Abstract
We compared haemodynamic and peripheral vasomotor responses to lower body negative pressure (LBNP) in cardiac transplant recipients who had undergone bicaval anastomoses, involving right atrial deafferentation (−RA), and the conventional procedure in which some atrial baroreceptor afferents remain intact (+RA). We measured mean forearm blood flow (FBF) responses using Doppler/ultrasound during three randomised trials involving 0 (baseline), −20 and −40 mmHg LBNP in 15 transplant recipients (9 −RA, 6 +RA) and in eight healthy matched controls. A significant effect of LBNP on FBF existed between control and transplant groups (P < 0.05; two-way ANOVA). Mild LBNP (−20 mmHg), significantly decreased FBF by 29.7 ± 10.0% relative to baseline in +RA subjects (P < 0.05), whereas the 17.7 ± 10.3% decrease in −RA subjects was not significant. In response to −40 mmHg LBNP, FBF significantly decreased in control (42.4 ± 4.6%, P < 0.05) and +RA subjects (33.3 ± 11.4%, P < 0.05) with no significant change in the −RA group. The response of systolic blood pressure (SBP) to −40 mmHg significantly differed between groups (P < 0.05): −RA subjects decreased significantly (P < 0.05) whilst the decrease in SBP in +RA subjects did not achieve significance and control subjects exhibited an increase. The heart rate increase from baseline to −40 mmHg was significantly attenuated in −RA relative to controls and the +RA group (P < 0.05). The present study demonstrates that atrial deafferentation impairs reflex vasomotor control of the circulation in response to low- and high-level LBNP, indicating that atrial deafferentation may contribute to abnormal arterial pressure regulation.
Cardiac transplantation has been an established treatment for end-stage cardiac failure for several decades. Because cardiac failure impairs reflex cardiovascular function (Goldsmith et al. 1983; Mohanty et al. 1986), much interest has focused on the effects of cardiac transplantation on reflex sympathetic responses to physiological stimuli such as orthostatic stress. In healthy humans, lower body negative pressure (LBNP)-induced decreases in venous return result in increased vascular resistance without altering arterial pressure. The findings of Mohanty et al. (1987) indicate that cardiac deafferentation attenuates the normal vasoconstrictor response to LBNP, implicate unloading of cardiopulmonary baroreceptors, possibly located in the atria, ventricles, coronary arteries and pulmonary veins, in the reflex vasoconstriction in response to LBNP. In contrast, Jacobson et al. (1993) suggested that sinoaortic baroreflexes are more important than their ventricular counterparts in regulating sympathetic outflow and resultant peripheral vasoconstrictor tone during LBNP. This study did not consider the role of atrial receptors in mediating the responses to LBNP as the subjects involved had undergone transplantation with the conventional technique developed by Lower and Shumway (1960) in which afferents arising from the recipient atrial remnant and venoatrial junctions remain intact. Indeed, animal studies suggest that atrial baroreceptors, which densely innervate the venoatrial junction of both left and right atria, are important in reflex responses to physiological stimuli (Hainsworth, 1991), raising the possibility that cardiopulmonary receptors may, in fact, play an important role in reflex responses to orthostasis and decreased venous return.
Although the standard technique for transplantation may preserve peripheral sympathetic vasomotor responses to LBNP (Jacobsen et al. 1993), it has also been reported to alter atrial dimensions and function (Angermann et al. 1990) and cause early postoperative arrhythmias (Milano et al. 2000) and mitral regurgitation (Stevenson et al. 1987). To prevent these common postoperative complications, the bicaval anastomoses technique, involving right atrial and ventricular denervation, was popularized (Webb et al. 1959; Sievers et al. 1991). However, the reflex vascular effects of the bicaval anastomoses and the Lower and Shumway techniques have not been compared.
In the present study, we compared two groups of cardiac transplant recipients with distinct baroreflex anatomy to determine the contribution of right atrial baroreceptor afferents to reflex vasomotor and haemodynamic responses to decreased venous return. We report haemodynamic and peripheral vascular responses to LBNP in both standard and bicaval cardiac transplant recipients.
Methods
Subject characteristics
Fifteen cardiac transplant recipients participated in the study (10 men, 5 women; mean age ± s.e.m., 53 ± 5 years; height, 1.72 ± 0.03 m; body mass, 81.2 ± 4.9 kg; body mass index (BMI), 27.3 ± 1.3 kg m−2; time from transplant, 50 ± 12 months). Physical and historical examination of each transplant recipient, including echo- and electrocardiograms, cardiac catheterization with endomyocardial biopsy provided evidence that none of the patients had allograft rejection or persistent cardiopulmonary disease at the time of study. However, previous episodes of rejection did not constitute a reason for exclusion. Transplant recipients were all treated with an immunosuppressant regimen and antihypertensive agents (Table 1), and medications remained unchanged on the study day. Transplant recipients were subdivided based on the transplantation technique (standard or bicaval). Six patients with standard transplants (+RA) participated (4 men, 2 women; age, 58 ± 3 years; height, 1.73 ± 0.03 m; body mass, 78.1 ± 5.0 kg; BMI, 26.0 ± 1.4 kg m−2; time from transplant, 74 ± 15 months) as well as nine patients (6 men, 3 women; age, 50 ± 5 year; height, 1.72 ± 0.03 m; body mass, 83.3 ± 5.1 kg; BMI, 28.1 ± 1.2 kg m−2; time from transplant, 34 ± 6 months) with bicaval transplants (−RA). No statistically significant difference was evident between +RA and −RA groups except for time from transplant (P = 0.04).
