Abstract
Allopregnanolone (AP) is a potent modulator of the GABAA receptor. Brain AP concentrations increase in response to stress, which is thought to provide neuroprotection by reducing excitation in the adult brain. Umbilical cord occlusion (UCO) causes hypoxia and asphyxia in the fetus, which are major risk factors associated with poor neurological outcome for the neonate, and may lead to adverse sequelae such as cerebral palsy. The aims of this study were as follows: (i) to determine the effect of 10 min UCO on AP concentrations in the extracellular fluid of the fetal brain using microdialysis, and (ii) to compare the content of the steroidogenic enzymes P450scc and 5α-reductase type II (5αRII) with brain and CSF neurosteroid concentrations. UCO caused fetal asphyxia, hypertension, bradycardia and respiratory acidosis, which returned to normal levels after 1–2 h. AP concentrations in dialysate samples from probes implanted in grey and white matter of the parietal cortex were significantly increased 1 h after UCO from control levels of 10.4 ± 0.4 and 12.4 ± 0.3 to 26.0 ± 5.1 and 27.6 ± 6.4 nmol l−1, respectively (P < 0.05), before returning to pre-occlusion levels by 3–4 h after UCO. When fetal brains were collected 1 h after a 10 min UCO, the relative increases of AP and pregnenolone content in the parietal cortex were similar to the increase observed in the extracellular (dialysate) fluid. AP, but not pregnenolone, was increased in CSF at this time. P450scc and 5αRII enzyme expression was significantly increased in the cerebral cortex in the UCO fetuses compared to control fetuses. These results suggest that the fetal brain is capable of transiently increasing neurosteroid production in response to asphyxia. The action of the increased neurosteroid content at GABAA receptors may serve to diminish the increased excitation due to excitotoxic amino acid release, and provide short-term protection to brain cells during such stress.
Neurosteroids that have potent sedative properties may be synthesized de novo in the brain or from precursors derived from the systemic circulation. Pregnane neurosteroids such as allopregnanolone (AP) interact with the γ-aminobutyric acid/benzodiazepine receptor–chloride ionophore (GABAa receptor), increasing GABAergic activity, and thus reducing CNS excitability (Majewska et al. 1986). Other steroids such as pregnenolone and pregnenolone sulphate bind to a distinct binding site on the GABAa receptor to reduce GABAergic inhibition and also act on the NMDA receptor, and may counteract the increased GABAergic inhibition produced by AP. This suggests that the balance of suppressive and excitatory neurosteroids may regulate CNS activity and the susceptibility of the brain to excitation-induced damage.
Enzymes that control the production of GABAa agonist steroids have been shown to be present in the fetal (Nguyen et al. 2003b), newborn (Nguyen et al. 2003b) and adult brain (Mellon & Deschepper, 1993). Immunoreactive cytochrome P450scc side-chain cleavage enzyme (P450scc), which catalyses the irreversible conversion of cholesterol to pregnenolone, has been shown in our previous studies to be expressed in the brains of both the fetal and neonatal sheep (Petratos et al. 2000; Nguyen et al. 2003b). The metabolism of progesterone to the GABAa-active metabolite AP requires 5α- and 3α-reduction by 5α-reductase and 3α-hydroxysteroid oxidoreductase, respectively. 5α-Reductase type-II, which converts progesterone into 5α-dihydroprogesterone, the immediate precursor for AP formation, has also been shown to be present in the fetal and neonatal brain (Petratos et al. 2000; Nguyen et al. 2003b).
Previous studies have shown that AP concentrations are increased following stress. In the adult rat, stressful stimuli of handling and swim stress have been shown to increase AP content in the brain and plasma (Purdy et al. 1991; Barbaccia et al. 1994). The fetal brain while in utero may also be subjected to a variety of stressors including acute hypoxia, asphyxia, or infection-induced inflammation, which may all cause permanent damage, leading to neurological impairment. The responses elicited in the fetal brain following stressful stimuli in utero remain unknown; however, the presence of elevated concentrations of AP in the fetal brain may be beneficial, as it would possibly prevent excessive excitation after periods of stress induced by hypoxia or asphyxia.
The aims of this study were firstly to determine the changes of AP in extracellular fluid following a brief (10 min) umbilical cord occlusion (UCO) using microdialysis probes placed in the grey and white matter of the fetal brain. Secondly, we sought to explain these changes by obtaining the fetal brain 1 h after UCO to determine the neurosteroid content of the tissue, and then to determine expression of two key steroidogenic enzymes, P450scc and 5α-reductase type II (5αRII), in the fetal brain. The results show that the fetal brain is capable of increasing production of the sedative neurosteroids in response to stressful stimuli, in utero.
Methods
All procedures were conducted in accordance with the Code of Practice for the Care and Use of Animals for Scientific Purposes of the National Health and Medical Research Council, and they had received prior approval from the Monash University Committee on Ethics in Animal Experimentation.
