Abstract
Hormone-sensitive lipase (HSL) catalyses the hydrolysis of myocellular triacylglycerol (MCTG), which is a potential energy source during exercise. Therefore, it is important to elucidate the regulation of HSL activity in human skeletal muscle during exercise. The main purpose of the present study was to investigate the role of 5′AMP-activated protein kinase (AMPK) in the regulation of muscle HSL activity and Ser565 phosphorylation (the presumed AMPK target site) in healthy, moderately trained men during 60 min bicycling (65% V̇o2peak). α2AMPK activity during exercise was manipulated by studying subjects with either low (LG) or high (HG) muscle glycogen content. HSL activity was distinguished from the activity of other neutral lipases by immunoinhibition of HSL using an anti-HSL antibody. During exercise a 62% higher (P < 0.01) α2AMPK activity in LG than in HG was paralleled by a similar difference (61%, P < 0.01) in HSL Ser565 phosphorylation but without any difference between trials in HSL activity or MCTG hydrolysis. HSL activity was increased (117%, P < 0.05) at 30 min of exercise but not at 60 min of exercise. In both trials, HSL phosphorylation on Ser563 (a presumed PKA target site) was not increased by exercise despite a fourfold increase (P < 0.001) in plasma adrenaline. ERK1/2 phosphorylation was increased by exercise in both trials (P < 0.001) and was higher in LG than in HG both at rest and during exercise (P = 0.06). In conclusion, the present study suggests that AMPK phosphorylates HSL on Ser565 in human skeletal muscle during exercise with reduced muscle glycogen. Apparently, HSL Ser565 phosphorylation by AMPK during exercise had no effect on HSL activity. Alternatively, other factors including ERK may have counterbalanced any effect of AMPK on HSL activity.
Triacylglycerol stored in skeletal muscle (myocellular triacylglycerol, MCTG) serves as an energy depot, which can be utilized during exercise. However, knowledge about the regulation of triacylglycerol hydrolysis in skeletal muscle is very limited, especially when it comes to molecular mechanisms involved in regulation of the activity of hormone-sensitive lipase (HSL), the enzyme thought to catalyse triacylglycerol hydrolysis in skeletal muscle. Most information about the regulation of HSL activity stems from adipocytes, where the regulation of triacylglycerol hydrolysis by HSL has been quite extensively studied (Holm et al. 2000). For instance, in adipocytes adrenaline-stimulated HSL activity was shown to be mediated by cAMP-dependent protein kinase (PKA), which phosphorylated HSL on Ser563, Ser659 and Ser660 (numbering refers to the rat sequence) (Holm et al. 2000). On the other hand, inhibition of HSL activity may be mediated by 5′AMP-activated protein kinase (AMPK). Thus, bovine adipocyte HSL was phosphorylated in vitro on Ser565 by AMPK, which led to inhibition of subsequent phosphorylation by PKA on Ser563 (Garton et al. 1989). Correspondingly, incubation of isolated rat adipocytes with 5-aminoimidazole-4-carboxamide-riboside (AICAR), which activated AMPK, inhibited lipolysis stimulated by the β-adrenergic agonist isoprenaline (Sullivan et al. 1994; Corton et al. 1995).
In rat skeletal muscle, HSL has only recently been detected and, furthermore, it was shown that HSL was activated during muscle contraction (Langfort et al. 2000). In human skeletal muscle total neutral lipase activation has been reported during exercise (Kjaer et al. 2000; Watt et al. 2003a) and it was recently confirmed that this exercise-induced increase in total neutral lipase activity could be ascribed to HSL (Watt et al. 2004b). However, the molecular mechanisms behind the activation of HSL in human skeletal muscle during exercise are still largely unknown but could be further elucidated by the investigation of site-specific HSL phosphorylation.
AMPK is an important fuel gauge, which is activated during various types of cellular stress (Hayashi et al. 2000) including exercise (Wojtaszewski et al. 2000). Activation of AMPK accelerates energy-providing pathways and inhibits energy-consuming pathways (Hardie & Carling, 1997). While in adipocytes pharmacological activation of AMPK with AICAR inhibits the HSL activation induced by β-adrenergic agents (Corton et al. 1995; Sullivan et al. 1994), it seems difficult to reconcile this with the view that activation of AMPK would inhibit HSL activity in skeletal muscle during exercise since this would decrease energy provision from MCTG. Nevertheless, while the present study was in progress Watt et al. (2004b) published evidence that the increase in HSL activity in human skeletal muscle induced by exercise at 70% V̇o2peak with normal muscle glycogen content and minimal α2AMPK activation was completely abolished when α2AMPK activation was high due to low muscle glycogen content. The authors also found that AICAR inhibited the HSL activation induced by adrenaline in L6 myotubes and therefore they concluded that β-adrenergic stimulation of HSL activity in skeletal muscle can be overridden by AMPK inhibition of HSL activity (Watt et al. 2004b). In the study by Watt et al. (2004b), the difference in HSL activation by exercise between the low glycogen and the control trials occurred in the face of different circulating glucose, fatty acid and adrenaline levels. Therefore, the isolated effect of AMPK on HSL activity during exercise was not readily deducible from that study (Watt et al. 2004b). Furthermore, it is still unknown whether AMPK can phosphorylate HSL on Ser565 in human skeletal muscle during exercise.
Therefore, in the present study we investigated the effect of moderate intensity exercise on HSL activity and MCTG hydrolysis in human skeletal muscle during two trials with markedly different muscle AMPK activity but with minimal differences in circulating levels of several metabolites and hormones. To obtain new information on the mechanisms involved in regulation of HSL activity we also studied phosphorylation of Ser563 and Ser565 on HSL. The difference between trials in AMPK activity during exercise was achieved via alteration of pre-exercise muscle glycogen stores to low (LG) or high (HG) levels by preceding exercise and dietary manipulation as previously described (Derave et al. 2000; Wojtaszewski et al. 2003). Potential differences between LG and HG trials in adrenaline-stimulated phosphorylation of HSL on Ser563 by PKA could influence HSL Ser565 phosphorylation (Garton et al. 1989) and/or HSL activity. Therefore, subjects ingested a light pre-exercise meal 4.5 h before exercise and glucose was infused intravenously during exercise to keep blood glucose concentrations constant and similar between trials and thereby minimize the difference in plasma adrenaline concentrations which might otherwise be inherent in the present study design (Wojtaszewski et al. 2003). It was our hypothesis that in human skeletal muscle, AMPK activation during exercise in the LG trial would increase HSL Ser565 phosphorylation but without any direct effect of AMPK on HSL activity.