Table 1.
Therapeutic regimens of transplant group
| No. of subjects | ||
|---|---|---|
| Therapy | +RA | −RA |
| Immunosuppression | ||
| Cyclosporine | 4 | 5 |
| Azathioprine | 3 | 1 |
| Prednisolone | 3 | 4 |
| Mycophenolate | 3 | 7 |
| Sirolimus | 1 | 4 |
| Tacrolimus | 1 | 1 |
| Antihypertensives | ||
| ACE inhibitors | 3 | 6 |
| Calcium channel blockers | 5 | 7 |
| Angiotensin II inhibitors | 5 | 7 |
| Prazosin | 0 | 3 |
| Frusemide | 1 | 1 |
| Aspirin | 5 | 7 |
| Hypolipidaemics | ||
| Statins | 6 | 9 |
| Diabetic medications | ||
| Metformin | 1 | 0 |
| Insulin | 0 | 1 |
ACE, angiotensin-converting enzyme.
Eight healthy unmedicated control subjects (5 men, 3 women) were matched to the transplant recipients according to age (49 ± 4 years), height (1.73 ± 0.03 m), body mass (81.8 ± 4.9 kg) and BMI (26.8 ± 1.1 kg m−2). All screening measures were similar between the control and transplant groups except for heart rate, which was significantly higher in both transplant groups compared to controls (P < 0.05; Table 2).
Table 2.
Resting screening measures
| Variable | Control | +RA | −RA | P value +RA versus −RA |
|---|---|---|---|---|
| Lipids (all mmol l−1) | ||||
| Total cholesterol | 4.9 ± 0.2 | 4.7 ± 0.2 | 4.6 ± 0.2 | 0.71 |
| LDL | 2.9 ± 0.2 | 2.6 ± 0.1 | 2.3 ± 0.3 | 0.34 |
| HDL | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 0.98 |
| Triglycerides | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.7 ± 0.4 | 0.39 |
| Blood pressure (mmHg) | ||||
| Systolic | 126 ± 2 | 125 ± 6 | 138 ± 4 | 0.10 |
| Diastolic | 76 ± 2 | 74 ± 5 | 82 ± 5 | 0.27 |
| Heart Rate (beats min−1) | 65 ± 2 | 78 ± 4 | 76 ± 4 | 0.68 |
| HbA1C (%) | 5.1 ± 0.1 | 5.4 ± 0.1 | 5.6 ± 0.2 | 0.35 |
| FBG (mmol l−1) | 5.0 ± 0.1 | 5.1 ± 0.2 | 4.7 ± 0.3 | 0.30 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein; HbA1C, glycosylated haemoglobin; FBG, fasting blood glucose.
All subjects were non-smokers and did not have current clinical evidence (or additionally, in controls, past evidence) of any cardiovascular, respiratory or neural disorder, including peripheral or autonomic neuropathy. Subjects were excluded based on the following criteria: pacemaker implantation, creatinine levels > 180 µg l−1, hepatic impairment, gout or more than slightly raised cholesterol levels (total cholesterol > 6.0 mmol l−1). The study was approved by the Ethics Committee of Royal Perth Hospital, and all procedures were performed in accordance with institutional guidelines and the Declaration of Helsinki. Prior to the study, each subject gave written informed consent to participate.
Experimental protocol
All subjects fasted for 4 h and abstained from caffeine, alcohol and exercise for 24 h prior to the study. Each study began with the subject supine and both arms supported perpendicular to the body at heart level. A 20-gauge 3-cm venous catheter (Becton Dickinson; Sandy, UT, USA) was then introduced into a vein of the right arm for blood sampling. After catheterization, the subject was gently secured in a custom-made LBNP box, placed over the subject from the iliac crest downwards. A commercial vacuum cleaner (Model UZ-930; Electrolux; Stockholm, Sweden) was attached to the box and connected to a voltage converter, which allowed graduated control of the vacuum intensity. Thus, negative pressure could be precisely controlled and set to 0, −20 and −40 mmHg with the aid of an industrial pressure gauge (Ambit Instruments; Wetherill Park, NSW, Australia).
After subject preparation, a 2-min familiarization session including application of LBNP was completed. Following a further 10-min rest, the study protocol ensued, consisting of three consecutive 5-min trials, separated by 10-min rest periods. Each trial involved the application of one of three levels of LBNP (0, −20, −40 mmHg). For 0 LBNP, the vacuum was turned on (sham effect) 30 s before the start of the trial. To minimize subject discomfort and movement at the commencement of negative pressure, the vacuum was applied gradually over 30 s (from 0 to −20 mmHg) or over 1 min (0 to −40 mmHg). The levels of LBNP were randomised.
Forearm blood flow assessment
Forearm blood flow was calculated from measurements using high-resolution vascular ultrasonography with synchronized Doppler velocity measurement. All parameters were recorded throughout each trial, with mean diameter, velocity and blood flow (FBF) measurements calculated from averages across the final 2 min of each trial. Mean vascular conductance (FVC) was calculated as (100 × FBF) mean arterial pressure−1 and expressed in arbitrary units. The brachial artery of the non-dominant arm was imaged in the distal third of the upper arm with a 10-MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (Acuson Aspen Advanced; Siemens; Malvern, PA, USA). Ultrasonic parameters were set to optimize longitudinal B-mode images of the lumen and arterial wall interface. Continuous Doppler velocity assessment was recorded using an insonation angle of 60 deg. Brachial artery diameter was assessed post test using custom-designed edge-detection and wall-tracking software as previously described (Woodman et al. 2001; Green et al. 2002).