Surgical procedures
Using aseptic techniques, surgery was performed on pregnant Border Leicester/Merino crossbred ewes carrying a single fetus at 124–126 days of gestation. General anaesthesia was induced by intravenous injection of thiopentone sodium (20 mg kg−1; Pentothal, Abbott Laboratories, North Ryde, NSW, Australia) with anaesthesia maintained by inhalation of 1–2% halothane (Fluothane; Astra Zeneca, Kernell, NSW, Australia). The fetus was partially exteriorized and a polyvinyl catheter was implanted into a brachial artery, with a second catheter implanted in the amniotic sac to record amniotic fluid pressure. Microdialysis probes (CMA/20; CMA Microdialysis, Sweden) were implanted in the parietal cortex at depths of 14 mm and 10 mm through 1 mm burr holes drilled 10 mm either side of the midline and 5 mm in front of the coronal suture. The probes were secured in place with cyanoacylate glue (Vetbond tissue adhesive no. 1469, 3M Animal Care Products, St Paul, Germany). The correct positioning of the microdialysis probes was verified at postmortem. Two inflatable silastic cuffs (In Vivo Medical, Ukiah, CA, USA) were also placed around the abdominal end of the umbilical cord and sutured to the fetal skin. The fetus was returned to the uterus, with the catheters and microdialysis tubing exteriorized through a maternal flank incision. After the repair of all incisions, the ewe was allowed to recover for 2–3 days before beginning the experimental procedures. Ewes received postoperative analgesia with buprenorphine (0.005 mg kg−1, i.m.) immediately after surgery.
Fetal monitoring, blood gas parameters and pH
Fetal arterial pressure, amniotic fluid pressure and fetal heart rate were recorded throughout each experiment using a Grass Polygraph (Model 7D Polygraph, Grass Instrument Co., Quincy, MA, USA) with signals passed through to an analog-to-digital converter (Maclab, ADInstruments, Castle Hill, NSW, Australia) for recording and storage. Pressures were recorded using disposable pressure transducers (Model 41500503 A, Maxxim Medical, Athens, TX, USA) Amniotic pressure was electronically subtracted from blood pressure to account for changes caused by uterine contractions in the position and movement of the ewe. Mean arterial pressure (MAP) was calculated electronically as the diastolic pressure + 1/3 (systolic–diastolic pressure). Blood samples (0.2 ml) were taken for the measurement of fetal arterial pH, arterial partial pressure of CO2 (PaCO2), arterial partial pressure of O2 (PaO2) and arterial O2 saturation (SaO2) using an ABL520 blood gas analyser (Radiometer, Copenhagen Denmark). Fetal blood gas measurements were corrected to the expected fetal temperature of 39.0 °C.
Experimental protocol
Each fetus was subjected to 10 min of complete UCO on postoperative days 2 and 6. The purpose of the first occlusion (day 4) was to determine the changes in extracellular fluid levels of AP for at least 24 h following UCO. To do this, microdialysis samples were collected at least 24 h before and for 24 h after the 10 min UCO. The purpose of the second occlusion (day 6) was to compare changes in AP concentrations in extracellular fluid (dialysate) with levels in the brain itself. To do this, microdialysate samples were collected up to 1 h after the UCO, and the fetus was killed to obtain brain tissue for neurosteroid measurement. Expression of 5αRII and P450scc was also determined in these samples. This protocol has the additional advantage of minimizing the number of animals required for this study.
Beginning 3 days after surgery, the microdialysis probes were perfused with artificial CSF (mm: 125 NaCl, 2.5 KCl, 27 NaHCO3, 0.5 NaH2PO4.H2O, 1.2 Na2HPO4, 0.5 Na2SO4, 1 MgCl2.6H2O) at a rate of 2 μl min−1 using a syringe pump (74900 Series, Cole Parmer Instrument Co., USA). Following a 6 h equilibration period, microdialysate samples were collected into plastic tubes and placed on ice for 1 h periods. The transit time for fluid to flow from the probe tip to the end of the outflow catheter was 1 h, calculated as half the time taken to completely fill the inflow and outflow catheters. After collecting each sample for 1 h, the samples were frozen in liquid N2 and stored at −70 °C until assayed for AP.
Collection of the dialysate samples commenced at 0600 h on the third postoperative day, and continued until the ewe and fetus were killed on day 6. During the first 24 h period (i.e. on day 3), a sham cord occlusion (i.e. cuff not inflated) was conducted at 1000 hours, and 2.5 ml of fetal and maternal arterial blood was collected at −30, +5, +10, +30 and +60 and +240 min, this time for analysis of blood gases and pH, and recovery of plasma for measurement of steroids. On day 4, the umbilical cord occluder was completely inflated for 10 min commencing at 1000 hours using a predetermined volume of sterile water that completely occluded flow in the umbilical cord; 2.5 ml blood samples were taken at the time intervals shown above. Collection of microdialysis samples continued throughout day 4 and all of day 5. On day 6, a second UCO was performed and the cuff was again inflated at 1000 hours for 10 min (thus, the period of microdialysis sampling between the first and second occlusion was 48 h). Fetal and maternal blood samples were again taken at −30, +5, +10, +30, and +60 mins with respect to the start of the second occlusion. Then pentobarbitone sodium was administered to the ewe (130 mg kg−1 i.v.) to rapidly kill both the ewe and the fetus. CSF was collected into a 2.5 ml syringe through a 23 gauge needle pushed through the dura beneath the atlanto-occiptal membrane; CSF samples contaminated with blood were discarded. The final microdialysate sample for the last hour was collected from the efflux tube. The fetal brain was then removed from the skull, divided into gross anatomical regions, and stored at −70 °C together with CSF and dialysate samples until analysis.
An additional group of seven sham-operated fetuses from singleton pregnancies, in which no UCO was performed, were killed at 132–134 days of gestation in order to obtain age-matched control fetal brain tissue to compare with the brains collected from fetuses 1 h after UCO. Sham-operated fetuses were confirmed at autopsy to be ‘healthy’ and did not show any signs of compromised development.
