at lower stimulation rates. This effect was reduced by the Ih blocker ZD7288. These data suggest that the decay time course of the action potential-induced hyperexcitability is determined by the membrane time constant.
In the mammalian cortex most axons have varying conduction and release functions along most of their length. A typical example is the axon branches of rat hippocampal CA3 neurones innervating CA1 cells: the Schaffer collaterals. In the rat, they lack myelin, branch extensively (Ishizuka et al. 1990; Li et al. 1994), and have pre-synaptic specializations (en passant boutons) every 3–5 μm (Westrum & Blackstad, 1962; Shepherd et al. 2002) connected by thin axonal segments with an average diameter of 0.17 μm (Shepherd & Harris, 1998).
In such axons the modulation of synaptic and axonal conduction properties may be closely related. One factor that theoretically could influence these axonal functions is the membrane potential changes following the action potential. However, the occurrence and properties of after-hyperpolarization (AHP) and after-depolarization (ADP) are largely unknown for cortical axons because their small dimensions have rendered them inaccessible to intracellular voltage recordings. However for such axons there are many descriptions of decreased and increased excitability following action potentials, which may correspond to AHP and ADP, respectively (Gasser & Erlanger, 1930; Grundfest & Gasser, 1938; Greengard & Straub, 1958; Gardner-Medwin, 1972; Merrill et al. 1978; Low & Bement, 1980; Wigström & Gustafsson, 1981).
Most knowledge about after-potentials in axons and their terminals derives from experiments on larger axons that have their conducting and transmitter release functions anatomically separated. Such axons can have AHPs (Hodgkin & Huxley, 1939; Weidmann, 1951) and ADPs as well (Gasser & Erlanger, 1930; Grundfest & Gasser, 1938; Frankenhaeuser & Hodgkin, 1956; Greengard & Straub, 1958; Blight & Someya, 1985; Bowe et al. 1987).
Recordings from large pre-synaptic terminals in invertebrates and mammalian CNS have demonstrated that AHPs and ADPs can follow the action potentials (Marsal et al. 1997; Wojtowicz & Atwood, 1983, 1984; Forsythe, 1994; Borst & Sakmann, 1996, 1998; Geiger & Jonas, 2000; Poage & Zengel, 2002). Furthermore, such pre-synaptic after-potentials can influence transmitter release. This has been demonstrated in the chick ciliary ganglion (Poage & Zengel, 2002) and at the crayfish neuromuscular junction (Wojtowicz & Atwood, 1983, 1984; Blundon et al. 1995; Vyshedskiy & Lin, 1997) where small hyperpolarizing or depolarizing pulses applied before the action potential influenced transmitter release.
Our main motivation for investigating spike-induced excitability changes in the Schaffer collaterals is that there are similarities between the time courses of the increased excitability (Wigström & Gustafsson, 1981) and the synaptic facilitation at the synapses made by these fibres (Cragg & Hamlyn, 1955; Andersen, 1960). The maximum of both these phenomena occurs around 30 ms, at least at room temperature, and their decay seems similar when comparing the figures in the above-mentioned articles.
However, one important question is whether any of these phenomena are influenced by experimental factors that are non-physiological. It has not, for example, been clear whether the hyperexcitability occurs only when an electrode simultaneously activates many fibres. This is an important concern because extracellular K+ accumulation contributes to a supernormal period in both cerebellar parallel fibres (Greengard & Straub, 1958; Kocsis et al. 1983; Malenka et al. 1981, 1983) and in Schaffer collaterals (Poolos et al. 1987).
We have re-examined the excitability changes occurring up to half a second after an action potential in rat hippocampal Schaffer collaterals. Single unit recordings and improved stimulation methods were required to show that individual spikes were followed by an initially reduced and subsequently increased excitability.
Methods
Animals and slice preparation
All procedures used were in accordance with regulations given by The National Animal Research Authority in Norway. Wistar rats of both sexes (age, 4–12 weeks) were anaesthetized with di-ethyl ether. After respiratory cessation the brain was quickly removed and submerged in ice-cold artificial cerebrospinal fluid (ACSF). The hippocampus was dissected free and cut in 400 μm transverse slices with a Vibroslicer (Campden Instruments) in ice-cold, oxygenated ACSF. During recording, the slices were kept submerged in a 3-ml tissue chamber with oxygenated ACSF flowing at 3.5 ml min−1. The temperature was kept at either 23–25°C (nominally 24°C) or 34–36°C (nominally 34°C) with a TC2bip temperature controller (Cell MicroControls, Norfolk, VA, USA). For compound action potential and single unit recordings, excitatory potentials were blocked by bath application (2 mm) of the unspecific glutamate receptor antagonist kynurenic acid (Sigma Aldrich). The composition of the ACSF was (mm): NaCl 124, KCl 2.0, KH2PO4 1.25, MgSO4 2.0, NaHCO3 26, CaCl2 2.0 and glucose 11. This solution was constantly equilibrated with 95%O2– 5%CO2.
Stimulation and recording
Field EPSPs, compound action potentials and single CA3 soma discharges were recorded with glass pipettes filled with ACSF, having a tip diameter of ∼5 μm. Action potentials were elicited with an insulated tungsten stimulation cathode.
For recording of field EPSPs and compound action potentials the stimulation and recording electrodes were positioned within stratum radiatum of CA1, equidistant from the soma layer with 500–1000 μm between the electrodes (as in Fig. 1A).
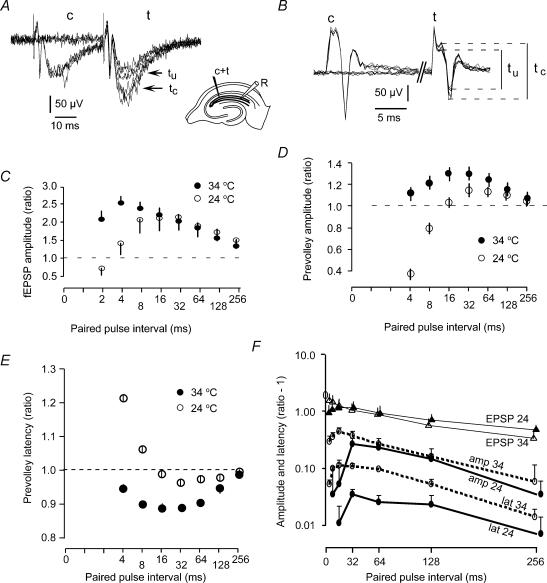
Figure 1. Paired-pulse facilitation and excitability changes at the CA3–CA1 synapse.
