Abstract
N- and P/Q-type Ca2+ channels are abundant in nerve terminals where they interact with proteins of the release apparatus, including syntaxin 1A and SNAP-25. In previous studies on N- or P/Q-type Ca2+ channels, syntaxin 1A co-expression reduced current amplitudes, increased voltage-dependent inactivation and/or enhanced G-protein inhibition. However, these studies were conducted in Ca2+ channels that exhibited significant voltage-dependent inactivation. We previously reported that N-type current in bovine chromaffin cells exhibits very little voltage-dependent inactivation and we identified the Ca2+ channel subunits involved. This study was undertaken to determine the effect of syntaxin 1A on this weakly inactivating Ca2+ channel. Co-expression of syntaxin 1A with the weakly inactivating bovine N-type Ca2+ channels in Xenopus oocytes did not appear to alter inactivation but dramatically reduced current amplitudes, without changing cell surface expression. To further understand the mechanisms of syntaxin 1A regulation of this weakly inactivating channel, we examined mutants of the α1B subunit, β2a subunit and syntaxin 1A. We determined that the synprint site of α1B and the C-terminal third of syntaxin 1A were necessary for the reduced current amplitude. In addition we show that enhanced G-protein-dependent modulation of the Ca2+ current by syntaxin 1A cannot explain the large suppression of Ca2+ current observed. Of most significance, syntaxin 1A increased voltage-dependent inactivation in channels containing mutant β2a subunits that cannot be palmitoylated. Our data suggest that changes in inactivation can not explain the reduction in current amplitude produced by co-expressing syntaxin and a weakly inactivating Ca2+ channel.
N- and P/Q-type Ca2+ channels (Cav2.2 and Cav2.1, respectively) are known to regulate neurotransmitter release (Hirning et al. 1988; Horne & Kemp, 1991; Luebke et al. 1993; Turner et al. 1993; Takahashi & Momiyama, 1993; Wheeler et al. 1994) and to bind to the SNARE proteins syntaxin 1A and SNAP-25 (Bennett et al. 1992; Leveque et al. 1994). The physical interaction between Ca2+ channels and SNARE proteins promotes functional interaction. Not only do Ca2+ channels initiate neurotransmitter release but SNARE proteins, in turn, modify Ca2+ channel function. For example, co-expression of syntaxin 1A with N-, P/Q- and in some cases, L-type Ca2+ channels results in decreased current amplitude, increased voltage-dependent inactivation and/or enhanced Gβγ-dependent inhibition (Bezprozvanny et al. 1995, 2000; Wiser et al. 1996, 1999; Jarvis et al. 2000; Trus et al. 2001; Jarvis & Zamponi, 2001; Jarvis et al. 2002). The modulation of Ca2+ channel function by syntaxin 1A has also been demonstrated in nerve terminals. Stanley & Mirotznik (1997) demonstrated that botulinum toxin, which cleaves syntaxin 1A, prevents G-protein-dependent modulation of pre-synaptic Ca2+ channels and induces a modest change in inactivation (Stanley, 2003). Although the mechanism(s) by which syntaxin 1A exerts its effects on Ca2+ channels are not well understood, it is likely that these types of interactions may have significant consequences for Ca2+ channel signalling and perhaps neurotransmitter release.
Previous studies that examined the effects of syntaxin 1A on Ca2+ channel function have been done largely with Ca2+ channels that exhibit significant voltage-dependent inactivation. Voltage-dependent inactivation, which is characterized by ever diminishing Ca2+ influx during prolonged depolarizations or in decreased channel availability at depolarized holding potentials, is an important form of Ca2+ channel regulation. Although the majority of neuronal N-type currents inactivate, non-inactivating (or weakly inactivating) currents have been described in bovine chromaffin cells and in pre-synaptic chick ciliary ganglion terminals (Stanley & Goping, 1991; Artalejo et al. 1992; Cahill et al. 2000). We previously showed that bovine α1B and β2a subunits expressed with α2δ subunit mimic the native ‘non-inactivating’ currents seen in chromaffin cells (Cahill et al. 2000).
Studies with inactivating Ca2+ channels found that syntaxin 1A promoted channel inactivation and greatly reduced peak current amplitude (Bezprozvanny et al. 1995, 2000; Wiser et al. 1996; Jarvis & Zamponi, 2001; Trus et al. 2001). We were interested to determine whether syntaxin 1A would have similar effects on weakly inactivating N-type channels. To address this question we have co-expressed syntaxin 1A and weakly inactivating N-type Ca2+ channels in Xenopus oocytes and tsA-201 cells and examined the effects of syntaxin 1A on Ca2+ currents. We found that peak current amplitude was greatly reduced but that inactivation was unaltered. We have also studied mutant forms of both the α1B Ca2+ channel subunit and syntaxin 1A in order to examine the mechanistic basis of the effects of syntaxin 1A on weakly inactivating N-type Ca2+ channel currents. Both mutations prevented syntaxin 1A from inhibiting the Ca2+ current. To determine whether the reduction in peak Ca2+ current observed with syntaxin 1A was due to modulation of channel properties or to a decrease in the number of N-type Ca2+ channels present in the membrane, we used binding of 125I-labelled ω-conotoxin GVIA to assess channel number. Our data show that the channel number was not affected.
Methods
All animal procedures were approved by the Animal Care and use Committee at Indiana University School of Medicine. Frogs were humanely killed after the last oocyte collection.
Clones
The bovine α1B (GenBank accession number AF173882), β2a (AF174417) and β1b (AF174415) Ca2+ channel subunit cDNAs were cloned from a bovine chromaffin cell library as previously described (Cahill et al. 2000). All bovine Ca2+ channel subunit cDNAs were subcloned into pcDNA3.1(+) for expression studies. The mutant β2a(Cys3,4Ser) subunit cDNA was a kind gift from Dr M. Hosey (Northwestern University). The human α2δ subunit was a generous gift from Dr R. J. Miller (Northwestern University).
Mutagenesis of α1B and syntaxin clones
The region of bovine α1B coding for the synprint site was deleted using the ‘splicing-by-overlap-extension’ method (Horton et al. 1989). The region surrounding the splice site was sequenced to confirm that the desired deletion had been accomplished without introducing any unintended mutations. This synprint-site deletion mutant is designated α1BΔ717-954 because it lacks amino acids 717–954 which correspond to the synprint site defined by Rettig et al. (1996).
