Abstract
Recent data have shown Ca2+-dependent activation of Rho-kinase by sustained depolarization of arterial smooth muscle. Visceral smooth muscles, however, contract phasically in response to action potentials and it is unclear whether Ca2+-dependent or -independent Rho-kinase activation occurs. We have therefore investigated this, under physiologically relevant conditions, in intact ureter. Action potentials, ionic currents, Ca2+ transients, myosin light chain (MLC) phosphorylation and phasic contraction evoked by action potentials in guinea-pig and rat ureter were investigated. In rat, but not guinea-pig ureter, three Rho-kinase inhibitors, Y-27632, HA-1077 and H-1152, significantly decreased phasic contractions and Ca2+ transients. Voltage- and current-clamp data showed that Rho-kinase inhibition reduced the plateau component of the action potential, inhibited Ca2+-channels and, indirectly, Ca2+-activated Cl− channels. The Ca2+ channel agonist Bay K8644 could reverse these effects. The K+ channel blocker TEA could also reverse the inhibitory effect of Y-27632 on the action potential and Ca2+ transient. Ca2+ transients and inward current, activated by carbachol-induced sarcoplasmic reticulum Ca2+release, were not affected by Rho-kinase inhibition. Rho-kinase inhibition produced a Ca2+-independent increase in the relaxation rate of contraction, associated with acceleration of MLC dephosphorylation, which was sensitive to calyculin A. These data show for the first time that: (1) Rho-kinase has major effects on Ca2+ signalling associated with the action potential, (2) this effect is species dependent and (3) Rho-kinase controls relaxation of phasic contraction of myogenic origin. Thus Rho-kinase can modulate phasic smooth muscle in the absence of agonist, and the mechanisms are both Ca2+-dependent, involving ion channels, and Ca2+-independent, involving MLC phosphorylation activity.
In smooth muscle, force can be modulated in Ca2+-dependent and Ca2+-independent mechanisms. Ca2+-independent pathways alter the activity of myosin light chain kinase (MLCK) and/or phosphatase (MLCP), and thus cause Ca2+ sensitization by changing the relation between [Ca2+] and force (Morgan & Morgan, 1984; Somlyo & Somlyo, 2000, 2003). In some smooth muscles, inhibition of MLCP is important in Ca2+ sensitization (Somlyo & Somlyo, 2003). The small GTPase, Rho-A, stimulates Rho-associated kinase (Rho-kinase), which phosphorylates the regulatory subunit of MLCP. Phosphorylation inhibits MLCP activity and thus potentiates force. Identification of this pathway has been aided by the use of relatively selective blockers of Rho-kinase, Y-27632, HA-1077 and H-1152 (Uehata et al. 1997; Fu et al. 1998; Yoshi et al. 1999; Sward et al. 2000; Somlyo & Somlyo, 2000; Ikenoya et al. 2002; Sasaki et al. 2002; Burdyga et al. 2003).
Until recently, Rho and Rho-kinase activation were considered to occur as a consequence of agonist binding. Recently, however, this assumption has been challenged. Mita et al. (2002) used high K+ to depolarize the caudal artery and found the contraction to be Rho-kinase sensitive and associated with inhibition of MLCP. Subsequent studies have confirmed this observation and shown the activation of Rho-kinase (Buyukafsar et al. 2003; Sakamoto et al. 2003; Asano & Nomura, 2003; Ghisdal et al. 2003; Urban et al. 2003). Sakurada et al. (2003) have shown that the activation of Rho and Rho-kinase by depolarization is Ca2+-dependent in vascular smooth muscle. Thus it appears that Rho and Rho-kinase are activated by two pathways, one Ca2+-independent and one Ca2+-dependent.
If some of the effects of Rho-kinase are Ca2+-dependent and activated by depolarization, the question of whether Rho-kinase can affect excitability and thus [Ca2+]i becomes important. There are a few reports of ion channels in a variety of tissues being regulated by Rho-kinase, e.g. anion channels (Nilius et al. 1999) and K+ channels (Cachero et al. 1998; Storey et al. 2002; Jones, 2003; Luykenaar et al. 2004). Effects of Rho-kinase on ionic currents in excitable tissues would have important effects on the action potential and [Ca2+]i. Although not frequent, some studies of Rho-kinase inhibition in smooth muscle have reported a fall in intracellular [Ca2+] ([Ca2+]i) (Takizawa et al. 1993; Ito et al. 2002; Maeda et al. 2003; Ghisdal et al. 2003). Thus it seems reasonable to ask if Rho-kinase inhibition is associated with decreased Ca2+ entry, and directly measure both [Ca2+]i and the Ca2+ current.
In the studies reviewed above, the smooth muscle under study is most often vascular. The tonic activity of blood vessels is very different from the phasic activity exhibited by visceral smooth muscle, including the fact that electrical activity is more important for phasic activity, and a rapid dephosphorylation of myosin light chains (MLCs) also occurs (Himpens et al. 1988; Gong et al. 1992; Murahashi et al. 1999; Murahashi et al. 1999). Thus it seems reasonable to hypothesize that the effects of Rho-kinase inhibition may differ in the two muscle types. There have been few studies of this in phasic smooth muscle, but the effects of Rho-kinase inhibition appear less marked compared to tonic smooth muscle (Kupittayanant et al. 2001; Kitazawa et al. 2003; Sward et al. 2003; Hashitani et al. 2004).
We have therefore investigated the role of Rho-kinase in electromechanical coupling in the ureter, i.e. a phasic smooth muscle, under myogenic action potential control. We have attempted to answer the following questions: (1) does Rho-kinase inhibition affect the action potential, Ca2+ signalling and phasic contractions? (2) does Rho-kinase affect ion channels? (3) is sarcoplasmic reticulum (SR) Ca2+ release targeted by Rho-kinase? (4) is Rho-kinase involved in the control of temporal relationships between MLC phosphorylation and force? and (5) are there Ca2+-dependent as well as Ca2+-independent actions of Rho-kinase in the ureter?
In rat ureter we found significant effects of Rho-kinase inhibition on Ca2+ signalling and force, as well as on the duration of the action potential. We also found that Rho-kinase targets at least two components of electromechanical coupling in ureteric smooth muscle, i.e. the activity of MLCP and ion channels, to modulate contractility.
An abstract of this work has been published (Burdyga et al. 2000).
Methods
Tissue and cells
Guinea-pigs (∼300–400 g) and rats (∼200 g) were humanely killed using CO2 anaesthesia followed by cervical dislocation, in accordance with UK legislation. The ureters were dissected, cleared of any fat and cut into 3–4 mm strips. Single isolated cells from the rat ureter were prepared as follows. The strips were incubated for 30 min in low-Ca2+ (40 μm) Hanks' solution (see below) and then transferred to Hanks' solution containing 0.75 mg ml−1 collagenase type I (Worthington; 183 U mg−1), 0.15 mg ml−1 protease E, 1 mg ml−1 BSA and 0.2 mg ml−1 Trypsin inhibitor. Strips were then placed into enzyme-free Kraft-Brühe (KB) medium (Klöckner & Isenberg, 1985) for 15–20 min and then triturated using a fire-polished Pasteur pipette to release the cells. The cells were stored in KB medium at 4 °C and were used on the day of isolation. All experiments were performed at 22–23 °C.