A, delivery of a conditioning pulse 32 ms prior to a test pulse of identical strength facilitated the test response. c, conditioning response; t, test response; tu and tc, unconditioned and conditioned test response, respectively. Three conditioned and three unconditioned responses are superimposed. The first biphasic response is the compound action potential, while the second negative wave is the field EPSP. Insert, electrode for conditioning and test stimulus (c + t) and recording electrode (R) in the Schaffer collateral area. B, the compound action potential response (tu) was increased (tc) when a stronger conditioning pulse (c) was presented 32 ms before the test pulse. Three traces with conditioning stimuli are superimposed on three without conditioning. c, t, tu, and tc, same as in A. C, time course of the paired-pulse facilitation of field EPSP (fEPSP) at 24°C (n = 7) and 34°C (n = 7). The ratio between conditioned and unconditioned test responses is plotted against the interstimulus interval (logarithmic axis). D, time course of the increase in compound action potential with stronger conditioning than test stimulus. At both 24°C (n = 7) and 34°C (n = 7) the increase in the amplitude of the compound action potential lasted for > 250 ms. An initial depression was observed at 24°C. E, time course of paired-pulse modulation of the latency of the compound action potential at 24°C (n = 5) and 34°C (n = 5). The ratio between conditioned and unconditioned test response latency was reduced for more than 100 ms following activity. At low temperature there was an initial latency increase. F, the data in C–E plotted with logarithmic y-axis and linear interval axis. One is subtracted from the ratios to make zero on the y-axis mean no change. The decay rates, estimated as the average decay in intervals 64–128 and 128–256 ms, were (in ms): −448 ± 74, −478 ± 36 (EPSP 24 and 34°C); −135 ± 24, −123 ± 13 (amplitude 24 and 34°C); −134 ± 28, −149 ± 20 (latency 24 and 34°C), respectively.
The Schaffer collaterals were either conditioned and tested by paired pulses of equal strength (duration, 140 μs), or conditioned by a pulse that was stronger than the test pulse (120 and 80 μs, respectively). The stimulation, given through an isolation unit, was adjusted in each experiment between 0.75 and 1.25 mA to give a compound action potential 5–15 times the peak-to-peak noise. The paired pulses were presented with increasing intervals (4, 8, 16, 32, 64, 128, 256, and 512 ms). Each pair was repeated at least four times with at least 2 s between the tests.
For single unit recordings the recording electrode was positioned in stratum pyramidale in CA3. Single CA3 units were activated anti-dromically by minimal stimulation of Schaffer collateral branches in stratum radiatum of CA1 and associational collaterals in stratum radiatum of the proximal CA3 (as in Fig. 3A). The single units were confirmed by controlling their all-or-none amplitude over a range of stimulation strengths, as previously described (Raastad, 1995). Collision tests determined whether the two anti-dromic responses originated from the same CA3 cell.
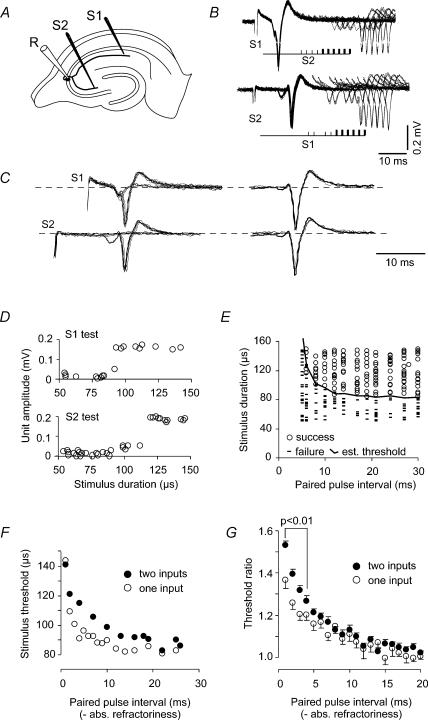
Figure 3. Single action potentials reduced the Schaffer axon excitability for 15 ms (at 24°C).
A, stimulation of two axonal branches (S1, S2) originating from a single CA3 pyramidal cell. B, collision tests confirmed that S1 and S2 originated from the same CA3 cell: a suprathreshold pulse given to one axonal branch (S1) caused a period of absolute refractoriness in the other input (S2), and vice versa. For both traces, the initial negative deflection represents the conditioning action potential, while the following peaks represent the response to stimuli at the test branch. Four stimulus intervals (thin bars) giving failures and six stimuli (thick bars) giving somatic discharge are shown. C, the somatic responses, both after S1 and S2 stimuli (left traces), were often mixed with responses from other smaller units. The background was isolated as the response that was left during the refractory periods. By averaging and subtracting this background, the somatic responses were very similar in shape, both from S1 and S2 stimuli (right traces). D, single unit recordings activated from two separate axonal branches show their all-or-none behaviour over a range of stimulation strengths. E, spike responses (○) and failures (–) of CA3 somata in response to Schaffer stimuli preceded by conditioning of a separate axonal branch. After the absolute refractory period (4 ms), the activation threshold was increased for another 10 ms. At each interstimulus interval, the activation threshold was estimated by calculating the average between the strongest stimulus giving failure and the weakest giving an anti-dromic action potential. F, estimated activation threshold for a Schaffer collateral branch following suprathreshold stimulation at the same (S1, ○) or a different axonal branch (S2, •). The threshold increase was smaller when both conditioning and test stimuli were given by the same electrode (S1). Both plots were horizontally shifted by subtracting the absolute refractory period. G, threshold change as a function of the paired-pulse interval. At all paired-pulse intervals the threshold change is given as a ratio between the conditioned and unconditioned thresholds. The period of increased threshold lasted up to 15 ms following a single action potential. During the first 4 ms the threshold increase was significantly lower (P < 0.01) when the suprathreshold pulse was delivered to the test branch, S1 (n = 20), compared to S2 (n = 33). At higher intervals the difference was not significant.
When we wanted to test a specific time interval by using separate-branch conditioning we added the conduction time between the conditioning electrode and soma, and the conduction time between the test electrode and soma, to the chosen interval. The assumption was that the axon branched relatively close to soma, which may not always have been the case, introducing an error in the test interval.
The activation threshold for the Schaffer collateral branch was determined by using a range of stimulation intensities. This is necessary in order to distinguish axonal activation failures from soma invasion failures. The current was kept constant and the pulse duration varied randomly within the range 80–140 μs. The threshold was defined as the mean value between the highest stimulus strength resulting in consistent soma failure and the lowest strength resulting in a regular response. The test stimulus was repeated at 0.5 Hz. Every other test was preceded by a suprathreshold conditioning pulse (constant strength) delivered to an associational collateral branch. The intervals between the conditioning and test pulses were varied between 4 and 50 ms, and for some tests kept constant at 30 ms plus the latency due to conduction in the associational branch.
All recordings were amplified with a DAM 50 differential amplifier (World Precision Instruments), filtered at 1 kHz, digitized at 10 kHz, and stored on the computer hard disk.
Measurements and statistical analysis
EPSP amplitudes were measured from the baseline to the most negative peak. For paired-pulse experiments at short intervals the amplitude of the tail of the first EPSP was subtracted from the amplitude of the second EPSP.
The amplitudes of the compound action potentials were measured from the initial positive to the most negative peak. Single unit amplitudes were measured from baseline to the most negative peak and were accepted only when they were > 3 times the peak-to-peak noise amplitude. Single unit and compound action potential latencies were measured from the start of the stimulus artefact to the most negative peak.
Unless noted otherwise, all measurements are given as mean and standard error of the mean (s.e.m.). In order to test differences of means, Student's t test, paired when relevant, was used.
Results
In the Schaffer collaterals the time course of the hyperexcitability following electrically activated action potentials (Wigström & Gustafsson, 1981) is similar to the time course of the excitatory synaptic facilitation (Andersen, 1960). In order to compare these time courses more accurately we estimated the paired-pulse modulation of synaptic efficacy at intervals between 5 ms and 500 ms at 24°C and 34°C. This time interval was chosen because the hippocampal pyramidal cells in vivo have spike intervals in this range (Renshaw et al. 1940; Ranck, 1973; Harris et al. 2001).