The H3 helical domain and membrane-spanning carboxyl terminus of rat syntaxin 1A were deleted by replacing the codon for glutamine-190 in syntaxin 1A cDNA with a stop codon using standard PCR techniques. A plasmid containing the coding sequence for rat syntaxin 1A (a kind gift from Dr R. W. Tsien, Stanford University) was used as the template. The PCR product was digested with restriction enzymes and ligated into pcDNA3.1(+)/Neo (Invitrogen, Carlsbad, CA, USA). The construct, referred to as syntrunc, was sequenced to confirm the presence of the stop codon and the absence of any PCR errors.
Xenopus oocyte expression and electrophysiology
Ca2+ channel proteins were expressed in Xenopus oocytes after injection of in vitro-transcribed mRNAs. Full-length cDNAs of each of the cloned subunits were used to generate mRNA from the T7 RNA polymerase promoter using an in vitro transcription kit (mMessage Machine; Ambion, Austin, TX). Oocytes were harvested from mature Xenopus laevis female frogs and separated from follicle cells with 2 mg ml−1 collagenase type IA (Sigma, St. Louis, MO). Oocytes were injected with 25 ng of each Ca2+ channel subunit mRNA and syntaxin 1A mRNA (5 ng) as indicated in a total of 50 nl DEPC-treated H20 using a Drummond automatic microinjector. Two-electrode voltage clamp experiments were conducted four and five days after RNA injection. In some experiments, syntaxin 1A mRNA (5 ng) was injected 3 days after Ca2+ channel subunit mRNAs. In these cases, experiments were conducted 48 h after syntaxin 1A injection. Oocytes were voltage-clamped at various potentials with two glass electrodes filled with 3 m KCl and having a resistance of 0.5–1.5 m Ω using an Axoclamp 2 A (Axon Instruments, Foster City, CA). Oocytes were initially superfused with ND96 (96 mm NaCl, 1.8 mm CaCl2, 2 mm KCl, 1 mm MgCl2 and 5 mm Hepes, pH 7.6) and N-type Ca2+ currents were measured in a Ba2+ recording solution [40 mm Ba(OH)2, 25 mm tetraethylammonium (TEA-OH), 25 mm NaOH, 2 mm CsOH and 5 mm Hepes, pH adjusted to 7.5 with methanesulphonic acid] designed to minimized the oocyte's endogenous Ca2+-dependent Cl− current. Membrane currents were recorded on a computer using pCLAMP software (Axon Instruments).
Ionic currents were elicited by step depolarizations of 50–200 ms duration from various holding potentials. Leak currents were subtracted on-line with a P/4 protocol. Inactivation was assessed by varying the holding potential. Each new holding potential was maintained for 60 s prior to depolarizing the oocyte. Inactivation of N-type Ca2+ channels is complicated with both fast and slow inactivation. In addition, there is a Ca2+-dependent component to inactivation. Sixty seconds at each holding potential will most likely not reveal true ‘steady-state’ channel availability. It is also possible that some form of ultra slow inactivation may have been missed. But we believe that the most likely possibility is that this combination of channel subunits shows little fast or slow inactivation. Even though slow inactivation can take minutes to reach steady state (Degtiar et al. 2000), it becomes apparent in just seconds, at depolarized potentials. Thus, if it were present, there should have been more evidence of slow inactivation when cells were held at either −40 mV or −30 mV for 60 s.
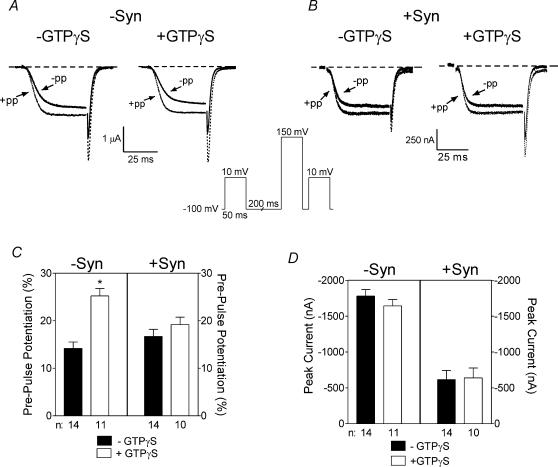
To indirectly assess the role of Gβγ in syntaxin 1A's effects, pre-pulse protocols (see Fig. 6) consisting of 50 ms steps to +10 mV before and 15 ms after a 50 ms step to 150 mV were used. In pre-pulse experiments, oocytes were injected with BAPTA (to a final concentration of 1–2 mm) or BAPTA and GTPγS (to a final concentration of 100–200 μm) 10 min before recording.
Figure 6. Effect of syntaxin 1A and GTPγS on pre-pulse potentiation in oocytes expressing weakly inactivating N-type channels.
A and B, pre-pulse protocol and representative current traces. The inset depicts the pre-pulse protocol that utilizes a 50 ms test pulse to +10 mV before and 15 ms after a 50 ms depolarizing pulse to +150 mV. A, the current traces are examples from oocytes expressing α1B, β2a and α2δ without syntaxin 1A and in the absence or presence of injected GTPγS as indicated. Our analysis program (pClamp) determined the peak current for the current preceded by the pre-pulse. Then the current with no pre-pulse was measured isochronally (please note that peak current occurred towards the end of the depolarization). B, the current traces are from oocytes expressing α1B, β2a and α2δ in the presence of syntaxin 1A and in the absence or presence of injected GTPγS as indicated. Continuous lines indicate the currents before the pre-pulse and dotted lines indicate the currents after the pre-pulse. C, comparison of percentage facilitation in oocytes expressing bovine α1B, β2a and α2δ in the absence or presence of syntaxin 1A and with or without injection of GTPγS as indicated. Data are calculated as the percentage change in peak current size after the pre-pulse compared to before the pre-pulse. D, comparison of peak current size in oocytes expressing bovine α1B, β2a and α2δ in the absence or presence of syntaxin 1A and with or without injection of GTPγS as indicated. Peak current size is measured during the first test depolarization before the pre-pulse. −syn, without syntaxin 1A; +syn, with syntaxin 1A.