Neurosteroid radioimmunoassay
Neurosteroids were extracted from brain tissue by a modification of the method of Barbaccia et al. (1992) as previously described for brain tissue (Nguyen et al. 2003b). Briefly, brain tissue was homogenized and extracted three times with 50% methanol containing 1% acetic acid. The extracts were applied to pre-primed Sep-Pak C18 cartridges (Waters Corp., Milford, MA, USA). Steroids were eluted with 100% methanol, and the collected fractions were dried under N2, and resuspended in 1.0 ml assay buffer (0.1 m PBS, pH 7.0). Recovery was assessed using parallel run samples with the addition of radiolabelled steroids. Recoveries were 66.2 ± 6.2% for AP (n = 7 extractions), 83.5 ± 4.6% for progesterone (n = 7 extractions) and 60.2 ± 2.6% for pregnenolone (n = 7 extractions). Corrections for losses during extraction were included in final calculations.
AP was measured by specific radioimmunoassay using a polyclonal antibody purchased from Dr R. H. Purdy (San Diego, CA, USA), which has been previously characterized (Bernardi et al. 1998) and used in studies with fetal sheep (Nguyen et al. 2003a,b). AP was measured from extracted samples as described above, while AP in CSF and microdialysis samples was measured directly without extraction. The limit of detection for AP was 0.20 ± 0.04 pmol per tube (n = 10) and the intra- and interassay coefficients of variance were 5% and 9%, respectively. Pregnenolone and progesterone were measured by radioimmunoassay using antibodies purchased from ICN Biomedicals (Seven Hills, NSW, Australia) and provided by Dr J. Malecki (Bairnsdale, Victoria, Australia), respectively, as previously described (Rice & Thorburn, 1986). The limit of detection for pregnenolone was 0.10 ± 0.03 pmol per tube (n = 10) and progesterone was 0.11 ± 0.02 pmol per tube (n = 10). The intra- and interassay coefficients of variance were 5% and 16% respectively for pregnenolone, and 8% and 19% respectively for progesterone. Cross reactivity of these antisera has been reported (Nguyen et al. 2003a).
In vitro AP probe recovery was determined prior to implantation and after removal from the fetuses at the end of the experiment. AP recovery was determined by placing the microdialysis probe into an artificial CSF solution containing a known concentration of AP, equivalent to that found in brain tissue of normal fetuses (Nguyen et al. 2003b). Probes were then perfused at a rate of 2 μl min−1 and the AP concentration in the collected dialysate was measured. The efficiency of AP transfer through the microdialysis membrane was found to be 20.8 ± 1.2% (n = 10), with no significant differences from recoveries measured prior to implantation or following removal for the fetuses. Corrections for the probe efficiency were included in final calculations of extracellular fluid concentration.
Immunoblotting
P450scc and 5αRII expression in the brain was determined by Western immunoblotting as previously described (Nguyen et al. 2003b). Briefly, frozen samples (∼0.1 g) were homogenized and concentrated by ammonium sulphate precipitation with protein content determined by the Bradford method (Bradford, 1976). A 15 μg quantity of protein was separated using SDS-PAGE on 15% separating gels and transferred onto 0.2 μm polyvinylidene difluoride membranes (Osmonics, Westborough, MA, USA) by electroblotting. Membranes were incubated with a 1: 3000 dilution of either the P450scc or the 5αRII antibody in Tris-buffered saline containing 0.2% Tween-20 for 1 h at 22°C. Specificities of the P450scc and 5αRII antibodies have been previously reported (Nguyen et al. 2003b). The immune complexes were visualized by chemiluminescence using the Amersham ECL detection system (Amersham, Buckinghamshire, UK) for 1 min and captured using BioMax ML autoradiograph film (Eastman Kodak, Rochester, NY, USA). Immunoblots were scanned and analysed using ImageQuaNT software (Amersham). The density of the bands was determined and individually corrected for background by subtracting the density of the blank background area immediately below each band.
Statistical analysis
Data are shown as mean ± standard error of the mean (s.e.m.). All data were analysed using SPSS statistical software (version 9.0, Chicago, IL, USA). Significance of changes in fetal MAP, heart rate, fetal blood gases/pH, AP concentration in microdialysate samples and plasma neurosteroid concentrations was determined by a two-way repeated measures ANOVA followed by a Student–Newman–Keul post hoc test. Significance of changes in brain neurosteroidogenic enzyme content, CSF neurosteroid levels and brain neurosteroid content was determined by Student's t test. P < 0.05 was considered to be statistically significant.
Results
Brain extracellular fluid AP concentrations: basal conditions
Allopregnanolone was detected in microdialysis efflux from both grey and white matter probes throughout the 24 h period preceding the first UCO. Concentrations ranged between 7.4 and 16.9 nmol l−1 in the grey matter and 10.6 and 19.7 nmol l−1 in white matter during the first 24 h preceding the first UCO. There was no evidence of a difference between day and night values. Mean concentrations over the 24 h were 10.7 ± 0.6 nmol l−1 in the grey matter and 13.6 ± 0.5 nmol l−1 in white matter, values which are not significantly different. The occasional increase and decrease of AP concentration in the dialysates were not obviously associated with changes of fetal arterial pressure or heart rate.