Briefly, the video signal was taken directly from the ultrasound machine and, using an IMAQ-PCI-1407 card, was directly encoded and stored as a digital DICOM file on the PC. Subsequent software analysis of this data, at approximately 20–30 frames s−1, was performed using an icon-based graphical programming language (LabVIEW 6.02, National Instruments, Austin, TX, USA) and toolkit (IMAQ, National Instruments) in which developers build software programs called virtual instruments (VIs). Vessel cross sectional area (CSA) was calculated from the software-derived arterial diameter measures using the equation: CSA =π radius2. Blood flow, calculated as the product of CSA and Doppler flow velocity (ν), was derived from Doppler/ultrasound measures, using the suite of VIs. Once the study has been acquired, a data ‘display’ VI plots a graph of the arterial diameters and velocities against time and uses this information to calculate and display the blood flow as a continuous plot across the cardiac cycle. Operator-controlled cursors can be used to select and zoom on the sections of data of interest (e.g. the last 2 min of each trial), and lastly the data is displayed as a graph and the data between the time points analysed. Our recent study indicated that this method of blood flow assessment is closely correlated with actual flow through a ‘phantom’ arterial flow system, that reproducibility of measurements is significantly better using the software than with manual methods and that it reduces observer error significantly, allowing studies to be capable of detecting significant changes with substantially fewer subjects (Woodman et al. 2001; Green et al. 2002).
Other measurements
Heart rate was continuously monitored using a three-lead ECG. Blood pressure was recorded using an automated auscultatory method (Dinamap; Critikon; Tampa, FL, USA). Blood samples (4 ml each) were taken at the end of each trial for measurement of plasma catecholamine (adrenaline and noradrenaline) levels, determined by HPLC with electrochemical detection (n = 5 for each group; Table 3). To maintain constant blood volume, the blood volume taken for sampling was immediately replaced by infusion of 4 ml saline.
Table 3.
Effects of LBNP on systemic haemodynamics, plasma catecholamines and mean blood flow and conductance
| Variable | Condition | Controls | +RA | −RA |
|---|---|---|---|---|
| Heart rate | 0 mmHg | 62 ± 2 | 76 ± 5 | 76 ± 5 |
| (beats min−1) | − 20 mmHg | 64 ± 2 | 81 ± 3* | 78 ± 4 |
| − 40 mmHg | 73 ± 2*† | 88 ± 4*† | 82 ± 4*† | |
| MAP | 0 mmHg | 89.7 ± 2.5 | 92.0 ± 4.4 | 97.1 ± 4.4 |
| (mmHg) | − 20 mmHg | 89.3 ± 3.3 | 90.8 ± 5.7 | 97.8 ± 4.6 |
| − 40 mmHg | 92.6 ± 3.7† | 89.8 ± 6.5 | 95.6 ± 3.9 | |
| SBP | 0 mmHg | 123 ± 4 | 122 ± 7 | 138 ± 4 |
| (mmHg) | − 20 mmHg | 124 ± 5 | 122 ± 8 | 135 ± 6 |
| − 40 mmHg | 127 ± 4 | 118 ± 7 | 131 ± 5* | |
| DBP | 0 mmHg | 73 ± 2 | 74 ± 3 | 77 ± 5 |
| (mmHg) | − 20 mmHg | 72 ± 3 | 73 ± 4 | 79 ± 5 |
| − 40 mmHg | 76 ± 3† | 76 ± 6 | 78 ± 4 | |
| Noradrenaline | 0 mmHg | 174 ± 32 | 157 ± 34 | 165 ± 44 |
| (pg ml−1) | − 20 mmHg | 282 ± 43* | 250 ± 51* | 252 ± 59* |
| − 40 mmHg | 323 ± 79* | 388 ± 111* | 467 ± 126* | |
| Adrenaline | 0 mmHg | 19 ± 2 | 47 ± 13§ | 14 ± 3 |
| (pg ml−1) | − 20 mmHg | 21 ± 8 | 49 ± 11§ | 15 ± 0 |
| − 40 mmHg | 30 ± 5 | 46 ± 10§ | 15 ± 0 | |
| FBF | 0 mmHg | 81.2 ± 16.3 | 92.3 ± 18.1 | 92.6 ± 18.2 |
| (ml min−1) | − 20 mmHg | 65.1 ± 19.4* | 61.8 ± 15.6* | 77.2 ± 17.0 |
| − 40 mmHg | 51.0 ± 13.9*† | 56.5 ± 14.1* | 76.2 ± 18.4 | |
| FVC | 0 mmHg | 89.5 ± 17.1 | 102.5 ± 19.7 | 94.9 ± 15.6 |
| (units) | − 20 mmHg | 72.2 ± 20.8* | 68.2 ± 16.0* | 79.3 ± 15.9 |
| − 40 mmHg | 54.9 ± 14.7*† | 62.0 ± 13.2* | 78.0 ± 16.5 | |
| ΔFBF (%) | −20 mmHg | −26.5 ± 7.3 | −29.7 ± 10.0 | −17.7 ± 10.3 |
| (Δversus 0 mmHg) | −40 mmHg | −42.4 ± 4.6† | −33.3 ± 11.4 | −20.9 ± 11.5 |
| ΔFVC (%) | −20 mmHg | −25.4 ± 7.3 | −28.9 ± 10.0 | −18.1 ± 10.1 |
| (Δversus 0 mmHg) | −40 mmHg | −43.6 ± 5.2† | −32.2 ± 10.7 | −20.4 ± 10.7 |
MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
P < 0.05 versus 0 within group
P < 0.05 versus −20 within group
P < 0.05 +RA versus control and −RA.