Methods
Subjects
Eight young, healthy, moderately trained men (means ± s.e.m.: age, 26 ± 1 years; height, 1.80 ± 0.02 m; body mass (BM), 76 ± 3 kg; body mass index, 23.5 ± 0.7 kg m−2; body fat, 14.6 ± 1.9%; V̇o2peak, 4.1 ± 0.2 l min−1; V̇o2peak per kg BM, 53.7 ± 1.6 ml kg−1 min−1) were recruited to participate in the study. All subjects were non-smokers and were engaged in 4–6 h per week of regular exercise such as running, bicycling and resistance training. Subjects were fully informed about the nature and possible risks of the study before they gave their written consent and were included in the study. The study was approved by the Copenhagen Ethics Committee (KF-01-078/01) and carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki II). The results presented in this study are part of a larger study on the regulation of lipid metabolism during exercise in human skeletal muscle, which has partly been published previously (Roepstorff et al. 2004).
Pre-experimental testing
All subjects initially performed an incremental exercise test on a mechanically braked Monark bicycle ergometer (Monark 839 Electronic Ergometer, Monark Exercise AB, Sweden) to determine their peak oxygen uptake (V̇o2peak). Respiratory measurements were performed with the Douglas bag technique. Furthermore, subjects filled out a questionnaire regarding habitual physical activity. Percentage body fat was measured by dual-energy X-ray absorptiometry (DEXA, Lunar Corp., Madison, WI, USA, DPX-IQ v. 4.6.6).
Experimental design
The subjects underwent two experimental trials separated by 2–3 weeks. In both trials the subjects initially completed 3–4.5 h of glycogen depletion bicycle exercise followed by a controlled diet for the rest of the day. The following day the subjects performed a 60 min exercise bout on a cycle ergometer at 65% V̇o2peak. In one trial the controlled diet consisted primarily of fat (LG) and in the other trial primarily of carbohydrate (HG) (see below). The order of the LG and HG trials was randomized and stratified.
Dietary control
Subjects determined their habitual energy and nutrient intake by a self-reported dietary record on the three days preceding the first trial. All food and beverage intakes were weighed to the accuracy of 1 g and recorded. The energy intake and composition of the habitual diet were then calculated using appropriate software (Dankost 2000, Danish Catering Center, Copenhagen, Denmark). On the three days preceding the second trial subjects closely followed their own registered diet as evidenced by highly matching self-reports.
Glycogen depletion protocol
On the day of the glycogen depletion trial the subjects arrived in the laboratory at 7.30 a.m. They had abstained from any physical activity on the preceding day. A breakfast meal was served consisting of 30% fat, 55% CHO and 15% protein. The energy content was 25% of each subject's daily energy intake (DEI) as reported from the 3-day dietary record. At 10 a.m. subjects embarked on an exercise regimen consisting of continuous submaximal bicycling, arm cranking, and a period of intermittent bicycle exercise as previously described (Roepstorff et al. 2004). The glycogen depleting exercise lasted for 3–4.5 h and was well tolerated by all subjects. For the following 6 h subjects were allowed to move freely around in the laboratory. Meals were served immediately after exercise and 1 h, 2 h, 3.5 h and 5 h afterwards. Before leaving the laboratory the subjects were given a light snack to ingest later at 11 p.m. The controlled diet ingested during recovery from the glycogen depleting exercise consisted of 85% fat, 2% CHO and 13% protein in the LG trial and 8% fat, 80% CHO, and 12% protein in the HG trial. The total energy intake during this period was calculated to ensure that subjects were in energy balance. The six meals which were consumed during recovery from exercise contained equal amounts of energy in LG and HG (16%, 10%, 16%, 10%, 43% and 5% of the total energy ingested immediately after and 1 h, 2 h, 3.5 h and 5 h after exercise and at 11 p.m., respectively). During the glycogen depletion trial and for the rest of the day subjects were allowed to drink water ad libitum.
Exercise experimental protocol
On the day following the glycogen depleting exercise the subjects arrived at the laboratory by car at 7.30 a.m. A light breakfast, which contained 10% of the individual DEI and consisted of 4% fat, 78% CHO and 18% protein, was served to avoid differences in blood metabolite and hormone concentrations otherwise inherent in the present study design (Wojtaszewski et al. 2003). After 30 min rest in the supine position a Teflon catheter was inserted under local anaesthesia into the femoral artery. During the second trial the femoral catheter was inserted into the femoral artery of the contralateral leg. A venous catheter was inserted into an antecubital vein for infusion of glucose.
Thereafter subjects rested for approximately 90 min in the supine position. Expired air was collected in a Douglas bag and arterial blood samples were obtained in duplicate for determination of resting blood concentrations. A muscle biopsy was obtained from the vastus lateralis muscle. Then subjects initiated a 60 min bicycle exercise test at approx. 65% V̇o2peak. Subjects exercised at the same absolute workload in LG and HG. A variable continuous infusion of glucose into an antecubital vein was initiated after 1 min of exercise to keep the blood glucose concentration constant and similar to the basal resting level, thereby avoiding large differences between trials in several other blood metabolite and hormone concentrations during exercise. Frequent arterial blood sampling and subsequent analysis of blood glucose concentration throughout the exercise period evaluated the infusion rate of glucose. Every 20 min during exercise expired air was collected in a Douglas bag and blood was drawn from the femoral artery. After 30 min of exercise subjects terminated exercise for < 120 s. During this break a biopsy was obtained from the vastus lateralis muscle within 15 s from termination of exercise. After completion of the 60 min bicycle test, another biopsy was obtained from the vastus lateralis muscle within 15 s from termination of exercise. All biopsies were obtained through separate incisions spaced at least 5 cm apart to avoid the stress response induced by repeated biopsy sampling and its possible effect on the proteins of interest (Aronson et al. 1998). Heart rate was monitored throughout the experiment with a Polar Vantage XL heart rate monitor (Polar Electro OY, Finland) and blood pressure was measured through the femoral arterial catheter connected to a pressure transducer (Spectramed P23XL, Statham, CA, USA). During the first trial subjects were offered water ad libitum and their water intake was recorded. During the second trial subjects repeated their water intake from the first trial.
Breath samples
Expired air in the Douglas bags was handled as previously described (Roepstorff et al. 2002).
Blood samples
Blood glucose concentrations were measured automatically (ABL510, Radiometer Medical A/S, Copenhagen, Denmark). Plasma FA concentrations were measured by a colourimetric commercial assay (Wako Chemicals, VA, USA) on a COBAS FARA autoanalyser (COBAS FARA 2, Roche Diagnostic, Switzerland). Concentrations of insulin (Pharmacia Insulin Radioimmunoassay 100, Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden) and adrenaline and noradrenaline (KatCombi Radioimmunoassay, Immuno-Biological Laboratories GmbH, Hamburg, Germany) in plasma were determined by radioimmunoassay.