Ca2+ measurement
For measurement of [Ca2+i], the intact ureters were incubated in the membrane-permeant form of Indo-1 (15 μm, Molecular Probes) with Pluronic F for 2 h at 22–23 °C. For measurement of [Ca2+i] in single cells, myocytes were incubated in the membrane-permeant form of either Indo-1 (3 μm) or Fluo-4 (5 μm) with Pluronic F for 30 min at 22–23 °C.
Force and electrical measurements
Tissues were rinsed and then placed in a 200 μl bath on the stage of an inverted microscope. One end of the tissue was fixed and the other attached to a force transducer. The tissue was stimulated around every minute, which exceeds the relative refractory period in this muscle (Cuthbert, 1965; Maggi & Giuliani, 1994), by Ag–AgCl electrodes, 5–7 V and 100 ms duration. This protocol directly stimulates the muscle and not neurotransmitter release (Golenhofen & Hannappel, 1973; Davidson & Lang, 2000). For simultaneous force, Ca2+ and electrical measurements, a modified tissue bath was used, as detailed elsewhere (Burdyga & Wray, 1997, 1999). Briefly a standard double-sucrose-gap chamber with a coverslip at its base, to enable the optical measurements to be made, was used. Action potentials were initiated by depolarizing rectangular current pulses of just suprathreshold size (in the order of 10−7 A). The pulse duration was short (20–100 ms) to avoid influence of the prolonged depolarization on the shape of the action potential. For Ca2+ measurement, the tissues were excited at 340 nm and the Indo-1 fluorescence emitted at 400 and 500 nm was recorded. The ratio of these signals (F400:F500) provides a measure of [Ca2+]i (Grynkiewicz et al. 1985; Burdyga et al. 1995; Burdyga & Wray, 1999). When Fluo-4 was used as the Ca2+-sensitive fluorescent indicator, cells were excited at 470 nm and the fluorescence emitted at 510 nm recorded.
Patch-clamp studies
The membrane potential of cells was maintained at −60 mV by conventional whole-cell patch clamp. Patch pipettes were fire polished and had a resistance of 3–6 MΩ. The basic pipette solution contained the following (mm): 130 CsCl or KCl, 10 Hepes, 5 MgCl2, 10 glucose, 5 ATP and 0.1 EGTA; pH was adjusted to 7.2 with NaOH. The capacitance currents were reduced electronically using the whole-cell parameter compensation facility of the Axopatch-200B amplifier (Axon Instruments). Membrane currents were low pass-filtered at 2 kHz and digitized with a sampling frequency of 2 kHz using Clampex 8 software (Axon Instruments). Data were analysed using Origin 6 (Microcal).
Quantification of MLC20 phosphorylation
Strips were fast frozen at different times during contraction. This was carried out by rapidly submerging the holders with the muscle attached in acetone, which had been pre-cooled with dry ice, in a dip tray (Hellam & Podolsky, 1969) that could change solution within a fraction of a second. After storage for ≥24–48 h at −80 °C in acetone containing 5% trichloroacetic acid (TCA) and 10 mm dithiothreitol (DTT), MLC phosphorylation was assayed as described in detail previously (Mitchell et al. 2001). Data were expressed as percentage phosphorylated MLC, determined as 100 times the ratio of the integrated area density of phosphorylated MLC spot to the sum of the integrated area densities for the phosphorylated and non-phosphorylated spots. The antibodies for MLC20 were from Sigma.
Solutions
Tissues and cells were superfused with oxygenated buffered Krebs solution (pH 7.4) of the following composition (mm): 120 NaCl, 5.9 KCl, 1.2 MgSO4, 2 CaCl2, 11.5 glucose, 11 Hepes. In some experiments Ca2+-free Ba2+ solution was used in which CaCl2 was replaced by BaCl2. In some experiments K+ currents were inhibited with tetraethylamonium (TEA, 10 mm). Y-27632, HA-1077, H-1152 (Calbiochem) and carbachol were dissolved in distilled water and used at concentrations detailed in text. Niflumic acid and calyculin A were dissolved in DMSO and used at 50 μm and 1 μm, respectively. Hank's solution (GibcoBRL; pH 7.4) had the following composition (mm): 136 NaCl, 5.4 KCl, 4.17 NaHCO3, 6.7 Na2HPO4, 0.44 KH2PO4, 5.5 glucose, 0.04 CaCl2, and was bubbled with O2. KB medium used during cell isolation had the following composition (mm): 40 KCl, 10 K2HPO4, 10 taurine, 10 TES, 11 glucose, 5 pyruvate, 5 creatine, 0.04 EGTA, 100 potassium glutamate; pH 7.4 adjusted with KOH. All chemicals were from Sigma unless stated otherwise.
Statistics
Values are given as means ±s.e.m. and n is the number of animals. Differences were taken as significant for P < 0.05 in the appropriate Student's t test or ANOVA. The IC50 values of the inhibitory action of Y-27632 on Ca2+ transients and force were obtained from the curves obtained using Origin Microcal sigmoidal fitting to the experimental points presented as means ± s.e.m.
Results
Effects of Rho-kinase inhibition on Ca2+ and force in ureter muscle
Rat ureter
Electrical field stimulation (EFS) of rat ureteric smooth muscle strips produced a regular pattern of Ca2+ transients associated with phasic contractions (Fig. 1A). As can be seen, Y-27632 (n= 11), produced a concentration- and time-dependent inhibition of this activity, apparent at 0.1 μm. There was a clear reduction in the amplitude of both parameters, but with force being more affected than [Ca2+]; the IC50 for force inhibition occurred at 0.3 ± 0.1 μm and for Ca2+ at 0.6 ± 0.1 μm (Fig. 1C, n = 7). The durations of both the force and the Ca2+ transient, measured at 50% of peak (t50), were also significantly reduced by Y-27632 (1 μm) from 3.6 ± 0.4 to 1.9 ± 0.1 s and from 3.3 ± 0.2 to 2.1 ± 0.4 s (n = 7), respectively.
Figure 1. The effects of Y-27632 on rat and guinea-pig ureter.
Concentration-dependent inhibition of force (top) and Ca2+ (Indo-1, F400/500; bottom) by Y27632 in rat (A) and guinea-pig (B) ureteric smooth muscle evoked by electrical field stimulation (EFS). C, dose–response curve of the inhibition of the Ca2+ transient (open symbols) and force (filled symbols) by Y-27632 in rat and guinea-pig ureter smooth muscle. All data were obtained at 22–23 °C. Muscle strips were stimulated by suprathreshold current pulses (7 V, 100 ms duration) in this and subsequent figures, unless indicated otherwise.
H-1152 produced inhibition of both Ca2+ transients and phasic contractions at submicromolar concentrations. A typical record (of seven others) of its effects at 0.1 μm is shown in Fig. 2A; it significantly decreased the amplitude of phasic contractions and Ca2+ transients to 21.8 ± 0.8% and 32.7 ± 0.7% of control, respectively. The duration of the Ca2+ transient was also decreased from t50 3.1 ± 0.9 to 1.9 ± 1.1 s. Similar effects were found with HA-1077 (Fig. 2B); At 10 μm it decreased the amplitude of the Ca2+ transient and phasic contraction to 10.2 ± 2.7 and 38.5 ± 5.5%, respectively, and the duration of the Ca2+ transient from 3.2 ± 1.3 to 1.8 ± 0.5 s (n= 4).