Data analysis
All data are reported as means ± s.e.m. for the last 2 min of each trial. Two-way repeated measures ANOVA was performed to compare the effect of LBNP levels between control and transplanted patients (+RA versus −RA versus controls at 0, −20 and −40 mmHg). Post hoc Student's t test analysis with Bonferroni correction was performed to identify differences between transplant type or level of LBNP. Comparisons between groups within each level of LBNP were analysed using one-way ANOVA or unpaired t tests.
Results
Haemodynamic and vasomotor changes during each level of LBNP are presented in Table 3. The sham LBNP trial (0 mmHg) was considered the baseline for mild (−20 mmHg) and high (−40 mmHg) level LBNP.
Vasomotor responses to LBNP
Absolute values for mean forearm blood flow (FBF) and vascular conductance (FVC) are presented in Table 3. In addition, we assessed vasomotor responses under each condition in terms of percentage change in flow and conductance from baseline for each subject (Fig. 1). This is the preferred way to compare interventions that cause vasodilatation or vasoconstriction under conditions in which marked baseline differences are evident (Lautt, 1989; O'Leary, 1991; Tschakovsky et al. 2002; Rosenmeier et al. 2003).
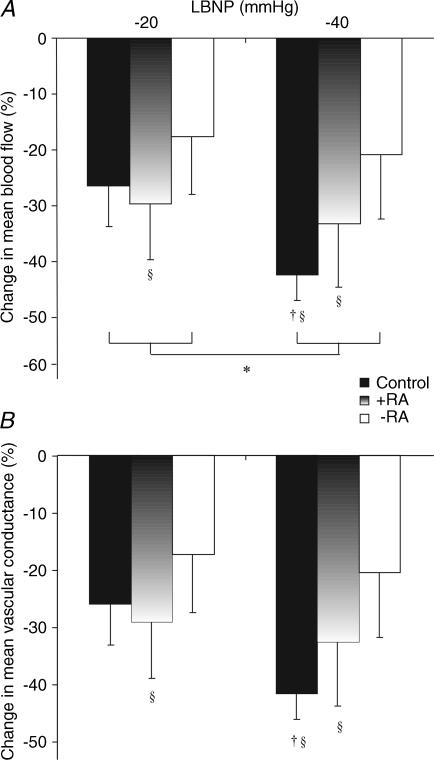
Figure 1. Mean blood flow and conductance responses to LBNP.
Mean forearm blood flow (A) and conductance (B) responses to mild and high-level LBNP, taken as a percentage change from baseline, are presented for control (filled bars), +RA (gradient bars) and −RA (open bars) subjects. *P < 0.05, two-way ANOVA, control versus +RA versus−RA, effect of LBNP −20 versus −40 mmHg; †P < 0.05, post hoc Student's paired t test, −20 versus −40 mmHg in control subjects; §P < 0.05 versus baseline responses, post hoc Student's t test.
A significant effect of LBNP on FBF existed when the control and transplant groups were compared by ANOVA (P < 0.05; Fig. 1). In control subjects, mild LBNP caused a reduction in FBF of 26.5 ± 7.3% and a reduction in FVC of 25.4 ± 7.3%. Absolute values of FBF and FVC were significantly different from those at baseline (P < 0.05, Table 3). Similarly, in the +RA group, FBF significantly decreased by 29.7 ± 10.0% (P < 0.05) and FVC by 28.9 ± 10.0% (P < 0.05), with absolute FBF and FVC data at this level of LBNP also significantly lower than at baseline (P < 0.05). The −RA group FBF response to −20 mmHg was −17.7 ± 10.3%, and FVC decreased by 18.1 ± 10.1%. These values were not significantly different from baseline and absolute FBF and FVC values at −20 mmHg also were not significantly different from preceding baseline values in this group.
In response to −40 mmHg LBNP, FBF decreased by 42.4 ± 4.6% and FVC by 43.6 ± 5.2% in controls, with both significantly different from −20 mmHg (P < 0.05). Absolute FBF and FVC levels in controls were significantly lower at −40 mmHg relative to both baseline and −20 mmHg (P < 0.05). In the +RA group, FBF and FVC decreased during −40 mmHg (by −33.3 ± 11.4% and −32.2 ± 10.7%, respectively), levels which were significantly lower compared to baseline (both P < 0.05), but there was no additional effect of −40 mmHg relative to −20 mmHg in this group. In −RA subjects, FBF and FVC levels during −40 mmHg did not differ from either baseline or −20 mmHg.