The time course of the modulation of synaptic efficacy and axonal excitability induced by conditioning stimuli
The stimulating and recording electrodes were positioned within the stratum radiatum of the CA1, at the same distance (∼100 μm) from the CA1 soma layer and with an interelectrode distance of 500 μm.
Figure 1A shows six traces where the Schaffer collaterals were activated every time by a test stimulus, and every other time also by a conditioning stimulus. The response to each stimulus consisted of a fast biphasic signal due to pre-synaptic action potentials and a slower downward signal corresponding to EPSPs. The conditioned test EPSP was larger than the unconditioned EPSP.
Figure 1B shows a similar experiment with same electrode positioning and test interval (32 ms), showing the effect of a conditioning stimulus on the compound action potential when all EPSPs were blocked by kynurenic acid. The conditioning stimulus was stronger than the test stimulus because, as described by others (Gardner-Medwin, 1972; Wigström & Gustafsson, 1981), the test response becomes larger only when preceded by a stronger stimulus. The interpretation presented by these authors is that a period of increased excitability induced by the first stimulus allows a larger population to be activated by the second weak stimulus than when no conditioning stimulus was applied.
The time courses of synaptic facilitation and axonal hyperexcitability at different time intervals at 24°C and 34°C is plotted in Fig. 1C and D, respectively. The time axis is logarithmic to facilitate evaluation at short and long intervals in the same plot. We notice certain similarities between these processes in that both last longer than 100 ms, that the maximal synaptic facilitation and axonal hyperexcitability occurs around the 32 ms interval at 24°C, and that the both processes became larger at intervals < 16 ms when the temperature was increased from 24 to 34°C.
Change in latency is often used as a measure for changes in excitability. Figure 1E shows that the latency change of the compound action potential followed a time course similar to the amplitude change (Fig. 1D), supporting the idea that both these measures reflect excitability.
Latency changes could theoretically be due to a change of the spike initiation point, but this is unlikely to contribute much here because there was a clear correlation between the initial latency (which was proportional to electrode distance) and the observed latency change (Pearson's correlation coefficient, r = 0.95; P < 0.001; n = 5; at 34°C), suggesting that changes in conduction velocity was the main reason for changes in latency.
The magnitude and decay of these phenomena are better compared using a linear time axis and a logarithmic axis for the relative effects (Fig. 1F). The hyperexcitability (evaluated as amplitude or latency change) was larger at 34°C than at 24°C. Of more importance, the hyperexcitability decayed with a strikingly similar rate at 24 and 34°C. The decay time constants (τ) between 64 and 256 ms were 135 ± 24 ms and 123 ± 13 ms for the amplitude at 24 and 34°C, respectively, and 134 ± 28 ms and 149 ± 20 ms for the latency at 24 and 34°C, respectively.
Compared to the axonal hyperexcitability, the EPSP facilitation decayed slower, with τ of 448 ± 74 and 477 ± 39 ms, at 24 and 34°C, respectively. This shows that there is not a simple linear relation between excitability and synaptic facilitation at these synapses.
Depression of the compound pre-synaptic action potential
The amplitude and latency changes around the 16 ms stimulation interval (Fig. 1D and E) suggest that both reduced and increased excitability contribute to the curves. The effect of reduced excitability on the amplitude measurement can be demonstrated by using two stimuli of equal strength (Fig. 2A). We never detected larger responses to the last of two equal stimuli, similar to earlier reports (Gardner-Medwin, 1972; Wigström & Gustafsson, 1981), suggesting that only the activated axons increase their excitability.
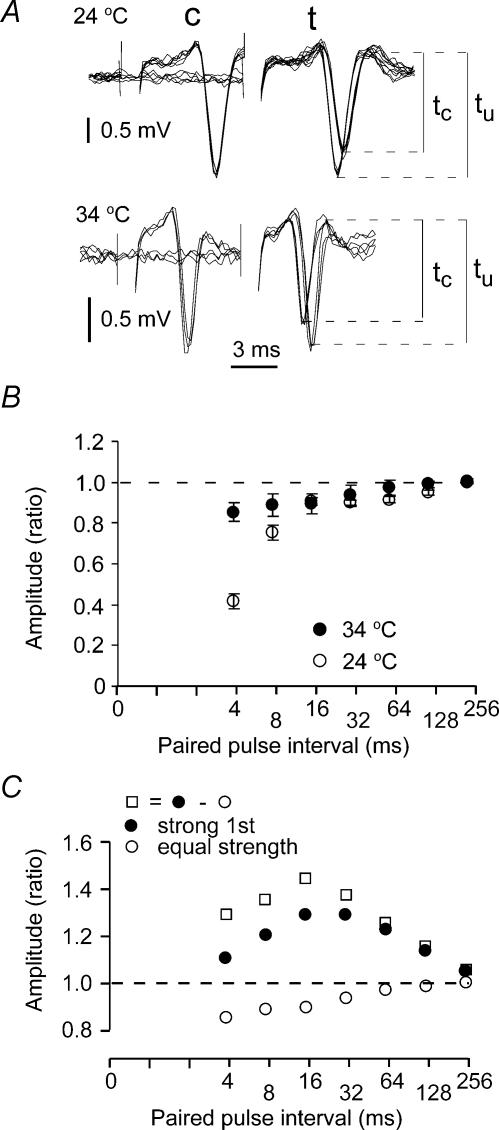
Figure 2. Paired-pulse modulation of the compound action potential.
A, a conditioning pulse 8 ms prior to a test pulse of identical strength prolonged the latency to the compound action potential at 24°C and reduced the latency at 34°C. The amplitude was reduced at both temperatures. c, conditioning response; t, test response; tu and tc, unconditioned and conditioned test response, respectively. Three conditioned and three unconditioned responses are superimposed. B, time course of the paired-pulse depression of the compound action potential amplitude at 24°C (n = 5) and 34°C (n = 5). The ratio between conditioned and unconditioned test response amplitudes is plotted against the interstimulus interval. C, time course of the estimated hyperexcitability following activity. The hyperexcitability effect is estimated by subtracting the overlapping depression illustrated in B from the response amplitudes in Fig. 1D (34°C).
The latency changes showed that a conditioning stimulus reduced the excitability at an interval of 10 ms (Fig. 2A) at 24°C, but increased the excitability at 34°C, as in Fig. 1D and E. However, the amplitude was reduced both at 24 and 34°C when two equal stimuli were used. This shows that the amplitude of the compound action potential is not an accurate measure for the excitability at intervals < 30 ms because factors other than excitability changes can change the amplitude.
The time course of the amplitude depression at 24°C and 34°C is shown in Fig. 2B. The absolute refractory period is probably the main depressing factor during the first 10 ms at 24°C, and the first 3–4 ms at 34°C, because it has been shown that these time periods fit well with the distribution of absolute refractory periods measured from single axon recordings (Raastad & Shepherd, 2003). In the present investigation we wanted to analyse the relative refractory period and have therefore excluded the first 10 ms in the analysis of the compound action potentials.