Figure 2. The effects of H-1152 and HA-1077 on rat and guinea-pig ureter.
Time-dependent inhibition of force (top) and Ca2+ (bottom) by H-1152 (0.1 μm) (A) and HA-1077 (10 μm) (B) in rat and guinea-pig ureteric smooth muscle evoked by EFS.
Guinea-pig ureter
All three Rho-kinase inhibitors had little or no effect on Ca2+ transients and force evoked by EFS in the guinea-pig ureter (Figs 1B and C, 2A and B). No significant changes in their amplitude occurred until a maximal concentration of any of the Rho-kinase inhibitors was used (Figs 1B and C, 2A and B). The differences between the effects of Y-27632 on rat and guinea-pig ureter can be seen in Fig. 1C where the dose–response effects are plotted. At 10 μm of HA-1077 (n= 4) and 0.1 μm H-1152 (n= 5), Ca2+ was 97.0 ± 4.3 and 98.2 ± 5.3%, respectively, and force was 93.7 ± 4.1 and 91.7 ± 2.2%, respectively, compared to 100% control.
Thus all three Rho-kinase inhibitors produced similar effects on Ca2+ transients and force in ureteric smooth muscle, and in the subsequent experiments, Y-27632 was used to investigate the mechanisms which produce these changes in the rat.
The effects of Y-27632 on the relaxation of the phasic contraction
Y-27632 had a marked stimulant effect on the rate of relaxation of contraction (Fig. 3A and B, n= 9). The rate constant, expressed as the reciprocal of the half-time (t½), in the presence of Y-27632 was significantly increased from 0.33 ± 0.03 to 0.48 ± 0.05 s−1 (n= 9). These effects were not paralleled by change in the Ca2+ transient, as seen in Fig. 3C. This effect of Y-27632 on force was fully reversed by the MLCP inhibitor, calyculin A (Fig. 3A). No effects on the t½ of relaxation of the Ca2+ transients were found with calyculin A (Fig. 3C, trace iv). Calyculin A produced summation of the individual phasic contractions and a gradual increase in the baseline force (Fig. 3A).
Figure 3. Effects of Y-27632 on the kinetics of the relaxation of force and Ca2+ transient in rat ureter muscle.
A, effect of Y-27632 (10 μm) on the Ca2+ transients and phasic contractions evoked by EFS in the absence and the presence of calyculin A (1 μm). B, superimposed traces of the Ca2+ transients (bottom) and force (top) recorded in control conditions (i), and at different times after addition of Y-27632 (ii and iii) and in the presence of Y-27632 and calyculin A (iv) (1 μm). C, superimposed records of normalized relaxation phases of force (top) and Ca2+ transients (bottom) recorded in control conditions (i), in the presence of Y-27632 (ii and iii), and Y-27632 and calyculin A (iv).
As inhibition of Rho-kinase decreased the amplitude and duration of force and Ca2+ transient (Ca2+-dependent mechanisms), and also acceleration of the relaxation of the phasic contraction with no change in the Ca2+ transient (Ca2+-independent mechanism), subsequent experiments were designed to elucidate the mechanisms involved.
Action potential and Ca2+ transient
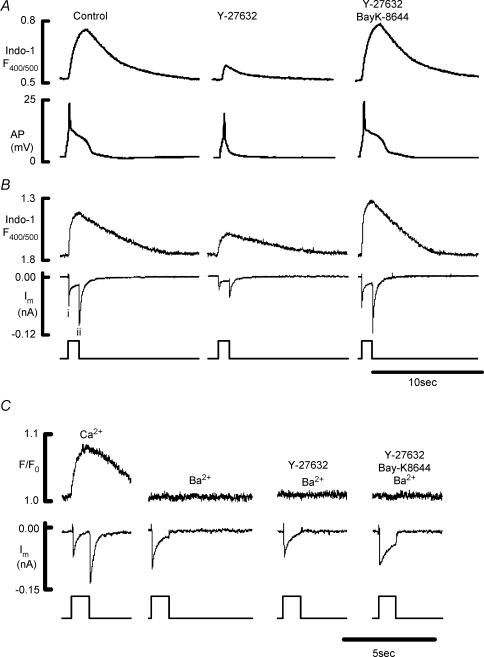
The action potential underlies Ca2+ transients and force production in rat ureter (Burdyga & Wray, 1997, 2002) and the effects of Y-27632 on it were examined (Fig. 4A). Y-27632 significantly reduced the duration of the action potential plateau; its duration (at t50) was 1.5 ± 0.1 s in the absence and 0.3 ± 0.1 s in the presence of Y-27632 (n= 5). Y-27632 produced a small but significant reduction of the amplitude of the spike component (89.4 ± 3.3%, compared to control 100%). The inhibitory effect of Y-27632 on the action potential and the Ca2+ transient could be reversed by the Ca2+-channel agonist Bay K8644 (Fig. 4A). Thus in the presence of Y-27632 and Bay K8644, the spike component was 111.5 ± 3.3%, the duration of the plateau component was 108.4 ± 6.3%, and the amplitude of the Ca2+ transient was 105.9 ± 3.3% of control (n= 5).
Figure 4. The effects of Y-27632 on the action potential, Ca2+ transient and inward current.
A, action potential (bottom) and Ca2+ transients (top) evoked by suprathreshold depolarizing current pulses in rat ureter in control conditions in the presence of Y-27632 (10 μm), and in the presence of Y-27632 (10 μm) and Bay K8644 (1 μm). B, inward current (bottom) and Ca2+ transients (top; measured with Ca2+ sensitive indicator Indo-1) evoked by depolarizing voltage step from a holding potential of −60 to 0 mV (pulse duration 1 s) in rat ureteric myocytes in control conditions in the presence of Y-27632 (10 μm), and in the presence of Y-27632 (10 μm) and Bay K8644 (1 μm). i, Peak Ca2+ current; ii, tail current. C, bottom trace, inward currents evoked by depolarizing voltage step from a holding potential of −60 to 0 mV (pulse duration 1 s) in rat ureteric myocytes, first in Ca2+-containing solution and then in Ba2+-containing solution under control conditions, in the presence of Y-27632 (10 μm), and in the presence of Y-27632 (10 μm) and Bay K8644 (1 μm); note that replacement of external Ca2+ with Ba2+ eliminates tail current (bottom trace) and fluorescent signal (top trace) measured with Ca2+-sensitive fluorescent indicator Fluo-4.
As the plateau component of the action potential is a strong determinant of [Ca2+]i, and hence force (Burdyga & Wray, 1997; Smith et al. 2002), the effects of Y-27632 on the Ca2+ signals and membrane currents were next studied.