Muscle biopsies
The biopsies were quick-frozen (< 10 s) in liquid nitrogen while still in the biopsy needle and stored at −80°C for subsequent biochemical analysis. Eighty milligrams wet weight of muscle tissue was freeze-dried and dissected free of all visible adipose tissue, connective tissue and blood under a microscope. The dissected muscle fibres were pooled and then divided into subpools for the respective analyses.
Muscle glycogen and MCTG
In the freeze-dried and dissected muscle tissue the glycogen concentration was determined by a fluorometric method (Lowry & Passonneau, 1972) and the concentration of MCTG was determined as previously described (Steffensen et al. 2002).
Muscle lysates
Freeze-dried and dissected muscle tissue was homogenized (1 : 80, weight: vol) in a buffer containing 50 mm Hepes (pH 7.5), 150 mm NaCl, 20 mm sodium pyrophosphate, 20 mmβ-glycerophosphate, 10 mm NaF, 2 mm sodium orthovanadate, 2 mm EDTA, 1% Noidet P-40, 10% glycerol, 2 mm PMSF, 1 mm MgCl2, 1 mm CaCl2, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, and 3 mm benzamidine. Homogenates were rotated end over end for 1 h at 4°C and then cleared by centrifugation at 17 500 g at 4°C for 1 h. Protein content in the supernatant was measured by the bicinchoninic acid method (Pierce Chemical Co., IL, USA).
Western blotting
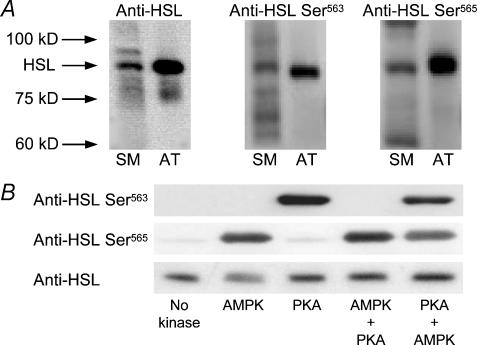
Expression of HSL and phosphorylation of αAMPK Thr172, HSL Ser563, HSL Ser565, ERK1 Thr202/Tyr204, and ERK2 Thr185/Tyr187 were detected by Western blotting on the muscle lysates. The lysates were boiled in Laemmli buffer before being subjected to SDS-PAGE and immunoblotting. Primary antibody was rabbit anti-HSL (kindly donated by Dr Cecilia Holm, Department of Cell and Molecular Biology, Lund University, Sweden) and rabbit anti-ERK1/2 (Cell Signalling Technology, Inc., Medinova Scientific A/S, Glostrup, Denmark). Primary phospho-specific antibodies were rabbit anti-αAMPK Thr172-phos (Upstate Biotechnology, Lake Placid, NY), sheep anti-HSL Ser563-phos, sheep anti-HSL Ser565-phos, and rabbit anti-ERK1 Thr202/Tyr204-phos/ERK2 Thr185/Tyr187-phos (Cell Signalling Technology, Inc., Beverly, MA, USA). The anti-HSL phosphospecific antibodies were raised in sheep using synthetic peptides coupled to keyhole limpet haemocyanin by protocols previously described (Sugden et al. 1999). The anti-HSL Ser563-phos antibody was raised against the peptide CTESMRRSpVSEAALAQP (cysteine plus residues 556–571 of rat HSL, Sp = phosphoserine) and the anti-HSL Ser565-phos antibody against CTESMRRSVSpEAALAQP. The human sequence is identical except it starts AEP rather than TES. Both antibodies were affinity purified by passing through columns containing the dephosphopeptide and then the phosphopeptide (Sugden et al. 1999). Secondary antibodies were horseradish peroxidase-conjugated anti-sheep and anti-rabbit (DAKO, Glostrup, Denmark and Zymed, CA, USA). Initially, the linearity range of each primary antibody, and thus the optimal sample protein concentration, was determined by Western blotting on varying amounts of extra protein from a few samples. Antigen–antibody complexes were visualized using enhanced chemiluminescence (ECL+, Amersham Biosciences, UK) and a Kodak Image Station E440CF (Kodak, Glostrup, Denmark). Quantification of antigen–antibody complexes was performed using appropriate software (Kodak 1D Scientific Imaging Systems, Kodak, Glostrup, Denmark). The anti-HSL revealed a distinct band in human skeletal muscle at the expected molecular weight (∼84 kDa) (Fig. 1A). The anti-HSL Ser563-phos and anti-HSL Ser565-phos revealed a distinct band in human skeletal muscle corresponding to the ∼84 kDa band detected by anti-HSL (Fig. 1A). To further test whether the ∼84 kDa band detected in human skeletal muscle corresponded to HSL, the anti-HSL, anti-HSL Ser563-phos, and anti-HSL Ser565-phos antibodies were applied in human paraumbilical adipose tissue, where all three antibodies revealed single bands corresponding to the ∼84 kDa band detected in skeletal muscle (Fig. 1A). Since HSL expression is manyfold higher in adipocytes than in skeletal muscle, we assumed with all three HSL antibodies that the ∼84 kDa band corresponded to HSL.
Figure 1. Testing of HSL antibodies.
A, representative Western blots of HSL total protein and of HSL phosphorylated on Ser563 or Ser565 in human skeletal muscle and in human paraumbilical adipose tissue sampled at rest. SM, skeletal muscle; AT, adipose tissue. B, specificity of anti-HSL Ser563-phos and anti-HSL Ser565-phos tested using recombinant rat HSL. HSL was incubated with MgATP either without added kinase, with AMPK, with PKA, with AMPK followed by PKA, or with PKA followed by AMPK. The protein was analysed by SDS-PAGE and blots were probed with anti-HSL Ser563-phos, anti-HSL Ser565-phos, or a non-phosphospecific anti-HSL antibody.