To use the amplitude change as a measure for excitability it would probably be most correct to compensate for the slowly decaying attenuation occurring after the absolute refractory period (Fig. 2B), and measure the amplitude increase in experiments with a strong conditioning stimulus (Fig. 1A) from an estimated baseline equal to this attenuation. Such an adjustment is shown in Fig. 2C for data at 34°C.
Single unit recordings confirm a period of reduced axonal excitability following the absolute refractory period
In order to measure the excitability change following immediately after the absolute refractory period it is necessary to record from individual units because both the conduction time (Soleng et al. 2003b) and the absolute refractory period (Raastad & Shepherd, 2003) differ for different axons. Therefore, we recorded extracellularly from individual pyramidal CA3 somata while activating selected axonal branches in the CA1 area.
Another problem is that the threshold at the stimulation point may change as a result of local, non-physiological factors such as K+ accumulation (Malenka et al. 1983). Therefore we compared two methods for single unit threshold detection. First, we activated the axon with conditioning and test stimuli delivered to the same point (S1 in Fig. 3A), and, second, we used a separate axonal branch for conditioning, usually in CA3 stratum radiatum in the hilus direction relative to the CA3 soma (S2 in Fig. 3A).
With two stimulating electrodes and collision tests (see Methods), we confirmed that the two tested branches belonged to the same neurone. Figure 3B shows a CA3 unit activated by S1 followed by S2 at intervals which started at 1 ms and increased with 1 ms steps. Below, the unit is activated from S2 followed by S1. For clarity, only four stimuli before the shortest successful stimulus and the following four successful stimuli are displayed. There was a long interval in which the second stimulus did not elicit somatic spikes. The only reasonable explanation is that the two stimuli interfered by activating the same neurone.
Background responses from other units were sometimes observed (Fig. 3B and C). However, the units we used always had amplitudes of three times the peak-to-peak amplitude of the background level. Also, when the background (obtained during the refractory period of the unit) was subtracted from the S1 and S2 responses (upper and lower traces of Fig. 3C), the responses had similar shapes (Fig. 3C, right), as expected for a single unit. The unitary nature of the soma response was also confirmed by spikes with all-or-none behaviour and stability in amplitude over a range of stimulus intensities (Fig. 3D).
The initial interval in which the second stimulus (at a separate branch) did not elicit any response was followed by an interval where the responses depended on the stimulation strength of the second stimulus. This relative refractory period was studied by giving a relatively strong and constant first stimulus followed by a second stimulus at different intervals and strengths. The result from one experiment is illustrated in Fig. 3E, where somatic responses are shown as circles and the failures as short bars. The first response required a strong stimulus. Weaker stimuli were sufficient to give responses at longer intervals. The weakest stimulus giving a response (threshold) is plotted against stimulus interval (absolute refractory period subtracted) as filled circles in Fig. 3F. The open circles give the corresponding threshold at the same intervals when both conditioning and test stimuli were given by the same electrode, showing a systematically lower threshold.
Figure 3G shows the average of all experiments. During the first 10–15 ms after the absolute refractory period there was a significantly increased threshold. This threshold increase was slightly larger when a separate axonal branch was used for the conditioning (n = 33) than when the same branch was used for both conditioning and testing (n = 20), significantly so for intervals < 4 ms (P < 0.01). The decay time constant of the increased threshold using different branch conditioning was 5.2 ms.
Single unit recordings with conditioning via a separate branch confirm that the long lasting hyperexcitability is induced by single action potentials
In order to avoid the possibility that synchronous activation of many axons at one point gave efflux of K+ or other neuroactive compounds reducing the threshold within this group of activated axons we used the same test method as described above, with conditioning and test stimuli delivered to two separate axonal branches.
Because the tests of the compound action potential showed that the threshold reduction was strongest around 30 ms we tested this interval only and used a range of intensities (x-axis in Fig. 4A) to determine the activation threshold for unconditioned and conditioned test pulses (upper and lower panels in Fig. 4A, respectively). The conditioning stimulus was in this case delivered to a branch within CA3 while a Schaffer collateral (in CA1) was tested. The conduction delays between each of these electrodes and soma was added to the 30 ms test interval to obtain approximately 30 ms between the arrival of the conditioning spike at the test electrode and the test stimulus. The threshold was clearly lower when there had been a previous action potential (Fig. 4A).
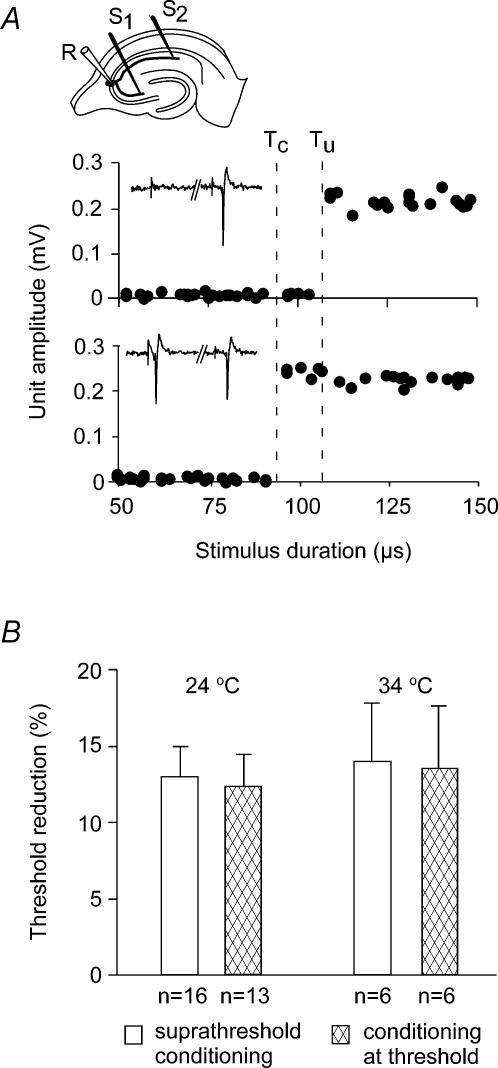
Figure 4. Activity-dependent threshold reduction in a single CA3 axonal branch conditioned by a separate branch.
A, a single CA3 unit, identified by its all-or-none response, was anti-dromically activated from two separate axonal branches, a Schaffer collateral and a CA3 associational branch. The Schaffer collateral activation threshold (Tu) was clearly reduced following a suprathreshold conditioning pulse (Tc) delivered to the separate branch. B, the threshold reduction at ∼30-ms interval was similar when comparing a constant suprathreshold conditioning to a conditioning of threshold strength (leading to intermittent failures) at 24°C (n = 16 and n = 13, respectively) and 34°C (n = 6 for both).
We also made experiments where the conditioning stimulus was kept constant at the threshold for activation, giving approximately 50% responses. We consider it likely that any local effect, such as K+ efflux, was relatively constant and uncorrelated with the firing of the single axon of the recorded cell. A comparison of the threshold for test stimuli with and without a preceding action potential showed that the threshold was reduced by 12.5 ± 2.0% and 13.6 ± 4.1% at 24°C (n = 13) and 34°C (n = 6), respectively. This was not significantly different from the results obtained with a suprathreshold conditioning stimulus (13.1 ± 1.9% and 14.0 ± 3.8% at 24°C (n = 16) and 34°C (n = 6), respectively, Fig. 4B. These results support the hypothesis that the observed reduction in activation threshold was the result of an action potential in individual axonal branches.