Effects of Y-27632 on the inward currents and Ca2+ transient
With CsCl in the pipette solution to eliminate K+ current, and stimulated at 20 s intervals to avoid cumulative inactivation of the inward current, depolarizing voltage steps to 0 mV were applied. In agreement with our previous data (Smith et al. 2002), Fig. 4B shows the nifedipine-sensitive inward Ca2+ current (Fig. 4Bi) and a global rise of [Ca2+]i with depolarization. Upon stepping back to the holding potential, a niflumic-acid-sensitive, large inward Ca2+-activated Cl− current (tail current) occurs (ii). Y-27632 significantly decreased the peak Ca2+ current (i) to 58.2 ± 6.7%, the amplitude of the Ca2+ transient to 61.4 ± 5.9%, and the amplitude of the tail current (ii) to 62.7 ± 7.7% of control (n= 11, Fig. 4B). These inhibitory effects of Y-27632 were reversed by Bay K8644 (Fig. 4B). In the presence of Y-27632 and Bay K8644, the amplitude of the peak Ca2+ current was 129.6 ± 9.3%, Ca2+ transient was 123.8 ± 8.8%, and the ClCa tail current was 134 ± 7.8% of control (n= 7, Fig. 4B). Figure 4C shows data obtained when Ba2+ replaced Ca2+ as the charge carrier. It can be seen that the inward Ba2+ current is still present, but no rise of [Ca2+]i occurs. There was also no tail current with Ba2+ (n= 5). Y-27632 reduced Ba2+ inward current to 63.6 ± 4.8% and Bay K8644 restored it to 101.2 ± 8.8% of control (n= 5).
Effects of Y-27632 on the I–V relationship
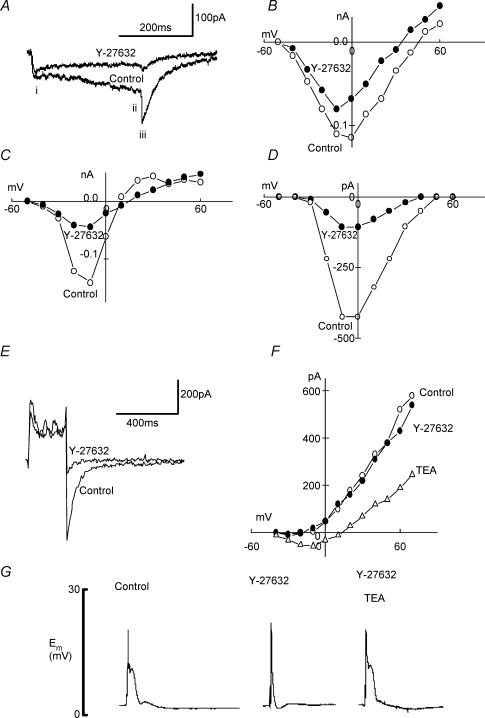
With CsCl in the pipette solution, cells were depolarized from −60 mV to different voltages by applying depolarizing pulses of 300 ms at 10 s intervals. Three types of current were generated: early L-type inward Ca2+ current (Figs 5Ai), late (measured at the end of the depolarizing pulse) current (Fig. 5Aii), which at below 0 mV was inward and above 0 mV was outward and reversed at around +8 mV, and inward tail current due to Ca2+-activated Cl− current (Smith et al. 2002; Fig. 5Aiii), recorded after stepping back to a holding potential (Fig. 5A). The I–V relationships for all three types of current obtained in the absence and the presence of Y-27632 are shown in Fig. 5B–D. Inhibition of Rho-kinase by Y-27632 significantly reduced peak Ca2+ current to 67.5 ± 8.7% (n= 9, Fig. 5A and B), the late current measured at the end of the depolarizing pulse to 31.9 ± 11.1% (n= 9, Fig. 5A and C) and the ClCa tail current to 26.1 ± 6.5% (n= 9, Fig. 5A and D).
Figure 5. Effects of Y-27632 on the current–voltage relationships.
A, superimposed original records of the ionic currents evoked by depolarizing voltage steps to −20 mV from a holding potential of −80 mV (pulse duration 300 ms) in the absence and the presence of Y-27632 (10 μm). B, C and D, current–voltage relationships for peak Ca2+ current (i), current measured at the end of the depolarizing pulse (ii), and the tail currents (iii). In these experiments CsCl was used in the pipette solution. E, superimposed original records of the total ionic currents evoked by depolarizing voltage steps to 0 mV from a holding potential of −80 mV (pulse duration 300 ms) in the absence and the presence of Y27632 (10 μm). F, current–voltage relationship for the outward K+ current in the absence and the presence of TEA (10 mm) and Y-27632 (10 μm). In these experiments KCl was used in the pipette solution and each curve represents an average of four measurements. Error bars are not shown for clarity reasons. G, action potential evoked by suprathreshold depolarizing current pulse in rat ureter in control conditions in the presence of Y-27632 (10 μm), and in the presence of Y-27632 (10 μm) and TEA (10 mm).
Outward current
A series of depolarizing pulses with 10 mV increments were applied every 10 s and the I–V relationships for the outward current, in the absence and presence of Y-27632 measured (Fig. 5E and F). The superimposed records of total ionic currents show Y-27632 had no significant effect on the outward K+ current and I–V curve (Fig. 5F), but again it inhibited the ClCa tail current (Fig. 5E). The reduction of outward current by TEA (10 mm) is shown in Fig. 5F. Figure 5G shows the ability of TEA to fully restore the action potential plateau, in the presence of Y-27632; 1.5 ± 0.3 (n= 5) compared to 1.5 ± 0.1 s.
To investigate if the effects of Y-27632 on ClCa were direct or indirect via a decrease in [Ca2+], we next studied the effect of Y-27632 on Ca2+ transients and ClCa current induced by Ca2+ released from the SR.
Effects of Y-27632 on SR Ca2+ release
Carbachol (10 μm) at a holding potential of −80 mV, with Ca2+ in the bathing solution, produced Ca2+ transients with phasic and sustained components (Fig. 6A, top trace, i and ii, respectively). After 100–300 ms delay, an inward current was activated, which after reaching peak amplitude, quickly declined to the baseline, despite cytosolic [Ca2+] remaining elevated (Fig. 6A, bottom trace). This carbachol-induced inward current was reversibly inhibited by 50 μm niflumic acid, a blocker of ClCa (n= 4, Fig. 6, inset), and had a reversal potential of 0 mV, with symmetrical concentration of Cl− on both sides of the cell membrane, suggesting that it was a ClCa current. Y-27632 had no significant effect on the Ca2+ transient and ClCa current evoked by carbachol and these were 98.9 ± 0.2 and 95.5 ± 0.4% of controls, respectively (n= 5).
Figure 6. Effect of Y-27632 on the Ca2+ transient, inward current and the action potential, evoked by carbachol in rat ureter.
A, Ca2+ transients (top) and inward currents (bottom) evoked by carbachol (CCh; 10 μm) in rat ureteric myocytes in the absence and presence of Y-27632 (10 μm) at a holding potential of −80 mV with CsCl in the pipette solution. B, action potentials (bottom) and Ca2+ transients (top) evoked by CCh in rat ureter under current-clamp conditions in the absence and the presence of Y-27632 (10 μm). Ai and ii, phasic and sustained component of the Ca2+ transient evoked by CCh under voltage-clamp conditions, Bi and ii, phasic (associated with the action potential) and sustained component of the Ca2+ transient evoked by CCh under current-clamp conditions. The insert shows the effect of 50 μm niflumic acid on the inward current evoked by CCh in voltage-clamped rat ureteric myocytes. The dotted line indicates the steepness of the Ca2+ transient.