Haemodynamic responses to LBNP
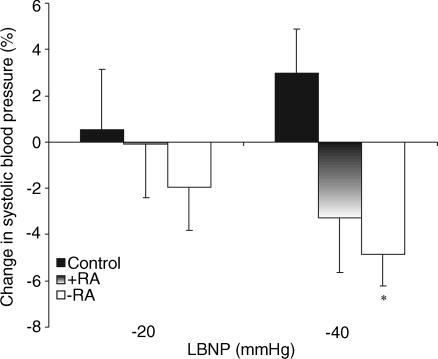
Although there were no significant differences in mean arterial pressures at baseline (i.e. 0 mmHg) between the groups, both transplant groups tended to possess higher values, as might be expected. LBNP data are therefore listed in Table 3 and also expressed as percentage change from 0 mmHg within groups (Fig. 2). Relative to baseline, no changes were evident within groups for pressures at −20 mmHg. In control subjects, −40 mmHg significantly increased mean arterial pressure relative to −20 mmHg data (P < 0.05, Table 3). In contrast, −40 mmHg was associated with a significant decrease in systolic pressure relative to baseline in the −RA subjects (Fig. 2). No significant change in systolic or mean arterial pressures was evident in the +RA group, although in contrast to the control subjects, data in this group tended to decrease at −40 mmHg (Fig. 2).
Figure 2. Systolic pressure responses to LBNP.
Percentage change in systolic pressure relative to baseline (0 mmHg) in control (fille bars), +RA (gradient bars) and −RA (open bars) subjects are shown during −20 and −40 mmHg LBNP. *P < 0.05 versus baseline within group.
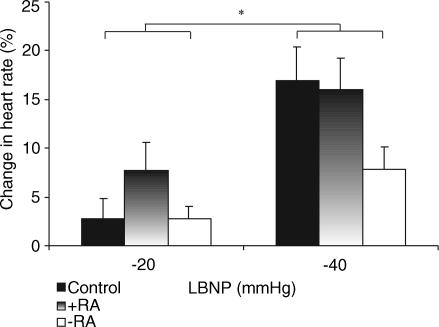
Although Table 3 indicates that baseline (i.e. 0 mmHg) heart rate data for each of the groups was within the ‘normal’ range, heart rate was significantly higher in both groups of transplant recipients versus controls (P < 0.05). We have therefore also presented data as percentage change from baseline within groups (Fig. 3). In response to −20 mmHg, heart rate in +RA subjects significantly increased relative to baseline (P < 0.05), whereas no changes were evident in control or −RA subjects. When all groups were compared, the increase in heart rate between −20 and −40 mmHg was blunted in both transplant groups relative to controls (Figs. 3; P < 0.05). In particular, heart rate during −40 mmHg was significantly attenuated in −RA subjects relative to both the control and +RA groups (all P < 0.05).
Figure 3. Heart rate responses to LBNP.
Percentage increase in mean heart rate relative to baseline (0 mmHg) in control (filled bars), +RA (gradient bars) and −RA (open bars) subjects are shown during −20 and −40 mmHg LBNP. *P < 0.05, two-way ANOVA, control versus +RA versus −RA, −20 versus−40 mmHg.
Discussion
The results of the present study indicate that transplant patients who have undergone bicaval anastamoses, with consequent atrial deafferentation, exhibit impaired peripheral vasomotor responses to mild orthostatic stress induced by −20 mmHg LBNP when compared to transplant patients with preserved right atrial afferents (standard transplantation). No changes in arterial pressure were evident in any group at this mild level of LBNP, indicating that sinoaortic baroreceptor unloading is unlikely to have occurred. These data indicate that right atrial cardiopulmonary afferents may contribute to reflex vasoconstrictor responses during moderate decreases in central venous pressure. At higher levels of LBNP (−40 mmHg), neither transplanted group exhibited further significant vasoconstrictor responses relative to the mild LBNP condition, whereas FBF and FVC decreased significantly between −20 and −40 mmHg in control subjects. The absence of a vasoconstrictor response was associated with decreased systolic blood pressure in both transplant groups, particularly in those without atrial innervation, whereas systolic pressure was maintained in healthy control subjects, probably due to the peripheral vasoconstriction.
Previous studies employing cardiac transplantation as a model to examine autonomic control of the circulation have produced disparate findings regarding the importance of cardiopulmonary and sinoaortic afferents in reflex responses to orthostatic stress. Early studies indicated that ventricular deafferentation was associated with attenuated increases in forearm vascular resistance in response to LBNP and decreased central venous pressure (Mohanty et al. 1987; Morgan et al. 1987). Other studies observed that maintenance of arterial pressure (Johnson et al. 1974) or carotid baroreflex stimulation with neck suction (Abboud et al. 1979) during high levels of LBNP did not abolish the typical vasoconstrictor responses observed. These studies, along with the observation that increases in forearm vascular resistance may be closely related to decreases in central venous pressure during ramp LBNP (Johnson et al. 1974) and the results of the present study, strongly imply a role for cardiopulmonary baroreceptors in reflex vasoconstrictor responses to orthostatic stress.
However more recently, Jacobsen and colleagues (1993) measured peroneal sympathetic nerve activity (SNA) and forearm blood flow responses during graded LBNP with and without intravenous phenylephrine infusion to prevent sinoaortic baroreceptor unloading in transplant recipients and control subjects. They observed similar SNA and vascular resistance responses to −15 and −40 mmHg LBNP in transplant and control subjects, suggesting that the neural stimulus for vasoconstriction is not attenuated after transplantation. They also abolished the increased SNA observed in transplant patients during mild (−15 mmHg) LBNP when phenylephrine was infused to prevent sinoaortic receptor unloading. This finding implies that the fall in arterial pressure mediating sinoaortic baroreceptor unloading in these transplant recipients was able to compensate for the loss of ventricular and/or coronary artery baroreceptors. Finally, Jacobsen et al. (1993) did not observe a close relationship between changes in SNA and changes in graded LBNP, indicating that LBNP may not be as closely associated with changes in central venous pressure as previously suggested (Johnson et al. 1974). Collectively, these data were interpreted as indicating that sinoaortic baroreceptors play the major role in reflex control of skeletal muscle blood flow during orthostatic stress in transplant recipients. Consistent with the data of Jacobsen et al. (1993), other studies have reported that combined heart and lung transplantation, with removal of the majority of cardiopulmonary afferents, does not impair forearm vasoconstriction during LBNP (Joyner et al. 1990) or tilt (Banner et al. 1990). In contrast, our results, indicating that at mild levels of LBNP the decrease in FBF observed was lower in the −RA group relative to +RA subjects, strongly suggest that atrial receptors participate in the organization of vasoconstrictor responses to decreases in arterial pressure. This may have implications for the interpretation of previous transplant studies that have enrolled only patients who have undergone the standard technique (Jacobsen et al. 1993) described by Lower & Shumway (1960).