Brain extracellular fluid AP concentrations: measured by microdialysis–UCO with recovery
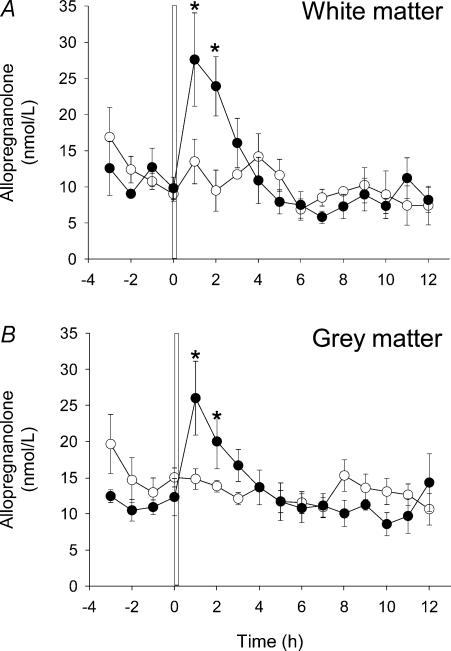
AP concentrations in both the grey and white matter immediately prior to the first UCO were not different from the mean values for the preceding 24 h. AP concentrations were significantly increased within 1 h following the first 10 min UCO in both the grey matter (26.0 ± 5.11 nmol l−1) and the white matter (27.6 ± 6.5 nmol l−1), with no difference in the AP concentration reached between the grey and white matter (Fig. 1). AP concentrations in both the grey and white matter had returned to basal levels by 3–4 h after UCO, and then did not differ significantly from the concentrations observed in the 24 h preceding UCO.
Figure 1. Allopregnanolone (AP) concentrations in fetal cerebral extracellular fluid before and after umbilical cord occlusion (UCO).
AP concentrations in fetal cerebral extracellular fluid (A, white matter; B, grey matter) measured by microdialysis probes. The probes were perfused continuously at 2 μl min−1 and samples were collected at hourly intervals (n = 5). A and B, open circles represent the control sampling period in which a sham UCO was performed; filled circles represent a period of sampling during which the first 10 min UCO was performed. The vertical bar in A and B represents the UCO period that commenced at time 0 (1000 hours) and was the first UCO of the experimental protocol (see text). Where no error bars exist, s.e.m. is within the area of the symbol. *P < 0.05 between the control and UCO samples for the same animal.
Fetal blood pressure, heart rate and blood gases/pH
Fetal blood pressure and heart rate (HR) were measured continuously along with regular blood sampling throughout the 24 h period preceding the first UCO. Fetal blood pressure, HR, PaO2 and PaCO2 did not vary significantly from mean values during the 24 h prior to the first UCO. The first UCO of 10 min duration produced hypertension and bradycardia, with severe asphyxia and respiratory acidosis (Table 1). There was a biphasic change in MAP with a transient increase after 5 min of UCO followed by a decline to below pre-UCO levels at 10 min. The initial rise in MAP was accompanied by a reduction in HR that returned to pre-UCO values by 10 min. On release of the cuffs at 10 min, HR increased slightly, but both MAP and HR had returned to normal levels by 1 h after UCO. Arterial pH decreased following UCO and was restored to normal levels by 2–3 h after the UCO. PaCO2 was significantly increased, while PaO2 and SaO2 decreased following the first UCO, with all three parameters returning to basal levels within 0.5–1 h after UCO.
Table 1. Fetal blood parameters before and after 10 min umbilical cord occlusion.
| Occlusion | Post occlusion | |||||
|---|---|---|---|---|---|---|
| Control −30 min | +5 min | +10 min | +40 min | +70 min | +250 min | |
| MAP (mmHg) | 33.5 ± 1.4 | 44.8 ± 5.5* | 30.3 ± 2.6 | 39.2 ± 1.7 | 37.2 ± 2.4 | 35.4 ± 3.8 |
| HR (beats min−1) | 158.5 ± 3.7 | 107.8 ± 13.9* | 128.7 ± 34.1 | 179.7 ± 18.0 | 178.9 ± 14.6 | 136.2 ± 10.6 |
| pH | 7.35 ± 0.02 | 7.02 ± 0.09* | 6.96 ± 0.09* | 7.21 ± 0.01* | 7.27 ± 0.01* | 7.36 ± 0.01 |
| PaCO2 (mmHg) | 48.1 ± 1.7 | 91.9 ± 12.0* | 94.2 ± 10.8* | 46.1 ± 2.7 | 43.3 ± 2.7 | 45.7 ± 1.5 |
| PaO2 (mmHg) | 21.5 ± 2.3 | 12.1 ± 1.9* | 11.7 ± 2.1* | 23.3 ± 3.0 | 23.9 ± 2.6 | 22.3 ± 1.5 |
| SaO2 (%) | 57.8 ± 6.8 | 14.5 ± 7.9* | 13.1 ± 6.1* | 55.2 ± 9.3 | 62.4 ± 7.8 | 63.9 ± 5.4 |
Fetal arterial pH, arterial partial pressure of CO2 (PaCO2), arterial partial pressure of O2 (PaO2), arterial O2 saturation (SaO2) and mean arterial pressure (MAP), heart rate (HR), pre (−30 min), during (+5 and +10 min) and after (+40, +70 and +250 min) the first 10 min UCO of the experimental protocol (see text). Data presented as mean ±s.e.m.
P < 0.05, significant difference from the pre-occlusion control value.
