Abstract
Ethylene, jasmonate, and salicylate play important roles in plant defense responses to pathogens. To investigate the contributions of these compounds in resistance of tomato (Lycopersicon esculentum) to the fungal pathogen Botrytis cinerea, three types of experiments were conducted: (a) quantitative disease assays with plants pretreated with ethylene, inhibitors of ethylene perception, or salicylate; (b) quantitative disease assays with mutants or transgenes affected in the production of or the response to either ethylene or jasmonate; and (c) expression analysis of defense-related genes before and after inoculation of plants with B. cinerea. Plants pretreated with ethylene showed a decreased susceptibility toward B. cinerea, whereas pretreatment with 1-methylcyclopropene, an inhibitor of ethylene perception, resulted in increased susceptibility. Ethylene pretreatment induced expression of several pathogenesis-related protein genes before B. cinerea infection. Proteinase inhibitor I expression was repressed by ethylene and induced by 1-methylcyclopropene. Ethylene also induced resistance in the mutant Never ripe. RNA analysis showed that Never ripe retained some ethylene sensitivity. The mutant Epinastic, constitutively activated in a subset of ethylene responses, and a transgenic line producing negligible ethylene were also tested. The results confirmed that ethylene responses are important for resistance of tomato to B. cinerea. The mutant Defenseless, impaired in jasmonate biosynthesis, showed increased susceptibility to B. cinerea. A transgenic line with reduced prosystemin expression showed similar susceptibility as Defenseless, whereas a prosystemin-overexpressing transgene was highly resistant. Ethylene and wound signaling acted independently on resistance. Salicylate and ethylene acted synergistically on defense gene expression, but antagonistically on resistance.
In nature, plants have to cope with abiotic and biotic stresses. Mechanisms have evolved that enable plants to resist drought and wounding but also attack by pathogenic microorganisms. Such mechanisms have been the subject of study for many years and recent results indicated striking similarities between biotic stress on the one hand, and senescence (Quirino et al., 2000), wounding (Romeis et al., 1999), and aging and drought stress (Langenkamper et al., 2001) on the other hand. The plant hormone ethylene is an important signal in many of such abiotic stress situations but also in plant-pathogen interactions (Boller, 1991; Bleecker and Kende, 2000). Production of ethylene can be induced by pathogen invasion, by fungal toxins as well as by race-specific and endogenous elicitors. Ethylene may activate plant defense-related processes such as the production of phytoalexins (Fan et al., 2000), pathogenesis-related (PR) proteins (Rodrigo et al., 1993; Tornero et al., 1994, 1997; van Kan et al., 1995), the induction of the phenylpropanoid pathway (Chappell et al., 1984), and cell wall alterations (Bell, 1981). Therefore, ethylene has been a target for studying resistance mechanisms in the last decades. The application of exogenous ethylene was found to induce resistance or susceptibility, or have no effect, depending on the plant-pathogen interaction studied (Esquerré Tugayé et al., 1979; El-Kazzaz et al., 1983; Elad, 1990; Marte et al., 1993; van Loon and Pennings, 1993). A similar variety of effects has been observed upon application of inhibitors of ethylene action or biosynthesis. The use of mutants in Arabidopsis, tobacco (Nicotiana tabacum), and soybean (Glycine max) demonstrated that both ethylene perception and signaling are required for resistance to some pathogens, but not to others (Knoester et al., 1998; Hoffman et al., 1999; Thomma et al., 1999). The role of ethylene in plant defense is apparently versatile.
The different results regarding the role of ethylene in plant defense could reflect its involvement in multiple physiological processes in the plant. Ethylene can accelerate senescence in leaves and ripening in fruits (Abeles et al., 1992). This might predispose the tissue for development of disease caused by some, mostly necrotrophic, pathogens. On the other hand, ethylene stimulates the development of necrosis (Lund et al., 1998) and in many cases the hypersensitive response (HR; Ciardi et al., 2001). HR is a defense phenomenon involving a rapid localized necrosis of plant cells at the infection site, followed by a local and systemic activation of defense-related genes (Pontier et al., 1998a). It can be envisaged that cell death during HR is able to restrict the proliferation of biotrophs because it deprives the pathogen of access to nutrient sources present in living cells (Cohn et al., 2001). A necrotroph, however, might benefit from HR because it feeds on dead plant cells. It has been reported that HR facilitates infection of Arabidopsis by necrotrophs such as Botrytis cinerea or Sclerotinia sclerotiorum (Govrin and Levine, 2000). It may be expected that necrotrophic pathogens are well adapted to deal with HR-based defense mechanisms that are active against biotrophs (Mayer et al., 2001). It has been proposed that different defense mechanisms are involved in resistance, each efficient against a particular range of pathogens (Thomma et al., 2001). In Arabidopsis, salicylic acid (SA), ethylene, jasmonic acid (JA), and the phytoalexin camalexin are, either alone or in different combinations, involved in defense against different pathogens. Resistance of Arabidopsis to B. cinerea was reported to involve an important contribution of a JA-/ethylene-mediated pathway (Thomma et al., 1999), whereas the role of an SA-mediated pathway was only minor (Zimmerli et al., 2001) or undetectable (Thomma et al., 1998). In tobacco and French bean (Phaseolus vulgaris), however, SA appears to be important for resistance against B. cinerea (De Meyer and Höfte, 1997; Murphy et al., 2000). Comprehensive studies with different hosts and comparison with results obtained in Arabidopsis might increase our knowledge on the roles of hormone signaling pathways in plant defense.
The fact that pathogen infection triggers a wound response, whereas wounding of the plant may sometimes facilitate infection, is frequently overlooked in the studies of the role of ethylene in plant disease resistance. Ethylene is released from wounded plant tissue and it participates in wound response signaling in concert with other compounds, such as oligogalacturonides (OGAs), JA, abscisic acid, hydrogen peroxide, and, in Solanaceae, the oligopeptide systemin (for review, see Ryan, 2000). The wound signaling pathway triggers defense mechanisms that usually act against herbivores, but in some cases they can be effective against pathogens as well (Bostock, 1999). It can be envisaged that B. cinerea induces the onset of a wound-like response because B. cinerea infection may result in release of OGAs through the action of fungal endopolygalacturonases (endoPGs) expressed during pathogenesis (ten Have et al., 1998, 2001).
Studies on the role of ethylene in disease resistance of tomato (Lycopersicon esculentum), mostly from Klee and coworkers (e.g. Lund et al., 1998; Ciardi et al., 2000), have provided a number of plant mutants (Klee et al., 1991; Lanahan et al., 1994). Extensive studies on tomato wound responses by Ryan and coworkers (for review, see Ryan, 2000) have also provided interesting plant mutants (McGurl et al., 1992, 1994; Howe et al., 1996). These ethylene and wound response mutants were tested in quantitative disease assays to evaluate the effect of the mutation on resistance against B. cinerea to unravel the signaling of resistance to B. cinerea in tomato. There appear to be similarities, but also important discrepancies, with results obtained in Arabidopsis.
RESULTS
The infection of tomato leaves by B. cinerea occurs in three phases, as described by Benito et al. (1998). The first phase occurs in the first 24 h postinoculation (hpi) and leads to the formation of primary necrotic lesions. It is followed by a quiescent period in which primary lesions remain restricted and do not expand. The third and final phase is characterized by an aggressive outgrowth from a small proportion of the primary lesions, typically 10% to 30%. We determined the proportion of expanding lesions, the lesion growth rate, and the fungal biomass as a measure of susceptibility to B. cinerea.