125I-labelled ω-conotoxin GVIA binding in Xenopus oocytes and tsA-201 cells
125I-labelled ω-conotoxin GVIA binding was conducted on intact non-injected (control) oocytes or oocytes expressing N-type channels in the absence or presence of syntaxin 1A. Oocyte binding was performed at room temperature in Ca2+-free ND96 containing 1 mg ml−1 bovine serum albumin. All oocytes (5 per group) were pre-incubated for 15 min at room temperature. Half of the oocytes were incubated for an additional 30 min in 100 nm ω-conotoxin GVIA to define non-specific binding, before incubating for 90 min in various concentrations of 125I-labelled ω-conotoxin GVIA in the absence or presence of 100 nm ω-conotoxin GVIA. Oocytes were rinsed three times for 2 min each in cold Ca2+-free ND96, before gamma counting. This experiment was repeated with oocytes from a different donor and the results were pooled. tsA-201 cells were transfected with α1B, β2a and α2δ cDNAs with and without syntaxin 1A cDNA using Lipofectamine Plus at a ratio of 3 : 1 : 3 : 1. After 3 days later, cells were gently removed from tissue culture plates and counted. Trypan Blue was used to verify that cells were intact. Equivalent numbers of cells were incubated at 37°C for 1 hour with various concentrations of 125I-labelled ω-conotoxin GVIA as previously described (Williams et al. 1992). The binding assay buffer contained (mm): Hepes 10, NaCl 140, KCl 5, glucose 12 and 1 mg ml−1 BSA; pH 7.4. The cells were washed three times in buffer containing (mm): Hepes 5, choline chloride 160, CaCl2 1.5 and 1 mg ml−1 BSA; pH 7.4. Non-specific binding was defined with 100 nmω-conotoxin GVIA. Binding data were analysed by non-linear regression. Data are presented as mean ± s.e.m. of triplicates.
Data analysis
For each experiment all conditions were completed (± syntaxin, ± GTPγS) in individual batches of oocytes and replicated at least three times in oocytes from different donors to confirm the reproducibility of the findings. For inactivation curves, currents from each cell at each holding potential were normalized to the peak current at the most hyperpolarized holding potential. The normalized data were averaged across cells and plotted as a function of holding potential. For purposes of illustration, some data were fitted with Boltzmann functions; however, the data from weakly inactivating currents were not well-fit by Boltzmann functions. Therefore, to analyse inactivation characteristics in the presence or absence of syntaxin 1A, we compared current size elicited from a holding potential of −60 mV to that produced from a holding potential of −120 mV and displayed this as percentage inactivation in the bar graphs. The peak current size as plotted in the bar graphs is defined as the peak Ca2+ current (ICa) elicited from the most hyperpolarized potential in the inactivation protocol and the data were averaged across cells. Pre-pulse potentiation is defined as (I−Pre− I+Pre)/I−Pre and expressed as a percentage (I−Pre is the current measured in the absence of a prepulse and I+Pre is the current measured after a prepluse). Unpaired student's t test was used to compare groups. Endogenous currents were approximately 50–75 nA in these solutions and therefore oocytes producing currents smaller than 200 nA were excluded from analysis.
Results
Effects of syntaxin 1A on inactivating N-type Ca2+ channels
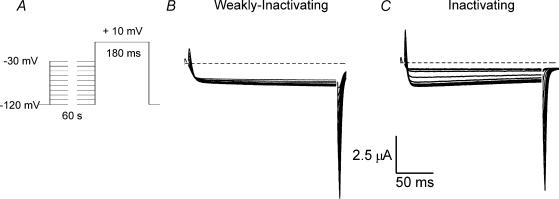
Previous studies have shown that syntaxin 1A enhances inactivation when expressed with N- or P/Q-type Ca2+ channels that exhibit significant voltage-dependent inactivation. We previously cloned an α1B (N-type) Ca2+ channel subunit and several β subunits from a bovine chromaffin cell library and determined that expression of the bovine α1B and β2a subunits with an α2δ subunit produced currents mimicking the non-inactivating N-type currents from chromaffin cells (Cahill et al. 2000). Channels constructed with other bovine chromaffin cell β subunits exhibited significant inactivation. Figure 1 shows examples of both inactivating and weakly inactivating N-type currents observed in oocytes expressing bovine α1B, human α2δ and different bovine β subunits. For each current record shown the test potential was constant at +10 mV, while the holding potential was changed in the range from −120 mV to −30 mV (Fig. 1A). Each holding potential was maintained for 60 s before depolarizing the oocyte. Weakly inactivating currents were recorded when β2a was expressed by the oocyte (Fig. 1B), while inactivating currents were observed when β1b subunits were expressed (Fig. 1C). Channel availability was not tested at very depolarized potentials (more than −30 mV) because these potentials activate Ca2+ currents in these cells. N-type current inactivation appears to be, in part, current-dependent (Cox & Dunlap, 1994; Liang et al. 2003) which would complicate our studies of voltage-dependent inactivation. Although it is now thought that N-type Ca2+ channel current-dependent inactivation requires the involvement of calmodulin (Liang et al. 2003), which is Ba2+ insensitive, it appears that current dependency can be observed, even in the presence of Ba2+. Larger currents tend to inactivate more quickly (our unpublished observation). This may be because Ca2+ pumps do not pump Ba2+. Under the experimental conditions used to study oocytes, there is no dialysis of the cytoplasm. During minute-long depolarizations to potentials that produce large maintained inward currents, cells will tend to accumulate Ba2+. Our impression has always been that any decline observed may have more to do with ion accumulation (and perhaps cell damage) than to channel inactivation. Thus, we study voltage-dependent inactivation exclusively at potentials where it can be unambiguously identified.
Figure 1. Examples of weakly inactivating and inactivating N-type Ca2+ currents produced by channels expressed in Xenopus oocytes.
A, the voltage protocol used to characterize voltage-dependent inactivation. The holding potential was varied in a range from −120 mV to −30 mV. Each holding potential was maintained for 60 s prior to 180 ms test depolarization to +10 mV. B, family of current traces elicited from an oocyte expressing bovine α1B, β2a and α2δ subunits. C, family of current traces elicited from an oocyte expressing bovine α1B, β1b and α2δ subunits.
Figure 2 shows the effect of co-expressing syntaxin 1A with inactivating N-type channels, containing the bovine α1B, β1b and α2δ subunits. Syntaxin 1A enhanced inactivation and reduced current amplitude (Fig. 2A and B) without affecting the current–voltage relationship (Fig. 2C). As shown in Fig. 2D, the average peak current amplitudes were reduced by 47% and the percentage inactivation at a holding potential of −60 mV, compared to −120 mV, was increased from 29 ± 3% to 47 ± 3% in the presence of syntaxin 1A. The results of this experiment are similar to those previously reported by others (Bezprozvanny et al. 1995; Jarvis & Zamponi, 2001), which demonstrated a 15–20 mV leftward shift in the inactivation curve when syntaxin 1A was co-expressed with inactivating N- or P/Q-type channels. Note that the channels produced by bovine chromaffin cell α1B, and the ‘inactivating’ β1b (with α2δ) subunit exhibit significantly less voltage-dependent inactivation than do other cloned N-type Ca2+ channels (for example, see Bezprozvanny et al. 1995); we believe this difference is due to the unique molecular structure of the bovine chromaffin channel (Cahill et al. 2000). Our results suggest that species or cell-type differences in the expressed clones do not alter the ability of syntaxin 1A to enhance inactivation.