Plasma pregnenolone and AP concentrations
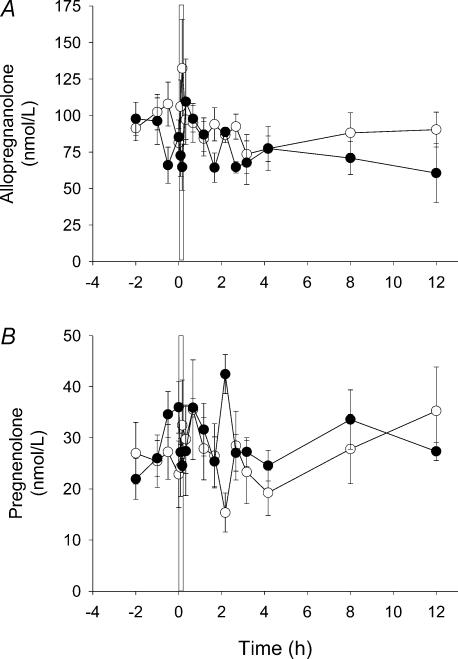
As for AP concentrations in the microdialysate samples, there was no significant variation of fetal plasma AP concentrations during the 24 h prior to the first UCO (Fig. 2A). However, whereas AP increased in the microdialysate samples following the UCO, plasma AP concentrations did not change significantly at any time following UCO. Fetal plasma pregnenolone concentrations also did not change significantly during the 24 h prior to the first UCO or in the 12 h after UCO (Fig. 2B). The occasional changes of AP and pregnenolone concentration in the fetal plasma were not significant, and they were not associated with changes in AP concentration in the microdialysate.
Figure 2. AP and pregnenolone concentrations in the fetal circulation before and after UCO.
Plasma AP (A) and pregnenolone (B) concentrations collected during the period of microdialysate sampling. A and B, open circles represent the control sampling period in which a sham UCO was performed; filled circles represent the sampling period during which the first 10 min UCO was performed. The vertical bar in A and B represents the UCO period that commenced at time 0 (1000 hours) and was the first UCO of the experimental protocol (see text). Where no error bars exist, s.e.m. is within the symbol. There were no significant differences between the control and UCO values within the same panel.
Neurosteroidogenic enzyme expression and neurosteroid content in brain and CSF following UCO
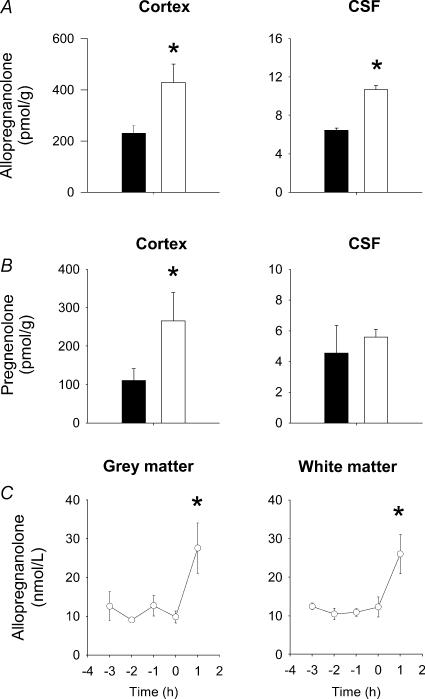
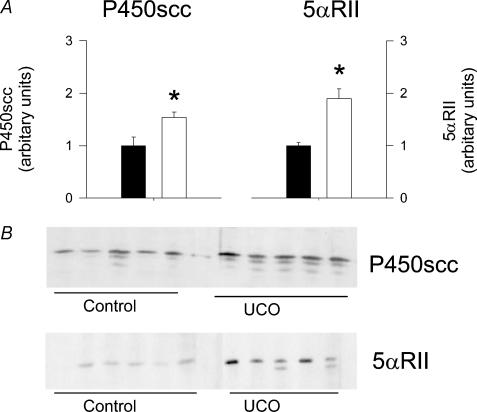
Cerebral cortex tissue was obtained 1 h after the second UCO on day 6 post surgery. The amounts of pregnenolone and AP in this tissue were significantly increased compared to the sham control (no occlusion) group (Figs 3A, B). The increase of AP relative to animals that did not receive a UCO was 2.18 ± 0.15-fold (P < 0.05). AP concentration in CSF was also increased after the second UCO (Fig. 3A), whereas the pregnenolone concentration was not changed (Fig. 3B). There was no change in progesterone content in the cortex tissue or in CSF following UCO compared to the control fetuses (data not shown). Microdialysis samples from grey and white matter were obtained at the same time as the tissues and CSF samples were collected, 1 h after the second UCO. The concentrations of AP in these samples were significantly increased compared to the concentrations in samples obtained immediately prior to the second UCO (Time (0), Fig. 3C) and with mean microdialysis AP levels during the 24 h preceding the second UCO (grey matter, 12.5 ± 0.5 nmol l−1; white matter, 10.5 ± 0.8 nmol l−1). Interestingly, the AP concentrations in the microdialysate after the second UCO were not significantly different from the elevated concentration values observed 1 h following the first UCO. The expression of both P450scc and 5αRII enzymes in the cortex collected 1 h after the second UCO was significantly increased compared to the levels observed in samples from age-matched control fetal brains (Fig. 4).
Figure 3. Fetal brain tissue and CSF AP concentrations 1 h after, and brain extracelluar fluid AP concentrations before and after a second UCO.