The specificity of the anti-HSL Ser563-phos and anti-HSL Ser565-phos antibodies against HSL in the relevant phosphorylated state was tested by incubation of recombinant rat HSL (0.02 mg ml−1) (kindly donated by Dr Cecilia Holm, Department of Cell and Molecular Biology, Lund University, Sweden) with ATP (200 μm) plus MgCl2 (5 mm) without added kinase, with AMPK (5 U ml−1), or with PKA (100 U ml−1) for 15 min at 30°C in 50 mm Na-Hepes, 1 mm dithiothreitol, 0.03% (w/v) Brij-35. The HSL was also incubated with AMPK (5 U ml−1) for 15 min followed by additional incubation with PKA (100 U ml−1) for 15 min or vice versa. The products were analysed by SDS-PAGE (0.16 μg HSL per lane) and blots probed with either anti-HSL Ser563-phos (200 ng ml−1) or anti-HSL Ser565-phos (50 ng ml−1) (Fig. 1B). Anti-HSL Ser563-phos detected HSL when incubated with PKA (which phosphorylates Ser563) but not when incubated with AMPK (which phosphorylates Ser565) (Fig. 1B). If HSL was incubated with AMPK prior to PKA, no signal was obtained. This was consistent with previous findings that phosphorylation of Ser563 by PKA and Ser565 by AMPK were mutually exclusive (Garton et al. 1989). Anti-HSL Ser565-phos revealed a strong band when HSL was incubated with AMPK (which phosphorylates Ser565) (Fig. 1B). In addition, when HSL was incubated with PKA prior to AMPK the signal was slightly decreased consistent with the finding that phosphorylation on Ser563 inhibited phosphorylation on Ser565 (Garton et al. 1989). Anti-HSL Ser565-phos also revealed a weak band when recombinant HSL was incubated with MgATP without added kinase, or with PKA (which phosphorylates Ser563). This could be due to a slight degree of phosphorylation on Ser565 in the recombinant protein, or it may be due to a slight detection of the dephosphoprotein by the HSL Ser565-phos antibody. Western blotting of a parallel gel with a non-phosphospecific anti-HSL revealed that the loadings were uniform in this experiment (Fig. 1B).
AMPK activity
α-Isoform-specific AMPK activity was determined in immunoprecipitates from muscle lysates as previously described (Wojtaszewski et al. 2003). Briefly, immunoprecipitates were prepared from 200 μg of muscle lysate protein using anti-α1 or anti-α2 antibodies previously described (Woods et al. 1996). AMPK activity was measured in the immunoprecipitates using SAMS-peptide (HMRSAMSGLHLVKRR, 200 μm) as previously described (Wojtaszewski et al. 2000).
HSL activity
Freeze-dried and dissected muscle tissue was homogenized as previously described (Donsmark et al. 2003) and the homogenate was centrifuged for 45 s (15 800 g, 4°C) in an Eppendorf microcentrifuge tube. The resulting supernatant containing ∼2.5 mg protein ml−1 was used for subsequent measurement of lipase activity. Lipase activity against tri[3H]olein was measured at pH 7.0, which is the pH optimum for HSL, with or without pre-incubation of the muscle supernatants with a chicken anti-HSL as previously described (Donsmark et al. 2003; Langfort et al. 2000). Pre-incubation of rat muscle supernatants with the chicken anti-HSL reduced the subsequently measured basal lipase activity against tri[3H]olein by ∼60% indicating the existence in muscle of other neutral lipases than HSL. However, pre-incubation with anti-HSL completely abolished the adrenaline- and contraction-induced increases in measured lipase activity in rat skeletal muscle (Langfort et al. 1999; Langfort et al. 2000). Therefore, in the present study HSL activity was calculated as neutral lipase activity measured in the absence of anti-HSL minus neutral lipase activity measured in the presence of anti-HSL. HSL activity was expressed in mU (mg protein)−1 where 1 unit of enzyme activity is equivalent to 1 μmol of fatty acids released per min at 37°C.
Indirect calorimetry
The relative contributions from fat and carbohydrate to the oxidative metabolism (as percentages of total oxygen uptake) were estimated from the respiratory exchange ratio (RER) as previously described (Roepstorff et al. 2002).
Statistical analysis
Data are presented as means ± s.e.m. For variables independent of time, Student's paired t test was performed to test for differences between LG and HG trials. For variables measured before and after exercise as well as variables measured before and during exercise, a two-way analysis of variance (ANOVA), with repeated measures for the time and trial factors, was performed to test for differences between trials or changes due to time. When a significant main effect of time was found, significant pairwise differences were detected using Tukey's post hoc test. Unless otherwise stated, a probability of 0.05 was used as the level of significance.
Results
Work load
Subjects exercised at the same power output in LG and HG trials (180 ± 7 W). This resulted in a difference between trials in exercise intensity, expressed as V̇o2peak as a percentage of V̇o2peak, which averaged 68 ± 1 and 62 ± 1% V̇o2peak in LG and HG, respectively (P < 0.001).
Respiratory exchange ratio
The respiratory exchange ratio (RER) was markedly lower in LG than in HG at rest and during the bicycle exercise test (Table 1).
Table 1.
RER and arterial blood or plasma metabolite and hormone concentrations before and during 60 min of bicycle exercise at 65% V̇o2peak with low (LG) or high (HG) muscle glycogen
| Rest | 20 min exercise | 40 min exercise | 60 min exercise | ||
|---|---|---|---|---|---|
| Respiratory exchange ratio | LG | 0.76 ± 0.02‡‡ | 0.84 ± 0.01 | 0.83 ± 0.02 | 0.82 ± 0.01 |
| HG** | 0.83 ± 0.03‡‡ | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.91 ± 0.01 | |
| Blood glucose (mm) | LG | 4.9 ± 0.2 | 4.8 ± 0.1 | 4.5 ± 0.2‡ | 4.8 ± 0.2 |
| HG | 5.1 ± 0.1 | 4.9 ± 0.1 | 4.6 ± 0.1‡ | 4.9 ± 0.1 | |
| Plasma FA (μm) | LG | 674 ± 110† | 577 ± 77 | 573 ± 70 | 703 ± 58†§ |
| HG* | 596 ± 112† | 417 ± 36 | 479 ± 38 | 618 ± 58†§ | |
| Plasma insulin (μU ml−1) | LG | 6.8 ± 1.0‡‡‡ | 4.2 ± 0.3 | 4.0 ± 0.2 | 3.4 ± 0.2 |
| HG | 6.6 ± 0.6¶ | 5.4 ± 0.4# | 4.5 ± 0.3 | 4.2 ± 0.3# | |
| Plasma adrenaline (nm) | LG | 0.6 ± 0.1‡‡‡ | 1.9 ± 0.3 | 2.5 ± 0.5 | 2.9 ± 0.5† |
| HG | 0.5 ± 0.1‡‡‡ | 1.6 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.3† | |
| Plasma noradrenaline (nm) | LG | 1.2 ± 0.3‡‡‡ | 15.2 ± 1.3 | 16.1 ± 1.8 | 16.1 ± 1.9 |
| HG | 1.5 ± 0.3‡‡‡ | 10.6 ± 0.8## | 12.2 ± 1.1## | 11.0 ± 1.3## |
Data are means ± s.e.m. Main effect of trial
P < 0.01
P < 0.001; different from LG
P < 0.05
P < 0.01; different from all other time points within trial
P < 0.05
P < 0.01
P < 0.001
different from 20 min within trial, P < 0.05
different from 40 min within trial, P < 0.05
different from 40 and 60 min within trial, P < 0.001.