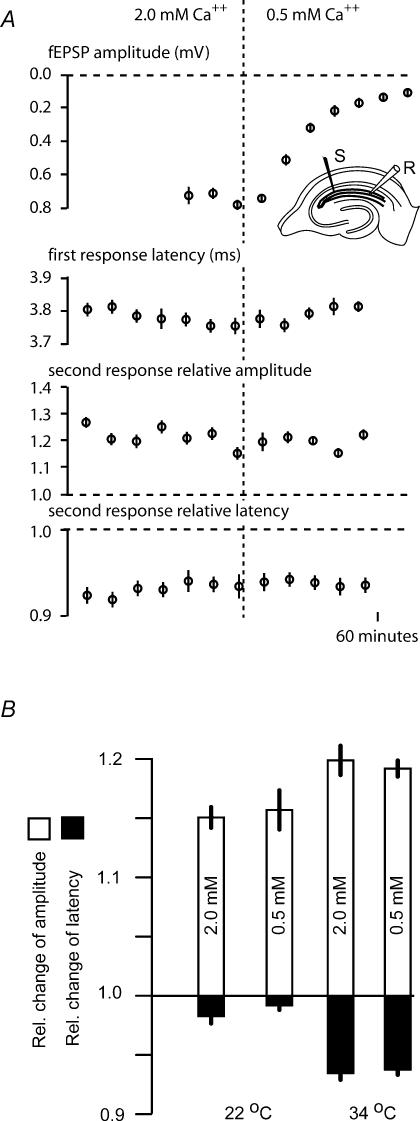
Changes in extracellular Ca2+ concentration did not change the hyperexcitability
To test the influence of Ca2+ currents on the hyperexcitability we compared the amplitude and latency of the conditioned and unconditioned compound action potential with 2.0 mm Ca2+−2.0 mm Mg2+ and 0.5 mm Ca2+−3.5 mm Mg2+ in the bath solution.
The low Ca2+ solution almost abolished the field EPSP after 20 min (Fig. 5A), but did not change the basal excitability, tested as the stability of the latency at 0.2 Hz stimulation rate (Fig. 5A, second plot).
Figure 5. The hyperexcitability was similar with 2.0 and 0.5 mm Ca2+ in extracellular solution.
A, while the field EPSP (fEPSP; first plot) was almost abolished by reducing the extracellular Ca2+ concentration from 2.0 to 0.5 mm, the latency of the compound action potential was not changed (second plot). The relative change in latency (third plot) and amplitude (fourth plot) in paired-pulse experiments (32 ms interval, with strong first stimulus) was not changed either. B, the average latency reduction and amplitude increase in five experiments similar to the one in A. The measurements were taken during the last 10 min with 2.0 mm Ca2+, and between 15 and 25 min after changing to 0.5 mm Ca2+. The hyperexcitability is a little stronger (more amplitude increase and latency decrease) at 34 than 24°C, but results are not significantly different with 2.0 or 0.5 mm Ca2+.
We then tested the spike-induced hyperexcitability as the latency and amplitude change of the compound action potential volley when a conditioning stimulus was given at 32-ms interval at 34°C. Switching to low Ca2+ solution did not change either the amplitude or the latency modulation (Fig. 5A, two lower plots).
Measuring the amplitude and latency change at 32-ms conditioning interval before and > 30 min after switching to low Ca2+ solution in five such experiments did not show any Ca2+-dependent effects (Fig. 5B).
From this we conclude that the hyperexcitability was not a result of transmitter release, and that transmembrane Ca2+ flux was not important for the recorded hyperexcitability.
Manipulating the decay rate of the hyperexcitability
In the reported experiments the hyperexcitability appeared as a rather constant phenomenon, larger at high than low temperature, but with unchanged decay time constant (Fig. 1F). Most pumps and ion channels work faster with increasing temperature, suggesting that the hyperexcitability is due to a passive repolarization of the late part of the action potential, and that it follows the membrane time constant.
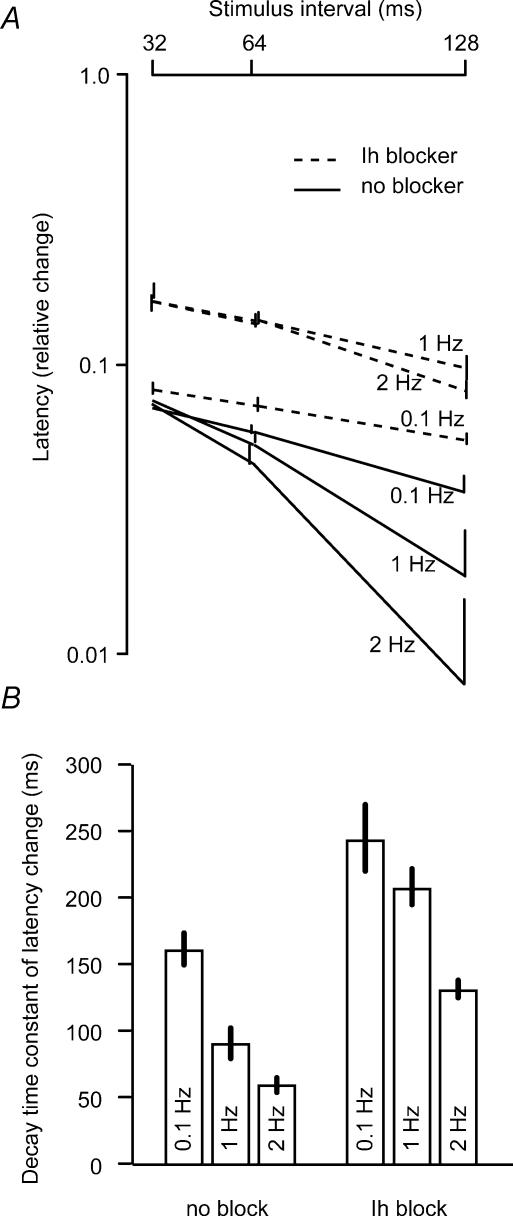
This suggestion may be tested by changing the membrane resistance. We were not successful using K+ channel blockers because low concentrations of 4-aminopyridine (4-AP) and TEA gave bursting behaviour of the CA3 cells, precluding interpretations of the effects of the conditioning stimulus. However, previous experiments had suggested that the Schaffer collateral axons activated Ih when repeatedly activated above 1 Hz (Soleng et al. 2003a). We therefore measured the decay rate of the hyperexcitability (as change in latency, 34°C) during 0.1, 1 and 2 Hz activation (Fig. 6A and B).
Figure 6. Manipulation of the decay rate of the hyperexcitability by stimulation frequency and Ih blocker.
A, the hyperexcitability was measured as the relative change in latency (logarithmic axis) induced by a conditioning stimulus at 32, 64 and 128 ms intervals at 34°C. The paired-pulse tests were repeated at 0.1, 1 and 2 Hz. The lowest frequency gave a decay similar to that in Fig. 1F, but the faster frequencies gave faster decays. With 25 μm ZD7288, the decays were slower at all frequencies (dashed lines). B, the average time constant of the decay of the latency change at the two intervals (32–64 and 64–128 ms) was reduced by increasing the stimulation frequency, and the decay was slower at all frequencies with the Ih blocker ZD7288.