Under current-clamp conditions, application of carbachol produced small depolarizations of the cell (3–7 mV), which upon reaching threshold triggered action potentials (Fig. 6B, bottom trace). This was associated with a fast rise of the Ca2+ transient, superimposed on the sustained component (Fig. 6B, top trace, i and ii, respectively). Y-27632 selectively inhibited the phasic but not sustained component of the Ca2+ transient (Fig. 6B). Y-27632 decreased the duration of the plateau component from 2.0 ± 0.1 to 0.5 ± 0.3 s (n= 5), and the amplitude of the Ca2+ transient to 45 ± 5.7%. The amplitude of the sustained component of the Ca2+ transient was 95%± 7.7% of control. Although not investigated rigorously, Fig. 6B also shows that Y-27632 slowed the rise of Ca2+, as indicated by the dotted line. Thus the effect of Y-27632 on the ClCa channels is not direct but results from a decrease in [Ca2+]i.
Y-27632 and force–Ca2+ relationship during phasic contractions restored by BAY K8644
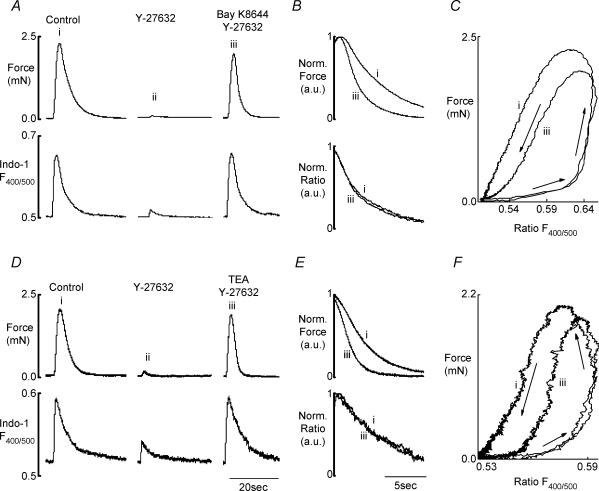
Figure 7A shows that despite Bay K8644 fully restoring the parameters of the Ca2+ transient, the amplitude of force remained significantly less than under control conditions (87.5 ± 5.8%, n= 7). Force peaked earlier and relaxed more rapidly (1.7 ± 0.2 times relative to control, n= 5), as seen in the phase-plane plots (Fig. 7C). As already described, this increase in the rate of relaxation was not accompanied by similar changes in the relaxation phase of the Ca2+ transient (Fig. 7B). As a result of the accelerating effect of the Y-27632 on the rate of relaxation, the force–Ca2+ relationship during the relaxation phase shifted to a higher level of Ca2+. This is shown in phase-plane plots (Fig. 7Ci and iii). There were no effects on the force−Ca2+ relationship during the rising phase of contraction.
Figure 7. The effects of Y-27632 on Ca2+ and force in the absence and the presence of Bay K864 or TEA.
A and D, Ca2+ transient (bottom trace) and force (top trace) in the rat ureter smooth muscle evoked by EFS in normal Krebs solution (i); 10 min after addition of 10 μm Y-27632 to normal Krebs solution (ii), and 10 min after addition of 1 μm Bay K8644 or 10 mm TEA (iii) to Krebs solution containing 10 μm Y-27632. B and E, superimposed records of the normalized relaxation phases of the phasic contractions (top) and Ca2+ transients (bottom) obtained in the presence Y-27632 and Bay K8644 (B) or TEA (E). C and F, phase-plane plots of the force−Ca2+ relationship during the development of the phasic contraction in control (i) and restored (iii) by Bay K8644 and TEA, respectively. The arrows show the time-dependent development of the phasic contraction. Note the shift in the force−Ca2+ relationship during the relaxation phase to higher level of Ca2+.
Y-27632 and TEA
Figure 7D shows that TEA was able to restore Ca2+ transients to near those found under control conditions, 94.3 ± 8.4% (n= 18). In 14/18 of the preparations, TEA restored Ca2+ to control values (99.8 ± 6.5%), and these preparations were therefore used to provide information on any Ca2+-independent action of Y-27632. In these 14 preparations, the amplitude of phasic contraction was still significantly less than control (90.1 ± 3.3%; Fig. 7D).
Y-27632 did not affect the rising phase of force, but it significantly increased the rate of relaxation of the phasic contraction, as can be seen from the superimposed, normalized records (Fig. 7Ei and iii). The t½ for relaxation in control preparations was 3.0 ± 0.1 s and in Y-27632 with TEA it was decreased to 1.7 ± 0.1 s (n= 14), but the Ca2+ transients were unchanged at 2.4 ± 0.3 and 2.4 ± 0.4 s, respectively. Thus, as seen in the phase-plane plots (Fig. 7F), Y-27632 had no effect on the force−Ca2+ relationship during the rising phase of contraction, but markedly shifted it during the relaxation phase to higher [Ca2+]i (Fig. 7Fi and iii), as was also the case in the presence of Bay K8644 (Fig. 7Ci and iii).
Effect of calyculin A
The effects of Y-27632 on the rate of relaxation of the phasic contractions suggest an increased activity of MLCP, and therefore an MLCP inhibitor, calyculin A, was used and Ca2+ and force measured. Calyculin A produced time-dependent increases in the amplitude of the phasic contraction without affecting the amplitude and duration of the Ca2+ transient (Fig. 8A and B). Superimposed records of force and Ca2+ recorded in control conditions (Fig. 8Bi) after restoration by TEA without (Fig. 8Bii) and with calyculin A (Fig. 8Biii), illustrate the ability of calyculin A to reverse the effects of Y-27632.
Figure 8. Effects of Y-27632 on force and myosin light chain (MLC) phosphorylation in the absence and the presence of calyculin A.
A, effect of calyculin A (1 μm) on Ca2+ transients (bottom traces) and phasic contractions (top traces) evoked by EFS in the presence of Y-27632 (10 μm) and TEA (10 mm). B, superimposed records of Ca2+ transients (bottom traces) and phasic contractions (top traces) evoked by EFS in the rat ureter in the absence of Y-27632 (i), 10 min after addition of 10 mm TEA (ii), and 5 min after addition of 1 μm of calyculin A (iii) in the continued presence of Y-27632 and TEA. C, superimposed scatter plots of force (top) and MLC phosphorylation (bottom) in individual control preparations (open squares), in the presence of Y-27632 and 10 mm TEA (filled squares), and the combined action of Y-27632, TEA and calyculin A (filled circles). The continuous lines are the sigmoidal fit of the relaxation phase of force and dephosphorylation part of the curves in each plot. D, representative Western blots showing separation of the phosphorylated and non-phosphorylated MLC20 by urea/gel electrophoresis. Lanes 1 and 2, during the rising phase (about 50 and 90%) of the phasic contraction in the absence (top blots) and the presence of Y-27632 and TEA (middle blots); Lane 3, during the relaxation of the phasic contraction (about 50% of control) in control preparations (top blots), in the presence of Y-27632 and TEA (middle blots), and in the presence of Y-27632, TEA and calyculin A (bottom blots).