A, content of AP in cerebral cortex tissue and CSF. B, content of pregnenolone in the cortex and CSF. Brain and CSF samples were collected from age-matched fetuses that did not receive a UCO (n = 7, filled columns) and from fetuses 1 h after the second 10 min UCO of the experimental protocol (see text) (n = 5, open columns). Note the difference in scale for cortex content and CSF concentrations. C, AP concentrations in fetal cerebral extracellular fluid from grey and white matter. Extracellular fluid samples were obtained from microdialysis probes in grey and white matter and values shown are from 3 h before and 1 h after the second 10 min UCO (n = 5). Note, the microdialysis samples collected 1 h after UCO were obtained at the same time as the brain tissue and CSF samples were collected (see text). Values are means ±s.e.m.A and B, *P < 0.05, significant difference between samples from control fetuses and fetuses after UCO. C, *P < 0.05, AP concentrations 1 h after UCO were significantly higher compared to pre-UCO values at time 0 and to mean AP concentrations in microdialysate during the 24 h preceding UCO.
Figure 4. Fetal brain P450scc and 5α-reductase II expression 1 h following a second UCO.
P450scc and 5αRII expression in the cortex. A, densitometric values for enzyme expression in samples collected from control fetuses that did not receive the UCO (n = 7, filled columns) and from fetuses 1 h after the second 10 min UCO (n = 5, open columns) of the experimental protocol (see text). Each bar represents the mean ±s.e.m.*P < 0.05, significant difference between samples from control fetuses and fetuses after UCO. B, Western immunoblot showing P450scc and 5αRII expression in control and UCO fetuses.
Discussion
The principal finding of this study was that occlusion of the umbilical cord for 10 min, a procedure that causes profound but reversible systemic asphyxia, resulted in increased AP concentrations in the extracellular fluid in both the grey and white matter of the late gestation fetal sheep brain. We also found that the rise in AP concentration in microdialysates from both the grey and white matter following a second UCO was accompanied by an increase in AP concentration in the cortex and CSF, consistent with increased AP production within the brain. These changes in brain occurred without corresponding increases in plasma AP or pregnenolone, and together with the increased expression of the steroidogenic enzymes P450scc and 5αRII in the brain, support the contention that there is an increase in the synthesis of AP in the fetal brain following UCO. These observations indicate that the fetal brain is capable of an acute upregulation of AP production initiated within 60 min of a brief asphyxial insult.
The rise in AP concentrations following the asphyxia challenge is consistent with our previous finding of responses to hypoxic stress in newborn lambs (Billiards et al. 2002) and of others of responses to stress in adult animals (Barbaccia et al. 1996). We previously found that lambs of 10–26 days postnatal age subjected to 2 h of hypoxia displayed a dramatic elevation in brain AP content within 1 h (Billiards et al. 2002), while 1 min of CO2 inhalation in adult rats has been shown to cause an increase in AP within 30 min in the brain (Barbaccia et al. 1994). Furthermore, brain and spinal cord injury has also been shown to result in the accumulation of neurosteroids, including AP around the focal point of injury (Di Michele et al. 2000). In contrast to these observations, we have previously shown that prolonged hypoxemia produced by partial embolization of the placental circulation does not lead to an increase in AP content within the brain, although there was a small increase observed in the plasma (Nguyen et al. 2003a). Together, these results suggest that the fetal brain is capable of the upregulation of AP production following an acute insult; however, it is unable to maintain elevated levels during long-term hypoxaemia. This may be due to the reduction in levels of neurosteroid pre-cursors such as pregnenolone, which was observed to be decreased with prolonged hypoxaemia (Nguyen et al. 2003a). Thus, the fetal brain may be particularly vulnerable to long-term hypoxaemia due to insufficient AP concentrations.
The finding that plasma AP and one of its important precursors, pregnenolone, did not change following UCO, in contrast to the changes seen in the brain extracellular fluid, supports the contention that mechanisms within the brain are primarily responsible for the rise in AP concentrations following UCO. Our previous findings with neonates (Billiards et al. 2002) and in the fetus (Nguyen et al. 2004) suggest that adrenal synthesis contributes to plasma AP concentrations. The present observations suggest that these mechanisms do not contribute to the changes seen immediately after an asphyxial challenge. However, these processes may become important in responses to challenges of longer duration and increased frequency.
The increase in AP content in the cortex and CSF was accompanied by a rise in pregnenolone in the brain tissue. As pregnenolone is a key precursor in the steroidogenic pathway that leads to AP production, this increase in the availability of pregnenolone may drive the rise in the production of AP. The finding of increased P450scc expression suggests that stimulation of the synthesis of this enzyme in the brain may account for increased pregnenolone concentration. In the adrenal glands, trophic hormones such as ACTH induce steroidogenesis by increasing pregnenolone synthesis in two distinct ways. Chronic stimulation persisting for one to several hours can induce P450scc gene (CYP11A1) transcription leading to increased P450scc protein and consequently increased steroidogenic capacity (Miller et al. 1999). Acute upregulation, occurring within minutes, occurs through the phosphorylation of pre-existing steroidogenic acute regulatory (StAR) protein and the rapid synthesis of new StAR protein. StAR increases the flow of cholesterol into mitochondria, thus increasing substrate availability for P450scc enzyme. However, our previous studies have indicated that increasing concentrations of ACTH do not affect brain P450scc content in the fetus (Nguyen et al. 2004). P450scc is highly expressed in astrocytes, and astrocyte activation following UCO may be an alternative explanation for the increased P450scc expression in the brain. Thus, while the mechanisms that control P450scc expression in the brain requires further investigation, these mechanisms appear to be distinct from those controlling the expression of this enzyme in the adrenal glands.