Ethylene Modulates Resistance to B. cinerea
Tomato cv Moneymaker plants were pretreated with either ethylene or an ethylene perception inhibitor: 1-methylcyclopropene (MCP) or 2,5-norbornadiene (NBD). MCP and NBD act as inhibitors of ethylene perception by binding to the ethylene receptor. MCP can be regarded as an irreversible inhibitor, whereas NBD is readily released from the receptor (for review, see Sisler and Serek, 1999).
Plants that were treated with ethylene before inoculation were less susceptible to B. cinerea than untreated control plants (Table I). The proportion of apparently uninfected plants at 96 hpi was increased by the ethylene pretreatment and reduced by MCP or NBD pretreatment, when compared with the control. The (partial) resistance induced by ethylene pretreatment was also reflected by a statistically significant reduction of the number of expanding lesions per plant, as well as a reduced growth rate of the expanding lesions. Pretreatment with MCP and NBD, on the other hand, increased the number of expanding lesions (Table I) without affecting the lesion growth rate. At 15 d postinoculation, one-half of the ethylene-pretreated plants still looked healthy overall, whereas all the control plants, as well as all the NBD- and MCP-pretreated plants, were severely infected, showing colonization of the stems by the fungus.
Table I.
Effect of ethylene, MCP, and NBD pretreatment on B. cinerea infection of tomato cv Moneymaker
| Pretreatment | Healthy Plantsa | Expanding Lesions per Planta | Lesion Expansion Rateb |
|---|---|---|---|
| % | % | mm/day | |
| Control | 6.7 | 14.7 (2.9) b | 5.7 (0.6) b |
| Ethylene 1 μL L−1 | 53.3 | 4.2 (1.5) a | 3.0 (1.1) a |
| MCP 10 nL L−1 | 0 | 27.2 (4.1) c | 6.3 (0.4) b |
| NBD 5 μL L−1 | 0 | 24.7 (3.0) c | 6.6 (0.4) b |
ses are shown in brackets. Different letters within the same column indicate a significant difference (P < 0.05) after ANOVA and Duncan's test. Data were pooled from two independent experiments, with 15 plants per treatment. Each plant was considered as a replicate.
At 96 hpi.
Calculated over the period from 48 to 72 hpi.
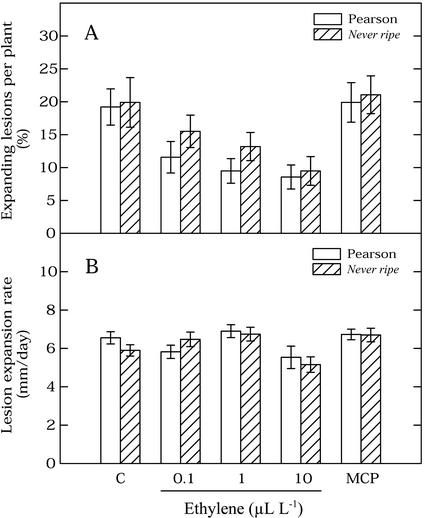
To assess whether the induction of resistance by ethylene pretreatment was truly dependent on ethylene perception, we tested the susceptibility of the tomato mutant Never ripe, which was initially reported to be insensitive to ethylene (Lanahan et al., 1994). Subsequent studies have indicated that Never ripe is not entirely insensitive, but rather is severely reduced in ethylene sensitivity (Aloni et al., 1998; Clark et al., 1999). The untreated Never ripe mutant was as susceptible as the wild-type tomato cv Pearson. Ethylene pretreatment also reduced susceptibility to B. cinerea in the Never ripe mutant (Fig. 1). Figure 1A shows that the proportion of expanding lesions decreased with the concentration of ethylene that was applied in the pretreatment, both in the wild-type tomato cv Pearson and in the Never ripe mutant. Two-way ANOVA test revealed that both lines were affected by ethylene with a similar trend. There were no significant differences between the two lines, either for the number of expanding lesions (Fig. 1A) or the lesion expansion rate (Fig. 1B). All ethylene pretreatments significantly reduced the proportion of expanding lesions, as compared with the untreated control and MCP pretreatment (P < 0.05). The effects on the proportion of expanding lesions were not significantly different among the three ethylene concentrations tested. However, the lesion expansion rate after the 10 μL L−1 ethylene pretreatment was significantly lower than in the other pretreatments (P < 0.05), both in tomato cv Pearson and Never ripe (Fig. 1B). MCP pretreatment did not affect any of the disease parameters in tomato cv Pearson or Never ripe.
Figure 1.
Effect of pretreatments with different ethylene concentrations and with MCP on B. cinerea infection of tomato cv Pearson and the ethylene-insensitive mutant Never ripe. A, Percentage of expanding lesions at 96 hpi. B, Lesion expansion rate over the period from 48 to 72 hpi. Bars show that se data were pooled from three independent experiments, with a total of 18 plants per treatment. Each plant was considered as a replicate. C, Untreated control; MCP, 10 nL L−1 MCP.
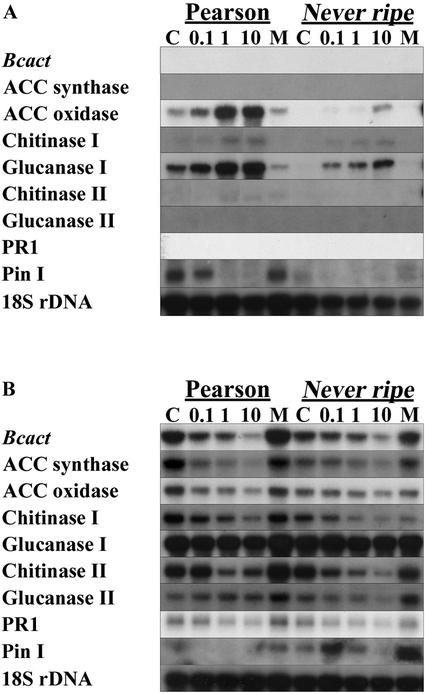
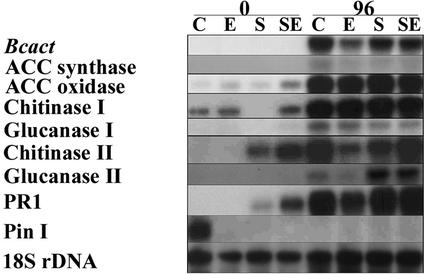
RNA hybridization analysis was performed to determine whether a decrease in the percentage of expanding lesions is accompanied by a decrease in fungal biomass (Fig. 2). Hybridization was performed with a probe for the B. cinerea actin gene (BcactA), a marker for actively growing B. cinerea (Benito et al., 1998). A positive correlation was observed between the BcactA hybridization intensity (Fig. 2B) and the percentage of expanding lesions (Fig. 1A). Pretreatment with increasing amounts of ethylene resulted in a reduction of both disease symptoms and BcactA hybridization intensity. The same RNA samples were hybridized with a set of probes derived from plant genes involved in ethylene biosynthesis and in defense responses. Ethylene is produced from S-adenosyl-Met by 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase (Yang and Hoffman, 1984). Both enzymes are encoded by multigene families that are differentially regulated (Oetiker et al., 1997; Nakatsuka et al., 1998). Pretreatment of wild-type tomato cv Pearson with increasing amounts of ethylene resulted in a nonlinear increase of ACC oxidase transcript (Fig. 2A). In the Never ripe mutant, ACC oxidase transcript levels were lower when compared with wild type but increased with the ethylene concentration used (Fig. 2A). The Never ripe mutant is apparently responsive to ethylene. The expression of ACC synthase 2 was reported to be induced by ethylene in fruit (Rottmann et al., 1991) but not in leaf tissue (van Kan et al., 1995). ACC synthase 2 expression was not induced by any of the ethylene pretreatments. Expression of both ACC synthase and ACC oxidase was induced in B. cinerea-infected leaves at 96 hpi and their hybridization intensity followed the pattern of BcactA (Fig. 2B). Because ethylene pretreatment reduced susceptibility, we analyzed the expression patterns of a number of plant defense-related genes, both after pretreatments but before inoculation and at 96 hpi. Glucanase I and chitinase I transcripts were induced by pretreatment with ethylene, whereas glucanase II, chitinase II, and PR-1 transcripts were not induced (Fig. 2A). Analogous to the ACC oxidase expression pattern, the glucanase I and chitinase I genes were also induced by ethylene pretreatment of the Never ripe mutant line, albeit to a lesser extent than in the wild type (Fig. 2A). Glucanase I transcript levels in tomato cv Pearson were induced by ethylene, and repressed by pretreatment with MCP (Fig. 2A). A longer exposure of the blot hybridized with the PR-1 probe revealed a slight induction by ethylene (not shown). Proteinase inhibitor I transcript levels in tomato cv Pearson were reduced by ethylene pretreatment. In Never ripe, proteinase inhibitor I transcripts were around the detection limit. In B. cinerea-inoculated tomato cv Pearson (Fig. 2B), proteinase inhibitor I transcript levels were below the detection limit, except in case that the plants were pretreated with MCP before inoculation. In contrast, expression of proteinase inhibitor I mRNA was readily detected in the Never ripe mutant upon B. cinerea infection. In B. cinerea-infected tissue, the transcript levels of chitinase I, chitinase II, glucanase II, and PR-1 showed a pattern similar to BcactA at 96 hpi, although differences in relative intensities occurred. Only the glucanase I transcript seemed to be expressed to similar (high) levels in all infections (Fig. 2B).