Figure 2. Effect of co-expressing syntaxin 1A with inactivating N-type currents.
A, voltage-dependent inactivation curves from oocytes expressing bovine α1B, β1b and α2δ subunits in the absence (−syn) or presence (+syn) of syntaxin 1A. The graph shows peak current amplitude as a function of holding potential and data are presented as mean ± s.e.m. B, same data as in A but normalized by dividing the peak current observed at each holding potential by the peak current observed at the most hyperpolarized holding potential of −120 mV. For A and B, the data are fitted with Boltzmann functions. The inactivation midpoints were significantly different (−syn, −54.2 ± 0.8 mV; +syn, −62.3 ± 1.4 mV), but the slopes of the inactivation curves do not differ significantly in the absence or presence of syntaxin (−syn, −7.9 ± 0.6; +syn, −10.9 ± 1.2). The 95% confidence intervals for the midpoints are: −syn, −56.14 to −52.34; +syn, −65.57 to −59.01. The 95% confidence intervals for Boltzmann slopes are: −syn, −9.43 to −6.29; +syn, −13.92 to −7.93. C, examples of the current−voltage relationship in the absence or presence of syntaxin 1A as indicated. Peak currents are plotted as a function of test potential. D, comparison of peak current size and percentage inactivation in the absence and presence of syntaxin 1A. The percentage inactivation is the amount of inactivation at a holding potential of −60 mV compared with −120 mV. *Significant difference between groups (t test, P < 0.05). HP, holding potential.
In contrast to a previous report (Michaelevski et al. 2003), we did not detect the presence of endogenous syntaxin 1A in Xenopus oocytes. However Western blots did identify the expressed syntaxin 1A and the syntrunc mutant (see below) at the appropriate molecular weight (data not shown). This result indicates that endogenous syntaxin 1A levels are quite low in oocytes.
Effects of syntaxin 1A on weakly inactivating N-type Ca2+ channels
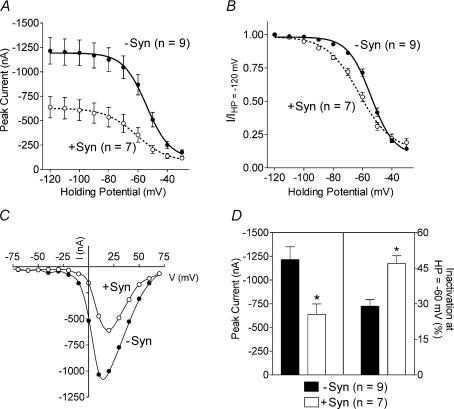
We next examined the effect of co-expressing syntaxin 1A with weakly inactivating N-type channels composed of bovine α1B, β2a and α2δ subunits. As seen in Figs 1 and 3, currents produced by this combination of subunits exhibit very little inactivation, upon changing the holding potential of the cell. Inactivation protocols (see Fig. 1A) done in the presence and absence of syntaxin 1A (Fig. 3A) demonstrated that syntaxin 1A reduced current size across the entire holding potential range. Normalizing the data shows that co-expression of syntaxin 1A did not alter the voltage dependence of inactivation when it is expressed with this weakly inactivating channel (Fig. 3B). In addition, syntaxin 1A did not alter the current–voltage relationship (Fig. 3C). As shown in Fig. 3D, syntaxin 1A reduced the average peak Ca2+ current by ∼75% without altering inactivation.
Figure 3. Effect of co-expressing syntaxin 1A with weakly inactivating N-type currents.
A, voltage-dependent inactivation curves from oocytes expressing bovine α1B, β2a and α2δ subunits in the absence (−syn) or presence (+syn) of syntaxin 1A. The graph shows peak current amplitude as a function of holding potential (HP) and data are presented as mean ± s.e.m. B, same data as in A but normalized by dividing the peak current observed at each holding potential by the peak current observed at the most hyperpolarized holding potential of −120 mV. C, examples of the current–voltage relationship in the absence and presence of syntaxin 1A as indicated. Peak currents are plotted as a function of test potential. D, comparison of peak current size and percentage inactivation in the absence or presence of syntaxin 1A. The percentage inactivation is the amount of inactivation at the holding potential of −40 mV compared with −120 mV. *Significant difference between groups (t test, P < 0.05).
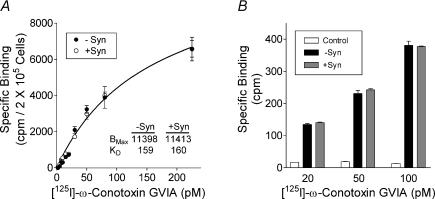
The most prominent effect of syntaxin 1A on the weakly inactivating N-type current is a large reduction in current size. The mechanism by which syntaxin 1A decreases current size is unknown. It is possible that syntaxin 1A inhibits Ca2+ influx either by modulating channel properties or by decreasing the number of Ca2+ channels in the plasma membrane. Some evidence suggests that syntaxin 1A may be important in trafficking membrane proteins to the cell surface (Peters et al. 1999; Horton & Quick, 2001), whereas others (Degtiar et al. 2000) have suggested that syntaxin 1A may alter synthesis or processing of the channel in expression systems. To ascertain whether syntaxin 1A alters the number of N-type Ca2+ channels reaching the cell's surface, we used binding of 125I-labelled ω-conotoxin GVIA to intact tsA-201 cells and Xenopus oocytes to assess the number of N-type channels in the cell membrane. The saturation binding isotherm (Fig. 4A) indicates no difference in the number of 125I-labelled ω-conotoxin GVIA binding sites in cells transfected with α1B, β2a and α2δ in the absence or presence of syntaxin 1A. The calculated KD values (160 nm) are similar to that published by Williams et al. (1992). Data are from a representative experiment, which was repeated six times with independent transfections. No significant differences in maximal binding (BMAX) or KD were seen in the pooled data. No specific binding was detected in untransfected cells (data not shown). 125I-labelled ω-conotoxin GVIA binding was also conducted in Xenopus oocytes. Figure 4B depicts specific binding to control oocytes (non-injected) and oocytes expressing α1B, β2a and α2δ in the absence or presence of syntaxin 1A. No differences in binding were seen between the groups despite the fact that the Ca2+ current amplitudes were significantly smaller in syntaxin 1A-expressing cells (– Syn, 1132 ± 125 nA; + Syn, 265 ± 36 nA; n = 8 for both). Binding was negligible in non-injected oocytes. These results demonstrate that syntaxin 1A does not reduce peak Ca2+ current amplitude by reducing the number of N-type channels, as defined by ω-conotoxin GVIA binding, on the cell surface.