Intravenous glucose infusion
The intravenous glucose infusion rate averaged 39.4 ± 5.3 and 14.8 ± 5.8 μmol (kg BM)−1 min−1 during the bicycle exercise test in LG and HG, respectively (P < 0.01).
Circulating metabolites
The arterial blood glucose concentration at rest was not significantly different between trials (Table 1). Due to the controlled and variable intravenous glucose infusion, the arterial blood glucose concentration during exercise was similar between trials and kept almost constant at the resting level.
The arterial plasma FA concentration decreased (P < 0.05) from rest to 20 min of exercise and subsequently increased (P < 0.05) throughout exercise (Table 1). The arterial plasma FA concentration was higher in LG than in HG at rest and during exercise (P < 0.01).
Plasma hormones
The arterial plasma insulin concentration at rest did not differ significantly between LG and HG (Table 1). In both trials the arterial plasma insulin concentration decreased (P < 0.001) continuously during the bicycle exercise test. The arterial plasma insulin concentration was lower in LG than in HG at 20 and 60 min of exercise (P < 0.05).
At rest the arterial plasma adrenaline concentration was similar in LG and HG (Table 1). From rest to 20 min of exercise an increase (P < 0.001) was seen after which a further continuous increase (P < 0.05) occurred. During exercise the arterial plasma adrenaline concentration did not differ significantly between LG and HG.
The resting arterial plasma noradrenaline concentration did not differ significantly between LG and HG (Table 1). At the onset of exercise it increased (P < 0.001) and then remained constant throughout exercise. During exercise the arterial plasma noradrenaline concentration was higher in LG than in HG (P < 0.01).
Muscle substrates
Concentrations of glycogen and myocellular triacylglycerol (MCTG) in the vastus lateralis muscle before and after 60 min of bicycle exercise are shown in Table 2. The glycogen concentration before and after exercise was markedly lower in LG than in HG (P < 0.001). A significant breakdown of glycogen was observed during exercise in both trials (P < 0.001) and the total breakdown was lower in LG than in HG (99 ± 21 and 210 ± 30 mmol (kg d.w.)−1 in LG and HG, respectively, P < 0.01). The MCTG concentration was higher in LG than in HG before and immediately after the exercise test (P < 0.05). The decline in MCTG concentration during exercise was not significant (P = 0.06).
Table 2.
Glycogen and MCTG concentrations in the vastus lateralis muscle before and at 60 min of bicycle exercise at 65% V̇o2peak with low (LG) or high (HG) muscle glycogen
| Rest | 60 min of exercise | ||||
|---|---|---|---|---|---|
| LG | HG | LG | HG | ||
| Glycogen (mmol (kg dw)−1) | 197 ± 21 | 504 ± 25** | # | 98 ± 19 | 294 ± 43** |
| MCTG (mmol (kg dw)−1) | 57 ± 10 | 40 ± 7* | 43 ± 7 | 30 ± 5* | |
Data are means ± s.e.m. dw, dry weight. Different from LG
P < 0.05
P < 0.001
difference between rest and exercise, P < 0.001.
AMPK
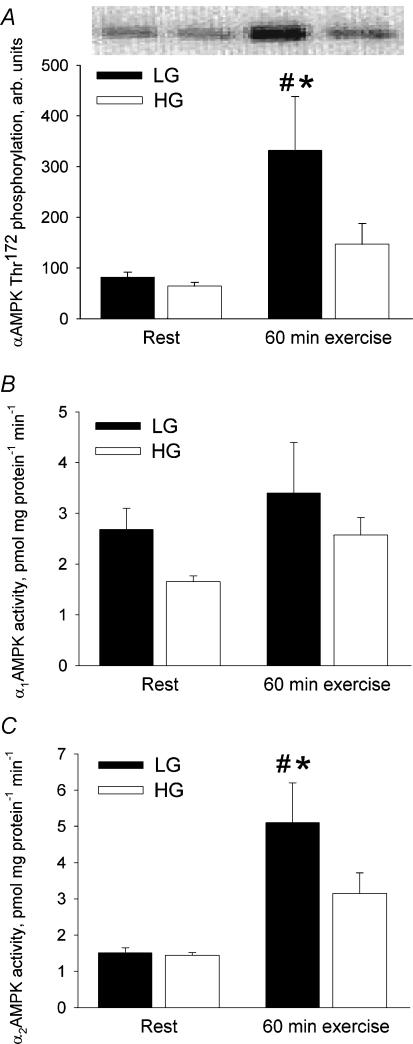
αAMPK Thr172 phosphorylation in the vastus lateralis muscle at rest did not differ significantly between trials (Fig. 2A). From rest to 60 min of exercise a 306% increase (P < 0.01) occurred in αAMPK Thr172 phosphorylation in LG whereas no significant change was observed in HG. At 60 min of exercise αAMPK Thr172 phosphorylation was 126% higher (P < 0.01) in LG than in HG.
Figure 2. AMPK phosphorylation and activity in the vastus lateralis muscle at rest and after 60 min bicycling at 65% V̇o2peak with low (LG) or high (HG) muscle glycogen.
A, αAMPK Thr172 phosphorylation. B, α1AMPK activity. C, α2AMPK activity. *Different from HG, P < 0.01; # different from rest, P < 0.01.
α1AMPK activity in the vastus lateralis muscle did not differ significantly between trials at rest or at 60 min of exercise (Fig. 2B). Neither did it increase significantly from rest to 60 min of exercise (P = 0.06).
α2AMPK activity was similar between the two trials at rest (Fig. 2C). A 238% increase (P < 0.01) from rest to 60 min of exercise was observed in LG whereas the exercise-induced 119% increase in α2AMPK activity in HG was not significant (P = 0.06). At 60 min of exercise α2AMPK activity was 62% higher in LG than in HG (P < 0.01).