MLC phosphorylation
Determination of light chain phosphorylation in control and treated tissues, using TEA to restore the level of force to 90–95% of control in the presence of Y-27632, showed that TEA had no effect on MLC phosphorylation at the steady-state evoked by high-K+ depolarization (S. Shabir & T. Burdyga unpublished observation). Figure 8C shows superimposed scatter plots of force (top panel) and MLC phosphorylation (bottom panel) recorded in control (open squares, n= 26), preparations treated with Y-27632 and 10 mm TEA (filled squares, n = 18) or TEA, Y-27632 and calyculin A (filled circles, n = 7), recorded at different times of the contractions. There were no differences in the amplitude of force or MLC phosphorylation during the rising phase of contraction (Fig. 8C). This can also be seen from the representative chemilumigrams of MLC Western blots, taken at around 50% of maximal force during the rising phase (Fig. 8D, lane 1). In contrast, there was a significant difference in force generated and the level of MLC phosphorylation during relaxation (Fig. 8D, lane 3). By applying a sigmoidal fit to the scatter points of force and MLC phosphorylation (control and Y-27632 plus TEA) during the relaxation phase, the t½ for relaxation of force and MLC dephosphorylation in the absence and the presence of Y-27632 were defined. Thus for the relaxation phase of the contractions, the corresponding values of t½ were 3.1 s and 1.7 s, respectively, and for the MLC dephosphorylation, 4.3 s and 2.3 s, respectively. These data give rate constants of MLC dephosphorylation of 0.2 and 0.4 s−1 and relaxation rates of 0.3 and 0.6 s−1, for control and Y-27632, respectively. Thus, at unchanged [Ca2+], Y-27632 significantly accelerated MLC dephosphorylation and relaxation of force.
Figure 8C shows that the accelerating effect of Y-27632 on the relaxation of the phasic contraction and MLC dephosphorylation in the presence of Y-27632 and TEA could be completely reversed after administration of calyculin A. Thus at a time which corresponded to about 50% of relaxation, the level of MLC phosphorylation was around 30%. In the presence of Y-27632 and TEA the level of force was 10–16% of control and the level of MLC phosphorylation was virtually 0 (Fig. 8C). After addition of calyculin A, the rate of relaxation of force and MLC dephosphorylations in the presence of Y-27632 and TEA were markedly reduced (Figs 8C and D, lane 3). Thus the level of force and MLC phosphorylation during the relaxation phase of the phasic contraction exceeded those seen in control preparations (Fig. 8C).
Discussion
Rho-kinase inhibition and phasic ureteric contractions
Pacemaker activity within ureteric cells in the renal pelvis leads to membrane excitation, action potential firing, Ca2+ entry, a global rise in [Ca2+]i and stimulation of the calmodulin–MLCK pathway (Burdyga & Wray, 1997, 1999). The end product is a phasic contraction and a wave of peristalsis down the ureter to the bladder. This activity is intrinsic to the ureteric cells, i.e. it is myogenic, although in vivo agonists may modify this activity (Golenhofen & Hannappel, 1973; Maggi et al. 1987). Our experiments were designed to examine the role of Rho-kinase, in the absence of agonists, on this myogenic activity.
Before discussing our data it is necessary to examine what evidence we have of Rho-kinase inhibition? Our data are based on three different drugs, all considered to inhibit Rho-kinase, Y-27632, HA-1077 and H-1152 (Somlyo & Somlyo, 2000; Uehata et al. 1997; Ishizaki et al. 2000). The effects of all three drugs were qualitatively similar and, along with the acceleration of the calyculin-A-sensitive myosin light chain (MLC20) dephosphorylation and relaxation of force, were consistent with an action on Rho-kinase targetting on MLCP, although it should be noted that they all work as competitive inhibitors at the ATP-binding site. The sensitivity of their effects in terms of increasing potency was H-1152 >> Y-27632 ≥ HA-1077. All three drugs are described as selective inhibitors of Rho-kinase, but at high concentrations of these drugs, PKC may be affected (Davies et al. 2000; Sasaki et al. 2002). It is unlikely, however, that our data result from PKC inhibition, as we were able to show that phorbol ester phorbol dibutyrate (PDBu), a PKC activator, stimulated contractile activity in the presence of Y-27632 (unpublished observation, S. Shabir & T. Burdyga). In addition, phorbol esters do not activate Rho, but do produce contraction in vascular smooth muscle (Fu et al. 1998).
Thus we conclude that our data in rat ureter can be discussed in terms of the role of Rho-kinase on electromechanical coupling. The lack of effect on guinea-pig ureter suggests that Rho or Rho-kinase may not be expressed or functionally coupled. Our data appear to be the first showing a species-dependent effect of Rho-kinase inhibition.
Rho-kinase and excitation–contraction (E–C) coupling in rat ureter smooth muscle
In rat ureter, Y-27632 markedly reduced the duration of the plateau component of the action potential evoked by electrical stimulation or carbachol, reduced Ca2+ signals and hence had deleterious effects on force production. Restoration of the action potential by Bay K8644 or TEA could overcome the effects of Y-27632 on the Ca2+ transient and significantly restore the amplitude of force. The significant effects of Y-27632 on the action potential were unexpected and this is the first report of such an action. Our data suggest that Ca2+ channels are a target of Rho-kinase.
Inhibition of Rho-kinase by Y-27632 produced decreases in the peak Ca2+ and ClCa tail currents, but had no effect on the outward K+ current. Our data suggest the effects of Y-27632 on ClCa is indirect and is caused by the decrease in [Ca2+]i, caused by the partial inhibition of the voltage-gated Ca2+ channels; thus (i) Bay K8644 could fully restore peak Ca2+ current, Ca2+ transient and ClCa, (ii) Y-27632 had no effect on ClCa activated by Ca2+ release from the SR by carbachol, and (iii) Ba2+ inward-current, which is carried through the L-type Ca2+ channel, was also reduced by Y-27632. These data are consistent with the Bay K8644 reversing the effects of Y-27632. As far as we can determine, these are the first data directly supporting a role for Rho-kinase in L-type Ca2+ entry. Ghisdal et al. (2003) suggested that Rho-kinase is involved in Ca2+ entry distinct from voltage or capacitative Ca2+ entry, and concluded that this was a cationic current. In other studies referred to in the Introduction, anion channels and K+ channels have been shown to be modulated by Rho or Rho-kinase. We found no effect on steady-state outward current or K+I–V relationship, making it unlikely that these were the target. Further studies to provide a detailed insight into the mechanism whereby Rho or Rho-kinase affect Ca2+ channel activity should provide much useful information. Our data are consistent with Rho-kinase affecting voltage-gated Ca2+ channels and thereby affecting the Ca2+ signalling pathways in smooth muscle, in agreement with some other studies (Takizawa et al. 1993; Maeda et al. 2003; Ghisdal et al. 2003). However, many studies of smooth muscle have reported that Y-27632 has no effect on Ca2+ signals irrespective of the mode of stimulation (Sward et al. 2000; Mita et al. 2002; Hashitani et al. 2004). Notwithstanding that few studies have simultaneously measured force and [Ca2+]i, it seems reasonable to suggest that Rho-kinase may not influence [Ca2+]i in some tissues.