Figure 4. Effect of syntaxin 1A on the number of N-type channels observed on the cell surface in Xenopus oocytes and tsA-201 cells.
A, saturation binding isotherms of 125I-labelled ω-conotoxin GVIA binding to intact tsA-201 cells transfected with α1B, β2a and α2δ in the absence or presence of syntaxin 1A as indicated. Data are from a representative experiment, which was repeated six times with independent transfections. Data are mean ± s.e.m. of triplicates. BMAX and KD values were calculated with computer-assisted non-linear regression analysis. B, 125I-labelled ω-conotoxin GVIA binding to intact oocytes expressing α1B, β2a and α2δ in the absence (−syn) or presence (+syn) of syntaxin 1A and in non-injected oocytes (controls). Bars indicate specific binding at the indicated concentration of 125I-labelled ω-conotoxin GVIA as defined by subtracting total binding from binding in the presence of 100 nm conotoxin GVIA and are the means of data pooled from two independent experiments.
Domains of α1B and syntaxin 1A required for functional interaction
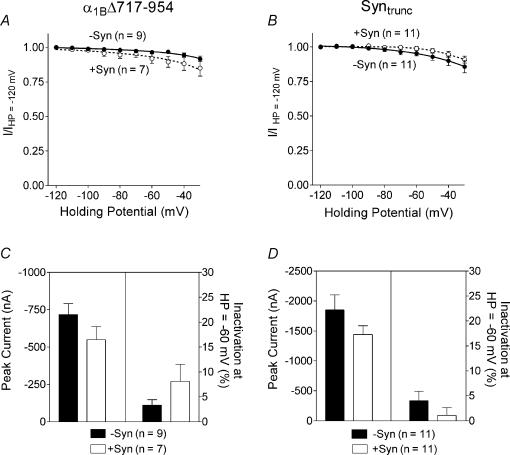
Previous studies have identified a region of the α1B subunit that is involved in the physical interactions between syntaxin 1A and α1B (Sheng et al. 1994; Rettig et al. 1996). This domain located in the intracellular loop between homologous repeats II and III has been termed the synprint site for synaptic protein interaction site. We constructed a bovine α1B mutant lacking the synprint site (α1BΔ717-954) and examined the effects of syntaxin 1A on the current properties. In the absence of syntaxin 1A, the voltage-dependent inactivation characteristics (Fig. 5A) and current–voltage relationships (data not shown) of the mutant α1BΔ717-954 channel did not differ from the wild-type α1B channel. In addition, as seen in Fig. 5A and C, syntaxin 1A had no significant effect on peak current amplitude or inactivation in cells expressing the α1B subunit lacking the synprint site.
Figure 5. The role of the α1B synprint site and the C-terminus of syntaxin 1A on the regulation of Ca2+ channels by syntaxin 1A.
A and C, effect of syntaxin 1A on N-type channels lacking the synprint site (α1BΔ717-954). B and D, effect of truncated syntaxin 1A on weakly inactivating N-type channels. A, shows normalized peak current amplitude as a function of holding potential from oocytes expressing bovine α1BΔ717-954, β2a and α2δ subunits in the absence or presence of syntaxin 1A. B, shows normalized peak current amplitude as a function of holding potential from oocytes expressing bovine α1B, β2a and α2δ subunits in the absence and presence of syntrunc. C, comparison of peak current size and percentage inactivation in oocytes expressing bovine α1BΔ717-954, β2a and α2δ subunits in the absence and presence of syntaxin 1A. The percentage inactivation is the amount of inactivation at the holding potential of −60 mV compared with −120 mV. D, comparison of peak current size and percentage inactivation in oocytes expressing bovine α1B, β2a and α2δ subunits in the absence and presence of syntrunc in the absence and presence of syntaxin 1A. HP, holding potential; −syn, without syntaxin 1A; +syn, with syntaxin 1A.
The H3 helix and the short transmembrane domain on the C-terminus of syntaxin 1A have been identified as regions important for the regulatory effects of syntaxin 1A on Ca2+ channels (Bezprozvanny et al. 1995, 2000; Wiser et al. 1996; Trus et al. 2001). Therefore, we prepared a truncated syntaxin 1A (syntrunc) that is missing both the H3 helix and the transmembrane domain, and we examined the effects of this mutant on the wild-type N-type channel containing β2a and α2δ. Syntrunc did not alter peak current size or inactivation of the weakly inactivating N-type channel (see Fig. 5B and D). The results of these two experiments with mutant syntaxin 1A and α1B subunits indicate that the effects of syntaxin 1A require the synprint site on the α1B subunit as well as the C-terminal region of syntaxin 1A.
Effects of syntaxin 1A on G-protein-dependent regulation of N-type Ca2+ channels
Syntaxin 1A appears to be necessary for G-protein-dependent modulation of Ca2+ currents in nerve terminals (Stanley & Mirotznik, 1997) and may facilitate G-protein-dependent modulation of Ca2+ currents in expression studies (Jarvis et al. 2000; Jarvis & Zamponi, 2001). Jarvis and colleagues postulated that syntaxin 1A might aid in co-localization of Ca2+ channels and Gβγ subunits as syntaxin 1A can bind the α1B subunit and Gβγ subunits simultaneously. Hallmarks of Gβγ-dependent inhibition are slowed activation and inactivation rates and decreased current size. Gβγ-dependent inhibition is voltage-dependent and can be relieved with a strong depolarizing pre-pulse (Dolphin, 1998; Zamponi & Snutch, 1998). The reduced current size mediated by syntaxin 1A seen in our study could be due to enhanced Gβγ-dependent inhibition. Therefore we examined the effect of syntaxin 1A on G-protein-dependent inhibition in oocytes. Non-injected oocytes or oocytes expressing N-type Ca2+ channels containing β2a subunits were subjected to a pulse protocol where currents were elicited by a step to +10 mV before and 15 ms after a 50 ms pre-pulse to +150 mV, designed to relieve G-protein-mediated inhibition. Peak currents before and after the pre-pulse were compared to determine the extent of inhibition. Non-injected oocytes exhibited no pre-pulse potentiation of the small endogenous currents with or without injection of GTPγS (a non-hydrolysable analogue of GTP that irreversibly activates G-proteins); that is, the current amplitude after the pre-pulse was always smaller than the current amplitude before the pre-pulse (data not shown). As seen in the current traces in Fig. 6A and B, activation rates were increased after the pre-pulse, most notably in the presence of GTPγS. The pre-pulse potentiated the basal current by approximately 14 ± 1% in the absence of syntaxin 1A (Fig. 6C). In oocytes injected with GTPγS, pre-pulse potentiation was significantly increased to 25 ± 2% in the absence of syntaxin 1A. In the presence of syntaxin 1A, pre-pulse potentiation was 17 ± 2% and when both syntaxin 1A and GTPγS were present the pre-pulse potentiation was similar (19 ± 2%) and the effects of syntaxin 1A and GTPγS were not additive. In this weakly inactivating N-type channel, syntaxin 1A enhancement of G-protein-dependent inhibition does not appear to account for the large reduction in peak current amplitude seen in our studies as most of the inhibition produced by syntaxin 1A on the weakly inactivating N-type Ca2+ channel appears to be voltage-insensitive.