Figure 2.
Gene expression in tomato Never ripe after pretreatments and infection by B. cinerea. Tomato cv Pearson and Never ripe plants were pretreated for 20 h with either 0.1, 1, or 10 μL L−1 ethylene as indicated, or with 10 nL L−1 MCP (M); C indicates a control treatment without added gas. A, Gene expression after pretreatment, but before inoculation. B, Gene expression after B. cinerea infection (96 hpi). Bcact, Actin probe of B. cinerea reflecting fungal biomass. Pin I, Proteinase inhibitor I. Autoradiographs of duplicate blots hybridized with probes as indicated in the left margin.
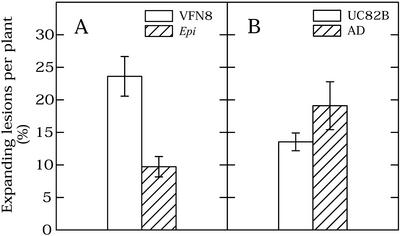
To investigate the role of ethylene responses on resistance to B. cinerea in more detail, inoculation assays were conducted on two additional tomato genotypes affected in ethylene responses or in ethylene biosynthesis (Fig. 3). The mutant Epi (Epinastic), initially reported to overproduce ethylene (Fujino et al., 1988), was demonstrated recently to be constitutively activated in a subset of ethylene responses (Barry et al., 2001). Susceptibility of Epi to B. cinerea was compared with that of its wild-type progenitor (VFN8). Epi showed a significant reduction (P < 0.05) in the percentage of expanding lesions as compared with the wild-type progenitor (Fig. 3A), whereas no difference in lesion expansion rate was observed (not shown). A transgenic line expressing ACC deaminase (accession no. UC8338), producing negligible amounts of ethylene (Klee et al., 1991), was more susceptible to B. cinerea infection than its non-transgenic progenitor, UC82B. ACC deaminase-expressing plants showed 29% more expanding lesions as compared with the non-transgenic line (Fig. 3B), but the difference was not statistically significant (P = 0.09). No difference in lesion expansion rate was observed (not shown).
Figure 3.
B. cinerea infection of tomato ethylene-responsive mutant (Epi) or transgene impaired in ethylene synthesis (AD) as compared with wild-type progenitor lines. A, Percentage of expanding lesions at 96 hpi for Epi and its progenitor line VFN8. B, Percentage of expanding lesions at 96 hpi for ACC deaminase (AD) and its progenitor line UC82B. Bars show se. Data were pooled from two independent experiments, with a total of 12 plants per treatment. Each plant was considered as a replicate.
Jasmonate and Wound Signaling Act in Resistance of Tomato to B. cinerea Independently of Ethylene
Both JA and ethylene have been reported to be required for the induction of a functional defense response in Arabidopsis toward B. cinerea (for review, see Thomma et al., 2001). Both compounds are required for the development of the wound response in tomato and other Solanaceous species, although the role of ethylene in the induction of wound-responsive genes is still obscure (Ryan, 2000). Wounding results in the cleavage of prosystemin into systemin, which is believed to transduce the wound signal via JA and OGAs, eventually resulting in the onset of defense genes like proteinase inhibitor I (for review, see Ryan, 2000). Because B. cinerea expresses endoPG genes during pathogenesis (ten Have et al., 1998, 2001), we envisaged that the release of OGAs by B. cinerea endoPG activity might induce the wound signaling response of tomato. This led us to test a jasmonate-deficient mutant, def1 (Defenseless) or JL5 (Howe et al., 1996), as well as transgenic plants that overexpress prosystemin, designated PSoe (McGurl et al., 1994), or that have reduced levels of prosystemin by antisense expression, designated PSas (McGurl et al., 1992). The prosystemin-overexpressing line presents a constitutive wound/herbivore defense response, whereas the def1 mutant and the prosystemin antisense line are impaired in such a response.
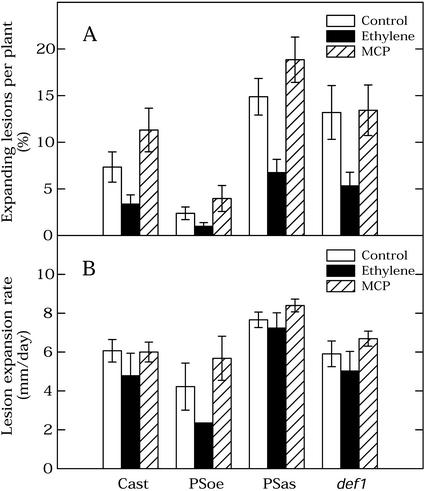
Figure 4 demonstrates that both def1 and the prosystemin antisense line were more susceptible to B. cinerea than the wild-type progenitor tomato cv Castlemart, whereas the prosystemin-overexpressing line was highly resistant. Ethylene and MCP pretreatments before inoculation had similar effects in all the lines: Ethylene reduced the percentage of expanding lesions in comparison with the untreated control, whereas MCP caused an increase (Fig. 4A). The effect of both pretreatments on the lesion expansion rate in any particular genotype was generally small (Fig. 4B). A two-way ANOVA test revealed no statistically significant interaction between lines and pretreatments in any of the disease parameters. Therefore, the data were tested for main effects. The two-way ANOVA confirmed that all differences among lines and among each of the separate pretreatments were statistically significant (P < 0.05), with one exception. There was no statistically significant effect of pretreatments on the growth rate of expanding lesions (P = 0.06).
Figure 4.