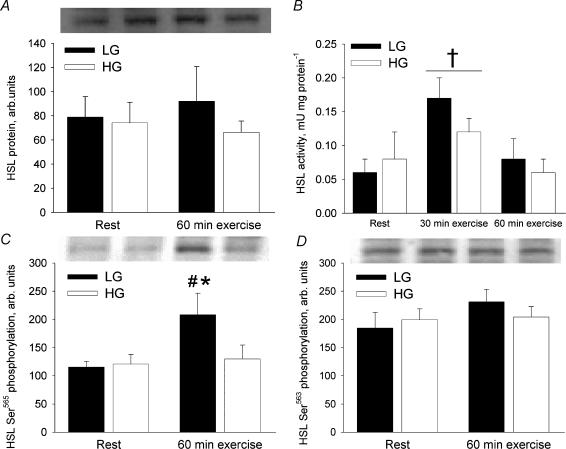
HSL
HSL activity was measured at rest and after 30 and 60 min of exercise whereas HSL protein content and phosphorylation were measured only at rest and after 60 min of exercise. This limitation was necessary due to insufficient muscle tissue obtained at 30 min of exercise. HSL Ser565 phosphorylation did not differ significantly between trials at rest (Fig. 3C). In LG the HSL Ser565 phosphorylation increased 80% from rest to 60 min of exercise (P < 0.01) whereas in HG it remained unchanged from rest to exercise. At 60 min of exercise HSL Ser565 phosphorylation was 61% higher in LG than in HG (P < 0.01).
Figure 3. HSL total protein, activity and phosphorylation in the vastus lateralis muscle at rest and after 60 min bicycling at 65% V̇o2peak with low (LG) or high (HG) muscle glycogen.
A, HSL protein. B, HSL activity calculated as neutral lipase activity measured in the absence of anti-HSL antibody minus neutral lipase activity measured in the presence of anti-HSL antibody. C, HSL Ser565 phosphorylation. D, HSL Ser563 phosphorylation. *Different from HG, P < 0.01; # different from rest, P < 0.01; † different from rest and 60 min exercise, P < 0.05.
HSL Ser563 phosphorylation was similar between the two trials at rest and no significant changes occurred from rest to 60 min of exercise (Fig. 3D).
The total amount of HSL protein in the vastus lateralis muscle was similar between LG and HG and no significant changes occurred in HSL protein amount from rest to 60 min of exercise (Fig. 3A).
Due to lack of muscle tissue, HSL activity was only measured in seven of the eight subjects. Total neutral lipase activity against tri[3H]olein increased 39% (P < 0.05) from rest (0.34 ± 0.04 and 0.39 ± 0.04 mU (mg protein)−1 in LG and HG, respectively) to 30 min of exercise (0.54 ± 0.10 and 0.46 ± 0.04 mU (mg protein)−1 in LG and HG, respectively) and then decreased (P < 0.05) to resting levels at 60 min of exercise without any significant differences between LG and HG trials. When muscle supernatants were pre-incubated with anti-HSL, neutral lipase activity against tri[3H]olein was reduced by ∼20% at rest. The increase in total neutral lipase activity against tri[3H]olein seen from rest to 30 min of exercise was completely abolished by pre-incubation with anti-HSL. HSL activity, calculated as neutral lipase activity inhibited by pre-incubation with anti-HSL, is shown in Fig. 3B. At rest HSL activity was similar in LG and HG. A mean increase of 117% was observed from rest to 30 min of exercise (P < 0.05). At 60 min of exercise HSL activity had returned to resting levels in both trials.
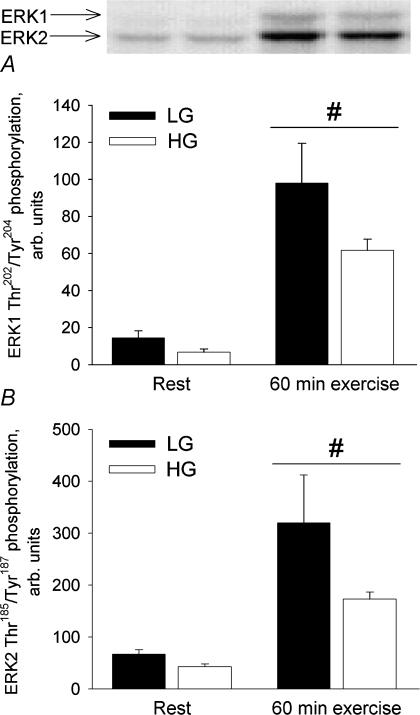
ERK1/2 phosphorylation
ERK1 Thr202/Tyr204 phosphorylation as well as ERK2 Thr185/Tyr187 phosphorylation increased significantly (P < 0.01) from rest to 60 min of exercise in both trials (Fig. 4). The difference in ERK1 Thr202/Tyr204 phosphorylation and ERK2 Thr185/Tyr187 phosphorylation between LG and HG was not significant (P = 0.06).
Figure 4. ERK1/2 phosphorylation in the vastus lateralis muscle at rest and after 60 min bicycling at 65% with low (LG) or high (HG) muscle glycogen.
A, ERK1 Thr202/Tyr204 phosphorylation. B, ERK2 Thr185/Tyr187 phosphorylation. #Different from rest, P < 0.01.
Discussion
The present study provides the first information on HSL Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. α2AMPK activity was manipulated during exercise, by varying pre-exercise muscle glycogen content, and parallel changes in phosphorylation of HSL on the AMPK target site Ser565 were observed, suggesting that α2AMPK phosphorylates HSL on Ser565 during exercise in humans. However, despite different levels of HSL Ser565 phosphorylation at 60 min of exercise in LG and HG trials, HSL activity and MCTG hydrolysis did not differ between these trials during exercise. Furthermore, while at 60 min of exercise α2AMPK activity and HSL Ser565 phosphorylation were increased from the resting level in the LG trial, HSL activity had returned to basal levels. In accordance with our hypothesis, the present findings therefore suggest that in human skeletal muscle HSL phosphorylation on Ser565 is not a primary regulator of HSL activity during exercise. Due to insufficient amounts of muscle tissue collected at 30 min of exercise AMPK activity was not measured at this time point. However, in our recent study, with similar exercise intensity and duration to those in the present study, it was shown that muscle α2AMPK activity was already increased after 10 min of exercise with low pre-exercise muscle glycogen and remained at this level at 60 min of exercise (Wojtaszewski et al. 2003). Thus, in the present study the increase in HSL activity from rest to a similar level in both trials at 30 min of exercise most likely occurred despite markedly higher α2AMPK activity at 30 min of exercise in the LG than in the HG trial, which further supports the notion that in human skeletal muscle α2AMPK activation during exercise does not inhibit HSL activity. This conclusion is also in accordance with the findings by Donsmark et al. (2004) in isolated contracting rat soleus muscle, where it was shown that HSL activity may vary in the face of constant AMPK activity and HSL Ser565 phosphorylation. In contrast to our conclusion that AMPK does not inhibit muscle HSL activity during moderate exercise, Watt et al. (2004b) recently published a paper reaching the opposite conclusion. Thus, based on a study in which α2AMPK activity during moderate intensity exercise was manipulated by altering pre-exercise muscle glycogen levels, as in the present study, they concluded that AMPK activity could inhibit β-adrenergic stimulation of muscle HSL activity during exercise. This finding was supported by studies in L6 myotubes in which stimulation of AMPK activity by AICAR inhibited adrenaline-stimulated HSL activation (Watt et al. 2004b). Surprisingly, in that study the lack of HSL activation by exercise in the low glycogen trial was seen together with significant MCTG hydrolysis, whereas the HSL activation during the control trial did not induce any MCTG hydrolysis (Watt et al. 2004b). The quite different results pertaining to the role of AMPK in regulation HSL activity during exercise obtained in the two studies may possibly be explained by differences in study design. Thus, in contrast to the study by Watt et al. (2004b), we sought to avoid confounding influence on HSL activity and HSL phosphorylation by minimizing the differences between the two exercise trials in the concentrations of several blood metabolites and hormones that might also influence HSL activity. This was accomplished by serving a light meal 4.5 h before exercise and by infusing glucose intravenously during exercise to keep the arterial blood glucose concentration at the resting level and similar between the two trials. In contrast, in the study by Watt et al. subjects were fasted overnight and therefore they differed markedly between trials in their plasma concentrations of glucose, FA and adrenaline (Watt et al. 2004b).