The finding that the elevation in AP concentrations in the brain was also accompanied by an increase expression of 5αRII, suggests that this increase may be responsible for the short-term elevation of AP in the brain and microdialysate. These observations suggest that increased pregnenolone precursor availability, together with elevated 5αRII enzyme activity, may together contribute to the rise of AP concentration following UCO. The mechanisms that regulate the activity and expression of the 5αRII enzyme/gene in the brain are poorly understood. Analysis of this gene in mouse brain suggests the presence of a progesterone regulatory element (Matsui et al. 2002). However, there was no change in brain progesterone content in the present study, suggesting that progesterone is unlikely to regulate 5αRII expression following UCO. Thus, the control of 5αRII content in the fetal brain will require further study.
AP has potent neuroprotective functions (Majewska et al. 1986). It has been demonstrated to increase cell viability in studies with rat hippocampal slices and in primary hippocampal cultures as a result of the attenuation of glutamate-induced increases of intracellular Ca2+ (Frank & Sagratella, 2000). This action arises through the modulation of the GABAA receptor leading to increased GABAergic inhibitory neurotransmission that reduces excitation and suppresses excitotoxicity induced by excessive glutamate stimulation. GABAA receptors are present in the late-gestation fetal sheep brain at concentrations equivalent or higher to those in the adult brain (Crossley et al. 2000). The concentrations of AP reached following UCO are sufficient to strongly stimulate the GABAa receptors and increase GABAergic activity (Crossley et al. 2000). AP markedly potentiates the action of GABA at GABAa receptors, resulting in further GABAa receptor stimulation. The increase in AP could have a neuroprotective effect as proposed by Majewska et al. (1986). However, attributing a protective effect to the transient rise in AP following UCO would require studies in which the increase in AP after UCO had been prevented. This could be achieved using a blocker of neurosteroidogenesis, such as the 5α-reductase inhibitor finasteride, followed by measurement of GABAergic activity and the assessment of the extent of cell damage (apoptosis, lipid peroxidation, etc.). Previous studies have shown that asphyxia/hypoxia–ischaemia in fetal sheep results in a 3–5-fold increase in GABA concentration in the brain (Hagberg et al. 1987). In rat neonates, asphyxia results in a 40% increase in GABA (Iversen et al. 1983). Therefore increases in AP and GABA concentrations would strongly enhance GABAergic inhibitory activity during periods of hypoxia and asphyxia. It is possible that the short-term increase in AP abrogates the rapid, necrotic cell death that occurs immediately following severe insults. It seems unlikely that the relatively transient increase in AP observed in this study could affect the later secondary waves of cell damage that have been observed following hours to days (Gluckman et al. 2001); however, the immediate protective effects of AP may allow many cells to survive subsequent metabolic challenges that appear to be a feature of the perinatal brain response to asphyxic shock.
Previous studies have shown that UCO for 10 min or more leads to brain injury in the late-gestation fetal sheep (Mallard et al. 1992) and primates (Myers, 1975). These findings suggest that the observed brain AP response may be involved in ameliorating the extent of brain injury following asphyxial insults during development. The present observations support the contention that the increase in AP concentrations in both the grey and white matter following the acute asphyxia may provide a degree of neuroprotection from excitotoxic stimulation that may subsequently occur as a result of the asphyxial insult. Importantly, the AP response elicited following both the first and second occlusions were similar (Fig. 3C), suggesting that the increase in AP levels following a second UCO was not reduced by potential preconditioning effects arising from the first occlusion 48 h previously. However, we cannot exclude the possibility that conditioning effects on the AP response may occur with more frequent episodes of occlusion. Indeed, we have found that chronic hypoxia, induced by placental insufficiency, does not result in elevated AP concentrations in the fetal brain (Nguyen et al. 2003a). In the case of infrequent cord occlusion, at least, the AP response may serve to limit the initial degree of neuronal injury, neurodegeneration and death initiated by: (i) excitotoxic release of glutamate, (ii) loss of Ca2+ homeostasis, (iii) cellular energy failure, or (iv) oxidative stress. This is in contrast with the brain damage that occurs over the ensuing hours to days through secondary and tertiary episodes of damage that have been previously observed with studies employing longer periods of UCO (Ikeda et al. 1998).
We conclude that AP synthesis in the fetal brain increases rapidly in response to acute asphyxia in utero. At least three important implications arise from this finding. Firstly, the increased amount of this neurosteroid available in extracellular fluid in the brain is likely to modify GABAa receptor activity, providing an inhibitory counter-balance to the release of excitatory neurotransmitters, which is also caused by asphyxial events. Secondly, GABAa-receptor subunit expression may be altered, especially expression of the α4 subunit, as it has been shown for the adult rodent brain that steroids can induce changes of subunit composition of the receptor (Concas et al. 1998, 1999; Smith, 2002). Such effects have not yet been shown for the developing brain. Finally, impairment of this neurosteroid modulation of GABAergic inhibitory activity, such as a fall in the supply of precursor from the circulation or placenta, may make the fetal brain more vulnerable to excitotoxic injury.
Acknowledgments
We thank Alex Satragno for expert surgical assistance and Kylie Scott for technical assistance. The work was supported by National Health and Medical Research Council grants to Dr J. J. Hirst and Dr D. W. Walker (no. 143503 and no. 284239).