Effect of ethylene and MCP pretreatments on susceptibility of tomato lines overexpressing prosystemin (PSoe), impaired in prosystemin (PSas) or jasmonate (def1) synthesis in comparison with their wild-type progenitor tomato cv Castlemart (Cast). A, Percentage of expanding lesions at 96 hpi. B, Lesion expansion rate over the period from 48 to 72 hpi. Treatments were with 1 μL L−1 ethylene or 10 nL L−1 MCP. Data were pooled from three independent experiments, with a total of 18 to 21 plants per treatment. Each plant was considered as a replicate. Bars show se.
Leaf material was sampled and used for RNA hybridization analysis analogously to the experiments presented in Figure 2. Figure 5 shows that the expression patterns observed after the pretreatment with ethylene or MCP before infection displayed a similar trend in the genetic background of tomato cv Castlemart as observed in cv Pearson, with one exception. Induction of chitinase I transcript levels by ethylene pretreatment was more prominent in tomato cv Castlemart than in cv Pearson. In all three tomato cv Castlemart mutant lines (transgenes PSoe and PSas and mutant def1), the transcript levels of ACC oxidase, chitinase I, and glucanase I were more strongly induced by ethylene pretreatment than in the wild-type progenitor tomato cv Castlemart. In PSas, the chitinase II and PR1 mRNA also were more strongly induced by ethylene pretreatment than in the wild-type progenitor tomato cv Castlemart. ACC synthase and glucanase II mRNAs were barely detectable in any of these lines after any treatment. Proteinase inhibitor I transcript was barely detectable in the wild-type tomato cv Castlemart, the PSoe transgene, and the def1 mutant upon any treatment. However, proteinase inhibitor I transcript was inducible in the PSoe line by MCP pretreatment before inoculation (Fig. 5A) or by B. cinerea infection (Fig. 5B). Upon infection of the four lines with B. cinerea, the transcript level of BcactA at 96 hpi correlated to the extent of infection (compare the top of Fig. 5B with Fig. 4). ACC oxidase, ACC synthase, and most of the PR protein transcript levels paralleled the pattern of BcactA, in agreement with the results presented in Figure 2B.
Figure 5.
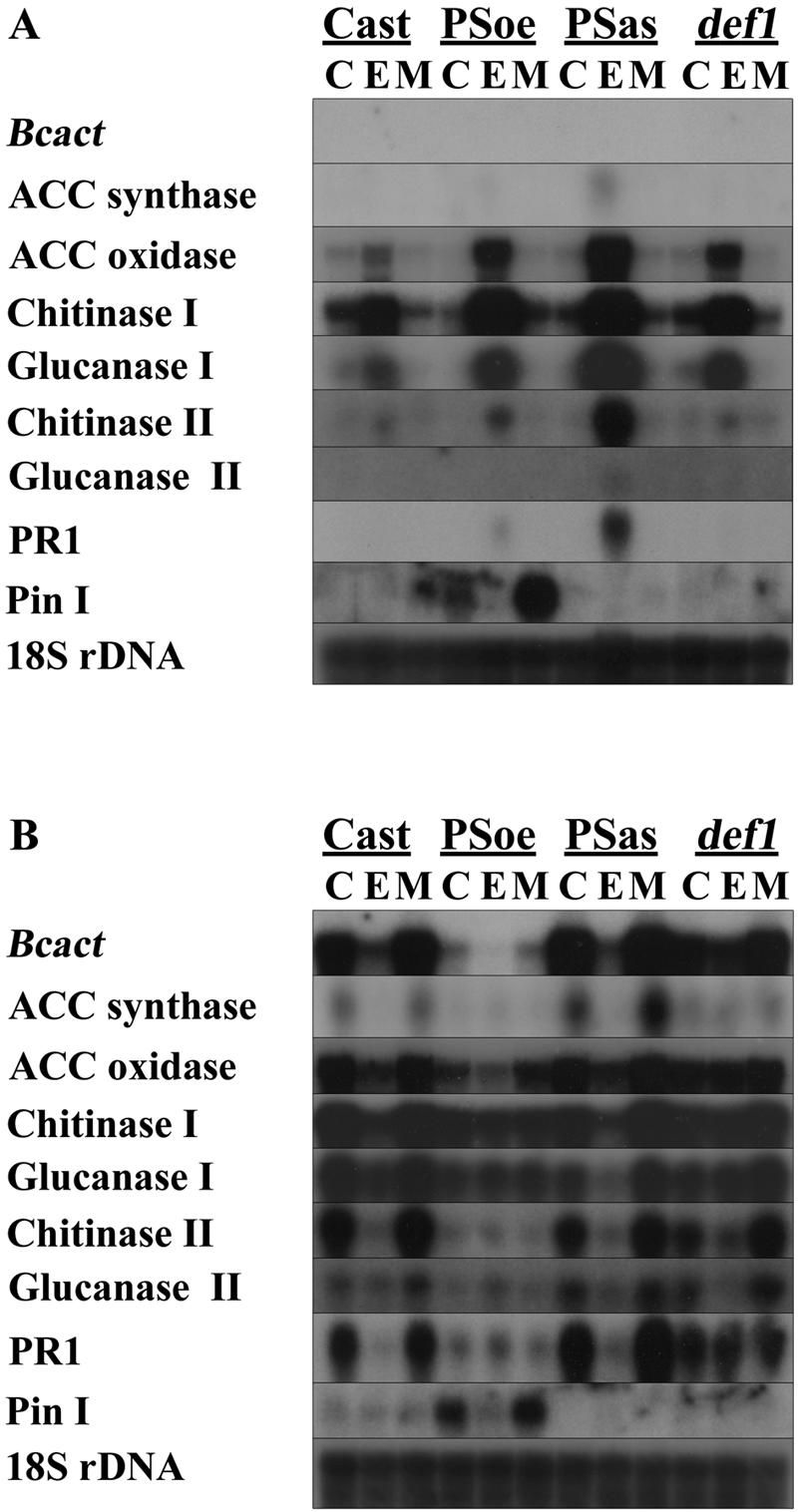
Gene expression in tomato def1 and prosystemin transgenes after pretreatments and infection with B. cinerea. Plants, control tomato cv Castlemart, PSoe, PSas, and mutant def1, were pretreated with either 1 μL L−1 ethylene (E) or with 10 nL L−1 MCP (M) for 20 h. C, Control treatment without added gas. A, Gene expression after pretreatment, but before inoculation. B, Gene expression after B. cinerea infection (96 hpi). Bcact indicates the actin probe of B. cinerea reflecting fungal biomass. Pin I, Proteinase inhibitor I. Autoradiographs of duplicate blots hybridized with probes as indicated in the left margin.
Salicylate- and Ethylene-Induced Responses to B. cinerea Are Antagonistic
The role of SA in resistance of tomato to B. cinerea was investigated by exogenous application of 4 mm SA to tomato cv Pearson plants. Pretreatments with ethylene, either alone or in combination with SA, were included to assess interaction between the two signaling pathways. Table II shows that independent pretreatments with either ethylene or SA alone caused a decrease in the percentage of expanding lesions. The joint application of both ethylene and SA before inoculation led to an equal percentage of expanding lesions as the untreated control. One-way ANOVA and Duncan's test indicated that the differences in percentage of expanding lesions were not statistically significant. The lesion expansion rate was significantly reduced by ethylene pretreatment as compared with the control (P < 0.05), but was unaffected by SA pretreatment.
Table II.