In the present study we showed that in contrast to phosphorylation on HSL Ser565, phosphorylation on HSL Ser563 was not increased during exercise in spite of a fourfold and a tenfold increase in plasma adrenaline and noradrenaline concentrations, respectively, during exercise. This was a surprising finding since PKA in vitro clearly phosphorylated HSL on Ser563 (Fig. 1B and Garton et al. 1989). Therefore, the present findings suggest that Ser563 on human skeletal muscle HSL may not be a target for PKA during exercise in vivo. Alternatively, HSL in human skeletal muscle may be constitutively phosphorylated on Ser563 by resting plasma catecholamine levels, and/or higher plasma catecholamine concentrations than observed in the present study are needed to increase skeletal muscle HSL Ser563 phosphorylation during exercise. This interpretation is in agreement with the notion based on studies in adipocytes that PKA-stimulated HSL activity may not necessarily depend on HSL Ser563 phosphorylation (Anthonsen et al. 1998). Two other sites on HSL (Ser659 and Ser660) were shown to be phosphorylated by PKA in isolated rat adipocytes (Anthonsen et al. 1998). However, the phosphorylation state on these two sites was not determined in the present study since phospho-specific antibodies against HSL phosphorylated on Ser659 and Ser660 have not yet become available to us.
The present study used an anti-HSL antibody to distinguish between activity of HSL and activity of other neutral lipases in human skeletal muscle. The pre-incubation of supernatants prepared from resting muscle with anti-HSL inhibited total neutral lipase activity against tri[3H]olein by 20%. This suggests that other triacylglycerol lipases are also present in human skeletal muscle as was previously indicated in rat skeletal muscle (Langfort et al. 2000). However, the 39% increase in total neutral lipase activity seen from rest to 30 min of exercise in the present study was completely abolished when muscle supernatants were pre-incubated with anti-HSL. Therefore, the increase in total neutral lipase activity seen from rest to 30 min of exercise could be completely accounted for by the increase in HSL activity (Fig. 3B) and the activity of other neutral lipases therefore did not increase in response to exercise in the present study. Thus, the present study directly shows that HSL activity, but not the activity of other neutral lipases, increases in human skeletal muscle during exercise. This is in accordance with a recent study where it was shown that the exercise-induced increase in total neutral lipase activity in human skeletal muscle could be completely ascribed to HSL activation (Watt et al. 2004b).
In the present study, HSL activity was on average 117% higher after 30 min of exercise compared to the resting level, irrespective of muscle glycogen content (Fig. 3B). However, by 60 min of exercise HSL activity had returned to resting levels, suggesting that the MCTG hydrolysis seen in both trials (P = 0.06) occurred primarily during the first 30 min of exercise. In accordance with this, it has previously been shown that MCTG hydrolysis occurs early during continuous exercise (Essen et al. 1977). The activation of HSL during the initial stage of exercise seen in the present study supports previous findings in isolated rat soleus muscle where the contraction-induced increase in HSL activity was transient (Langfort et al. 2000). Furthermore, Watt et al. (2003a,b) have shown that total neutral lipase activity against tri[3H]olein increased rapidly at initiation of exercise but then declined during continued exercise. The factor(s) causing HSL activity to decrease during the later stages of prolonged submaximal exercise are not known. The use of kinase inhibitors in isolated contracting rat skeletal muscle suggests that HSL activation in contracting skeletal muscle is mediated by PKC and partly via ERK (Donsmark et al. 2003). Ca2+, which activates some PKC isoforms, may therefore regulate HSL activity during exercise. Conflicting results have been obtained on the effect of Ca2+ on HSL activity (Donsmark et al. 2003; Watt et al. 2003c). Thus, caffeine, which increases intracellular Ca2+ in the absence of contraction, has been shown to increase HSL activity in isolated rat skeletal muscle at rest (Donsmark et al. 2003). Conversely, caffeine or cyclopiazonic acid, a sarcoplasmic reticulum Ca2+-ATPase inhibitor that increases intracellular Ca2+, both reduced HSL activity in isolated rat skeletal muscle at rest (Watt et al. 2003c). Nonetheless, regulation of HSL activity during exercise may be different from the resting condition so it could be speculated that intracellular Ca2+ flux between the sarcoplasmic reticulum and the cytoplasm may change with time during prolonged moderate intensity exercise, leading to diminished cytosolic Ca2+ even with constant workload, and consequently reduced Ca2+-dependent signalling (e.g. PKC activation of HSL).
In the present study ERK was phosphorylated, and thereby presumably activated, during exercise in both trials (Fig. 4) and an effect of muscle glycogen content was observed, with ERK phosphorylation being higher in LG than in HG (P = 0.06). To our knowledge this is the first study showing that ERK activation in human skeletal muscle during exercise is more pronounced when the muscle glycogen concentration is low compared with high. In contrast, it was recently shown that ERK phosphorylation in human skeletal muscle was higher both at rest and during exercise when the muscle glycogen content was normal compared with low (Watt et al. 2004b). It is difficult to explain this apparent discrepancy between the present study and the study by Watt et al. (2004b). ERK phosphorylation is induced by cellular stress and is higher in human skeletal muscle during high-intensity than during low-intensity exercise (Widegren et al. 2000; Widegren et al. 2001). Therefore, the higher ERK phosphorylation seen during exercise with low muscle glycogen content in the present study is in line with the general view of ERK being activated by muscle stress and/or disturbances in cellular homeostasis (Widegren et al. 2001). It has been suggested that ERK acts upstream of AMPK during AICAR-stimulation of AMPK (Chen et al. 2002). The parallel activation of ERK and AMPK during exercise in the LG compared to the HG trial suggests that there may also be a connection between ERK and AMPK activation in skeletal muscle during exercise.