SR role?
In endothelial cells RhoA has been reported to interact with inositol 1,4,5-trisphosphate (IP3) receptors on the SR, and stimulate Ca2+ entry through a capacitative pathway (Mehta et al. 2003). We therefore asked if the SR and its Ca2+ store could be contributing to the effects of Rho-kinase inhibition? Our data with carbachol make this unlikely – Y-27632 had no effect on Ca2+ transients or currents evoked by SR Ca2+ release. This conclusion agrees with the report of Y-27632 in A7r5 cells not affecting IP3-evoked responses (Ghisdal et al. 2003). Thus we conclude that our data are consistent with a direct action of Rho-kinase on plasmalemma Ca2+ channels, and not the SR.
Ca2+-independent effects: relaxation of force
In the rat ureter, the amplitude of force and particularly the relaxation phase of the phasic contractions are also modulated by Rho-kinase in a Ca2+-independent manner. This was evident in the increased sensitivity of force to Rho-kinase inhibitors compared to Ca2+ transients. Also, full restoration of the Ca2+ transient in the presence of Y-27632 was unable to fully restore the amplitude of force. Force also peaked earlier and showed premature relaxation, shifting the force−Ca2+ relationship during the relaxation phase to higher level of Ca2+. The data obtained here indicate, for the first time, that in the rat ureter, Rho-kinase is involved in control of relaxation of the phasic contraction in a Ca2+-independent way. These data also show that agonists are not required for activation of rho-kinase (ROK) pathways to inhibit MLCP and produce Ca2+ sensitization, in agreement with Ayman et al. (2003) who studied anococcygeus smooth muscle.
The lack of effect of Y-27632 on the relaxation phase of the phasic contraction, in the presence of calyculin A, a MLCP inhibitor, suggests that the acceleration of the relaxation of the phasic contraction by Y-27632 can be best explained by stimulation of the MLCP activity.
MLC phosphorylation
Several previous studies have shown that Y-27632, by inhibiting Rho- kinase, can affect light-chain phosphorylation (Fu et al. 1998; Sward et al. 2000; Miyazaki et al. 2002; Oh et al. 2003). Consistent with this is our detection of decreased light-chain phosphorylation in its presence. By ensuring that the Ca2+ signal was unchanged during Y-27632 treatment, the effects of changes in dephosphorylation rates can be directly related to force. The enhanced relaxation rates were seen to be correlated with the increased rate at which light chains were dephosphorylated. Similarly calyculin A reversed the stimulant action of Y-27632 on the MLC dephoshorylation and relaxation. These data therefore suggest that the parameters of phasic contraction controlled by the action potential, can be greatly modulated by Rho-kinase via targeting of MLCP.
Phasic activity and physiological implications
From the few studies that have been made of Rho-kinase involvement in phasic smooth muscle, it was anticipated that force would not be greatly affected by Rho-kinase inhibition (Kupittayanant et al. 2001; Sward et al. 2003). It now seems unproductive to generalize about phasic muscles, as we found that rat ureter is greatly affected by Rho-kinase inhibition, but guinea-pig ureter is not. In a study of detrussor smooth muscle undergoing phasic activity, Hashitani et al. (2004) concluded, as we do, that action potential and Ca2+ entry are vital to phasic activity, but that the Rho-kinase pathway can modulate this activity. However, Hashitani et al. (2004) found no evidence for a Ca2+ dependence of their effects, nor did Y-27632 affect electrical activity. Our findings are in agreement and support the conclusions of others on arterial muscle (Sakurada et al. 2003; Urban et al. 2003) and extend them to phasic smooth muscle and physiological stimulation. A recent study of two phasic muscles, vas deferens and portal vein (Kitazawa et al. 2003), reported evidence of Ca2+ sensitization via phosphorylation of MLCP, that was independent of G-proteins and Rho-kinase.
Our data adds to and extends the evidence that there is a Ca2+-dependent Rho, Rho-kinase pathway present in some smooth muscles, that can be activated by depolarization and, as we show, by action potentials. This will be part of the mechanism, along with the Ca2+–calmodulin–MLCK pathway, that determines the characteristics of the normal phasic activity in the rat ureter but not guinea pig. The Ca2+-dependent Rho, Rho-kinase pathway targets Ca2+ entry through voltage-gated Ca2+ channels to enhance both the Ca2+ transient and force. If this pathway is inhibited, then both Ca2+ current and transient fall, and the action potential is shortened. Thus, in rat ureter, Rho-kinase plays an important role in the control of E–C coupling. The Ca2+-independent Rho, Rho-kinase pathway in rat ureter works ‘conventionally’ to prolong contraction; its inhibition reduces the duration of the phasic activity by accelerating the rate of the fall of force. Thus Rho, Rho-kinase activity without agonists, modulates myogenic phasic contraction in a species-dependent manner in intact ureteric smooth muscle, in both Ca2+-dependent and -independent mechanisms, and hence both MLCK and MLCP activity should be considered important for peristalsis in the urinary tract.
Acknowledgments
We are grateful to the MRC, Mersey Kidney Research Fund and Wellcome Trust for funding this work. We thank Dr R. Mitchell (Chicago University, USA) and Dr E. Babiychuk (Berne, Switzerland) for help with Western blotting.