Effects of syntaxin 1A on N-type Ca2+ channels containing a palmitoylation-deficient β2a subunit
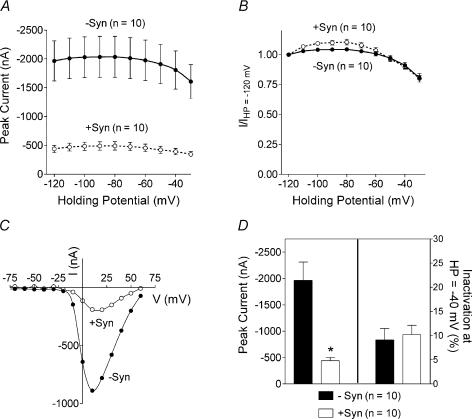
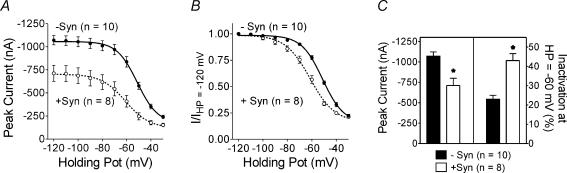
It has previously been shown that the lack of inactivation observed in N-type channels containing β2a subunits is dependent on palmitoylation of two N-terminal cysteines in this subunit (Qin et al. 1998). N-type channels containing palmitoylation-deficient β2a subunits, that is where the two palmitoylated cysteines have been mutated to serines, exhibit significant voltage-dependent inactivation (Qin et al. 1998; Hurley et al. 2000). Figure 7 shows the effect of syntaxin 1A on N-type channels containing palmitoylation-deficient β2a subunits. Co-expression of syntaxin 1A shifted the voltage dependence of inactivation towards more hyperpolarized voltages and significantly decreased peak current amplitudes at all holding potentials in N-type channels containing the palmitoylation-deficient β2a subunit (Fig. 7A and B). Figure 7C shows that the average peak current amplitude was reduced by ∼30%. Note that although the depression of current size by syntaxin 1A varied somewhat from experiment to experiment the current amplitude was always significantly reduced in N-type channels containing α1B subunits with intact synprint sites. Most striking is that inactivation at −60 mV was increased in the presence of syntaxin 1A from 23 ± 2% to 43 ± 4%. In contrast to the wild-type palmitoylated β2a subunit, syntaxin 1A can enhance inactivation of N-type channels containing the palmitoylation-deficient β2a subunit.
Figure 7. Effect of co-expressing syntaxin 1A with N-type channels containing palmitoylation-deficient β2a subunits.
A, shows peak current amplitude as a function of holding potential from oocytes expressing bovine α1B, β2aCys3,4Ser and α2δ subunits in the absence or presence of syntaxin 1A. B, same data as in A but normalized by dividing the peak current observed at each holding potential by the peak current observed at the most hyperpolarized holding potential of −120 mV. For A and B, the data are fitted with Boltzmann functions. C, comparison of peak current size and percentage inactivation in the absence and presence of syntaxin 1A. The percentage inactivation is the amount of inactivation at a holding potential of −60 mV compared with −120 mV. HP, holding potential; −syn, without syntaxin 1A; +syn, with syntaxin 1A.
Discussion
N-type Ca2+ channels are modulated by a number of mechanisms including phosphorylation, binding of G-proteins, and interaction with proteins of the exocytotic apparatus such as syntaxin 1A and SNAP-25. It is the modulation of N-type Ca2+ channels by syntaxin 1A that is the focus of this work. Previously it has been demonstrated that syntaxin 1A binds to the α1B subunit of the N-type channel (Bennett et al. 1992; Sheng et al. 1994; Leveque et al. 1994) and that this binding has functional effects on Ca2+ currents. When expressed in Xenopus oocytes with N-, P/Q- or L-type channels, syntaxin 1A reduces peak Ca2+ currents, shifts the voltage-dependence of inactivation, alters the kinetics of activation and inactivation, and enhances Gβγ-dependent inhibition (Bezprozvanny et al. 1995, 2000; Wiser et al. 1996, 1999; Jarvis et al. 2000, 2002; Jarvis & Zamponi, 2001; Trus et al. 2001). These earlier studies used Ca2+ channels that exhibited voltage-dependent inactivation. Our study explored the effect of syntaxin 1A on a weakly inactivating N-type Ca2+ channel in order to determine whether syntaxin 1A could induce inactivation and if not, whether syntaxin 1A would still alter channel activity.
We have previously shown that bovine N-type Ca2+ channels were either inactivating or weakly inactivating depending on the subunit composition of the channel (Cahill et al. 2000). Channels incorporating bovine chromaffin cell α1B and β2a subunits were weakly inactivating while channels incorporating other β subunits showed significant voltage-dependent inactivation. In this study we have shown that co-expression of syntaxin 1A reduced the peak current amplitude of the inactivating N-type channel and shifted the voltage-dependence of inactivation in the hyperpolarizing direction. These results are in agreement with the published literature (Bezprozvanny et al. 1995, 2000; Wiser et al. 1996, 1999; Jarvis et al. 2000, 2002; Jarvis & Zamponi, 2001; Trus et al. 2001) and validate our cDNA constructs, oocyte expression system, and Ca2+ current measurements. These results also suggest that bovine α1B subunits possess a functional synprint site and can be modulated by syntaxin 1A. In contrast the weakly inactivating N-type channel consisting of bovine α1B, β2a and α2δ subunits exhibited a reduction in peak current but no change in inactivation when co-expressed with syntaxin 1A. Thus although syntaxin 1A can promote inactivation of an inactivating N-type channel it cannot cause a weakly inactivating channel to inactivate.