ERK has been shown to activate HSL in 3T3-L1 adipocytes (Greenberg et al. 2001) and in isolated rat soleus muscle (Donsmark et al. 2003) presumably via phosphorylation of Ser600 on HSL (Greenberg et al. 2001). Therefore, we cannot exclude the possibility that, with respect to HSL regulation, the ∼75% higher ERK phosphorylation may have been opposed by the ∼65% higher α2AMPK activity in LG compared with HG. If so, AMPK may have inhibited HSL activity in the LG trial. However, during the later stages of prolonged submaximal exercise with ingestion of nicotinic acid, α2AMPK activity was higher than at rest whereas ERK phosphorylation was at the resting level and, nevertheless, total neutral lipase activity against tri[3H]olein did not change (Watt et al. 2004a), suggesting that AMPK does not regulate HSL activity during exercise even in the absence of ERK activation.
In summary, the present study demonstrates that the high AMPK activation, seen during prolonged submaximal exercise in human skeletal muscle with reduced glycogen, is paralleled by high HSL Ser565 phosphorylation. Apparently, AMPK activation and HSL Ser565 phosphorylation had no effect on HSL activity and MCTG hydrolysis, although it cannot be ruled out that other factors may have opposed an effect of AMPK on HSL activity. HSL Ser563 phosphorylation was not increased during exercise despite an increase in plasma adrenaline concentrations. In vitro studies on adipocyte HSL have shown that pharmacological activation of AMPK with AICAR leads to phosphorylation of Ser565 and inhibition of the HSL activation induced by β-adrenergic agents (Garton et al. 1989; Sullivan et al. 1994; Corton et al. 1995). Also in myotubes, AICAR abolished the HSL activation induced by adrenaline although no direct effect of AICAR was observed (Watt et al. 2004b). As AMPK is thought to be a general fuel sensor, which increases fuel availability when energy status is perturbed, it would seem counterproductive if AMPK decreased the hydrolysis of MCTG via inhibition of HSL in vivo at least during moderate exercise where lipid combustion covers a large fraction of energy provision. In this context, the present study provides important information showing that in human skeletal muscle in vivo, AMPK activation during moderate exercise does not impair HSL activity in agreement with its role as a fuel provider rather than a fuel inhibitor.
Acknowledgments
We thank Dr Cecilia Holm, Department of Cell and Molecular Biology, Lund University, Sweden, for kindly providing the recombinant rat HSL and the anti-HSL antibody. We acknowledge the skilled technical assistance of Irene Bech Nielsen, Betina Bolmgren, and Winnie Taagerup. This study was supported by The Danish National Research Foundation (Grant 504-14), The Copenhagen Muscle Research Centre, The Novo Nordisk Research Foundation, The Danish Diabetes Association, a Research and Technological Development Project (QLG1-CT-2001-01488) funded by the European Commission, The Danish Sports Research Council, The Carlsberg Foundation (J.N.N), The Wellcome Trust (UK) (K.A.G and D.G.H), The Danish Medical Research Council (J.F.P.W.), and a Hallas Møller Fellowship from The Novo Nordisk Foundation (J.F.P.W.).
References
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Aronson D, Wojtaszewski JFP, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol Cell Physiol. 1998;44:C555–C561. doi: 10.1152/ajpcell.1998.275.2.C555. [DOI] [PubMed] [Google Scholar]
- Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Farese RV. Activation of the ERK pathway and atypical protein kinase C Isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside – A specific method for activating amp-activated protein-kinase in intact-cells. European J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Contractions activate hormone-sensitive lipase in rat muscle by protein kinase C and mitogen-activated protein kinase. J Physiol. 2003;550:845–854. doi: 10.1113/jphysiol.2003.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Contractions induce phosphorylation of the AMPK site Ser565 in hormone-sensitive lipase in muscle. Biochem Biophys Res Commun. 2004;316:867–871. doi: 10.1016/j.bbrc.2004.02.140. [DOI] [PubMed] [Google Scholar]
- Essen B, Hagenfeldt L, Kaijser L. Utilization of blood-borne and intramuscular substrates during continuous and intermittent exercise in man. J Physiol. 1977;265:489–506. doi: 10.1113/jphysiol.1977.sp011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport – Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutrition. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Howlett K, Langfort J, Zimmerman-Belsing T, Lorentsen J, Bulow J, Ihlemann J, Feldt-Rasmussen U, Galbo H. Adrenaline and glycogenolysis in skeletal muscle during exercise: a study in adrenalectomised humans. J Physiol. 2000;528:371–378. doi: 10.1111/j.1469-7793.2000.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282:E435–E447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Roepstorff K, Kiens B. Regulation of plasma long-chain fatty acid oxidation in relation to uptake in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2004 doi: 10.1152/ajpendo.00001.2004. (in press DOI: 10.1152/ajpendo.00001.2004) [DOI] [PubMed] [Google Scholar]
- Steffensen CH, Roepstorff C, Madsen M, Kiens B. Myocellular triacylglycerol breakdown in females but not in males during exercise. Am J Physiol Endocrinol Metab. 2002;282:E634–E642. doi: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J. 1999;19:433–439. doi: 10.1046/j.1365-313x.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein-kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJF, O'Neill M, Spriet LL. Hormone-sensitive lipase activity and fatty acyl-CoA content in human skeletal muscle during prolonged exercise. J Appl Physiol. 2003a;95:314–321. doi: 10.1152/japplphysiol.01181.2002. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJF, Spriet LL. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. J Physiol. 2003b;547:301–308. doi: 10.1113/jphysiol.2002.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004a;287:E120–E127. doi: 10.1152/ajpendo.00542.2003. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Chan S, Garnham A, Kemp BE, Febbraio MA. β-Adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signaling. FASEB J. 2004b;18:1445–1446. doi: 10.1096/fj.03-1067fje. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Heigenhauser GJF, Spriet LL, Dyck DJ. Hormone-sensitive lipase activity and triacylglycerol hydrolysis are decreased in rat soleus muscle by cyclopiazonic acid. Am J Physiol Endocrinol Metab. 2003c;285:E412–E419. doi: 10.1152/ajpendo.00023.2003. [DOI] [PubMed] [Google Scholar]
- Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172:227–238. doi: 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Widegren U, Wretman C, Lionikas A, Hedin G, Henriksson J. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflugers Arch. 2000;441:317–322. doi: 10.1007/s004240000417. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha 1 and alpha 2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]