References
- Asano M, Nomura Y. Comparison of inhibitory effects of Y-27632, a Rho kinase inhibitor, in strips of small and large mesenteric arteries from spontaneously hypertensive and normotensive Wistar-Kyoto rats. Hypertens Res. 2003;26:97–106. doi: 10.1291/hypres.26.97. 10.1291/hypres.26.97. [DOI] [PubMed] [Google Scholar]
- Ayman S, Wallace P, Wayman CP, Gibson A, McFadzean I. Receptor-independent activation of Rho-kinase mediated calcium sensitisation in smooth muscle. Br J Pharmacol. 2003;139:1532–1538. doi: 10.1038/sj.bjp.0705394. 10.1038/sj.bjp.0705394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga TV, Bowler E, Shabir S, Wray S. The effects of the Rho-associated kinase inhibitor Y-27632 on force and Ca2+ in ureteric muscle are species dependent. J Physiol. 2000;527.P:64–65p. [Google Scholar]
- Burdyga T, Mitchell RW, Ragozzino J, Ford LE. Force and myosin light chain phosphorylation in dog airway smooth muscle activated in different ways. Respir Physiol Neurobiol. 2003;137:141–149. doi: 10.1016/s1569-9048(03)00143-5. [DOI] [PubMed] [Google Scholar]
- Burdyga TV, Taggart MJ, Wray S. Major difference between rat and guinea-pig ureter in the ability of agonists and caffeine to release Ca2+ and influence force. J Physiol. 1995;489:327–335. doi: 10.1113/jphysiol.1995.sp021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. Simultaneous measurements of electrical activity, intracellular [Ca2+] and force in intact smooth muscle. Pflugers Arch. 1997;435:182–184. doi: 10.1007/s004240050499. [DOI] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. The relationship between the action potential, intracellular calcium and force in intact phasic, guinea-pig ureteric smooth muscle. J Physiol. 1999;520:867–883. doi: 10.1111/j.1469-7793.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. What Is the Role of the SR in Smooth Muscle. Wiley Press for the Novartis Foundation; 2002. SR function and contractile consequences in ureteric smooth muscles; pp. 208–220. [PubMed] [Google Scholar]
- Buyukafsar K, Levent A, Ark M. Expression of Rho-kinase and its functional role in the contractile activity of the mouse vas deferens. Br J Pharmacol. 2003;140:743–749. doi: 10.1038/sj.bjp.0705479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero TG, Morielli AD, Peralta EG. The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell. 1998;93:1077–1085. doi: 10.1016/s0092-8674(00)81212-x. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW. The relation between response and the interval between stimuli of the isolated guinea-pig ureter. J Physiol. 1965;180:225–238. doi: 10.1113/jphysiol.1965.sp007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson ME, Lang RJ. Effects of selective inhibitors of cyclo-oxygenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2) on the spontaneous myogenic contractions in the upper urinary tract of the guinea-pig and rat. Br J Pharmacol. 2000;129:661–670. doi: 10.1038/sj.bjp.0703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammaS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. 10.1016/S0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- Ghisdal P, Vandenberg G, Morel N. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J Physiol. 2003;551:855–867. doi: 10.1113/jphysiol.2003.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen K, Hannappel J. Normal spontaneous activity of the pyeloureteral system in the guinea-pig. Pflugers Arch. 1973;341:257–270. doi: 10.1007/BF00592794. [DOI] [PubMed] [Google Scholar]
- Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors on tonic and phasic smooth muscle. J Biol Chem. 1992;267:14662–14668. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam DC, Podolsky RJ. Force measurements in skinned muscle fibres. J Physiol. 1969;200:807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B, Matthijs G, Somlyo AV, Butler TM, Somlyo AP. Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle. J Gen Physiol. 1988;92:713–729. doi: 10.1085/jgp.92.6.713. 10.1085/jgp.92.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- Ito S, Kume H, Yamaki K, Katoh H, Honjo H, Kodama I, Hayashi H. Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by rho-kinase in tracheal smooth muscle. Am J Respir Cell Mol Biol. 2002;26:491–498. doi: 10.1165/ajrcmb.26.4.4701. [DOI] [PubMed] [Google Scholar]
- Jones SV. Role of the small GTPase Rho in modulation of the inwardly rectifying potassium channel Kir2.1. Mol Pharmacol. 2003;64:987–993. doi: 10.1124/mol.64.4.987. 10.1124/mol.64.4.987. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–889. doi: 10.1113/jphysiol.2002.029306. 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflügers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kupittayanant S, Burdyga TV, Wray S. The effects of inhibiting Rho-associated kinase on force and intracellular calcium in human myometrium. Pflugers Arch. 2001;443:112–114. doi: 10.1007/s004240100668. [DOI] [PubMed] [Google Scholar]
- Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286:H1088–1100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Hirano K, Nishimura J, Sasaki T, Kanaide H. Rho-kinase inhibitor inhibits both myosin phosphorylation-dependent and -independent enhancement of myofilament Ca2+ sensitivity in the bovine middle cerebral artery. Br J Pharmacol. 2003;140:871–880. doi: 10.1038/sj.bjp.0705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S. Calcitonin gene-related peptide (CGRP) regulates excitability and refractory period of the guinea-pig ureter. J Urol. 1994;152:520–524. doi: 10.1016/s0022-5347(17)32786-6. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Abelli L, Meli A. Visceromotor responses to calcitonin gene-related peptide (CGRP) in the rat lower urinary tract: evidence for a transmitter role in the capsaicin-sensitive nerves of the ureter. Eur J Pharmacol. 1987;143:73–82. doi: 10.1016/0014-2999(87)90736-9. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J. 2002;364:431–440. doi: 10.1042/BJ20020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, Seow C, Burdyga TV, Maass-Moreno R, Pratusevich V, Ragozzino J. Relationship between myosin light chain phosphorylation and contractile capability of canine airway smooth muscle. J Appl Physiol. 2001;90:2460–2465. doi: 10.1152/jappl.2001.90.6.2460. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Yano T, Schmidt DJ, Tokui T, Shibata M, Lifshitz LM, Kimura S, Tuft RA, Ikebe M. Rho-dependent agonist-induced spatio-temporal change in myosin phosphorylation in smooth muscle cells. J Biol Chem. 2002;277:725–734. doi: 10.1074/jbc.M108568200. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murahashi T, Fujita A, Kitazawa T. Ca2+-induced Ca2+ desensitization of myosin light chain phosphorylation and contraction in phasic smooth muscle. Mol Cell Biochem. 1999;190:91–98. [PubMed] [Google Scholar]
- Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J Physiol. 1999;516:67–74. doi: 10.1111/j.1469-7793.1999.067aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JH, You SK, Hwang MK, Ahn DS, Kwon SC, Taggart MJ, Lee YH. Inhibition of rho-associated kinase reduces MLC (20) phosphorylation and contractility of intact myometrium and attenuates agonist-induced Ca2+ sensitization of force of permeabilized rat myometrium. J Vet Med Sci. 2003;65:43–50. doi: 10.1292/jvms.65.43. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Hori M, Izumi M, Oka T, Kohama K, Ozaki H, Karaki H. Inhibition of high K+-induced contraction by the ROCKs inhibitor Y-27632 in vascular smooth muscle: possible involvement of ROCKs in a signal transduction pathway. J Pharm Sci. 2003;92:56–69. doi: 10.1254/jphs.92.56. [DOI] [PubMed] [Google Scholar]
- Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline) sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Smith RD, Borisova L, Wray S, Burdyga TV. Characterisation of the ionic currents in freshly isolated rat ureter smooth muscle cells: evidence for species-dependent currents. Pflugers Arch. 2002;445:444–453. doi: 10.1007/s00424-002-0941-7. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Storey NM, O'Bryan JP, Armstrong DL. Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr Biol. 2002;12:27–33. doi: 10.1016/s0960-9822(01)00625-x. [DOI] [PubMed] [Google Scholar]
- Sward K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitisation of myosin phosphorylation and force in guinea-pig ileum. J Physiol. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- Takizawa S, Hori M, Ozaki H, Karaki H. Effects of isoquinoline derivatives, HA1077 and H-7, on cytosolic Ca2+ level and contraction in vascular smooth muscle. Eur J Pharmacol. 1993;250:431–437. doi: 10.1016/0014-2999(93)90030-l. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;398:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol Cell Physiol. 2003;285:C1377–1385. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- Yoshi A, Izuka K, Dobashi K, Horie T, Harada T, Nakazawa T, Mori M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+-sensitization. Am J Respir Cell Mol Biol. 1999;20:1190–1200. doi: 10.1165/ajrcmb.20.6.3441. [DOI] [PubMed] [Google Scholar]