The inactivation properties of voltage-gated Ca2+ channels appear to be largely controlled by which β subunit is incorporated into the channel (Hering et al. 2000). N-, P/Q- and R-type channels containing the β2a subunit exhibit slowed inactivation while those containing β1a, β1b, β3a subunits show enhanced inactivation (Ellinor et al. 1993; Olcese et al. 1994; Qin et al. 1998). The lack of inactivation with β2a has been attributed to the presence of two palmitoylated cysteines in the N-terminus that are not present in other β subunits (Qin et al. 1998). It has been suggested that palmitoylated β2a subunits may be anchored in the membrane in a way that impedes movement of the proposed inactivation gate of the α1 subunit (Restituito et al. 2000). If the N-terminus cysteines of the β2a subunit are mutated to Ser (β2aCys3,4Ser), this β2a subunit can no longer be palmitoylated, and channels containing this mutant β2a exhibit voltage-dependent inactivation (Fig. 7 and Qin et al. 1998). We find that syntaxin 1A can modulate inactivation of N-type channels containing β2aCys3,4Ser and suggest that the determinants of the actions of syntaxin 1A on inactivation are present in bovine N-type channels containing β2a subunits but may be masked or inaccessible when the β2a subunit is anchored to the membrane by the palmitoylated cysteines. One previous study examined the effects of syntaxin 1A on a rat brain N-type channel containing a β2a subunit (Wiser et al. 1996). The channel in that study exhibited diminished inactivation in the absence of syntaxin 1A, but did not show the marked lack of inactivation as seen with the bovine channel.
In this study syntaxin 1A significantly reduced the peak current of both inactivating and weakly inactivating N-type channels. One possible explanation for this is that syntaxin 1A altered the expression or trafficking of the channel to the plasma membrane, as it does with the GABA transporter and cystic fibrosis transmembrane conductance regulator (CFTR) (Peters et al. 1999; Horton & Quick, 2001). We investigated this possibility by measuring binding of ω-conotoxin GVIA to intact tsA-201 cells or Xenopus oocytes expressing α1B, β2a and α2δ in the absence or presence of syntaxin 1A. ω-conotoxin GVIA binding did not differ in cells with or without syntaxin 1A, suggesting that syntaxin 1A did not alter cell surface expression of the N-type Ca2+ channels but rather modulated the activity of the channel.
To investigate the molecular domains of syntaxin 1A and the Ca2+ channel α1B subunit that may be involved in modulation of channel function we used mutant forms of these subunits lacking regions believed to be important for their interaction. The synprint site of α1B and α1A subunits binds syntaxin 1A and has been clearly defined within the II–III loop (Sheng et al. 1994; Rettig et al. 1996). This domain may serve as a stable anchoring site for syntaxin 1A, whereas other sites within the channel may be important for modulation by syntaxin 1A (Bezprozvanny et al. 2000). As expected, the peak current of the α1BΔ717-954 (synprint deletion mutant) was not reduced by syntaxin 1A, suggesting that the synprint site is necessary for the effects of syntaxin 1A on current size. Our result is in agreement with that of Zamponi and colleagues (Spafford et al. 2003) who showed that syntaxin 1A did not regulate an invertebrate Ca2+ channel that did not have a synprint site. The region of syntaxin 1A that binds the synprint site is somewhat unclear. It appears that the short transmembrane C-terminus domain (Sheng et al. 1994; Bezprozvanny et al. 1995, 2000,Wiser et al. 1996) and more specifically two highly conserved amino acids (Trus et al. 2001) within it (Cys271 and Cys272) are important for the regulatory effects of syntaxin 1A. The H3 helical domain and two amino acids within it (Ala240 and Val244) have also been implicated in the modulatory effects of syntaxin 1A (Bezprozvanny et al. 2000). A more recent study points to the N-terminus of syntaxin 1A in binding to the synprint site of α1A and α1B subunits (Jarvis et al. 2002). The syntaxin 1A truncation mutant used in this study is lacking almost all of the H3 helix as well as the C-terminus transmembrane domain. This truncated syntaxin 1A failed to reduce the current amplitude of weakly inactivating channels (Fig. 5), confirming the proposed importance of the C-terminus in the modulatory effects of syntaxin 1A on Ca2+ channels. However, this result must be interpreted with caution, as we cannot be certain whether the lack of effect is due to inability of the mutant syntaxin 1A to insert into the membrane (Masaki et al. 1998) or loss of a Ca2+ channel interaction site.
A role for syntaxin 1A in facilitating Gβγ-dependent inhibition of Ca2+ channels has been demonstrated in nerve terminals (Stanley & Mirotznik, 1997) and in cell lines transfected with syntaxin 1A (Jarvis et al. 2000, 2002; Jarvis & Zamponi, 2001). It was of interest to determine whether syntaxin 1A would promote G-protein-dependent inhibition of N-type channels containing β2a subunits because R-type Ca2+ channels consisting of α1E and β2a with a2δ were less susceptible to Gβγ inhibition (Qin et al. 1998) and slow the recovery and activation kinetics of G-protein-inhibited α1B channels (Lu et al. 2001; Feng et al. 2001). We tested the ability of syntaxin 1A to facilitate Gβγ inhibition. The data appears to show that syntaxin 1A does not induce as much G-protein-dependent inhibition as the injection of GTPγS. It appears as if the small amount of G-protein-dependent modulation seen with syntaxin 1A precludes any further modulation by GTPγS. Although the data are somewhat difficult to explain, they cannot account for the reduction in current amplitude induced by syntaxin 1A. Overall, voltage-dependent Gβγ inhibition may be less prominent in the oocytes due to low concentrations of Gβγ proteins (Lu et al. 2001) relative to the exogenously expressed Ca2+ channel and syntaxin 1A (see Bourinet et al. 1996; in this study G-protein mRNA was introduced into oocytes to make responses more consistent). Our results show that most of the inhibition produced by syntaxin 1A is not relieved by a strongly depolarizing pre-pulse, as would be expected for a Gβγ-mediated effect. Syntaxin 1A may inhibit channel activity by a mechanism involving direct block of the channel. Further investigation will require single channel recordings or studies of gating currents.
The effects of syntaxin 1A on inactivation and Gβγ inhibition appear to be separable (Jarvis & Zamponi, 2001; Jarvis et al. 2002), which would allow species or cell-type specificity and even plasticity. Our results suggest that the reduction in current amplitude is not strongly linked to the effects on Gβγ or inactivation. The effects of syntaxin 1A on current amplitude have not been fully explained especially in light of studies on the recently described α-neurexin knockout mice. α-neurexins (Missler et al. 1998) are a family of proteins that may physically link the pre- and postsynaptic compartments of synapses and they are necessary for Ca2+-stimulated neurotransmitter release. α-neurexin knockout mice (Missler et al. 2003) exhibit reduced Ca2+ current amplitudes with no apparent change in cell surface expression, which suggests a positive modulation of Ca2+ channel function as opposed to the negative modulatory actions of syntaxin 1A. Thus it appears that a variety of proteins that anchor Ca2+ channels can either up- or down-regulate their activity, opening the possibility that this form of regulation may be dynamic.