In the present study, heart rate responses to LBNP were relatively intact in subjects with preserved native right atrium, whereas subjects with atrial denervation exhibited impaired heart rate responsiveness. Several factors may have influenced this finding. The first, and most likely, is that intact atrial reflex innervation may play a role in the exaggerated heart rate response to LBNP in the +RA group. However, a second contributor to the increased heart rate responsiveness in the +RA group may be elevated catecholaminergic control (Gilbert et al. 1989). In the present study, whilst noradrenaline increased step-wise with the level of LBNP in each group, LBNP had no effect on adrenaline levels, and during all LBNP conditions adrenaline levels were significantly higher in the +RA group relative to controls, as previously demonstrated by Gilbert et al. (1989), and to the −RA group. The reason for increased plasma adrenaline levels in the +RA group is unclear, but may not be physiologically significant given that the magnitude of difference is modest compared to, for example, the effect of exercise on circulating concentrations (Pott et al. 1996; Kjær et al. 2004). Although this finding relating to sympathoadrenal control may reflect longer time from transplant and possibly reinnervation of the sinus node (Gilbert et al. 1989; van de Borne et al. 2001), it is unlikely because adrenaline levels did not significantly correlate with time from transplant (r = 0.701, P = NS). In any event, the fact that the +RA group had a greater vasoconstrictor response to LBNP than the −RA group in the presence of a greater baseline concentration of adrenaline, a β2-agonist which would oppose the sympathetic vasoconstriction of the LBNP, provides further evidence of the involvement of the cardiopulmonary baroreflex.
While the main focus of the present study was to detect the effects of right atrial denervation on vasomotor responses to LBNP, the results provide some additional insights into the contributions of other cardiac reflexes. Despite denervated right atrium and ventricles, some residual vasoconstriction was evident in the −RA group in response to decreased venous return (−20 mmHg). This suggests that some baroreceptor function persists in these subjects and it is possible that afferents arising from the remnant recipient left atrium, vena cavae or pulmonary vasculature may play a role. It would be interesting to further investigate this possibility by comparing patients in this study to a group who have undergone heart–lung transplantation resulting in left atrium and pulmonary receptor denervation. In one preliminary study of three subjects, reported in abstract form, some residual vasoconstrictor function persisted following the heart–lung procedure (Joyner et al. 1990), suggesting that vena caval afferents may play a role in response to decreased venous return.
In clinical terms, the current study has important implications. Our data indicate that patients who have undergone bicaval transplantation do not possess intact vasoconstrictor responses when venous return is reduced. Indeed, the bicaval subjects were the only group to exhibit a significant fall in blood pressure during LBNP, possibly indicating that preservation of the right atrium may therefore produce some cardiovascular benefits post transplantation. However, these favourable effects must be balanced against the purported benefits of the bicaval approach in terms of decreasing the risk of rhythm abnormalities (Kaye et al. 1992; Deleuze et al. 1995; Leyh et al. 1995; Aziz et al. 1999) and tricuspid regurgitation (Angermann et al. 1990; Laske et al. 1995).
There are several limitations in the present study. Time from transplantation differed significantly between the groups, reflecting the contemporary popularity of the bicaval technique. Because post-transplantation time is associated with increases in sympathetic activity (van de Borne et al. 2001), this raises the possibility that more physiological disturbance may have evolved over time in the +RA group, though this seems unlikely as these subjects exhibited relatively preserved vasomotor responsiveness to LBNP compared to the more recently transplanted −RA group. It is also possible that more reinnervation may have occurred over time in the +RA group and that this, rather than presence of intact afferents from the remnant atria, may have been responsible for the relatively preserved cardiopulmonary baroreflex function in these subjects. However, the removal of the +RA subject with the longest post-transplantation time (132 months), which normalizes the difference in post-transplantation time between +RA versus −RA groups, did not change our findings in terms of heart rate, mean arterial, systolic and diastolic blood pressures or absolute mean blood flow and conductance. In addition, reinnervation is unlikely because (i) considerable evidence suggests that post-transplant reinnervation does not occur (Stinson et al. 1972; Mohanty et al. 1987; Yusuf et al. 1987; Arrowood et al. 1995), (ii) no differences were evident between the transplant groups in terms of resting heart rate or FBF and (iii) cardiac reinnervation, if it is apparent, probably relates to intrinsic rather than extrinsic cardiac nerves (Yusuf et al. 1987). A second limitation is that our group of transplant recipients were administered a range of drug regimens and doses, and we did not stop these during the study for ethical reasons. Our data therefore reflect the reflex responses of a group of transplant recipients on typically administered contemporary therapies, and we cannot exclude the possibility that impaired vasomotor responses to high-level LBNP may result, in part, from drug effects. However, it seems unlikely that differences between the transplant patients can be explained by drug effects, as both groups were managed on similar medications. Furthermore, when statistical comparison between the groups was performed excluding the three patients in the −RA group to whom prazosin was administered, no difference was evident in either the pattern or significance of difference in vasomotor responses between the groups (P = 0.048 for n = 6 versus P = 0.047 for n = 9 in −RA, two-way ANOVA versus +RA). Finally, it remains a possibility that transplant patients possess impaired responses to all physiological stimuli and that our data are simply a manifestation of this generalized reflex impairment. However, we think this unlikely because increases in FBF during chemoreflex activation with hypoxia, which we assessed separately in each group using a methodological approach we have detailed previously (Weisbrod et al. 2001, 2004), were similar between groups (normoxia to hypoxia: controls, 81.2 ± 16.3 to 101.4 ± 37.5 ml min−1; +RA, 92.3 ± 18.1 to 111.8 ± 22.8 ml min−1; −RA, 92.6 ± 18.2 to 101.9 ± 19.4 ml min−1). These data strongly suggest that both transplant groups have intact chemoreflex responses and imply that the differences in vasomotor responses to LBNP we observed are a specific consequence of differences in atrial contribution to cardiopulmonary baroreflex control.