References
- Barbaccia ML, Roscetti G, Trabucchi M, Ambrosio C, Massotti M. Cyclic AMP-dependent increase of steroidogenesis in brain cortical minces. Eur J Pharmacol. 1992;219:485–486. doi: 10.1016/0014-2999(92)90495-p. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Cuccheddu T, Concas A, Biggio G. Neurosteroids in the brain of handling-habituated and naive rats: effect of CO2 inhalation. Eur J Pharmacol. 1994;261:317–320. doi: 10.1016/0014-2999(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998;138:316–321. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Walker DW, Canny BJ, Hirst JJ. Endotoxin increases sleep and brain allopregnanolone concentrations in newborn lambs. Pediatr Res. 2002;52:892–899. doi: 10.1203/00006450-200212000-00014. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley KJ, Walker DW, Beart PM, Hirst JJ. Characterisation of GABAA receptors in fetal, neonatal and adult ovine brain: region and age related changes and the effects of allopregnanolone. Neuropharmacology. 2000;39:1514–1522. doi: 10.1016/s0028-3908(99)00222-1. [DOI] [PubMed] [Google Scholar]
- Di Michele F, Lekieffre D, Pasini A, Bernardi G, Benavides J, Romeo E. Increased neurosteroids synthesis after brain and spinal cord injury in rats. Neurosci Lett. 2000;284:65–68. doi: 10.1016/s0304-3940(00)00965-4. [DOI] [PubMed] [Google Scholar]
- Frank C, Sagratella S. Neuroprotective effects of allopregnenolone on hippocampal irreversible neurotoxicity in vitro. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:1117–1126. doi: 10.1016/s0278-5846(00)00124-x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Pinal CS, Gunn AJ. Hypoxic-ischemic brain injury in the newborn: pathophysiology and potential strategies for intervention. Semin Neonatol. 2001;6:109–120. doi: 10.1053/siny.2001.0042. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Andersson P, Kjellmer I, Thiringer K, Thordstein M. Extracellular overflow of glutamate, aspartate, GABA and taurine in the cortex and basal ganglia of fetal lambs during hypoxia-ischemia. Neurosci Lett. 1987;78:311–317. doi: 10.1016/0304-3940(87)90379-x. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Murata Y, Quilligan EJ, Choi BH, Parer JT, Doi S, Park SD. Physiologic and histologic changes in near-term fetal lambs exposed to asphyxia by partial umbilical cord occlusion. Am J Obstet Gynecol. 1998;178:24–32. doi: 10.1016/s0002-9378(98)70621-0. [DOI] [PubMed] [Google Scholar]
- Iversen K, Hedner T, Lundborg P. GABA concentrations and turnover in neonatal rat brain during asphyxia and recovery. Acta Physiol Scand. 1983;118:91–94. doi: 10.1111/j.1748-1716.1983.tb07247.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Gunn AJ, Williams CE, Johnston BM, Gluckman PD. Transient umbilical cord occlusion causes hippocampal damage in the fetal sheep. Am J Obstet Gynecol. 1992;167:1423–1430. doi: 10.1016/s0002-9378(11)91728-1. [DOI] [PubMed] [Google Scholar]
- Matsui D, Sakari M, Sato T, Murayama A, Takada I, Kim M, Takeyama K, Kato S. Transcriptional regulation of the mouse steroid 5alpha-reductase type II gene by progesterone in brain. Nucleic Acids Res. 2002;30:1387–1393. doi: 10.1093/nar/30.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Miller WL, Strauss JF III. Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- Myers RE. Fetal asphyxia due to umbilical cord compression. Metabolic and brain pathologic consequences. Biol Neonate. 1975;26:21–43. doi: 10.1159/000240714. [DOI] [PubMed] [Google Scholar]
- Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5alpha-pregnane steroids and neurosteroidogenic enzyme expression in fetal sheep with umbilicoplacental embolization. Pediatr Res. 2003a;54:840–847. doi: 10.1203/01.PDR.0000088066.47755.36. [DOI] [PubMed] [Google Scholar]
- Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5alpha-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res. 2003b;53:956–964. doi: 10.1203/01.PDR.0000064905.64688.10. [DOI] [PubMed] [Google Scholar]
- Nguyen PN, Young IR, Walker DW, Hirst JJ. Allopregnanolone in the brain and blood after disruption of the hypothalamic-pituitary-adrenal axis in fetal sheep. J Endocrinol. 2004;182:81–88. doi: 10.1677/joe.0.1820081. [DOI] [PubMed] [Google Scholar]
- Petratos S, Hirst JJ, Mendis S, Anikijenko P, Walker DW. Localization of p450scc and 5alpha-reductase type-2 in the cerebellum of fetal and newborn sheep. Brain Res Dev Brain Res. 2000;123:81–86. doi: 10.1016/s0165-3806(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Thorburn GD. Characterization of particle associated choriomammotrophin and progesterone in ovine placentomes. J Endocrinol. 1986;111:217–223. doi: 10.1677/joe.0.1110217. [DOI] [PubMed] [Google Scholar]
- Smith SS. Withdrawal properties of a neuroactive steroid: implications for GABAA receptor gene regulation in the brain and anxiety behavior. Steroids. 2002;67:519–528. doi: 10.1016/s0039-128x(01)00170-2. [DOI] [PubMed] [Google Scholar]