Effect of ethylene and salicylic acid pretreatment on B. cinerea infection of tomato cv Pearson
| Pretreatment | Expanding Lesions per Planta | Lesion Expansion Rateb |
|---|---|---|
| % | mm/day | |
| Control | 17.9 (2.7) a | 7.2 (0.3) b |
| Ethylene 1 μL L−1 | 12.8 (2.1) a | 5.6 (0.6) a |
| SA 4 mm | 14.3 (2.9) a | 7.4 (0.6) b |
| SA 4 mm+ Ethylene 1 μL L−1 | 17.6 (3.9) a | 6.6 (0.4) ab |
ses are shown in brackets. Different letters within the same column indicate a significant difference (P < 0.05) after ANOVA and Duncan's test. Data were pooled from two independent experiments, with 14 plants per treatment. Each plant was considered as a replicate.
At 96 hpi.
Calculated over the period from 48 to 72 hpi.
RNA hybridization analysis (Fig. 6) showed that ethylene or SA pretreatment before inoculation resulted in a slight decrease in the transcript level of BcactA at 96 hpi (Fig. 6, top). Transcript levels of ACC oxidase and chitinase I increased slightly before inoculation as a result of ethylene (but not SA) pretreatment, whereas levels of PR-1 and chitinase II (but not glucanase II) transcripts were increased upon SA (but not ethylene) pretreatment. Combined pretreatment with SA and ethylene resulted, before inoculation, in further elevated transcript levels of ACC oxidase, PR-1, and chitinase II. In B. cinerea-infected tissue, the transcript levels of all defense-related genes, except for glucanase II, followed the pattern of the BcactA gene, although large differences in hybridization intensity were observed (Fig. 6).
Figure 6.
Gene expression in tomato after SA or ethylene pretreatment, before and after infection with B. cinerea. Tomato cv Pearson plants were pretreated with either 1 μL L−1 ethylene (E), 4 mm SA (S), or the combination of compounds (se) for 20 h before inoculation with B. cinerea. C, Control treatment. The first set of four lanes (indicated with 0) show gene expression after pretreatment, but before inoculation, whereas the second set of four lanes (indicated with 96) show gene expression after B. cinerea infection at 96 hpi. Bcact, Actin probe of B. cinerea reflecting fungal biomass. Pin I, Proteinase inhibitor I. The panels represent autoradiographs of duplicate blots hybridized with probes as indicated in the left margin.
DISCUSSION
Ethylene, JA, and SA all independently contribute to resistance of tomato toward B. cinerea. Although there is ample information on resistance signaling in Arabidopsis (Clarke et al., 2000; Govrin and Levine, 2000; Dickman et al., 2001; Kachroo et al., 2001; Thomma et al., 2001), knowledge on resistance signaling in tomato is scarce and has mostly originated from the work of Klee and coworkers. Lund et al. (1998) reported that ethylene-insensitive tomato Never ripe shows reduced disease symptoms upon infection by the bacterial pathogens Pseudomonas syringae pv tomato and Xanthomonas campestris pv vesicatoria. Recently, it was proclaimed that this effect is mainly mediated by ethylene-dependent SA biosynthesis, ultimately leading to an altered cell death response (O'Donnell et al., 2001). Never ripe was more resistant against the fungal wilt pathogen Fusarium oxysporum (Lund et al., 1998), whereas Audenaert et al. (1999) reported that detached leaves of Never ripe were slightly, but significantly, more susceptible to B. cinerea. We observed no significant difference in susceptibility to B. cinerea between Never ripe and wild-type tomato cv Pearson, when tested on leaves of intact plants. In our experiments, ethylene pretreatment induced resistance against B. cinerea (Table I; Fig. 1). Ethylene and SA pretreatments had a synergistic effect on PR gene expression (see Fig. 6), but the effect on resistance was antagonistic (Table II). JA and systemin, components of a wound signaling pathway in tomato, were also found to be involved in resistance against B. cinerea, and they appear to act independent from the ethylene-mediated pathway.
The Role of Ethylene
Pretreatment of tomato plants with ethylene resulted in an increased, yet partial, resistance to B. cinerea, both at the level of the percentage of expanding lesions and, in some cases, the lesion expansion rate. The severity of symptom development was well correlated with the extent of fungal biomass, reflected by the B. cinerea actin mRNA level (see Fig. 2B). Pretreatments with the strong, irreversible ethylene perception inhibitor MCP resulted in a significant increase of disease in both tomato cv Moneymaker and cv Castlemart, indicating that ethylene perception is required for resistance signaling, whereas no increase was found in tomato cv Pearson. We have no explanation for the discrepancy among cultivars. Our results contradict the results reported by Elad (1990, 1993), who showed that supply of exogenous ethylene promotes B. cinerea infection in tomato. In those experiments, specific inhibitors of ethylene perception or synthesis (NBD and CoSO4) did not confer significant protection in tomato leaves. However, it should be noted that the experiments of Elad were conducted in polyethylene bags, which are known to release ethylene into the environment.
Never ripe plants were equally susceptible to B. cinerea infection as the wild-type progenitor. The Never ripe mutant was initially considered insensitive to ethylene on the basis of organ response assays, such as triple response of seedlings, leaf epinasty, flower senescence, and fruit ripening. It was reported that the insensitivity of this mutation was partial in several tomato lines, including tomato cv Ailsa Craig (Lanahan et al., 1994) and tomato cv Pearson (Aloni et al., 1998; Clark et al., 1999). The concentration of ethylene required to achieve the same induction of gene expression was higher in the Never ripe mutant (Fig. 2A), but the residual ethylene sensitivity in Never ripe was sufficient to trigger enhanced resistance. Our results clearly confirm that residual responsiveness to ethylene is retained in tomato cv Pearson Never ripe leaves at this stage of development, i.e. 3- to 4-week-old plants with two to three true leaves. At this stage, neither tomato cv Pearson nor Never ripe showed any discernible signs of senescence upon ethylene treatment. Senescing plants are often very susceptible to gray mold development.
We further studied the role of ethylene by inoculating additional mutant lines. Reduction of ethylene production to negligible levels in the ACC deaminase-expressing transgenic plants (Klee et al., 1991) led to higher susceptibility. The mutant line Epinastic was initially reported to be an ethylene-overproducing mutant (Fujino et al., 1988), but was recently demonstrated to be constitutively activated in a subset of ethylene responses (Barry et al., 2001). Epinastic showed a significant level of partial resistance (Fig. 3). This is in full agreement with the hypothesis that in tomato, as in Arabidopsis, ethylene responses are involved in the induction of defense against B. cinerea.
The Role of the Wounding Pathway
The tomato wound response (Ryan, 2000) is mediated by a signal that is transduced from systemin and JA, via an endogenous endoPG and OGA, toward the systemic release of hydrogen peroxide, inducing transcription of defense-related genes like the proteinase inhibitor I gene (Orozco Cardenas et al., 2001). We envisaged the possibility that B. cinerea induces wound responses by means of OGAs released by fungal endoPGs. This would imply that PSas plants, def1 and their wild-type progenitor (tomato cv Castlemart) would be equally susceptible to B. cinerea. The results in Figure 4 indicate, however, that PSas plants and def1 showed a marked increase in susceptibility as compared with the wild-type progenitor, whereas the transgenic prosystemin overexpressor line was less susceptible. The precise role of OGAs in determining the outcome of the interaction between B. cinerea and tomato remains to be clarified.
Very recently, Audenaert et al. (2002) reported that the mutant def1 did not show increased susceptibility to B. cinerea, which seems to contradict our results. However, these experiments were conducted with detached leaves of plants that were considerably older than the plants that we used in our studies.