In summary, the present study has demonstrated for the first time that patients with atrial deafferentation exhibit abnormal reflex haemodynamic and vasomotor responses to LBNP, suggesting that right atrial baroreceptor afferents contribute significantly to cardiovascular control during orthostatic stress.
Acknowledgments
We thank Helen Hayes and Clare Wood for their assistance with recruitment and Gavin Lambert of the Human Neurotransmitter Laboratory, Baker Heart Research Institute, for assistance with catecholamine analysis. We especially thank the volunteers who participated in this study. C.J.W. was supported by a University of Western Australia Whitfeld Fellowship. D.J.M. is supported by the National Health and Medical Research Council of Australia and the Foundation for High Blood Pressure Research Australia.
References
- Abboud F, Eckberg D, Johannsen U, Mark A. Carotid and cardiopulmonary baroreceptor control of splanchnic and forearm vascular resistance during venous pooling in man. J Physiol. 1979;286:173–184. doi: 10.1113/jphysiol.1979.sp012612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermann C, Spes C, Tammen A, Stempfle H, Schutz A, Kemkes B, Theisen K. Anatomic characteristics and valvular function of the transplanted heart: transthoracic versus transesophogeal echocardiographic findings. J Heart Transplant. 1990;9:331–338. [PubMed] [Google Scholar]
- Arrowood J, Goudreau E, Minisi A, Davis A, Mohanty P. Evidence against reinnervation of cardiac vagal afferents after human orthotopic cardiac transplantation. Circulation. 1995;92:402–408. doi: 10.1161/01.cir.92.3.402. [DOI] [PubMed] [Google Scholar]
- Aziz T, Burgess M, El-Gamel A, Campbell C, Rahman A, Deiraniya A, Yonan N. Orthotopic cardiac transplantation technique: a survey of current practice. Ann Thorac Surg. 1999;68:1242–1246. doi: 10.1016/s0003-4975(99)00796-1. 10.1016/S0003-4975(99)00796-1. [DOI] [PubMed] [Google Scholar]
- Banner N, Williams T, Patel N, Chalmers J, Lightman S, Yacoub M. Altered cardiovascular and neurohumoral responses to headup tilt after heart-lung transplant. Circulation. 1990;82:863–871. doi: 10.1161/01.cir.82.3.863. [DOI] [PubMed] [Google Scholar]
- Deleuze P, Benvenutti C, Mazzucotelli J, Perdrix C, Le Besnerais P, Mourtada A, Hillion M, Patrat J, Jouannot P, Loisance D. Orthotopic cardiac transplantation with direct caval anastomosis: is it the optimal procedure? J Thorac Cardiovasc Surg. 1995;109:731–737. doi: 10.1016/S0022-5223(95)70355-1. [DOI] [PubMed] [Google Scholar]
- Gilbert E, Eiswirth C, Mealey P, Larrabee P, Herrick C, Bristow M. β-adrenergic supersensitivity of the transplanted human heart is presynaptic in origin. Circulation. 1989;79:344–349. doi: 10.1161/01.cir.79.2.344. [DOI] [PubMed] [Google Scholar]
- Goldsmith S, Francis G, Levine T, Cohn J. Regional blood flow response to orthostasis in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1391–1395. doi: 10.1016/s0735-1097(83)80041-2. [DOI] [PubMed] [Google Scholar]
- Green D, Cheetham C, Reed C, O'Driscoll G. Continuous assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during lower limb exercise. J Appl Physiol. 2002;93:361–368. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–649. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Jacobsen T, Morgan B, Scherrer U, Vissing S, Lange R, Johnson N, Ring W, Rahko P, Hanson P, Victor R. Relative contributions of cardiopulmonary and sinoaortic baroreflexes in causing sympathetic activation in the human skeletal muscle circulation during orthostatic stress. Circ Res. 1993;73:367–378. doi: 10.1161/01.res.73.2.367. [DOI] [PubMed] [Google Scholar]
- Johnson J, Rowell L, Niederberger M, Eismann M. Human splanchnic and forearm constrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–524. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- Joyner M, Suwarno N, Seals D, Shepherd J. Effects of graded lower body negative pressure on forearm blood flow in patients after heart-lung transplantation. FASEB J. 1990;4:A428. [Google Scholar]
- Kaye D, Anderson S, Federman J. Electrocardiographic and echocardiographic features of left atrial size after orthotopic cardiac transplantation. Am J Cardiol. 1992;70:1096–1099. doi: 10.1016/0002-9149(92)90371-5. 10.1016/0002-9149(92)90371-5. [DOI] [PubMed] [Google Scholar]