Acknowledgments
This work was supported by an NIH grant to APF and a Showalter Research Trust grant to JHH.
References
- Artalejo CR, Perlman RL, Fox AP. Omega-conotoxin GVIA blocks a Ca2+ current in bovine chromaffin cells that is not of the ‘classic’ N type. Neuron. 1992;8:85–95. doi: 10.1016/0896-6273(92)90110-y. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Zhong P, Scheller RH, Tsien RW. Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc Natl Acad Sci U S A. 2000;97:13943–13948. doi: 10.1073/pnas.220389697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci U S A. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill AL, Hurley JH, Fox AP. Coexpression of cloned α1B, β2a, and α2δ subunits produces non-inactivating calcium currents similar to those found in bovine chromaffin cells. J Neurosci. 2000;20:1685–1693. doi: 10.1523/JNEUROSCI.20-05-01685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH, Dunlap K. Inactivation of N-type calcium current in chick sensory neurons: calcium and voltage dependence. J Gen Physiol. 1994;104:311–336. doi: 10.1085/jgp.104.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtiar VE, Scheller RH, Tsien RW. Syntaxin modulation of slow inactivation of N-type calcium channels. J Neurosci. 2000;20:4355–4367. doi: 10.1523/JNEUROSCI.20-12-04355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinor PT, Zhang JF, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Arnot MI, Doering CJ, Zamponi GW. Calcium channel β subunits differentially regulate the inhibition of N-type channels by individual Gβ isoforms. J Biol Chem. 2001;276:45051–45058. doi: 10.1074/jbc.M107784200. [DOI] [PubMed] [Google Scholar]
- Hering S, Berjukow S, Sokolov S, Marksteiner R, Weiss RG, Kraus R, Timin EN. Molecular determinants of inactivation in voltage-gated Ca2+ channels. J Physiol. 2000;528:237–249. doi: 10.1111/j.1469-7793.2000.t01-1-00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Horne AL, Kemp JA. The effect of ω-conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro. Br J Pharmacol. 1991;103:1733–1739. doi: 10.1111/j.1476-5381.1991.tb09855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Horton N, Quick MW. Syntaxin 1A up-regulates GABA transporter expression by subcellular redistribution. Mol Membr Biol. 2001;18:39–44. [PubMed] [Google Scholar]
- Hurley JH, Cahill AL, Currie KP, Fox AP. The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc Natl Acad Sci U S A. 2000;97:9293–9298. doi: 10.1073/pnas.160589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SE, Barr W, Feng ZP, Hamid J, Zamponi GW. Molecular determinants of syntaxin 1 modulation of N-type calcium channels. J Biol Chem. 2002;277:44399–44407. doi: 10.1074/jbc.M206902200. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Magga JM, Beedle AM, Braun JE, Zamponi GW. G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gβγ. J Biol Chem. 2000;275:6388–6394. doi: 10.1074/jbc.275.9.6388. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J Neurosci. 2001;21:2939–2948. doi: 10.1523/JNEUROSCI.21-09-02939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque C, el Far O, Martin-Moutot N, Sato K, Kato R, Takahashi M, Seagar MJ. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin. A complex implicated in synaptic vesicle exocytosis. J Biol Chem. 1994;269:6306–6312. [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- Lu Q, AtKisson MS, Jarvis SE, Feng ZP, Zamponi GW, Dunlap K. Syntaxin 1A supports voltage-dependent inhibition of alpha1B Ca2+ channels by Gβγ in chick sensory neurons. J Neurosci. 2001;21:2949–2957. doi: 10.1523/JNEUROSCI.21-09-02949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Masaki R, Yamamoto A, Akagawa K, Tashiro Y. Important roles of the C-terminal portion of HPC-1/syntaxin 1A in membrane anchoring and intracellular localization. J Biochem. 1998;124:311–318. doi: 10.1093/oxfordjournals.jbchem.a022113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Chikvashvili D, Tsuk S, Singer-Lahat D, Kang Y, Linial M, Gaisano HY, Fili O, Lotan I. Direct interaction of target SNAREs with the Kv2.1 channel. Modal regulation of channel activation and inactivation gating. J Biol Chem. 2003;278:34320–34330. doi: 10.1074/jbc.M304943200. [DOI] [PubMed] [Google Scholar]
- Missler M, Fernandez-Chacon R, Sudhof TC. The making of neurexins. J Neurochem. 1998;71:1339–1347. doi: 10.1046/j.1471-4159.1998.71041339.x. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. α-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;424:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel beta subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Peters KW, Qi J, Watkins SC, Frizzell RA. Syntaxin 1A inhibits regulated CFTR trafficking in xenopus oocytes. Am J Physiol. 1999;277:C174–C180. doi: 10.1152/ajpcell.1999.277.1.C174. [DOI] [PubMed] [Google Scholar]
- Qin N, Platano D, Olcese R, Costantin JL, Stefani E, Birnbaumer L. Unique regulatory properties of the type 2a Ca2+ channel β subunit caused by palmitoylation. Proc Natl Acad Sci U S A. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restituito S, Cens T, Barrere C, Geib S, Galas S, De Waard M, Charnet P. The β2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci. 2000;20:9046–9052. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of the α1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci U S A. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- Spafford JD, Chen L, Feng ZP, Smit AB, Zamponi GW. Expression and modulation of an invertebrate presynaptic calcium channel α1 subunit homolog. J Biol Chem. 2003;278:21178–21187. doi: 10.1074/jbc.M302212200. [DOI] [PubMed] [Google Scholar]
- Stanley EF. Syntaxin I modulation of presynaptic calcium channel inactivation revealed by botulinum toxin C1. Eur J Neurosci. 2003;17:1303–1305. doi: 10.1046/j.1460-9568.2003.02536.x. [DOI] [PubMed] [Google Scholar]
- Stanley EF, Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991;11:985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF, Mirotznik RR. Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels. Nature. 1997;385:340–343. doi: 10.1038/385340a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Trus M, Wiser O, Goodnough MC, Atlas D. The transmembrane domain of syntaxin 1A negatively regulates voltage-sensitive Ca2+ channels. Neuroscience. 2001;104:599–607. doi: 10.1016/s0306-4522(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Turner TJ, Adams ME, Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci U S A. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of an ω-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Wiser O, Bennett MK, Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]