In Arabidopsis, JA and ethylene are considered to act synergistically in the response to pathogens (Thomma et al., 1999). In tomato, however, these signals seem to act in an independent manner. Ethylene pretreatment of mutant lines, altered in JA or systemin signaling, as well as the wild-type tomato cv Castlemart consistently resulted in a similar increase in resistance. The opposite was observed for MCP pretreatment. JA- and prosystemin-mediated responses thus appear to act independently from ethylene-induced resistance of tomato against B. cinerea. Ethylene was proclaimed to be required for proteinase inhibitor 2 gene expression, which was used as marker for the wound response (O'Donnell et al., 1996). The results in Figure 2A show that proteinase inhibitor 1 mRNA was expressed in the wild-type tomato cv Pearson, but to much lower levels in the Never ripe mutant. Application of exogenous ethylene resulted in reduced proteinase inhibitor 1 mRNA levels. In addition, proteinase inhibitor 1 transcripts were only detected in B. cinerea-infected plants that are disturbed in ethylene perception, i.e. Never ripe and MCP-pretreated tomato cv Pearson. Furthermore, MCP pretreatment led to a strongly increased proteinase inhibitor 1 mRNA level in prosystemin-overexpressing plants, even in the absence of B. cinerea infection (Fig. 5A). Clearly, the effects of ethylene on proteinase inhibitor 1 gene expression depend on ethylene concentration and the sensitivity of the plant to ethylene. Distinctions should be made between experiments with mutant plants and inhibitors of ethylene biosynthesis or perception. The complex interactions between ethylene and its receptors are studied in detail by Klee and coworkers (Ciardi et al., 2000, 2001; Tieman et al., 2000).
Interactions between SA and Ethylene
Exogenous application of SA induced partial resistance in tomato against B. cinerea. The effect was small, but it might be increased by optimizing the concentration and timing of SA application. This result provides further support for the involvement of SA in resistance to B. cinerea, as was first put forward by the observation that transgenic nahG-expressing tomato plants were more susceptible than a wild-type line (Audenaert et al., 1999, 2002). In our experiments, we compared the resistance level accomplished by SA with that induced by ethylene and the interaction between both signals. Interestingly, both the SA and the ethylene pretreatment independently induced a small partial resistance, which was reverted by the joint application of both compounds, to the level of untreated plants. With respect to resistance, SA and ethylene appear to act antagonistically. At the level of gene expression, however, SA and ethylene acted synergistically. Genes previously identified as SA inducible did respond to SA pretreatment, whereas genes previously identified as ethylene inducible did respond to ethylene (van Kan et al., 1995). Simultaneous pretreatment with SA and ethylene resulted in notably higher transcript levels of PR-1, glucanase II, and ACC oxidase, when compared with the induction achieved by either compound alone (Fig. 6).
The results obtained with the SA and ethylene pretreatments point to a paradox. Ethylene and SA each induce the expression of a subset of PR protein genes and each compound independently induces (partial) resistance to B. cinerea. Infection by B. cinerea also induced PR protein expression. The transcript levels of PR protein genes correlated with the fungal biomass, clearly indicating that the induced PR proteins are not active against this necrotrophic fungus. A further increase of gene expression mediated by joint application of SA and ethylene does not elevate resistance, but abolishes the induction of partial resistance achieved by either compound alone. Analogously, the proteinase inhibitor I gene expression was induced by MCP pretreatment (Fig. 5A), which resulted in an increased susceptibility (Fig. 4).
Defense Mechanisms Contributing to Resistance toward B. cinerea
If PR proteins would contribute to resistance of tomato to B. cinerea, one would expect to observe an inverse correlation between PR protein gene expression and the fungal biomass. All tomato genes that were examined, except for glucanase I and proteinase inhibitor I, showed similar patterns of expression as the marker for B. cinerea biomass BcactA, encoding actin (Benito et al., 1998). This indicates that expression levels of PR protein encoding genes are correlated to the disease severity. Thus, PR proteins truly act as PR proteins and not as “defense-related” proteins. Therefore, the increased disease resistance resulting from pretreatments or mutations (e.g. Figs. 1A and 3A; Table II) is unlikely to be mediated by overexpression of PR proteins, suggesting that these genes are not major players in the resistance mechanism. It remains to be unraveled which components of the tomato defense response contribute to growth inhibition of B. cinerea.
HR is a defense mechanism active against biotrophic pathogens complying with the gene-for-gene hypothesis. B. cinerea is a typical necrotroph and does not comply with the gene-for-gene hypothesis. Evidence is accumulating that B. cinerea infection not only induces a HR but also might benefit from HR. Inoculation of B. cinerea induced an oxidative burst and hypersensitive cell death in Arabidopsis (Govrin and Levine, 2000). Arabidopsis mutants that display an intensification of HR are more resistant to biotrophic pathogens, but more susceptible to B. cinerea (Govrin and Levine, 2000). This increased susceptibility seems to be mediated by an oxidative burst. Dickman et al. (2001) presented further evidence for a crucial contribution of the HR and concomitant cell death to susceptibility of plants to B. cinerea. Transgenic tobacco plants expressing animal genes that negatively regulate apoptosis were more resistant than untransformed plants against several necrotrophs, including B. cinerea (Dickman et al., 2001). The situation in tomato is as yet less clear. LeHSR203, a commonly used marker transcript indicative for HR in tomato (Pontier et al., 1998b), showed high transcript levels in B. cinerea-infected tomato leaves (A. ten Have, unpublished data), indicating that the fungus induces an HR in tomato. It remains to be determined whether HR is beneficial for B. cinerea in facilitating infection in tomato. In analogy to Arabidopsis and tobacco, it will be informative to generate mutants of tomato, positively or negatively affected in HR, and evaluate their resistance to a range of (biotrophic and necrotrophic) pathogens. Mutations in HR may be combined with defined mutations in the hormone-mediated defense signaling pathways that we have exploited here, to study the complex interactions between pathways in relation to disease resistance toward different pathogens.
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum Mill.) seedlings were obtained by germinating seeds in vermiculite. One to 2 d after emergence, the seedlings were transferred to a mixture of potting soil:vermiculite (3:1 [v/v]). During all the experiments, plants were grown with a 16-h photoperiod, at 25°C in the light period and 18°C in the dark.
Several tomato lines were used in the experiments. Tomato cv Moneymaker was used in inhibitor experiments. Tomato cv Pearson, the homozygous mutant Nr/Nr in the cv Pearson background, VFN8, and the mutant Epi in the VFN8 background were obtained from Rick Davies (Tomato Genetic Resources Center, University of California, Davis). A transgenic line (UC8338), expressing ACC deaminase and its wild-type progenitor (UC82B), were provided by Harry J. Klee (University of Florida, Gainesville). The transgenic lines overexpressing prosystemin, expressing a PSas construct, the def1 mutant, and their wild-type progenitor (tomato cv Castlemart) were provided by Clarence A. Ryan (Washington State University, Pullman).
Inoculation Assays
Botrytis cinerea Pers.:Fr. strain B05.10 was cultured and inoculum was prepared as described by Benito et al. (1998). Conidia were suspended at a density of 106 mL−1 in Gamborg's B5 medium (Duchefa Biochemie bv, Haarlem, The Netherlands) supplemented with 10 mm Glc and 10 mm potassium phosphate (pH 6). The suspension was pre-incubated without shaking for 2 to 3 h. Two-microliter droplets of the suspension were placed on the first and second true leaves of 3-week-old plants.