The decay became faster with 1 Hz (90 ± 11 ms) compared to 0.1 Hz (159 ± 13 ms), and even faster with 2 Hz activation (59 ± 6 ms), without much change in peak latency change (at 32 ms interval, n = 14). The time constant was longer at all frequencies when Ih was reduced by addition of 25 μm ZD7288 (n = 11) 241 ± 27 ms, 205 ± 15 ms and 130 ± 7 ms at 0.1, 1.0 and 2 Hz, respectively, supporting the hypothesis that the decay was faster because of an activation of Ih.
Discussion
We have found that a single action potential in an individual Schaffer collateral axon is followed by excitability changes; an early reduction and a later increase of excitability. These findings are in agreement with earlier studies of excitability changes in autonomic nerves and Schaffer collaterals (Greengard & Straub, 1958; Kocsis et al. 1979; Wigström & Gustafsson, 1981; Bartesaghi, 1987) and the morphologically similar cerebellar parallel fibres (Gardner-Medwin, 1972).
Our main additional contribution is that we have reduced the number of possible interpretations and causes of these excitability changes. Furthermore, we argue that the time course of the hyperexcitability is given by the membrane time constant of the axon.
A period of reduced excitability
To distinguish the early period of reduced excitability following an action potential from the strong depression caused by the absolute refractory period it was necessary to study the threshold of individual axons. The amplitude of the compound action potential is relatively insensitive to small changes in threshold because, in this situation, most participating axons are activated with stimuli well above their threshold. Combined with the fact that the absolute refractory period may not be identical in all axons and that spike amplitude of individual axons is reduced for at least 10 ms after a conditioning stimulus (Raastad & Shepherd, 2003) it becomes clear that spike population measurements at short stimulation intervals are difficult to interpret.
With single unit recordings it was possible to adjust the stimulus strength to fit the threshold of an individual axonal branch, and to compensate for different latencies in different axonal branches (Soleng et al. 2003b). Using such methods, we could observe that the absolute refractory period was followed by a second period of reduced excitability decaying with a time constant of 5.2 ms. This is similar to mammalian C fibres (Grundfest & Gasser, 1938), but longer than reported for the cerebellar parallel fibres (Gardner-Medwin, 1972; Kocsis et al. 1979, 1983).
The threshold increase following individual spikes was somewhat less when the conditioning and test stimulus were given to the same point than when the conditioning stimulus was delivered to another axonal branch. This difference may be due to an increase in extracellular K+, but changes in Ca2+ or other neuroactive compounds cannot be excluded (Frankenhaeuser & Hodgkin, 1956; Malenka et al. 1983). The local effect of the conditioning stimulus lasted for only ∼5 ms and was small. However, this result may depend on the type of tissue and electrodes being used, and should therefore not be used to argue against longer lasting K+ accumulation in other kinds of experiments.
Causes of reduced excitability after spikes
The initially reduced excitability may be due to an AHP. Both peripheral unmyelinated axons (Frankenheuser & Hodgkin, 1956; Greengard & Straub, 1958) and some pre-synaptic terminals (Forsythe, 1994; Poage & Zengel, 2002) show AHPs after single action potentials.
The boutons on the Schaffer collaterals have action potential-activated K+ currents (Haas et al. 1983; Hu et al. 2001) and channels responsible for a medium long AHP in pyramidal cell somata are found also in some pre-synaptic terminals in hippocampal cultures (Obermair et al. 2003). This supports the hypothesis that the excitability reduction could be due to AHPs. Unfortunately, the K+ current blockers we tested gave spontaneous firing of CA3 units, preventing us from drawing conclusions from these experiments. It is therefore difficult to exclude alternative explanations for the reduced excitability, for example a long lasting Na+ channel inactivation in pre-synaptic terminals (Brody & Yue, 2000; He et al. 2002, Meeks & Mennerick, 2004).
A period of increased excitability
A supernormal period following an axonal action potential has been observed in a variety of axons (Gasser & Erlanger, 1930; Gasser & Grundfest, 1936; Grundfest & Gasser, 1938; Gardner-Medwin, 1972; Merrill et al. 1978; Low & Bement, 1980; Stöhr, 1981; Wigström & Gustafsson, 1981; Bartesaghi, 1987; Bowe et al. 1987).
The interpretation of the supernormal period in Schaffer collaterals (Wigström & Gustafsson, 1981) and cerebellar parallel fibres (Gardner-Medwin, 1972) was that the excitability was increased only for the activated axons. This conclusion was based on the finding that, during the increased excitability, two stimuli of equal strength seemed to activate the same number of fibres. However, the authors also considered the extracellular accumulation of K+ from a large activated fibre population as a possible explanation. This could be the case if the K+ accumulation was large enough to influence the membrane potential in the bundle of activated fibres, but too small to spread outside this bundle. The K+ accumulation hypothesis was strengthened by a correlation between extracellular K+ concentration and the threshold changes (Malenka et al. 1983).
Furthermore, blocking excitatory synaptic activity reduced the extracellular K+ concentration and the excitability in parallel (Malenka et al. 1981; Kocsis et al. 1983; Poolos et al. 1987). This observation led to the hypothesis that most activity-induced increase in K+ came from postsynaptic neurones, although an increased probability for retrogradely activated CA3 soma response after a conditioning stimulus of the Schaffer collaterals has been reported also during block of excitatory transmission (Storm & Lipowski, 1994).
These considerations show that an essential step in the analysis of the reduced axonal threshold is to determine whether it also occurs in individual axons following single action potentials. This can be done by intracellular soma recordings and anti-dromic activation of their axonal branches (Kocsis & VanderMaelen, 1979), but also by using a simpler extracellular approach as described above.
The experiment using constant threshold stimulation of an axonal branch far away from the test branch strongly supported the hypothesis that the supernormal period occurred after single action potentials in individual axons. The experimental conditions were constant and the response varied randomly between failure and success. A threshold reduction in the test branch was only observed following a conditioning spike, showing that it was the spike and not the stimulus that caused the threshold reduction. It is important that the threshold reduction was tested by applying a range of stimulus intensities, leading to the conclusion that it was the threshold at the stimulus site (the axon) and not the probability for anti-dromic action potential invasion of the soma that changed.
Causes of increased excitability after spikes
For some myelinated axons, intracellular voltage measurements have shown that individual spikes are followed by a membrane depolarization (Barrett & Barrett, 1982; Bowe et al. 1987) that may explain the reduced threshold after a spike. In such axons, a passive discharge of the membrane (Barrett et al. 1988; Davis et al. 1995) is the main contribution to the hyperexcitable period. However, a high resistance between the membrane and the myelin sheet is important to explain the slow decay, so this explanation cannot be directly used for the unmyelinated Shaffer collaterals in rat.
In peripheral nerves it is possible to measure intracellular voltage changes even in thin, unmyelinated axons by using grease- or sucrose-gap techniques. In such preparations, for example mammalian C fibres, the supernormal period (Grundfest & Gasser, 1938; Greengard & Straub, 1958) corresponds to a depolarization.