- Kjær A, Appel J, Hildebrandt P, Petersen C. Basal and exercise-induced neuroendocrine activation in patients with heart failure and in normal subjects. Eur J Heart Fail. 2004;6:29–39. doi: 10.1016/S1388-9842(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Laske A, Carrel T, Niederhauser U, Pasic M, von Segesser L, Jenni R, Turina M. Modified operation technique for orthotopic heart transplantation. Eur J Cardiothorac Surg. 1995;9:120–126. doi: 10.1016/s1010-7940(05)80057-0. [DOI] [PubMed] [Google Scholar]
- Lautt W. Resistance or conductance for expression of arterial vascular tone. Microvasc Res. 1989;37:230–236. doi: 10.1016/0026-2862(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Leyh R, Jahnke A, Kraatz E, Sievers H. Cardiovascular dynamics and dimensions after bicaval and standard cardiac transplantation. Ann Thorac Surg. 1995;59:1495–1500. doi: 10.1016/0003-4975(95)00185-n. [DOI] [PubMed] [Google Scholar]
- Lower R, Shumway N. Studies on orthotopic homotransplantation in the canine heart. Surg Forum. 1960;11:18–19. [PubMed] [Google Scholar]
- Milano C, Ashish S, Van Trigt P, Jaggers J, Davis D, Glower D, Higgenbotham M, Russell S, Landolfo K. Evaluation of early postoperative results after bicaval versus standard cardiac transplantation and review of the literature. Am Heart J. 2000;140:717–721. doi: 10.1067/mhj.2000.111105. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Thames M, Arrowood J, Sowers J, McNamara C, Szentpetery S. Impairment of cardiopulmonary baroreflex after cardiac transplantation in humans. Circulation. 1987;75:914–921. doi: 10.1161/01.cir.75.5.914. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Thames M, Sowers J, McNamara C, Beck F. Reflex effects of lower body negative pressure in patients with congestive heart failure. Circulation. 1986;74:430. [Google Scholar]
- Morgan B, Rahko P, Pease M, Berkoff H, DeBoer L, Hanson P. Vasoconstrictor response to lower body negative pressure is attenuated after cardiac transplantation. Circulation. 1987;76(Suppl. 4):IV–59. [Google Scholar]
- O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol. 1991;29:H632–H637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- Pott F, Jensen K, Hansen H, Christensen N, Lassen N, Secher N. Middle cerebral artery blood velocity and plasma catecholamines during exercise. Acta Physiol Scand. 1996;158:349–356. doi: 10.1046/j.1365-201X.1996.564325000.x. [DOI] [PubMed] [Google Scholar]
- Rosenmeier J, Dinenno F, Fritzlar S, Joyner M. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers H, Weyand M, Kraatz E, Bernhard A. An alternative technique for orthotopic cardiac transplantation, with preservation of the normal anatomy of the right atrium. Thorac Cardiovasc Surg. 1991;39:70–72. doi: 10.1055/s-2007-1013934. [DOI] [PubMed] [Google Scholar]
- Stevenson L, Dadourian B, Kobashigawa J, Child J, Clark S, Laks H. Mitral regurgitation after cardiac transplantation. Am J Cardiol. 1987;60:119–122. doi: 10.1016/0002-9149(87)90997-0. [DOI] [PubMed] [Google Scholar]
- Stinson E, Griepp R, Schroeder J, Dong EJ, Shumway N. Hemodynamic observations one and two years after cardiac transplantation in man. Circulation. 1972;45:1183–1194. doi: 10.1161/01.cir.45.6.1183. [DOI] [PubMed] [Google Scholar]
- Tschakovsky M, Sujirattanawimol K, Ruble S, Valic Z, Joyner M. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Borne P, Neubauer J, Rahnama M, Jansens J, Montano N, Porta A, Somers V, Degaute J. Differential characteristics of neural circulatory control. Early versus late after cardiac transplantation. Circulation. 2001;104:1809–1813. doi: 10.1161/hc4101.097248. [DOI] [PubMed] [Google Scholar]
- Webb W, Howard H, Neely W. Practical methods of homologous cardiac transplantation. J Thorac Surg. 1959;37:361–366. [PubMed] [Google Scholar]
- Weisbrod C, Eastwood P, O'Driscoll J, Walsh J, Best M, Green D. Vasomotor responses to hypoxia in type 2 diabetes. Diabetes. 2004;53:2073–2078. doi: 10.2337/diabetes.53.8.2073. [DOI] [PubMed] [Google Scholar]
- Weisbrod CJ, Minson C, Joyner MJ, Halliwill J. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Green D. Improved analysis of brachial artery ultrasound images using a novel edge-detection software system. J Appl Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Theodoropoulos S, Mathias C, Dhalla N, Wittes J, Mitchell A, Yacoub M. Increased sensitivity of the denervated transplanted human heart to isoprenaline both before and after beta-adrenergic blockade. Circulation. 1987;75:696–704. doi: 10.1161/01.cir.75.4.696. [DOI] [PubMed] [Google Scholar]