Plant Pretreatments
Before inoculation, plants were subjected to different pretreatments with chemical compounds, namely ethylene and two inhibitors of ethylene perception, NBD and MCP (Sisler and Serek, 1999). Plants were exposed overnight to the chemical in sealed containers. Ethylene and MCP are gases. They were taken from concentrated stocks and injected into the containers using a syringe. The final concentration for MCP was 10 nL L−1, and ethylene was used at 0.1, 1, and 10 μL L−1, depending on the experiment. NBD was applied as a cooled liquid on a glass petri dish inside the container (5 μL of NBD per liter of air). NBD is volatile at room temperature. It readily evaporated in the container atmosphere. Control plants were kept overnight in sealed containers without any added chemical. Except for the ethylene pretreatments, KMnO4 was included in the containers to prevent ethylene accumulation. Containers were opened after 20 h of treatment and fresh air was allowed to replace the chemicals. Then, plants were inoculated as described above.
SA pretreatment was performed 20 h before inoculation by watering the plants with a solution of 4 mm SA in 20 mm sodium phosphate buffer (pH 7.0). Watering the plants with the buffer alone was used as control pretreatment.
RNA Extraction and RNA-Blot Analysis
RNA was extracted from frozen tissue samples as described previously (ten Have and Woltering, 1997), glyoxylated, electrophoresed on agarose gels as described (van der Vlugt-Bergmans et al., 1997), and subsequently blotted onto Hybond N+ (Amersham, Buckinghamshire, UK) membranes using 0.025 m phosphate buffer (pH 8). Blots were hybridized as described (van der Vlugt-Bergmans et al., 1997) with probes radioactively labeled using α-32P-dATP (Amersham) and the Prime a Gene labeling kit according to the manufacturer's description (Promega, Madison, WI). DNA fragments used for labeling were a 730-bp EcoRI-HindIII fragment containing most of the fifth exon of the B. cinerea actA gene (Benito et al., 1998); the entire EcoRI-XhoI inserts of cDNA clones GLUA (glucanase II), GLUB (glucanase I), P6 (PR1; van Kan et al., 1992), CHI3 (chitinase II), and CHI9 (chitinase I; Danhash et al., 1993); a 400-bp EcoRI-HindIII fragment of pACS2 (ACC synthase; Rottmann et al., 1991); a 1.1-kb AccI-HindIII of pACO3 (ACC oxidase; Hamilton et al., 1991); a 700-bp EcoRI-HindIII fragment of pTI24 (proteinase inhibitor I; Graham et al., 1986); and a 1.7-kb EcoRI fragment of the radish (Raphanus sativus) 18S rDNA gene (Grellet et al., 1989).
Statistical Analyses
All statistical analysis were performed using Statgraphics Plus for Windows 4.0 Professional Version (Statistical Graphics Corp., Englewood Cliffs, NJ). In experiments with only one independent variable, a one-way ANOVA was performed. If ANOVA showed significant differences, Duncan's tests were carried out to compare the treatments by pairs. In experiments with two independent variables, two-way ANOVA was initially performed to check for interactions between the independent variables and for main effects (Dytham, 1999). Duncan's test was used as post hoc analysis. To get normality of the data and avoid heteroscedasticity, an arc-sin square root transformation was performed on the percentage of expanding lesions presented in Table I, whereas log transformation was performed on the results presented in Figure 3.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Clarence A. Ryan (Washington State University) for providing the mutant defenseless1 and the PSoe transgenes as well as for fruitful discussions, to Dr. Harry J. Klee (University of Florida) and Monsanto (St. Louis) for providing the ACC deaminase plants (UC8338), and to Rick Davies (Tomato Genetic Resources Center) for providing the tomato cv Pearson Never ripe and Epi mutants. Dr. Ernst Woltering (ATO-DLO, Waginengin, The Netherlands) is acknowledged for providing ethylene and MCP, and for fruitful discussions.
Footnotes
This work was supported in part by the Technology Foundation STW (The Netherlands), the applied science division of the Netherlands Organization for Scientific Research and the technology program of the Ministry of Economic Affairs; and by the Area Sociocultural Caixavigo and Secretaría Xeral de Investigación e Desenvolvemento, Xunta de Galicia, Spain (fellowships to J.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001453.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit MEJ. Ethylene in Plant Biology. San Diego: Academic Press; 1992. [Google Scholar]
- Aloni R, Wolf A, Feigenbaum P, Avni A, Klee HJ. The Never ripe mutant provides evidence that tumor-induced ethylene controls the morphogenesis of Agrobacterium tumefaciens-induced crown galls in tomato stems. Plant Physiol. 1998;117:841–849. doi: 10.1104/pp.117.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, De Meyer G, Höfte M. Pathways involved in control of B. cinerea via induced resistance. Med Fac Landbouwwet Rijksuniv Gent. 1999;64:477–488. [Google Scholar]
- Audenaert K, De Meyer G, Höfte M. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Fox EJ, Yen H, Lee S, Ying T, Grierson D, Giovannoni JJ. Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiol. 2001;127:58–66. doi: 10.1104/pp.127.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AA. Biochemical mechanisms of disease resistance. Annu Rev Plant Physiol. 1981;32:21–81. [Google Scholar]
- Benito EP, ten Have A, van't Klooster JW, van Kan JAL. Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur J Plant Pathol. 1998;104:207–220. [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:13–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Boller T. Ethylene in pathogenesis and disease resistance. In: Mattoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC; 1991. pp. 293–314. [Google Scholar]
- Bostock RM. Signal conflicts and synergies in induced resistance to multiple attackers. Physiol Mol Plant Pathol. 1999;55:99–109. [Google Scholar]
- Chappell J, Hahlbrock K, Boller T. Rapid induction of ethylene biosynthesis in cultured parsley cells by fungal elicitor and its relationship to the induction of phenylalanine ammonia-lyase (Phytophthora megasperma) Planta. 1984;161:475–480. doi: 10.1007/BF00394581. [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Jones JB, Klee HJ. Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol Plant-Microbe Interact. 2001;14:487–495. doi: 10.1094/MPMI.2001.14.4.487. [DOI] [PubMed] [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ. Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol. 2000;123:81–92. doi: 10.1104/pp.123.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. Root formation in ethylene-insensitive plants. Plant Physiol. 1999;121:53–60. doi: 10.1104/pp.121.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong XN. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn J, Sessa G, Martin GB. Innate immunity in plants. Curr Opin Immunol. 2001;13:55–62. doi: 10.1016/s0952-7915(00)00182-5. [DOI] [PubMed] [Google Scholar]
- Danhash N, Wagemakers CAM, van Kan JAL, de Wit PJGM. Molecular characterization of four chitinase cDNAs obtained from Cladosporium fulvum-infected tomato. Plant Mol Biol. 1993;22:1017–1029. doi: 10.1007/BF00028974. [DOI] [PubMed] [Google Scholar]
- De Meyer G, Höfte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87:588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- Dickman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R. Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acad Sci USA. 2001;98:6957–6962. doi: 10.1073/pnas.091108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dytham C. Choosing and Using Statistics: A Biologist's Guide. Oxford: Blackwell Science Ltd.; 1999. [Google Scholar]
- Elad Y. Production of ethylene by tissues of tomato, pepper, French-bean and cucumber in response to infection by Botrytis cinerea. Physiol Mol Plant Pathol. 1990;36:277–287. [Google Scholar]
- Elad Y. Regulators of ethylene biosynthesis or activity as a tool for reducing susceptibility of host plant tissues to infection by Botrytis cinerea. Neth J Plant Pathol. 1993;99:105–113. [Google Scholar]