Both passive and active membrane mechanisms may give long-lasting depolarization. Although the time course of the supernormal period is similar to the decay of intracellular Ca2+ concentration after action potentials in neocortical pyramidal cell axons (Koester & Sakmann, 2000), the hyperexitability was not significantly changed when the bath concentration of Ca2+ was reduced from 2.0 to 0.5 mm. This makes it unlikely that Ca2+-mediated mechanisms were important. Presynaptic glutamate effects could not be important either, because the glutamate release was strongly reduced with such low Ca2+ concentrations.
The constant decay rate of the hyperexcitability at 24 and 34°C, and at 2.0 and 0.5 mm Ca2+ makes it unlikely that changes in membrane resistance, channel kinetics or extracellular Ca2+ concentration (Frankenhaeuser & Hodgkin, 1957; Malenka et al. 1983) were important factors in the hyperexcitability.
The membrane time constant, given by the presumably constant membrane capacitance and membrane resistance, seems to be the simplest explanation for the relatively temperature-insensitive decay time constant of about 135 ms at 24 and 34°C. Although the membrane resistance would not be constant over the temperature range (Hille, 2001), it usually changes much less than channel and pump kinetics. This suggestion was supported by the obvious shortening of the decay time constant at activation frequencies > 1 Hz. This effect was significantly reduced by the Ih blocker, supporting the hypothesis that Ih is activated as the axons hyperpolarize at frequencies > 1Hz (Soleng et al. 2003a).
The application of ZD7288 had a detectable effect on the decay time of the hyperexcitability even at 0.1 Hz, suggesting activation of Ih at rest (0.1 Hz activation). Is this compatible with our previous finding (Soleng et al. 2003a) where the latency changes during 0.5 Hz stimulus trains were not influenced by Ih block? One possibility is that the amplitude and latency changes are less sensitive to changes in Ih than the decay time of the hyperexcitability. An additional possibility is that a reduction of Ih can have two opposite effects on the conduction velocity: a tendency to increase the conduction velocity because of higher membrane resistance; and a tendency to slow the conduction because of hyperpolarization. The block of the depolarizing effect may dominate at more hyperpolarized potentials that are seen only at spike frequencies > 1 Hz.
If we assume that the hyperexcitability was due to an ADP, and that the decay of this ADP was due to passive repolarization, there are still unresolved questions about the cause of the initial part of the depolarization. One possibility is that fast repolarizing K+ currents were too small to restore the resting membrane potential. Another possibility is that depolarizing currents, for example Na+ currents with slow inactivation, were still active after the repolarizing K+ currents. Intracellular current and voltage control is probably necessary to distinguish between these alternatives. However, even in the somatic compartment the analysis of ADPs is complicated and a variety of underlying mechanisms have been shown (see Jung et al. 2001, and references therein).
Functional considerations
Action potential-induced changes of excitability and conduction velocity may have a different impact in different types of axons. Changes in conduction velocity may be important in long peripheral axons, but in the intrahippocampal short axons only small changes of the arrival time of the impulses at the synapses would be expected. Because pyramidal cell axons show few conduction failures (Frenguelli & Malinow, 1996; Mackenzie et al. 1996; Cox et al. 2000; Forti et al. 2000; Koester & Sakmann, 2000; Raastad & Shepherd, 2003), the theoretical reduction of such failures by increased excitability is probably of limited importance.
The most interesting interpretation of the threshold and conduction velocity changes after action potentials is that they are caused by hyperpolarizations and depolarizations because such afterpotentials may influence transmitter release.
Although the decay rate of the synaptic facilitation was slower than that of the hyperexcitability, a depolarization could theoretically contribute, for example to Ca2+ influx. Experiments from other preparations show that transmitter release can be influenced by small changes in the pre-synaptic potential. In the crayfish neuromuscular synapse, 7–15 mV depolarizing pre-pulses dramatically increased the transmitter release (Wojtowicz & Atwood, 1983), but in the squid giant synapse such pulses have the opposite effect (Miledi & Slater, 1966). Also in the pre-synaptic terminals of the chick ciliary ganglion the AHP is important for transmitter release. With repeated action potentials the AHP is reduced in amplitude and the postsynaptic potential increases in amplitude with a parallel time course (Poage & Zengel, 2002).
The fact that the Schaffer collaterals and other unmyelinated cortical axons have pre-synaptic boutons spaced only 3–5 μm apart along the entire axonal path (Westrum & Blackstad, 1962; Shepherd & Harris, 1998) makes the excitability changes relevant for the analysis of both axon conduction and synaptic properties.
References
- Andersen P. Interhippocampal impulses II. Apical dendritic activation of CA1 neurons. Acta Physiol Scand. 1960;48:178–208. doi: 10.1111/j.1748-1716.1960.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Morita K, Scappaticci KA. Effects of tetraetylammonium on the depolarizing after-potential and passive properties of lizard myelinated axons. J Physiol. 1988;402:65–78. doi: 10.1113/jphysiol.1988.sp017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartesaghi R. Supernormal excitability of fibers of the dorsal hippocampal commissure. Exp Neurol. 1987;96:208–213. doi: 10.1016/0014-4886(87)90182-8. [DOI] [PubMed] [Google Scholar]
- Blight AR, Someya S. Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Neuroscience. 1985;15:1–12. doi: 10.1016/0306-4522(85)90118-6. [DOI] [PubMed] [Google Scholar]
- Blundon JA, Wright SN, Brodwick MS, Bittner GD. Presynaptic calcium-activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995;73:178–189. doi: 10.1152/jn.1995.73.1.178. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:393–394. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium current during a single action potential in large presynaptic terminal of the rat brainstem. J Physiol. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe CM, Kocsis JD, Waxman SG. The association of the supernormal period and the depolarizing afterpotential in myelinated frog and rat sciatic nerve. Neuroscience. 1987;21:585–593. doi: 10.1016/0306-4522(87)90144-8. [DOI] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci. 2000;20:2480–2494. doi: 10.1523/JNEUROSCI.20-07-02480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Denk W, Tank DW, Svoboda K. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc Natl Acad Sci U S A. 2000;97:9724–9728. doi: 10.1073/pnas.170278697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg BG, Hamlyn LH. Action potentials of the pyramidal neurones in the hippocampus of the rabbit. J Physiol. 1955;129:608–627. doi: 10.1113/jphysiol.1955.sp005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G, Modney B, Scappaticci KA, Barrett JN, Barrett EF. Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J Physiol. 1995;489:141–157. doi: 10.1113/jphysiol.1995.sp021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti L, Pouzat C, Llano I. Action potential-evoked Ca2+ signals and calcium channels in axons of developing rat cerebellar interneurones. J Physiol. 2000;527:33–48. doi: 10.1111/j.1469-7793.2000.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956;131:341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli BG, Malinow R. Fluctuations in intracellular calcium responses to action potentials in single en passage presynaptic boutons of layer V neurons in neocortical slices. Learn Mem. 1996;3:150–159. doi: 10.1101/lm.3.2-3.150. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin AR. An extreme supernormal period in cerebellar parallel fibres. J Physiol. 1972;222:357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser HS, Erlanger J. The ending of the action potential and its relation to other events in nerve activity. Am J Physiol. 1930;94:247–277. [Google Scholar]
- Gasser HS, Grundfest H. Action and excitability in mammalian A fibers. Am J Physiol. 1936;117:113–133. [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Greengard P, Straub RW. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958;144:442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundfest H, Gasser HS. Properties of mammalian nerve fibers of slowest conduction. Am J Physiol. 1938;123:307–318. [Google Scholar]
- Haas HL, Wieser HG, Yasargil MG. 4-Aminopyridine and fiber potentials in rat and human hippocampal slices. Experientia. 1983;39:114–115. doi: 10.1007/BF01960661. [DOI] [PubMed] [Google Scholar]
- Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsaki G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron. 2001;32:141–149. doi: 10.1016/s0896-6273(01)00447-0. [DOI] [PubMed] [Google Scholar]
- He Y, Zorumski CF, Mennerick S. Contribution of presynaptic Na(+) channel innactivation to paired-pulse synaptic depression in cultured hippocampal neurons. J Neurophysiol. 2002;82:925–936. doi: 10.1152/jn.00225.2001. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, Massachusets: Sinauer Associates Inc; 2001. [Google Scholar]
- Hodgkin AL, Huxley AF. Action potentials recorded from inside a nerve fibre. Nature. 1939;144:710–711. [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jung H-Y, Staff NP, Spruston N. Action potential bursting in subicular pyramidal neurons is driven by a calcium tail current. J Neurosci. 2001;21:3312–3321. doi: 10.1523/JNEUROSCI.21-10-03312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Malenka RC, Waxman SG. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J Physiol. 1983;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Swadlow HA, Waxman SG, Brill MH. Variation in conduction velocity during the relative refractory and supernormal periods: a mechanism for impulse entrainment in central axons. Exp Neurol. 1979;65:230–236. doi: 10.1016/0014-4886(79)90263-2. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, VanderMaelen CP. A supernormal period in central axons following single cell stimulation. Exp Brain Res. 1979;36:381–386. doi: 10.1007/BF00238919. [DOI] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Low WC, Bement SL. Enhancement of afferent fiber activity in hippocampal slices. Brain Res. 1980;198:472–477. doi: 10.1016/0006-8993(80)90763-5. [DOI] [PubMed] [Google Scholar]
- Mackenzie PJ, Umemiya M, Murphy TH. Ca2+ imaging of CNS axons in culture indicates reliable coupling between single action potentials and distal functional release sites. Neuron. 1996;16:783–795. doi: 10.1016/s0896-6273(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD, Ransom BR, Waxman SG. Modulation of parallel fiber excitability by postsynaptically mediated changes in extracellular potassium. Science. 1981;214:339–341. doi: 10.1126/science.7280695. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD, Waxman SG. The supernormal period of the cerebellar parallel fibers effects of [Ca2+]o and [K+]o. Pflugers Arch. 1983;397:176–183. doi: 10.1007/BF00584354. [DOI] [PubMed] [Google Scholar]
- Marsal J, Ruiz-Montasell B, Balsi J, Moreira JE, Contreras D, Sugimori M, Llinás R. Block of transmitter release by botulinum C1 action on syntaxin at the squid giant synapse. Proc Natl Acad Sci U S A. 1997;94:14871–14876. doi: 10.1073/pnas.94.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J Neurosci. 2004;24:197–206. doi: 10.1523/JNEUROSCI.4845-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Wall PD, Yaksh TL. Properties of two unmyelinated fibre tracts of the central nervous system: lateral Lissauer tract, and parallel fibres of the cerebellum. J Physiol. 1978;284:127–145. doi: 10.1113/jphysiol.1978.sp012531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Slater CR. The action of calcium on neuronal synapses in the squid. J Physiol. 1966;184:473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Kaufmann WA, Knaus HG, Flucher BE. The small conductance Ca2+-activated K+ channel SK3 is localized in nerve terminals of excitatory synapses of cultured mouse hippocampal neurons. Eur J Neurosci. 2003;17:721–731. doi: 10.1046/j.1460-9568.2003.02488.x. [DOI] [PubMed] [Google Scholar]
- Poage RE, Zengel JE. Repolarization of the presynaptic action potential and short-term synaptic plasticity in the chick ciliary ganglion. Synapse. 2002;46:189–198. doi: 10.1002/syn.10135. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Mauk MD, Kocsis JD. Activity-evoked increase in extracellular potassium modulates presynaptic excitability in the CA1 region of the hippocampus. J Neurophysiol. 1987;58:404–416. doi: 10.1152/jn.1987.58.2.404. [DOI] [PubMed] [Google Scholar]
- Raastad M. Extracellular activation of unitary excitatory synapses between hippocampal CA3 and CA1 pyramidal cells. Eur J Neurosci. 1995;7:1882–1888. doi: 10.1111/j.1460-9568.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Raastad M, Shepherd GM. Single-axon action potentials in the rat hippocampal cortex. J Physiol. 2003;548:745–752. doi: 10.1113/jphysiol.2002.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Renshaw B, Forbes A, Morison BR. Activity of isocortex and hippocampus: electrical studies with microelectrodes. J Neurophysiol. 1940;3:74–105. [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Raastad M, Andersen P. General and variable features of varicosity spacing along unmyelinated axons in the hippocampus and cerebellum. Proc Natl Acad Sci U S A. 2002;99:6340–6345. doi: 10.1073/pnas.052151299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Chiu K, Raastad M. Unmyelinated axons in rat hippocampus hyperpolarize and activate an H-current when spike frequency exceeds 1 Hz. J Physiol. 2003a;15:459–470. doi: 10.1113/jphysiol.2003.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Raastad M, Andersen P. Conduction latency along CA3 hippocampal axons from rat. Hippocampus. 2003b;13:953–961. doi: 10.1002/hipo.10141. [DOI] [PubMed] [Google Scholar]
- Stöhr M. Activity-dependent variations in threshold and conduction velocity of human sensory fibers. J Neurol Sci. 1981;49:47–54. doi: 10.1016/0022-510x(81)90187-8. [DOI] [PubMed] [Google Scholar]
- Storm JF, Lipowski R. Evidence that excitability changes in presynaptic fibers may affect paired-pulse facilitation in hippocampal slices. Soc Neurosci Abstr. 1994;20:42. [Google Scholar]
- Vyshedskiy A, Lin JW. Activation and detection of facilitation as studied by presynaptic voltage control at the inhibitor of the crayfish opener muscle. J Neurophysiol. 1997;77:2300–2315. doi: 10.1152/jn.1997.77.5.2300. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical characteristics of Sepia axons. J Physiol. 1951;114:372–381. doi: 10.1113/jphysiol.1951.sp004628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum LE, Blackstad TW. An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA1) with particular emphasis on synaptology. J Comp Neurol. 1962;119:281–309. doi: 10.1002/cne.901190303. [DOI] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B. Increased excitability of hippocampal unmyelinated fibres following conditioning stimulation. Brain Res. 1981;229:507–513. doi: 10.1016/0006-8993(81)91013-1. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Atwood HL. Maintained depolarization of synaptic terminals facilitates nerve-evoked transmitter release at a crayfish neuromuscular junction. J Neurobiol. 1983;14:385–390. doi: 10.1002/neu.480140506. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Atwood HL. Presynaptic membrane potential and transmitter release at the crayfish neuromuscular junction. J Neurophysiol. 1984;52:99–113. doi: 10.1152/jn.1984.52.1.99. [DOI] [PubMed] [Google Scholar]