- El-Kazzaz MK, Sommer NF, Kader AA. Ethylene effects on in vitro and in vivo growth of certain postharvest fruit-infecting fungi. Phytopathology. 1983;73:998–1001. [Google Scholar]
- Esquerré Tugayé MT, Lafitte C, Mazau D, Toppan A, Touze A. Cell surfaces in plant-microorganism interactions: II. Evidence for the accumulation of hydroxyproline-rich glycoproteins in the cell wall of diseased plants as a defense mechanism. Plant Physiol. 1979;64:320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XT, Mattheis JP, Roberts RG. Biosynthesis of phytoalexin in carrot root requires ethylene action. Physiol Plant. 2000;110:450–454. [Google Scholar]
- Fujino DW, Burger DW, Yang SF, Bradford KJ. Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. cultivar VFN8) Plant Physiol. 1988;88:774–779. doi: 10.1104/pp.88.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A. The hypersensitive reaction facilitates plant infection by the necrotrophic fungus Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Graham JS, Hall G, Pearce G, Ryan CA. Regulation of synthesis of proteinase inhibitors I and II mRNAs in leaves of wounded tomato plants. Planta. 1986;169:399–405. doi: 10.1007/BF00392137. [DOI] [PubMed] [Google Scholar]
- Grellet F, Delcasso-Tremousaygue D, Delseny M. Isolation and characterization of an unusual repeated sequence from the ribosomal intergenic spacer of the crucifer Sisymbrium irio. Plant Mol Biol. 1989;12:695–706. doi: 10.1007/BF00044160. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Bouzayen M, Grierson D. Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proc Natl Acad Sci USA. 1991;88:7434–7437. doi: 10.1073/pnas.88.16.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman T, Scott Schmidt J, Zheng X, Bent AF. Isolation of ethylene insensitive soybean mutants that are altered in pathogen-susceptibility and gene-for-gene disease resistance. Plant Physiol. 1999;119:935–949. doi: 10.1104/pp.119.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (jl5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Hayford MB, Kretzmer KA, Barry GF, Kishore GM. Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell. 1991;3:1187–1194. doi: 10.1105/tpc.3.11.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJM. Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. The never ripe mutation blocks ethylene perception in tomato. Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenkamper G, Manac'h N, Broin M, Cuine S, Becuwe N, Kuntz M, Rey P. Accumulation of plastid lipid-associated proteins (fibrillin/CDSP34) upon oxidative stress, ageing and biotic stress in Solanaceae and in response to drought in other species. J Exp Bot. 2001;52:1545–1554. doi: 10.1093/jexbot/52.360.1545. [DOI] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marte M, Buonaurio R, Dellatorre G. Induction of systemic resistance to tobacco powdery mildew by tobacco mosaic virus, tobacco necrosis virus or ethephon. J Phytopathol. 1993;138:137–144. [Google Scholar]
- Mayer AM, Staples RC, Gil-ad NL. Mechanisms of survival of necrotrophic fungal plant pathogens in hosts expressing the hypersensitive response. Phytochemistry. 2001;58:33–41. doi: 10.1016/s0031-9422(01)00187-x. [DOI] [PubMed] [Google Scholar]
- McGurl B, Orozco-Cardenas M, Pearce G, Ryan CA. Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc Natl Acad Sci USA. 1994;91:9799–9802. doi: 10.1073/pnas.91.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurl B, Pearce G, Orozco Cardenas ML, Ryan CA. Structure, expression and antisense inhibition of the systemin precursor gene. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Holcombe LJ, Carr JP. Characteristics of salicylic acid-induced delay in disease caused by a necrotrophic fungal pathogen in tobacco. Physiol Mol Plant Pathol. 2000;57:47–54. [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 2001;25:315–323. doi: 10.1046/j.1365-313x.2001.00968.x. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Olson DC, Shiu Oi Y, Yang Shang F. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Mol Biol. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- Orozco Cardenas ML, Narvaez Vasquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Pontier D, Balague C, Roby D. The hypersensitive response. A programmed cell death associated with plant resistance. CR Acad Sci III. 1998a;321:721–734. doi: 10.1016/s0764-4469(98)80013-9. [DOI] [PubMed] [Google Scholar]
- Pontier D, Tronchet M, Rogowsky P, Lam E, Roby D. Activation of hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol Plant-Microbe Interact. 1998b;11:544–554. doi: 10.1094/MPMI.1998.11.6.544. [DOI] [PubMed] [Google Scholar]
- Quirino BF, Noh YS, Himelblau E, Amasino RM. Molecular aspects of leaf senescence. Trends Plant Sci. 2000;5:278–282. doi: 10.1016/s1360-1385(00)01655-1. [DOI] [PubMed] [Google Scholar]
- Rodrigo I, Vera P, Tornero P, Hernandez Yago J, Conejero V. cDNA cloning of viroid-induced tomato pathogenesis-related protein P23: characterization as a vacuolar antifungal factor. Plant Physiol. 1993;102:939–945. doi: 10.1104/pp.102.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang SQ, Klessig DF, Hirt H, Jones JDG. Rapid Avr9- and Cf-9-dependent activation of map kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–962. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Compounds controlling the ethylene receptor. Bot Bull Acad Sin. 1999;40:1–7. [Google Scholar]
- ten Have A, Woltering EJ. Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Mol Biol. 1997;34:89–97. doi: 10.1023/a:1005894703444. [DOI] [PubMed] [Google Scholar]
- ten Have A, Mulder W, Visser J, van Kan JAL. The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant-Microbe Interact. 1998;11:1009–1016. doi: 10.1094/MPMI.1998.11.10.1009. [DOI] [PubMed] [Google Scholar]
- ten Have A, Oude Breuil W, Wubben JP, Visser J, van Kan JAL. Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet Biol. 2001;33:97–105. doi: 10.1006/fgbi.2001.1269. [DOI] [PubMed] [Google Scholar]
- Thomma B, Penninckx I, Broekaert WF, Cammue BPA. The complexity of disease signaling in Arabidopsis. Curr Opinion Immunol. 2001;13:63–68. doi: 10.1016/s0952-7915(00)00183-7. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert W. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DV, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors nr and Leetr4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA. 2000;97:5663–5668. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P, Conejero V, Vera P. A gene encoding a novel isoform of the PR-1 protein family from tomato is induced upon viroid infection. Mol Gen Genet. 1994;243:47–53. doi: 10.1007/BF00283875. [DOI] [PubMed] [Google Scholar]
- Tornero P, Gadea J, Conejero V, Vera P. Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol Plant-Microbe Interact. 1997;10:624–634. doi: 10.1094/MPMI.1997.10.5.624. [DOI] [PubMed] [Google Scholar]
- van der Vlugt-Bergmans CJB, Wagemakers CAM, van Kan JAL. Cloning and expression of the cutinaseA gene of Botrytis cinerea. Mol Plant-Microbe Interact. 1997;10:21–29. doi: 10.1094/MPMI.1997.10.1.21. [DOI] [PubMed] [Google Scholar]
- van Kan JAL, Cozijnsen T, Danhash N, de Wit PJGM. Induction of tomato stress protein mRNAs by ethephon, 2,6-dichloroisonicotinic acid and salicylate. Plant Mol Biol. 1995;27:1205–1213. doi: 10.1007/BF00020894. [DOI] [PubMed] [Google Scholar]
- van Kan JAL, Joosten MHAJ, Wagemakers CAM, van den Berg-Velthuis GCM, de Wit PJGM. Differential accumulation of mRNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol Biol. 1992;20:513–527. doi: 10.1007/BF00040610. [DOI] [PubMed] [Google Scholar]
- van Loon LC, Pennings GGH. Involvement of ethylene in the induction of systemic acquired resistance in tobacco. In: Fritig B, Legrand M, editors. Mechanisms of Plant Defense Responses. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 156–159. [Google Scholar]
- Yang FS, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Zimmerli L, Métraux J-P, Mauch-Mani B. β-aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol. 2001;126:517–523. doi: 10.1104/pp.126.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]